Abstract
The impact of fructose, commonly consumed with sugars by humans, on blood pressure and uric acid has yet to be defined. A total of 267 weight‐stable participants drank sugar‐sweetened milk every day for 10 weeks as part of their usual, mixed‐nutrient diet. Groups 1 and 2 had 9% estimated caloric intake from fructose or glucose, respectively, added to milk. Groups 3 and 4 had 18% of estimated caloric intake from high fructose corn syrup or sucrose, respectively, added to the milk. Blood pressure and uric acid were determined prior to and after the 10‐week intervention. There was no effect of sugar type on either blood pressure or uric acid (interaction P>.05), and a significant time effect for blood pressure was noted (P<.05). The authors conclude that 10 weeks of consumption of fructose at the 50th percentile level, whether consumed as pure fructose or with fructose‐glucose–containing sugars, does not promote hyperuricemia or increase blood pressure.
Despite decades of significant effort, important advances in science, and numerous educational campaigns, hypertension remains common and is increasing at a significant rate in the United States.1, 2 Hypertension affects more than 60 million Americans and more than 1 billion individuals worldwide.3 It is the most common, readily identifiable, and reversible risk factor for stroke, cardiovascular disease, renal disease, aortic dissection, peripheral vascular disease, and death. High blood pressure (BP) accounts for 10% of the total annual health budget in developed countries.4 The global burden of hypertension is rising and is projected to affect more than 1.5 billion persons (one third of the world's population) by the year 2025.5 Hypertension, therefore, remains one of the world's great public health problems.
Dietary factors that may increase BP are of significant interest to physicians and public health officials. While a number of dietary factors have been associated with high BP, including salt intake, saturated fat intake, and overconsumption of calories,1 recent attention has focused on intake of added sugars in general and fructose‐containing sugars in particular.6, 7
Johnson and colleagues6 have proposed a mechanism linking fructose consumption to increases in BP and other manifestations of the metabolic syndrome. According to the theory postulated by these investigators, the metabolism of fructose leads to consumption of adenosine triphosphate, which is subsequently degraded into adenosine monophosphate and ultimately uric acid. Uric acid then is postulated to lead to endothelial dysfunction, ultimately causing increases in BP. These investigators have supported this theory with both animal8, 9, 10, 11 and human data.7 Other investigators, however, have questioned the relevance of this basic mechanism and have criticized these studies in particular for using excessive amounts of fructose well beyond those normally consumed in the human diet.12, 13, 14 Furthermore, several recent systematic reviews and meta‐analyses and cohort studies have not demonstrated that fructose consumption, when substituted for other carbohydrates in isocaloric conditions, results in elevations in BP15 or uric acid.16
Elevated uric acid levels have been associated with an increased risk for developing hypertension.17, 18, 19, 20, 21, 22, 23, 24, 25 In multiple prospective observational studies, uric acid level has been demonstrated to be an independent risk factor for the development of hypertension.17, 18, 19, 20, 21, 22, 23, 24, 25
The study reported here was undertaken to explore the relationship between consumption of fructose‐containing sugars, BP, and uric acid at the normal range of consumption of fructose in the normal fructose‐containing sugars in the human diet, namely, high fructose corn syrup (HFCS) and sucrose, compared with a fructose‐only condition and glucose‐only control. We postulated that at normal levels of consumption, in the normal ways that fructose is consumed in the human diet (ie, in the presence of glucose such as in HFCS or sucrose), there would be no increases in BP or uric acid.
Materials and Methods
Study Design
This was a prospective, blinded, randomized controlled trial comparing three different fructose‐containing sugars including HFCS, sucrose, and fructose itself delivered at the 50th percentile population consumption level of fructose (18% calories from HFCS or sucrose or 9% of calories from fructose)26 to a glucose control (9% of calories). The sugars were delivered in 1% fat milk (Tetrapak, Denton, TX) as a component of the participants' usual mixed‐nutrient diet.
Both participants and staff members were blinded as to whether participants were consuming sucrose, HFCS, fructose, or glucose. Staff members were, however, aware of the amount of calories from sugar that each participant was consuming since this information was needed in order to prescribe the correct amount of milk. This research protocol was approved by the Western Institutional Review Board. Prior to enrollment, all participants were required to sign informed consent.
Participants
A total of 366 men and women between the ages of 20 and 60 years with a body mass index (BMI) between 23 kg/m2 to 35 kg/m2 were recruited and randomly divided into the following four groups: 9% of calories from fructose, 18% of calories from HFCS, 18% of calories from sucrose, and 9% of calories from glucose. A total of 267 individuals completed the 10‐week trial (27% dropout). Only data from those individuals who completed the study are reported here. Dropouts occurred for a variety of reasons including dissatisfaction with time commitment for the study, moved from area, change of job or home location, cost to follow‐up, or withdrawal from study by investigators for failure to comply with milk consumption. Dropouts did not differ from completers in any relevant parameter. This information is presented in Figure 1.
Figure 1.
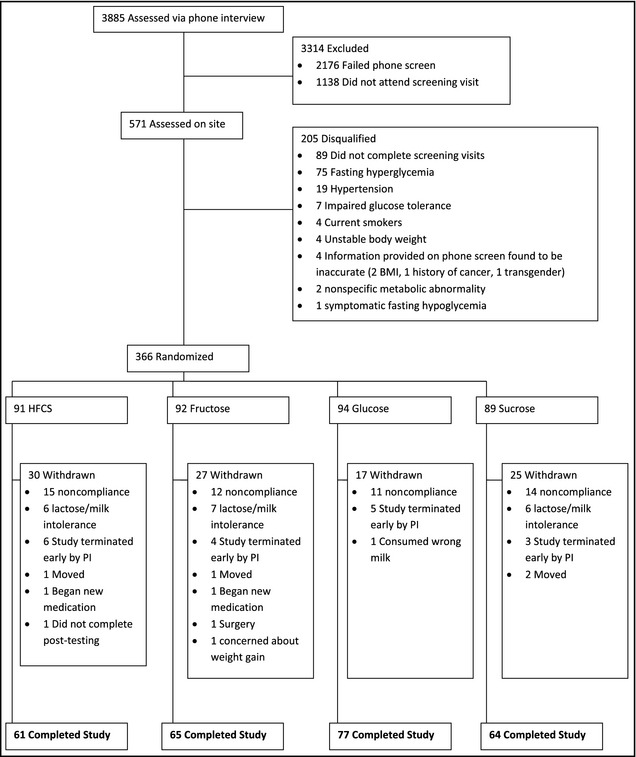
Study schematic. BMI indicates body mass index; PI, principal investigator.
Individuals could not have been currently enrolled in a commercial weight loss program or taking weight loss medication or supplements. Individuals were excluded if they had experienced a weight change >5 pounds in the prior 30 days. They could not have been using tobacco products or have utilized such products in the past year. Individuals who had thyroid disease and either stopped taking medication or had a dosage change within the prior 6 months or a history of major surgery or surgical procedure for weight loss within the 3 months prior to enrollment or a history of heart problems (eg, myocardial infarction and bypass surgery) within 3 months of enrollment were also excluded. Individuals diagnosed with type 1 or type 2 diabetes or uncontrolled hypertension (≥140/90 mm Hg) were excluded. Individuals with orthopedic limitations that would interfere with moderate regular physical activity were also excluded, as were individuals with a history of gastrointestinal disorders or diagnosed eating disorders, history or presence of cancer, or congestive heart failure. Any individuals consuming ≥14 alcoholic drinks per week were excluded, as were women who were pregnant, lactating, or trying to become pregnant. Individuals with a known allergy to sucrose, HFCS, fructose, or glucose who had participated in another clinical trial within 30 days prior to enrollment, as well as lactose intolerance or any other significant food allergy were also excluded.
Participant Screening and Measurements
Prospective participants completed a standardized screening form (online or via phone conversation) based on self‐reported data. Qualified participants reported to the clinic in a rested and fasted state for verification of eligibility. Height and weight were measured to ensure BMI was in the required range. Participants had resting BP measured using standard procedures. A fasting blood sample was collected and tested for glucose. Participants also performed a 2‐hour oral glucose tolerance test to rule out impaired glucose tolerance.
Dietary Plans
Sugar‐sweetened, low‐fat milk was given to all participants to consume daily in the context of a free‐living, nonstructured diet. The energy intake required to maintain initial body weight was estimated using the Mifflin‐St Jeor equation and applying an appropriate activity factor determined from a self‐reported physical activity questionnaire. Participants were then randomly assigned to one of the following four groups:
HFCS: 18% of weight‐maintenance calories provided by added HFCS in milk.
Fructose: 9% of weight‐maintenance calories provided by added fructose in milk.
Glucose: 9% of weight‐maintenance calories provided by added glucose in milk.
Sucrose: 18% of weight‐maintenance calories provided by added sucrose in milk.
Participants were given recommendations on how to account for the liquid calories, but were instructed to otherwise make their usual food choices. Compliance with prescribed consumption levels were assessed by self‐reported checklists that had to be turned in during weekly clinic visits, during which weight was measured and a week's worth of milk distributed. Compliance was additionally verified by analysis of 3 days of food records at the beginning and end of the protocol. Participants were withdrawn if their cumulative consumption fell below 80% of the prescribed amount for 2 weeks.
Dietary intake was measured by analysis of 3‐day food records using the Nutrient Data System for Research (NDS‐R) software (University of Minnesota, Minneapolis, MN). These were completed prior to the intervention and again during the final week of the 10‐week intervention.
Uric Acid Measurements
The fasting blood sample was also used to measure fasting uric acid. However, a subset of 78 participants (HFCS=20, fructose=20, glucose=19, sucrose=19) underwent two 24‐hour stays (prior to the intervention and again after 10 weeks) in our metabolic unit, during which the acute response of uric acid was observed. The metabolic unit procedure has been previously described.27 In short, participants were given a standardized meal at 6 pm the evening before starting the metabolic unit portion of the study. They then reported to the metabolic unit between 7 am and 8 am after an over‐night fast. An intravenous catheter was inserted and a fasting blood sample obtained. Participants were then provided with calorie‐ and macronutrient‐controlled meals (breakfast, lunch, and dinner) that contained the appropriate form and amount of sugar sweetened milk. Breakfast was provided at 9 am and additional blood samples were obtained every 30 minutes until midnight, and hourly thereafter until 8 am. Physical activity was minimized during the visit and limited to walking through the clinic to go to the bathroom or to eat meals. Uric acid was measured on fasting samples and every 2 hours thereafter. Full‐day (23‐hour) area under the curve was calculated for each visit using the trapezoidal method, and post‐prandial responses to each of the three meals were also assessed.
Data Analysis
Data were checked for normalcy and analyzed using a two‐way (group and time) analysis of variance with repeated measure (ANOVA). Only data on participants who completed the intervention were included in the analyses. Significant time by group assignment interactions were probed by using paired t tests to assess the within‐subject change for each group independently. In addition, changes over the course of 10 weeks (week 10 minus baseline) were calculated and between‐group differences assessed by one‐way ANOVA with Tukey's post hoc as necessary. For analyses, the alpha was set at 0.05. All data were analyzed using SPSS Advanced Statistics V‐18 (IBM, Armonk, NY).
Results
Demographic Data
The average age of participants was 37.7 years with a BMI slightly above the healthy weight range (average BMI=26.3±3.3). Levels of BP, cholesterol, triglycerides, and glucose were all normal at baseline. Demographic data are summarized in Table 1.
Table 1.
Baseline Characteristics of 268 Participants Who Completed the Intervention
| Total | 18% HFCS | 9% Fructose | 9% Glucose | 18% Sucrose | |
|---|---|---|---|---|---|
| Age, y | 37.66±12.11 | 36.32±10.72 | 38.65±12.19 | 36.10±12.06 | 39.83±13.19 |
| Weight, lb | 162.19±27.24 | 161.91±25.94 | 166.61±30.90 | 159.13±25.88 | 161.66±27.26 |
| BMI | 26.26±3.29 | 26.25±3.13 | 26.52±3.33 | 25.66±3.14 | 26.75±3.51 |
| SBP, mm Hg | 109.18±10.15 | 108.69±9.65 | 107.71±10.51 | 109.17±10.09 | 111.14±10.28 |
| DBP, mm Hg | 69.78±8.69 | 69.50±9.64 | 65.67±8.83 | 68.96±8.34 | 71.14±8.02 |
| Glucose, mg/dL | 90.00±6.47 | 89.44±6.48 | 90.48±7.07 | 90.66±6.14 | 89.26±6.22 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure. Participants were randomly assigned to receive 18% of calories from high fructose corn syrup (HFCS) or sucrose or 9% of calories from fructose or glucose.
Dietary Intake and Weight
There was an overall increase in energy intake across the entire study population (2011.8±708.2 kcal vs 2254.6±647.7 kcal, P<.001), but this was not different among the four groups (interaction P>.05). The increase in energy intake resulted in an average 2‐pound weight increase in the entire population (weight pre=162.2±27.3 lb pre vs 164.2±28.1 lb post; P<.001). There were no differences in weight gain among the four sugar conditions (interaction P>.05). There was also an increase in protein intake (86.2±37.6 g vs 99.4±30.9 g, P<.001), while fat intake decreased (76.8±33.2 g vs 71.2±27.8 g, P<.01). Increases in total carbohydrates, sugars, and added sugars were observed in all groups (P<.001), but increases in the two 18% groups were greater than those in the two 9% groups for total and added sugars (P<.05), and the increase in carbohydrate in the 9% groups was significantly different than the 18% HFCS group only (P<.05). These data are summarized in Table 2.
Table 2.
Changes in Energy and Macronutrient Intake over the Course of 10 Weeks of Sugar‐Sweetened Milk Consumption
| Grp | Baseline | Week 10 | Time P Value | Interaction P Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Energy intake, Kcal | HFCS | 1929.4 | 733.7 | 2390.1 | 700.7 | <.001 | .053 |
| Fructose | 2065.8 | 722.9 | 2195.4 | 581.7 | |||
| Glucose | 1978.6 | 644.3 | 2182.2 | 636.6 | |||
| Sucrose | 2069.5 | 711.6 | 2277.5 | 668.8 | |||
| Total | 2011.8 | 708.2 | 2254.6 | 647.6 | |||
| Fat, g | HFCS | 72.0 | 29.9 | 72.3 | 29.0 | .008 | .210 |
| Fructose | 76.6 | 34.4 | 71.3 | 25.1 | |||
| Glucose | 77.6 | 35.6 | 73.3 | 29.1 | |||
| Sucrose | 80.2 | 32.3 | 67.9 | 28.2 | |||
| Total | 76.8 | 33.2 | 71.2 | 27.8 | |||
| Carbohydrate, g | HFCS | 232.9 | 110.1 | 334.8a | 100.2 | <.001 | <.001 |
| Fructose | 261.0 | 96.0 | 293.9b,c,a | 89.8 | |||
| Glucose | 239.9 | 87.2 | 286.0a,c | 87.6 | |||
| Sucrose | 253.7 | 92.5 | 328.4a | 90.8 | |||
| Total | 247.0 | 96.1 | 309.1 | 93.7 | |||
| Protein, g | HFCS | 88.9 | 55.2 | 105.7 | 36.2 | <.001 | .310 |
| Fructose | 88.9 | 31.3 | 99.2 | 28.2 | |||
| Glucose | 83.5 | 31.4 | 100.5 | 31.8 | |||
| Sucrose | 84.5 | 30.5 | 92.7 | 26.3 | |||
| Total | 86.2 | 37.6 | 99.4 | 30.9 | |||
| Total sugar, g | HFCS | 90.7 | 56.0 | 203.0a | 56.9 | <.001 | <.001 |
| Fructose | 113.8 | 50.9 | 171.6a,c,d | 63.6 | |||
| Glucose | 104.2 | 43.3 | 160.7a,c,d | 51.2 | |||
| Sucrose | 102.7 | 49.2 | 203.4a | 53.7 | |||
| Total | 103.3 | 52.7 | 183.2 | 59.2 | |||
| Added sugar, g | HFCS | 53.9 | 37.4 | 129.9a | 41.3 | <.001 | <.001 |
| Fructose | 68.8 | 40.3 | 95.5a,c,d | 39.3 | |||
| Glucose | 61.3 | 43.1 | 90.2a,c,d | 35.5 | |||
| Sucrose | 60.6 | 44.1 | 132.3a | 42.7 | |||
| Total | 61.3 | 41.5 | 110.5 | 43.9 | |||
Abbreviation: SD, standard deviation. aSignificant change within group (P<.001). bSignificant change within group (P<.01). cChange (post‐pre) significantly different than in the high fructose corn syrup (HFCS) group (P<.05). dChange (post‐pre) significantly different than in HFCS and sucrose groups.
Fasting and Resting Measurements
Resting BP decreased for the entire cohort of 268 milk drinkers (systolic 109.2±10.2 mm Hg vs 106.1±10.6 mm Hg [P<.001], diastolic: 69.8±8.7 mm Hg vs 68.1±9.7 mm Hg [P<.01]), but sugar type had no effect on this change for either measure (interaction P>.05). Fasting uric acid was unchanged over the course of 10 weeks (4.97±1.42 mg/dL vs 4.92±1.33 mg/dL, P>.05), and was also unaffected by the type of sugar consumed (interaction P>.05). These data are presented in Table 3.
Table 3.
Changes in Resting Blood Pressure and Fasting Uric Acid After 10 Weeks of Sugar‐Sweetened Milk Consumption
| Group | Baseline | Week 10 | Time P Value | Interaction P Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Systolic blood pressure, mm Hg | HFCS | 108.61 | 9.70 | 107.57 | 11.29 | <.001 | .081 |
| Fructose | 107.71 | 10.51 | 105.49 | 9.97 | |||
| Glucose | 109.17 | 10.09 | 104.60 | 9.78 | |||
| Sucrose | 111.14 | 10.28 | 107.25 | 10.55 | |||
| Total | 109.16 | 10.17 | 106.13 | 10.39 | |||
| Diastolic blood pressure, mm Hg | HFCS | 69.48 | 9.71 | 68.90 | 10.78 | .003 | .510 |
| Fructose | 69.68 | 8.83 | 67.82 | 8.91 | |||
| Sucrose | 68.96 | 8.34 | 66.12 | 9.35 | |||
| Glucose | 71.14 | 8.02 | 69.81 | 9.48 | |||
| Total | 69.78 | 8.70 | 68.05 | 9.68 | |||
| Uric acid, mg/dL | HFCS | 4.84 | 1.37 | 4.91 | 1.39 | .375 | .562 |
| Fructose | 5.08 | 1.37 | 5.02 | 1.33 | |||
| Sucrose | 5.06 | 1.48 | 4.94 | 1.38 | |||
| Glucose | 4.88 | 1.45 | 4.83 | 1.25 | |||
| Total | 4.97 | 1.42 | 4.92 | 1.33 | |||
Abbreviations: HFCS, high fructose corn syrup; SD, standard deviation. Blood pressure was measured in the right arm sitting after 5 minutes of quiet rest using standard techniques.
Metabolic Unit – Uric Acid
In the metabolic ward subset, 24‐hour AUC for uric acid was not different after 10 weeks of sugar‐sweetened milk consumption (93.6±25.8 h*mg/dL vs 96.1±29.1 h*mg/dL, P>.05). For all measures there was no effect of sugar type (interaction effects, P>.05). These data are presented in Figure 2.
Figure 2.
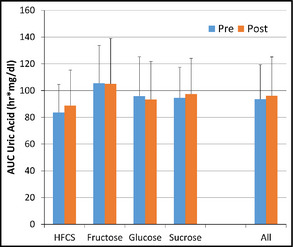
Entire day (23‐hour) area under the curve (AUC) for uric acid under standardized feeding conditions. HFCS indicates high fructose corn syrup.
Discussion
The results of this study confirmed our hypothesis that fructose‐containing sugars, when consumed over a 10‐week period at average consumption levels (50th percentile population consumption level for fructose),26 in comparison to glucose control did not result in increased BP or increases in uric acid.
Our findings that fructose‐containing sugars at the average population consumption level for fructose do not increase BP are consistent with the systematic review and meta‐analysis published by Ha and colleagues,15 which analyzed 13 isocaloric and two hypercaloric trials related to fructose on BP. These investigators found that fructose intake in isocaloric exchange for other carbohydrates significantly decreased diastolic BP and mean arterial pressure and did not significantly affect systolic BP. In the hypercaloric‐controlled feeding trials reported by Ha and colleagues there was, once again, no significant effect of fructose on overall mean arterial BP comparison with other carbohydrates.
Our findings also mirror those of Forman and coworkers,28 who prospectively investigated fructose consumption and the risk of developing hypertension in three large cohort studies: the Nurses' Health Study 1 (NHS1), the Nurses' Health Study 2 (NHS2), and the Health Professionals Follow‐up Study (HPFS), which together included >200,000 participants. These investigators found that there was no correlation between fructose consumption and risk of developing high BP.
Our findings are also consistent with reported findings from other research groups that did not find a relationship between fructose‐containing sugars and increases in uric acid at normal population consumption levels of fructose. Sun and colleagues29 performed logistic regression analysis utilizing US National Health and Nutrition Examination Survey (NHANES) data from a total of 9,381 patients between the ages of 20 and 80 years without diabetes, cancer, or heart disease and found that dietary fructose intake was not associated with increased hyperuricemia risk. Alcohol intake was significantly associated with hyperuricemia and increased fiber intake was significantly associated with decreased hyperuricemia risk. These same investigators analyzed a larger NHANES database from 1999 to 2006, which included 25,506 patients aged 12 to 80 years and found that fructose consumption was not associated with uric acid or indicators of the metabolic syndrome.30 Wang and colleagues16 performed a systematic review and meta‐analysis of controlled feeding trials and found that when fructose was substituted isocalorically for other carbohydrates, it was not associated with increased risk of elevated uric acid.
Some,31, 32, 33 but not all,34 epidemiologic studies have suggested that high levels of intake of added sugars may contribute to elevations in BP. In particular, some epidemiologic studies have suggested that the consumption of sugar‐sweetened beverages may result in increases in BP.32, 33 Whether this association is related to fructose in these beverages is uncertain. The Framingham Study data have demonstrated an association between consumption of carbonated soft drinks and increased odds for developing high BP.33 Stanhope and colleagues35 did not report adverse effects on BP from consumption of 25% of energy from pure fructose or pure glucose. Our current study suggests that fructose‐containing sugars, when consumed at average levels (50th percentile population consumption of fructose) compared with glucose control do not result in increases in BP.
Elevated uric acid levels have been independently associated with increased risk of developing hypertension.17, 18, 19, 20, 21, 22, 23, 24, 25 This association has been examined in 14 studies and all but two have documented it. Furthermore, uric acid has been associated with endothelial dysfunction in rat models.36 As already indicated, the theory by Johnson and associates6, 7 is that uric acid may cause endothelial dysfunction that contributes to hypertension. Some investigators have suggested that this model relating uric acid to endothelial dysfunction may not be the correct pathophysiologic model of development of high BP in human beings.28, 37 Forman and colleagues28 have argued that uric acid is a potent antioxidant and this attribute of uric acid may counteract any tendency for uric acid to create endothelial dysfunction in humans. Waring and coworkers38 reported that uric acid restores endothelial function in patients with type 1 diabetes and smokers. Thus, uric acid may not cause endothelial dysfunction in humans in contrast to rodents. Indeed, uric acid infusion in smokers and patients with type 1 diabetes has been demonstrated to improve endothelial dysfunction in humans, despite a two‐fold increase in serum uric acid concentration.38
Study Strengths and Limitations
The strengths of the current study involve the fact that it was the first blinded, randomized, controlled trial with a large sample size to our knowledge that explored normal population consumption levels of fructose compared with glucose. Weaknesses include that patients were followed for only 10 weeks and that those older than 60 years, children, and adolescents were excluded. Adolescents represent the single, highest fructose‐consuming group in the United States.26 In addition, office BP may not reflect true BP and 24‐hour ambulatory BP was not obtained, which should be taken into account when evaluating our results. Our study reports only BP response to average levels of fructose‐containing sugars. Responses to higher levels of sugars cannot be determined from this study. This level was chosen because of concern that higher levels of sugar consumption might lead to weight gain and potentially confound BP results. Even at the 50th percentile population consumption level of fructose, an average weight gain of 2 pounds occurred during the 10‐week trial across the entire population studied. We have reported elsewhere, however, similar results on cardiovascular risk factors from individuals consuming up to the 90th percentile population consumption level of fructose.39 We must also acknowledge that the interpretation of the metabolic unit uric acid data should be treated with more caution because of the much smaller sample size.
It should also be noted that all of the sugars in this study were delivered in 1% low‐fat milk. This may represent a potential confounder since the calcium or proteins40, 41 found in milk may be associated with decreased BP.
Finally, it should be noted that HFCS, sucrose, glucose, and fructose could not be measured directly in the diets, so the actual differences in intake of these two sugars remain unknown, which should also be taken into consideration when interpreting these data.
Conclusions
The findings from this randomized controlled trial suggest that fructose‐containing sugars do not raise BP or uric acid at average levels of human consumption. This study adds to the expanding literature that at normal human consumption levels of fructose‐containing sugars there are no increases in either BP or uric acid.
Disclosures
J. M. Rippe's research laboratory has received unrestricted grants and Dr Rippe has received consulting fees from ConAgra Foods, Kraft Foods, the Florida Department of Citrus, PepsiCo International, Coca Cola, the Corn Refiners Association, Weight Watchers International, and various publishers. TJ Angelopoulos, J Lowndes, and S Sinnett report no specific funding in relation to this research and no conflicts to disclose. This trial has been registered with the NIH Clinical Trial Registry: NCT01797042.
J Clin Hypertens (Greenwich). 2015;17:87–94. DOI: 10.1111/jch.12457. © 2014 Wiley Periodicals, Inc.
References
- 1. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 Report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Victor RG, Kaplan NM. Systemic hypertension: mechanisms and diagnosis. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald's Heart Disease, 9th ed. Philadelphia, PA: Elsevier; 2012:935–953. [Google Scholar]
- 4. Lawes CM, Van der Hoorn S, Law MR, et al. Blood pressure and the global burden of disease 2000. Part II: estimates of attributable burden. J Hypertens. 2006;24:423–430. [DOI] [PubMed] [Google Scholar]
- 5. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 6. Johnson RJ, Perez‐Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson R, Segal M, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. [DOI] [PubMed] [Google Scholar]
- 8. Catena C, Cavarape A, Novello M, et al. Insulin receptors and renal sodium handling in hypertensive fructose‐fed rats. Kidney Int. 2003;64:2163–2171. [DOI] [PubMed] [Google Scholar]
- 9. Dai S, McNeill JH. Fructose‐induced hypertension in rats is concentration‐ and duration‐dependent. J Pharmacol Toxicol Methods. 1995;33:101–107. [DOI] [PubMed] [Google Scholar]
- 10. Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose‐induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. [DOI] [PubMed] [Google Scholar]
- 11. Vasdev S, Prabhakaran VM, Whelan M, et al. Fructose‐induced hypertension, hypertriglyceridemia and elevated cytosolic calcium in rats: prevention by deuterium oxide. Artery. 1994;21:124–147. [PubMed] [Google Scholar]
- 12. Sievenpiper JL, de Souza RJ, Kendall CW, Jenkins DJ. Is fructose a story of mice but not men? J Am Diet Assoc. 2011;111:219–220; author reply 220–212. [DOI] [PubMed] [Google Scholar]
- 13. White JS. Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Adv Nutr. 2013;4:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rippe JM, Angelopoulos TJ. Sucrose, high‐fructose corn syrup, and fructose, their metabolism and potential health effects: what do we really know? Adv Nutr. 2013;4:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ha V, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on blood pressure a systematic review and meta‐analysis of controlled feeding trials. Hypertension. 2012;59:787–795. [DOI] [PubMed] [Google Scholar]
- 16. Wang DD, Sievenpiper JL, de Souza RJ, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr. 2012;142:916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alper AB Jr, Chen W, Yau L, et al. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34–38. [DOI] [PubMed] [Google Scholar]
- 18. Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. 2007;18:287–292. [DOI] [PubMed] [Google Scholar]
- 19. Forman Hunt SC, Stephenson SH, Hopkins PN, Williams RR. Predictors of an increased risk of future hypertension in Utah: a screening analysis. Hypertension. 1991;17:969–976. [DOI] [PubMed] [Google Scholar]
- 20. Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49:298–303. [DOI] [PubMed] [Google Scholar]
- 21. Masuo K, Kawaguchi H, Mikami H, et al. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. [DOI] [PubMed] [Google Scholar]
- 22. Mellen PB, Bleyer AJ, Erlinger TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48:1037–1042. [DOI] [PubMed] [Google Scholar]
- 23. Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48:1031–1036. [DOI] [PubMed] [Google Scholar]
- 24. Shankar A, Klein R, Klein BE, Nieto FJ. The association between serum uric acid level and long‐term incidence of hypertension: population‐based cohort study. J Hum Hypertens. 2006;20:937–945. [DOI] [PubMed] [Google Scholar]
- 25. Sundstrom J, Sullivan L, D'Agostino RB, et al. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. [DOI] [PubMed] [Google Scholar]
- 26. Marriott B, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:1228S–1235S. [DOI] [PubMed] [Google Scholar]
- 27. Yu Z, Lowndes J, Rippe J. High fructose corn syrup and sucrose have equivalent effects on energy regulating hormones at normal human consumption levels. Nutr Res. 2013;33:1043–1052. [DOI] [PubMed] [Google Scholar]
- 28. Forman JP, Choi H, Curhan GC. Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol. 2009;20:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun SZ, Flickinger BD, Williamson‐Hughes PS, Empie MW. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab. 2010;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun SZ, Anderson GH, Flickinger BD, et al. Fructose and non‐fructose sugar intakes in the US population and their associations with indicators of metabolic syndrome. Food Chem Toxicol. 2011;49:2875–2882. [DOI] [PubMed] [Google Scholar]
- 31. Feig D, Soletsky B, Johnson R. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension. JAMA. 2008;300:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen S, Choi HK, Lustig RH, Hsu C‐Y. Sugar‐sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle‐aged adults in the community [published correction appears in Circ. 2007;116:e557]. Circulation. 2007;116:480–488. [DOI] [PubMed] [Google Scholar]
- 34. van der Schaaf MR, Koomans HA, Joles JA. Dietary sucrose does not increase twenty‐four‐hour ambulatory blood pressure in patients with either essential hypertension or polycystic kidney disease. J Hypertens. 1999;17:453–454. [DOI] [PubMed] [Google Scholar]
- 35. Stanhope K, Schwarz J, Keim N, et al. Consuming fructose‐sweetened, not glucose‐sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kholsa UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. [DOI] [PubMed] [Google Scholar]
- 37. Waring WS, Adwani SH, Breukels O, et al. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart. 2004;90:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waring WS, McKnight JA, Webb DJ, Maxwell SRJ. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55:3127–3132. [DOI] [PubMed] [Google Scholar]
- 39. Lowndes J, Sinnett S, Yu Z, Rippe J. The effects of fructose‐containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients. 2014;6:3153–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dickinson HO, Mason JM, Nicolason DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–233. [DOI] [PubMed] [Google Scholar]
- 41. van Mierlo LA, Arends LR, Streppel MT, et al. Blood pressure response to calcium supplementation: a meta‐analysis of randomized controlled trials. J Hum Hypertens. 2006;20:571–580. [DOI] [PubMed] [Google Scholar]


