Abstract
The aim of this study was to estimate the prevalence and correlates of blood pressure (BP) according to somatic maturation in Southern Brazilian adolescents. A total of 1321 adolescents participated in the study (732 girls), aged between 10 and 16 years, enrolled in public schools. The assessment of BP was performed using oscillometric equipment. Measurements of body weight, height, waist circumference, and skinfold thickness were performed. Somatic maturation was estimated by the age at peak height velocity. Behavioral and hereditary variables were obtained using a questionnaire. Early‐maturing adolescents had the highest prevalence of high BP (28%; 95% confidence interval, 24.6–33.5) compared with other maturational groups (P=.003). In late‐maturing adolescents, the variables associated with BP were paternal hypertension (systolic BP: β=4.9; diastolic BP: β=5.3) and early physical activity (systolic BP: β=−4.0; diastolic DBP: β=−3.6). In average‐maturing adolescents, waist circumference (systolic BP: β=0.3), body mass index (diastolic BP: β=0.5), and mother's hypertension (diastolic BP: β=1.8) were positively related to BP. In early‐maturing adolescents, only waist circumference (systolic BP: β=0.3; diastolic BP: β=0.3) was associated with BP. The authors conclude that the prevalence of high BP is greater in adolescents with early maturity and the outcome appears to be related to biological indicators in this group. On the other hand, in late‐maturing adolescents, behavioral and hereditary variables are more related to BP.
Since the epidemiological transition, chronic diseases have been responsible for most cases of morbidity and mortality worldwide.1 Among the principle risk factors for these diseases, high blood pressure (BP) has been highlighted. This condition is characterized by consistent BP levels above the borderline range. According to the World Health Organization,2 9.4 million deaths worldwide are caused by high BP (HBP). However, although its main consequences are observed in adulthood,3 its etiology seems to have an early origin in childhood and adolescence.4 Thus, identifying the factors associated with BP in young populations could help in creating strategies to reduce the complications of hypertension in the medium and long term.
Biological, social, and behavioral variables have been related to BP in adolescence.5, 6 Even the growth and development processes, which are features of this stage of life, are linked to biological changes that can influence BP.7 Among these changes, a natural increase in BP values with enhancement in chronological age is related to body size, represented by increases in weight, height, and changes in body composition.8 However, young people of the same chronological age may present variation in relation to biological maturity status, which is a process characterized by changes in several human dimensions. This is consistently associated with BP,9 and different responses are presented when considering this outcome.
Therefore, biological maturation seems not to be simply an associated factor, but also a mediator of the relationship between biological and behavioral factors and BP. Despite previous investigations having adopted crude or adjusted models of biological maturation,10, 11 they have not elucidated the possible indirect interactions between biological variation and maturity. Thus, the stratification of biological maturation (early, on time, or late) could be an important factor for understanding the associations related to the pubertal process. Based on this information, the aim of this study was to analyze the prevalence and factors associated with BP according to the maturation status in Brazilian adolescents.
Methods
Sample
This cross‐sectional school‐based study was conducted in adolescents aged between 10 and 16 years enrolled in public schools in Londrina, Brazil. Located in the north of Paraná state, Londrina has 506,701 inhabitants and a Human Development Index and Gross Domestic Product of 0.824 and US$ 4,442,229.50, respectively.12
The sampling process was conducted in two stages: first, all public schools were separated into geographical locations (north, south, east, west, and center), after which two schools were randomly selected per region. Subsequently, classes were randomly selected from the previously selected schools and all students in these classes were invited to participate. Students who used prescription medication or were taking treatment for chronic diseases were excluded from the sample, as were those who did not return the consent form signed by their parents. All the study processes were approved by the local ethics research committee, according to the Declaration of Helsinki guidance for research involving humans.
The present study forms part of a larger project, in which the sample size was calculated according to the prevalence of the metabolic syndrome of 4%, α=0.05, with a margin of error of 2 percentage points, a design effect of 2.0, and an addition of 20% for any possible losses.
Blood Pressure
BP measurements were performed on the right arm, with a suitably sized cuff after a rest period of 10 minutes; the results were assessed using Omron HEM‐742 automatic equipment (Omron Healthcare, Inc, Lake Forest, IL), which had been previously validated for adolescents.13 Two measurements were realized with a 2‐minute interval between them and a third measurement when the difference between the first two was >10 mm Hg for both systolic BP (SBP) and diastolic BP (DBP). In this case, the two most proximal measurements were considered and the final BP was calculated from the arithmetic mean of both measurements using cutoffs from the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents14 according to sex and age groups.
Information on the parents was obtained through a self‐reported questionnaire given to the adolescents, which delivered to parents that related diagnoses of arterial hypertension dichotomous (yes or no).
Anthropometry
Body mass index (BMI) was obtained from weight and height measurements. Waist circumference was measured according to the recommendations of Katzmarzyk and colleagues15 using an anthropometric tape with a precision of 0.1 cm. The subscapular and tricipital skinfold thicknesses were measured by an experienced evaluator with a Lange caliper (Beta Technology, Santa Cruz, CA) properly calibrated (precision=0.5 mm), according to recommendations of Harrison and colleagues.16 For the quality control of measurements, the technical error of measurement (TEM) was calculated from a pilot project that measured 90 adolescents who did not compose the sample of the present study; however, their characteristics were similar with regard to socioeconomic status, race, somatic maturation, and body fat. The TEMs were between 4.8% and 3.5% for subscapular and tricipital skinfolds, respectively. From this information, the percentage of body fat was calculated according to the equations proposed by Boileau and colleagues.17
Somatic Maturity
Somatic maturity was estimated by the age at peak height velocity (APHV) proposed by Mirwald and colleagues.18 From the information on height, trunk‐head height, and length of the legs, the time to APHV was calculated. In addition, the chronological age was subtracted from the time to APHV for the classification of maturity status categories in both boys (late: >14.8 years; on time: 14.8–13.4 years; early: <13.4 years) and girls (late: >13.0 years; on time: 13.0–11.8 years; early: <11.8 years).
Behavioral Variables
The Baecke questionnaire19 was applied and the score proposed by the authors adopted as an indicator of physical activity. This questionnaire contemplates questions related to physical activity at school and formal sports in leisure time. The sum of these domains composed the indicator of habitual physical activity. For the quality control of this variable, the application was repeated on a representative portion of the total sample (10%) with an interval of 7 days, and the intraclass correlation coefficient (ICC) was 0.73. Adolescents were considered active who reported engagement in moderate to vigorous sporting activities for at least 240 min/wk in the previous 4 months. Moreover, experience of early sporting activities was obtained through the dichotomous indication of supervised practice for at least 1 year between the ages of 7 and 10 years (ICC=0.87).
The screen time (ICC=0.77) in minutes per week and on the weekend was adopted as an indicator of sedentary behavior. From the daily weighted mean, sedentary behavior was considered as more than 4 hours per day spent watching television. Information on alcohol consumption (ICC=0.74) and tobacco smoking (ICC=0.78) was obtained in a dichotomous manner through indication of use in the previous 30 days.
Socioeconomic Status
Socioeconomic status was assessed by means of the Brazilian Criterion for Economic Classification instrument,19 which considers the education of the household leader and certain possessions (televisions, radio, washing machine, number of bathrooms in the house, owning a car or not), providing a score based on this information. From information reported by the parents, it was possible to categorize the participants into classes (A1, A2, B1, B2, C, D, and E). Classification in classes C, D, and E was considered as low socioeconomic status.
Statistical Analyses
Mean, standard deviation, and relative frequency were adopted for categorization of the sample. Analysis of covariance (adjusted by chronological age and sex) and chi‐square test were used to compare the maturity status between the groups. For the verification of the crude relationship between the independent variables and SBP and DBP, we used partial correlation (adjusted by sex). Subsequently, multivariate linear regression was performed to verify the relationship between the independent variables and the outcome. All the analyses were processed using SPSS software 20.0 (IBM, Armonk, NY) with a significance level of 5%.
Results
Of the 1395 assessed patients, 74 did not return the consent form signed by their parents or did not complete all the data for this study and were excluded. Thus, the final sample was composed of 1321 adolescents. The study included 589 boys (late: 15.3%; on time: 71.3%; early: 13.4%) and 732 girls (late: 14.9%; on time: 70.9; early: 14.2%).
Table 1 shows the sample characteristics according to the maturity group. A linear relationship was observed between the presented indicators of biological risk (BMI, waist circumference, body fat percentage, and BP), in which early‐ and late‐maturing adolescents represented the upper and lower extremes, respectively (P<.05). On the other hand, in the late group, a higher prevalence of alcohol and tobacco consumption was observed. Regarding the prevalence of HBP (Figure), the early group presented higher values than the late and on time groups (P=.03).
Table 1.
Sample Characteristics According to Maturity Status
| Late (199) | On Time (939) | Early (183) | |
|---|---|---|---|
| Age | 14.5±0.1 | 12.8±0.4 | 12.0±0.1 |
| Height | 144.6±0.5a | 155.6±0.2 | 164.9±0.4 |
| BMI, kg/m2 | 17.5±0.3a | 19.5±0.1 | 24.1±0.3 |
| WC, cm | 60.6±0.6a | 65.9±0.2 | 76.3±0.5 |
| Body fat percentage | 18.7±0.5a | 21.7±0.2 | 27.9±0.5 |
| Systolic BP, mm Hg | 106.5±0.8a | 110.1±0.3 | 117.6±0.8 |
| Diastolic BP, mm Hg | 61.1±0.6a | 63.0±0.3 | 66.4±0.6 |
| Lowest SES | 72.7% (65.2–79.2) | 65.6% (62.7–68.9) | 67.4% (59.3–74.6) |
| Physically inactive | 77.5% (71.0–82.9) | 81.3% (78.7–83.7) | 78.3% (71.7–83.8) |
| Sedentary behavior | 44.5% (37.5–51.8) | 49.0% (45.8–52.3) | 49.4% (42.1–56.8) |
| Alcohol use | 24.9% (19.2–31.5) | 17.4% (15.1–20.0) | 12.8% (8.7–18.6) |
| Current smoker | 15.3% (10.9–21.2) | 6.8% (5.4–8.7) | 4.5% (2.1–8.7) |
| Previous PA | 63.0% (55.9–69.5) | 67.5% (64.4–70.5) | 64.8% (57.6–71.4) |
| HBP in mother | 20.4% (14.6–27.7) | 20.1% (17.2–23.2) | 16.9% (11.4–24.4) |
| HBP in father | 17.4% (11.6–25.2) | 14.7% (12.0–17.9) | 18.4% (12.1–26.7) |
Abbreviations: BMI, body mass index; BP, blood pressure; HBP, high blood pressure; PA, physical activity; SES, socioeconomic status; WC, waist circumference. aDifferences between all groups, adjusted by sex and chronological age. Values are expressed as mean±standard error or prevalence (95% confidence interval).
Figure 1.
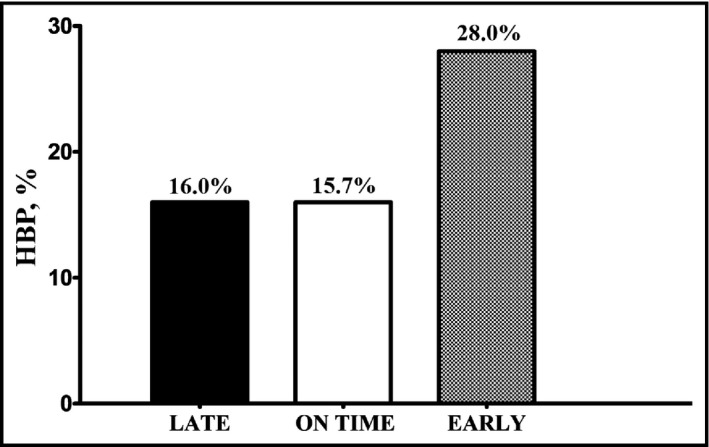
Prevalence of high blood pressure (HBP) according to maturity categories (P for trend, .003).
In the models for SBP and DBP, adjusted by biological maturation, but without separation by maturity status (early, on time, and late), the final models were composed by chronological age (β=2.737 [2.163–3.311]), waist circumference (β=0.195 [0.103–0.287]), and mother's hypertension (β=1.769 [0.200–3.338]) for SBP. In the same way, for DBP, the variables that composed the final model were waist circumference (β=0.215 [0.147–0.283]), height (β=0.096 [0.041–0.151]), habitual physical activity (β=−0.647 [−1.016 to −0.277]), and mother's hypertension (β=1.846 [0.595–3.096]).
Stratifying by maturity categories, the partial correlations between the independent variables and systolic and diastolic BP are presented in Table 2. Regardless of maturity status, the chronological age, height, BMI, and waist circumference were positively correlated with systolic and diastolic BP.
Table 2.
Partial Correlations Between Independent Variables and Blood Pressure According to Maturity Category
| Late (n=199) | On Time (n=939) | Early (n=183) | ||||
|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | SBP | DBP | |
| Age | 0.309 | 0.217 | 0.380 | 0.210 | 0.316 | 0.214 |
| Height | 0.296 | 0.254 | 0.391 | 0.201 | 0.298 | 0.153 |
| Socioeconomic status | 0.059 | 0.113 | −0.021 | −0.002 | 0.061 | −0.054 |
| Previous PA | −0.090 | −0.132 | −0.040 | −0.030 | −0.062 | −0.065 |
| Physical activity | −0.078 | −0.142 | −0.051 | −0.087 | −0.013 | −0.117 |
| Sedentary behavior | 0.079 | −0.022 | 0.015 | 0.045 | −0.101 | −0.008 |
| Alcohol consumption | 0.097 | −0.039 | 0.042 | −0.028 | −0.064 | −0.057 |
| Tobacco use | −0.028 | −0.040 | −0.021 | −0.016 | 0.010 | 0.013 |
| HBP in mother | 0.152 | 0.149 | 0.076 | 0.101 | −0.057 | 0.139 |
| HBP in father | 0.214 | 0.281 | 0.055 | 0.049 | −0.045 | 0.033 |
| Body mass index | 0.208 | 0.177 | 0.310 | 0.266 | 0.329 | 0.398 |
| Waist circumference | 0.229 | 0.206 | 0.336 | 0.259 | 0.341 | 0.418 |
| Body fat percentage | 0.078 | 0.195 | 0.159 | 0.197 | 0.142 | 0.289 |
Abbreviations: DBP, diastolic blood pressure; HBP, high blood pressure; PA, physical activity; SBP, systolic blood pressure. Bold values indicate P<.05. All models adjusted by sex.
After this stage, two multivariate models (systolic and diastolic) were created for each maturity category (Table 3). In late‐maturing adolescents, SBP was associated with paternal history of hypertension, physical activity in childhood, and age, while DBP was associated with paternal history of hypertension, physical activity in childhood, and height. As for the on‐time maturation group of adolescents, waist circumference (SBP), age, height, BMI, and maternal HBP (DBP) composed the models. In the adolescents with early‐onset puberty, only age and waist circumference explained both models. In the models, with the exception of physical activity in childhood, all the coefficients were positive. The highest coefficients of determination were found in DBP of adolescents who matured late and early and in SBP of adolescents who matured on time.
Table 3.
Regression Models to Predict Systolic and Diastolic BPs According to Maturity Category in Adolescents
| β | 95% CI | P Value | Adjusted R 2 | |
|---|---|---|---|---|
| Late | ||||
| Systolic BP | ||||
| Age | 2.103 | 0.948–3.258 | <.001 | 0.167 |
| High BP in father | 4.912 | 0.370–9.454 | .034 | |
| Previous physical activity | −3.991 | −7.655 to −0.327 | .033 | |
| Diastolic BP | ||||
| Height | 0.295 | 0.153–4.111 | <.001 | 0.207 |
| High BP in father | 5.295 | 1.811–8.778 | .003 | |
| Previous physical activity | −3.571 | −6.375 to −0.766 | .013 | |
| On time | ||||
| Systolic BP | ||||
| Age | 0.927 | 0.061–1.793 | .036 | 0.196 |
| Height | 0.234 | 0.120–0.348 | <.001 | |
| Waist circumference | 0.276 | 0.182–0.369 | <.001 | |
| Diastolic BP | ||||
| Height | 0.106 | 0.046–0.166 | .001 | 0.104 |
| Body mass index | 0.478 | 0.318–0.637 | <.001 | |
| High BP in mother | 1.765 | 0.358–3.171 | .014 | |
| Early | ||||
| Systolic BP | ||||
| Age | 2.797 | 1.400–4.193 | <.001 | 0.186 |
| Waist circumference | 0.323 | 0.178–0.469 | <.001 | |
| Diastolic BP | ||||
| Age | 1.054 | 0.132–1.976 | .025 | 0.196 |
| Waist circumference | 0.283 | 0.187–0.379 | <.001 | |
Abbreviations: BP, blood pressure; CI, confidence interval. All models adjusted by sex.
Discussion
The main findings of this paper demonstrate that the prevalence of HBP as well as other risk factors (BMI, waist circumference, and body fat) are higher in adolescents with early maturation and that the variables associated with systolic and diastolic BP differ according to maturity category. Considering the control for sex in the multivariate analyses, behavioral (infant physical activity) and hereditary (father's hypertension) variables were related to BP in late‐maturing adolescents, while only one biological variable (waist circumference) was related to the outcome in early‐maturing adolescents. In the adolescents who matured on time, waist circumference was related to SBP and BMI and mother's hypertension was related to DBP.
Because of the high prevalence of HBP in adolescents and its relationship with other damaging health outcomes in the medium and long term,20 studies have been performed to identify the factors related to BP in young people, with the aim of forming strategies for the prevention and intervention of this problem.14 The majority of studies have associated the variables in a crude manner, or with statistically adjusted models.10, 11 However, in the present study, we observed that somatic maturity could have an important mediating role in these relationships. Therefore, not only the adjustment but also the stratification of the statistical models could facilitate the practical application of the information.
Furthermore, it is necessary to highlight that the factors associated with BP may not be similar between the different maturity stages (early, on time, and late). The biological variability of the path toward maturity and the relationship between organic systems have been the subject of studies for some time.7 This variability has been associated with health indicators during adolescence and also, indirectly, with outcomes in adulthood.21 In addition to the biological explanation, which is the most recurrent, the maturation process also seems to influence social, behavioral, and emotional aspects, principally through the establishment of self‐conscience and self‐esteem.22 Thus, the principle factors associated with BP in adolescents, either biological or behavioral, interact with biological maturation.
Another relevant aspect that should be considered is the maturity indicator adopted. This can vary according to the studied biological system (nervous, sexual, somatic, and skeletal) and with their characteristic progress toward the maturity status (timing or tempo). In the present study, we opted for the age at peak height velocity (somatic maturity),18 since it is an accepted objective method, composed of simple measures to enable classification of maturity status.
To our knowledge, this is the first study to investigate the factors associated with BP according to somatic maturation, a fact that prevents, in part, comparison with the literature. Nevertheless, previous studies have pointed out the importance of some of the variables highlighted in the present study, although in a generalized form. The variable most related to BP in all stages of maturity was chronological age. In a recent study performed with more than 4000 healthy individuals aged between 20 and 75 years, it was observed that advances in age were associated with increased arterial hardness and BP.23
The parental history of hypertension is consistently evidenced as an important risk factor for HBP in adolescence.24, 25 The relationship between these variables has frequently been interpreted by means of genetic and environmental approaches. In the present study, we tried to control the possible environmental confounding factors and observed that parental history related directly with BP, especially in adolescents who experienced late or on‐time maturation.
Concerning the practice of physical activity during childhood (between the ages of 7 and 10 years), it was observed that, in late adolescents, those who reported the practice of physical activity during childhood presented lower values of BP in adolescence, independent of other variables. Considering that the current habitual physical activity level (adolescence) was not associated with BP, these results indicate a possible direct relationship, without necessarily being explained by the tracking of physical activity.26, 27 One possible physiological response, which could be expressed over time, is the fact that physical exercise contributes to the liberation of substances that relax the blood vessels (nitric oxide being one of these substances28), increasing their caliber and subsequently contributing to better blood flow. This, in turn, allows protection against arterial hypertension in adulthood.
With regard to the biological variables related to BP, both BMI and waist circumference have been considered the main factors associated with this outcome.29, 30, 31 Nevertheless, the results of the present study observed this only in adolescents who matured on time or early, going against the findings in generalized samples, principally because the proportion of late‐maturing adolescents was small in relation to the total adolescents who matured early or on time. Furthermore, early maturation seems to be associated with a larger deposition of body fat, a fact that could contribute to the tracking of obesity32 and subsequently to higher chances of developing hypertension in the future.
The indicator of central obesity (waist circumference) was demonstrated to be more associated with BP when compared with other indicators of total obesity, corroborating previous findings.5, 6 Central adipose tissue, in excess, seems to release large amounts of fatty acids that can promote changes such as hypertriglyceridemia and hyperinsulinemia,33 a framework that can lead to an increase in sodium reabsorption and sympathetic nervous activity, which contributes to elevated BP levels.34
In general, a clear difference was observed between the extreme groups of maturity status. The preliminary analyses contributed to understanding, in part, these differences. In the late‐maturing group, which presented the most positive risk profile, behavioral and hereditary variables were associated with BP. On the other hand, our data suggest that in early‐maturing adolescents, only one biological variable (waist circumference) was related to the outcome. This difference is probably related to the growth and development process. In addition to blood volume, stroke volume, mass of the heart, cardiac output, and peripheral vascular resistance, which can increase as a function of body size,8 the maturation process influences other factors such as the amount and distribution of body fat.9 In accordance with the present results, previous investigations reported that early‐maturing adolescents present an increased risk profile, especially in girls.35 In this way, if late‐maturing adolescents do not develop other medical conditions prior to HBP, the adolescents with early‐onset maturation probably will already have, for example, a higher body fat percentage. When this information is entered in multivariate statistical models, the influence of these biological risk variables tends to be higher in the early‐maturing adolescents, supplanting the relationship of behavioral and hereditary variables with BP. This information indicates the importance of interventions for prevention of overweight, especially in early‐maturing adolescents.
Study Limitations and Strengths
This study has limitations that should be pointed out. First, because of the sample size and consequently the statistical power of the analyses, it was not possible to stratify models according to sex––a fact that would aggregate information considering that changes in the maturation process are different in boys and girls. However, we adjusted all models to this variable. The instrument adopted to measure BP can also be considered a limitation; however, even though it is not the gold standard method, it had already been validated in this population.13 With regard to the questionnaire, even with good reproducibility, the subjective measurement of physical activity by the adolescents (previous and habitual) may have bias. The same can be pointed out for the questionnaire of hypertension answered by parents. Another point is the fact that the ethnicity of the adolescents interviewed was not reported; it is known that black adolescents tend to mature faster than white adolescents.36 However, we emphasize the difficulty in performing such an assessment in Brazil because of the high miscegenation of the population. Finally, the cross‐sectional design, which precludes the relationship of time between the variables, should be considered. Nevertheless, positive points should also be highlighted. Methodological care was applied in the sample selection, and the division into groups according to somatic maturation status enabled a different point of view for the identification of the variables associated with BP in adolescents and possible intervention strategies.
Conclusions
Adolescents with early maturation presented a higher prevalence of HBP, which was strongly related to central adiposity. In contrast, the BP in late‐maturing adolescents was more related to behavioral and hereditary factors. In two of the three maturation stages considered in this study, abdominal adiposity was associated with increased levels of BP. Based on these results, added to the relationship between biological maturation and other cardiovascular risk factors37 and the stable BP levels from childhood to adulthood,4 we suggest that preventative actions for obesity should be focused principally on early‐maturing adolescents.
Funding
Funding was provided by the National Council of Scientific and Technological Development (483867/2009‐8).
Disclosure
The authors have nothing to declare.
Author Contributions
Mr Werneck: analysis/interpretation of data and draft of the article and selection of manuscripts to discuss the results. Mr Silva: acquisition, analysis/interpretation of data, and drafting of the article and selection of manuscripts to discuss the results. Ms Tomeleri and Souza: acquisition, analysis, and interpretation of data. Drs Christofaro, Sardinha, and Coelho‐e‐Silva: interpretation of data and important review of the final version to be submitted. Drs Fernandes, Ronque, and Cyrino: conception and design of the study and review the final version. All authors read and approved the final manuscript.
Acknowledgments
The authors thank Alessandra Okino, Jair Oliveira, Danielle Venturini, and Décio Barbosa for research support; Mariana Carnelossi and Sandra Kawaguti for acquisition of data; and Raphael Ritti‐Dias, David Ohara, and Aline Gerage for contributions in the previous version of the manuscript; and Coordination for the Improvement of Higher Education Personnel (CAPES/BRAZIL) for scholarships (DRPS and CMT) and National Council of Scientific and Technological Development (CNPq/BRAZIL) for funding the project, and for scholarship productive research (ERVR, ESC, and RAF).
J Clin Hypertens (Greenwich). 2016;18:424–430. DOI: 10.1111/jch.12699. © 2015 Wiley Periodicals, Inc.
References
- 1. WHO . Global Status Report on Noncommunicable Diseases 2014. Geneva: World Health Organization; 2015. [DOI] [PubMed] [Google Scholar]
- 2. WHO . Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization; 2009. [Google Scholar]
- 3. Urbina EM, Khoury PR, McCoy C, et al. Cardiac and vascular consequences of pre‐hypertension in youth. J Clin Hypertens (Greenwich). 2011;13:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang Y, Mi J. Pubertal hypertension is a strong predictor for the risk of adult hypertension. Biomed Environ Sci. United States: 2011 The Editorial Board of Biomedical and Environmental Sciences. Published by Elsevier B.V; 2011;24:459–466. [DOI] [PubMed] [Google Scholar]
- 5. Christofaro DGD, Fernandes RA, Oliveira AR, et al. The association between cardiovascular risk factors and high blood pressure in adolescents: a school‐based study. Am J Hum Biol. 2014;26:518–522. [DOI] [PubMed] [Google Scholar]
- 6. Ying‐Xiu Z, Da‐Yong S, Jing‐Yang Z, et al. Blood pressure among children and adolescents with normal weight but large waist circumference in Shandong, China. Eur J Pediatr. 2014;173:285–289. [DOI] [PubMed] [Google Scholar]
- 7. Kotchen JM, McKean HE, Kotchen TA. Blood pressure trends with aging. Hypertension. 1982;4(5 Pt 2):III128–III134. [DOI] [PubMed] [Google Scholar]
- 8. Mahoney LT, Clarke WR, Burns TL, Lauer RM. Childhood predictors of high blood pressure. Am J Hypertens. 1991;4:608S–610S. [DOI] [PubMed] [Google Scholar]
- 9. Malina RM, Bouchard C, Bar‐Or O. Growth, Maturation, and Physical Activity. 2 ed. Champaign, Illinois: Human Kinetics; 2004. [Google Scholar]
- 10. Cho SYD, Mueller WH, Meininger JC, et al. Blood pressure and sexual maturity in adolescents: the Heartfelt Study. Am J Hum Biol. 2001;13:227–234. [DOI] [PubMed] [Google Scholar]
- 11. Chen X, Wang Y. The Influence of Sexual Maturation on Blood Pressure and Body Fatness in African‐American Adolescent Girls and Boys. Am J Hum Biol. 2009;21:105–112. [DOI] [PubMed] [Google Scholar]
- 12. Statistics BIoGa . Gross Domestic Product of Municipalities. http://www.ibge.gov.br/cidadesat/xtras/perfil.php?codmun=411370&search=parana. Published 2013. Accessed July 5, 2013.
- 13. Christofaro DGD, Fernandes RA, Gerage AM, et al. Validação do monitor de medida de pressão arterial Omron HEM 742 em adolescentes. Arq Bras Cardiol. 2009;92:10–15. [DOI] [PubMed] [Google Scholar]
- 14. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. United States2004. p. 555–576. [PubMed]
- 15. Katzmarzyk PT, Srinivasan SR, Chen W, et al. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114:e198–e205. [DOI] [PubMed] [Google Scholar]
- 16. Harrison G, Buskirk E, Carter L, et al. Skinfold thicknesses and measurement technique. In: Lohman T, Roche A, Martorell R, eds. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics Books; 1988. [Google Scholar]
- 17. Boileau RA, Lohman TG, Slaughter MH. Exercise body composition in children and youth. Scand J Sports Sci. 1985;7:17–27. [Google Scholar]
- 18. Mirwald RL, Baxter‐Jones ADG, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34:689–694. [DOI] [PubMed] [Google Scholar]
- 19. Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical‐activity in epidemiological‐studies. Am J Clin Nutr. 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 20. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 21. Gong TT, Wu QJ, Vogtmann E, et al. Age at menarche and risk of ovarian cancer: a meta‐analysis of epidemiological studies. Int J Cancer. 2013;132:2894–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sherar LB, Cumming SP, Eisenmann JC, et al. Adolescent biological maturity and physical activity: biology meets behavior. Pediatr Exerc Sci. 2010;22:332–349. [DOI] [PubMed] [Google Scholar]
- 23. Wen W, Luo R, Tang X, et al. Age‐related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis. 2015;238:147–152. [DOI] [PubMed] [Google Scholar]
- 24. Alpay H, Oezdemir N, Wuehl E, Topuzoglu A. Ambulatory blood pressure monitoring in healthy children with parental hypertension. Pediatr Nephrol. 2009;24:155–161. [DOI] [PubMed] [Google Scholar]
- 25. Kuschnir MC, Mendonca GA. Risk factors associated with arterial hypertension in adolescents. J Pediatr. 2007;83:335–342. [DOI] [PubMed] [Google Scholar]
- 26. Hallal PC, Victora CG, Azevedo MR, Wells JC. Adolescent physical activity and health: a systematic review. Sports Med. 2006;36:1019–1030. [DOI] [PubMed] [Google Scholar]
- 27. Fernandes RA, Zanesco A. Early physical activity promotes lower prevalence of chronic diseases in adulthood. Hypertens Res. 2010;33:926–931. [DOI] [PubMed] [Google Scholar]
- 28. Zanesco A, Antunes E. Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol Ther. 2007;114:307–317. [DOI] [PubMed] [Google Scholar]
- 29. Christofaro DG, Ritti‐Dias RM, Fernandes RA, et al. High blood pressure detection in adolescents by clustering overall and abdominal adiposity markers. Arq Bras Cardiol. 2011;96:465–470. [DOI] [PubMed] [Google Scholar]
- 30. Dulskiene V, Kuciene R, Medzioniene J, Benetis R. Association between obesity and high blood pressure among Lithuanian adolescents: a cross‐sectional study. Ital J Pediatr. 2014;40:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aounallah‐Skhiri H, El AJ, Traissac P, et al. Blood pressure and associated factors in a North African adolescent population. a national cross‐sectional study in Tunisia. Bmc Public Health. 2012;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Must A, Naumova EN, Phillips SM, et al. Childhood overweight and maturational timing in the development of adult overweight and fatness: the Newton Girls Study and its follow‐up. Pediatrics. 2005;116:620–627. [DOI] [PubMed] [Google Scholar]
- 33. Rubin DA, Hackney AC. Inflammatory cytokines and metabolic risk factors during growth and maturation: influence of physical activity. Med Sport Sci 2010;55:43–55. [DOI] [PubMed] [Google Scholar]
- 34. Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr Diabetes 2007;8:299–306. [DOI] [PubMed] [Google Scholar]
- 35. Mueller NT, Pereira MA, Demerath EW, et al. Earlier menarche is associated with fatty liver and abdominal ectopic fat in midlife, independent of young adult BMI: the CARDIA study. Obesity (Silver Spring). 2015;23:468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biro FM, McMahon RP, Striegel‐Moore R, et al. Impact of timing of pubertal maturation on growth in black and white female adolescents: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–643. [DOI] [PubMed] [Google Scholar]
- 37. Staiano AE, Broyles ST, Gupta AK, et al. Maturity‐associated variation in total and depot‐specific body fat in children and adolescents. Am J Hum Biol. 2013;25:473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]


