Abstract
The association between fruit and vegetables (FVs) consumption and hypertension risk remains controversial. A systematic search was performed in PubMed and the Web of Science for relevant articles published in English or Chinese up to April 2015. A total of 25 studies with 334,468 patients (41,713 cases) were included in the present meta‐analysis. When comparing the highest with the lowest consumption, the pooled relative risks of hypertension were 0.812 (95% confidence interval, 0.740–0.890) for FVs, 0.732 (95% confidence interval, 0.621–0.861) for fruit, and 0.970 (95% confidence interval, 0.918–1.024) for vegetables. A significantly inverse association between fruit consumption and hypertension risk was found in studies carried out in Asia (relative risk, 0.70; 95% confidence interval, 0.61–0.79). Influence analysis revealed that no individual study had an excessive influence on the pooled relative risks. The present meta‐analysis indicates that FV consumption might be inversely associated with hypertension risk, which still needs to be confirmed by prospective cohort studies.
According to reports of the World Health Organization, the global prevalence of hypertension was about 22% among adults in 2014 (http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence/en/), and the prevalence is projected to rise to 29.2% in 2025 without intervention.1 In addition, hypertension has been strongly associated with the risk of cardiovascular disease, stroke, and kidney failure.2, 3 Thus, it is indispensable to pay attention to the prevention and control of hypertension.
Several risk factors might be associated with hypertension, such as genetic factors, obesity, physical inactivity, and dietary factors.3, 4, 5, 6 Among dietary factors, alcohol and dietary fat have been shown to be significantly associated with an increased risk of hypertension.4, 7 In contrast, there were inverse associations between the consumption of garlic8 and soya protein9 and the risk of hypertension. As important components of diet, fruit and vegetables (FV) are rich in minerals, vitamins, and folic acid, which have been reported to have beneficial effects on endothelial function.10, 11 Endothelial dysfunction is also a potential risk factor for hypertension.12
Accordingly, many epidemiological studies have been performed to investigate the relationship between FV consumption and the risk of hypertension. However, the results of these studies have been inconsistent. While FV consumption has been found to be significantly associated with a decreased risk of hypertension in some studies,13, 14, 15, 16, 17, 18 Lin and colleagues19 found that FV consumption was significantly associated with an increased risk of hypertension, and no significant relationship was found between FV and risk of hypertension in other studies.20, 21, 22, 23 Therefore, we systematically conducted a meta‐analysis to assess the hypertension risk for highest vs lowest fruit and/or vegetables consumption, separately.
Method
Search Strategy
We searched PubMed and the Web of Science systematically up to April 2015, using the following search terms “fruit,” “fruits,” “vegetable,” “vegetables,” “blood pressure,” and “hypertension.” The search strategy in PubMed with Boolean terms is shown in Data S1. The articles were restricted to English or Chinese. We also reviewed the reference lists from retrieved articles to identify further available studies not captured by our databases. The detailed information is shown in Figure 1.
Figure 1.
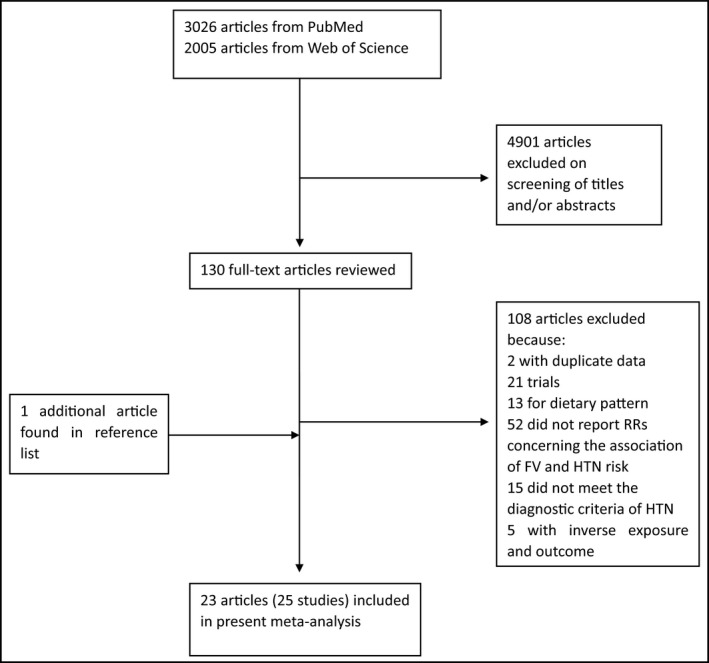
Flow diagram of literature search. RRs indicate relative risks; FV, fruit and vegetables; HTN, hypertension.
Inclusion Criteria
Studies were included if they met the following criteria: (1) an observational study (cohort, case‐control, cross‐sectional); (2) the exposure of interest was the consumption of fruit or/and vegetables; (3) the outcome of interest was hypertension (systolic blood pressure [SBP] ≥140 mm Hg or diastolic blood pressure [DBP] ≥90 mm Hg); (4) relative risk (RR) or odds or hazard ratios with 95% confidence interval (CI) provided; and (5) the object of the study was the general population (diabetes population excluded).
We chose the most recent studies if data from the same population were duplicated in more than one study.
Two investigators (Bingrong Li and Fang Li) searched articles and reviewed all retrieved studies independently. If the two investigators disagreed about the eligibility of an article, it was resolved by consensus with Dongfeng Zhang.
Data Extraction
Two investigators (Longfei Wang and Dongfeng Zhang) independently extracted the following data: first author's name, publication year, study location, sex, age, follow‐up years, study design (cohort or case‐control or cross‐sectional), sample size, number of cases, measurement of BP (measured by investigators or reported by participants), RRs (we presented all results with RRs for simplicity) with corresponding 95% CIs for the highest vs lowest categories of fruit or/and vegetables consumption and adjusted covariates. The RRs adjusted for the most confounders in the original studies were extracted.
Statistical Analysis
Pooled measurement was calculated as the inverse variance‐weighted mean of the logarithm of multivariate‐adjusted RRs with 95% CIs to assess the strength of associations between fruit and/or vegetables consumption and the risk of hypertension. The I² was used to assess heterogeneity among studies, and I² values of 0%, 25%, 50%, and 75% represent no, low, moderate, and high heterogeneity, respectively. The fixed‐effect model (FEM) was used if moderate or lower heterogeneity (I²<50%) was found; otherwise (I²≥50%), the random‐effect model (REM) was adopted. Meta‐regression with restricted maximum likelihood estimation was conducted to explore potential sources of heterogeneity, and P values from meta‐regression were calculated with a permutation test of 1000 to control the spurious findings.24 Small‐study effect was assessed with visual inspection of the funnel plot and Egger test. All statistical analyses were performed with STATA version 12.0 (StataCorp, College Station, TX, USA). All reported probabilities (P values) were two‐sided with P<.05 considered statistically significant.
Results
Literature Search
Initially, 3026 articles from PubMed and 2005 articles from the Web of Science were identified. A total of 4901 articles that were not relevant to the association of fruit and/or vegetables consumption and the risk of hypertension were excluded. We further excluded 108 articles after reviewing the 130 full‐text articles. The detailed reasons for the exclusion of the articles are presented in Figure 1. One additional article was included after reviewing the reference lists from retrieved articles.25 As a result, a total of 23 articles met the inclusion criteria.
Study Characteristics
A total of 25 studies from 23 available articles7, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 with 334,468 patients (41,713 cases) were included in the present meta‐analysis, because two articles included two studies independently.13, 17 Among these studies, two studies were carried out in the United States,20, 21 13 in Asia,7, 13, 15, 17, 19, 23, 27, 28, 29, 33, 36 five in Europe,14, 18, 22, 32, 35 and five in Africa.16, 25, 26, 31, 34 As for study design, there were three cohort studies, 20, 22, 35 two case‐control studies,7, 21 and 20 cross‐sectional studies13, 14, 15, 16, 17, 18, 19, 23, 25, 26, 27, 28, 29, 31, 32, 33, 34, 36. The baseline characteristics of the studies are shown in the Table.
Table 1.
Characteristics of the Studies Included on Consumption of Fruit and/or Vegetables and the Risk of Hypertension
| Reference | Country (Year) | Age/Follow‐Up, Years | Study Design | Sample Size (Cases) | RR (95% CI) | Blood Pressure Measurement | Adjustment for Covariates |
|---|---|---|---|---|---|---|---|
| Wang and colleagues20 | US (2012) | 39–89/12.9 | Cohort | 28,082 (13,633) |
1.03 (0.93–1.13) for FV and hypertension 0.95 (0.88–1.04) for fruit and hypertension 0.98 (0.91–1.06) for vegetables and hypertension |
Reported by participant | Age, race, total energy intake, randomized treatment, smoking, daily alcohol intake, exercise, postmenopausal status, menopausal hormone use, multivitamin supplement use, history of diabetes, history of hypercholesterolemia, intake of whole grains, red meats, low‐fat dairy products, and nuts, BMI |
| Uhernik and colleagues14 | Croatia (2009) | ≥18 | Cross‐sectional | 9070 (1651) | 0.7 (0.6–0.9) for FV and hypertension | Measured by investigator | Age, increased BMI, use of animal fat, drinking whole milk, moderate coffee consumption, high coffee consumption, eating smoked meat products, adding salt, moderate alcoholic beverage consumption, high alcoholic beverage consumption, smoking, leisure‐time physical activity |
| Tazi and colleagues26 | Moroccan (2009) | ≥20 | Cross‐sectional | 1802 (713) | 0.69 (0.48–1.01) for fruit and hypertension | Measured by investigator | Age, place of residence, sex, marital status, education level, cigarette smoking, alcohol drinking, walking, activity, BMI, waist size, waist‐hip ratio, diabetes, cholesterol, consumption of fish, consumption of lamb, chicken, beans, eggs, dried fruits |
| Song and colleagues27 | Korea (2014) | 19–64 | Cross‐sectional | 9791 (1038) | 0.76 (0.62–0.94) for fruit and hypertension | Measured by investigator | Age, BMI, fasting glucose, triglycerides, LDL‐C, HDL‐C, energy, sodium intake, smoking, alcohol consumption, physical activity, education, income, education for hypertension, survey year |
| Saeed and colleagues28 | Afghan (2014) | ≥40 | Cross‐sectional | 1183 (546) | 0.64 (0.47–0.86) for fruit and hypertension | Measured by investigator | Age, education level, having rice as meal, having chicken as meal, family history of diabetes, central obesity, general obesity as BMI cutoff of 30 |
| Nunez‐Cordoba and colleagues22 | Spain (2009) | 20–95/4.1 | Cohort | 8594 (426) |
0.78 (0.55–1.10) for FV and hypertension 0.85 (0.59–1.22) for fruit and hypertension 0.87 (0.55–1.39) for vegetables and hypertension |
Reported by participant | Age, sex, total energy intake, BMI, physical activity, alcohol, family history of hypertension, sodium intake, low‐fat dairy intake, whole grains intake, fish intake and smoking |
| Mundan and colleagues31 | Kenya (2013) | 18–58 | Cross‐sectional | 340 (170) |
0.55 (0.33–0.92) for fruit and hypertension 0.58 (0.33–1.02) for vegetables and hypertension |
Measured by investigator | Age, sex, and education level |
| Lin and colleagues19 | China (2012) | ≥30 | Cross‐sectional | 833 (228) | 1.64 (1.06–2.54) for FV and hypertension | Measured by investigator | Age, marital status, type of disability, disability level, BMI, waist circumference |
| La Vecchia and colleagues32 | Italia (1998) | ≥15 | Cross‐sectional | 46,693 (5295) | 0.98 (0.90–1.06) for vegetables and hypertension | Reported by participant | Age, sex, education,tobacco use, and alcohol consumption |
| Huang and colleagues15 | China (2010) | ≥35 | Cross‐sectional | 2438 (655) | 0.882 (0.784–0.992) for FV and hypertension | Measured by investigator | Predominantly sedentary work, positive family history, high education level, alcohol intake, salty diet, animal insides intake, regular physical exercise, overweight, abnormal BP |
| Helelo and colleagues34 | Southern Ethiopia (2014) | >31 | Cross‐sectional | 518 (116) | 0.43 (0.22–0.85) for vegetables and hypertension | Measured by investigator | Sex, age, salt use, number of days walking 10 min/wk, family history of hypertension, BMI |
| Gupta and colleagues13 | India (2013) | 20–75 | Cross‐sectional | 3371 (1476) |
0.6 (0.47–0.77) for FV and hypertension in men 0.54 (0.43–0.69) for FV and hypertension in women |
Measured by investigator | Educational status, socioeconomic status, depression, physical activity, high dietary fat, smoking and/or tobacco use, overweight/obesity, central obesity, high cholesterol, high triglycerides, metabolic syndrome, diabetes |
| Fan and colleagues21 | US (2010) | ≥45 | Case‐control | 198,642 (10,843) | 0.98 (0.92–1.05) for FV and hypertension | Reported by participant | Age, sex, race/ethnicity, education level, and marital status |
| de Ramirez and colleagues16 | Sub‐Saharan Africa (2010) | ≥18 | Cross‐sectional | 665 (138) | 0.47 (0.22–1.00) for FV and hypertension | Measured by investigator | Age, sex and village, sampling variables,BMI, television ownership, work‐related vigorous physical activity and other food group consumption |
| Camoes and colleagues35 | Portuguese (2010) | ≥40/3.8 | Cohort | 549 (160) | 0.61 (0.40–0.93) for FV and hypertension | Measured by investigator | Sex, age, education, BMI, physical activity level, and total energy intake (baseline) |
| Beegom and Singh17 | India (1997) | 25–64 | Cross‐sectional | 737 (255) |
0.85 (0.76–0.95) for FV and hypertension in men 0.91 (0.86–0.96) for FV and hypertension in women |
Measured by investigator | Age, BMI, coconut oil and butter, alcohol, salt, saturated fat consumption |
| Barron and colleagues18 | Ireland (2014) | ≥18 | Cross‐sectional | 10,364 (1306) | 0.77 (0.64–0.92) for FV and hypertension | Reported by participant | Age, BMI, smoking |
| Awoke and colleagues25 | Northwest Ethiopia (2012) | ≥35 | Cross‐sectional | 679 (192) | 1.43 (0.61–3.35) for vegetables and hypertension | Measured by investigator | Age, education level, marital status, occupation, BMI, self‐reported diabetes, family history of hypertension, walking status for 10 min |
| Shaoyan and colleagues23 | China (2011) | 31–57 | Cross‐sectional | 101 (17) | 2.31 (0.66–8.04) for FV and hypertension | Measured by investigator | Grains, meat, fish and other aquatic products, milk and dairy products, eggs and egg products, beans and bean products, sweets, fried foods, pickled and smoked food |
| Qi and colleagues29 | China (2011) | ≥18 | Cross‐sectional | 2699 (751) | 0.79 (0.70–0.88) for FV and hypertension | Measured by investigator | Age, occupation, frequency of bean products, pickled food, BMI |
| Jian‐Hui and colleagues33 | China (2009) | ≥25 | Cross‐sectional | 1256 (301) | 0.71 (0.6499–0.7713) for FV and hypertension | Measured by investigator | Pickled food, cooking oil |
| Bin and colleagues36 | China (2011) | 15–69 | Cross‐sectional | 961 (222) | 0.547 (0.368–0.814) for fruit and hypertension | Measured by investigator | Age, family history of cerebrovascular disease, alcohol, BMI, variety of fruit |
| Shouying and colleagues7 | China (2007) | 35–87 | Case‐control | 1605 (212) | 0.708 (0.564–0.887) for fruit and hypertension | Measured by investigator | Age, sex, BMI, educational status, socioeconomic status, smoking, alcohol, fat, eggs, family history of hypertension |
Abbreviations: BMI, body mass index; CI, confidence interval; FV, fruit and vegetables; RR, relative risk.
Quantitative Synthesis
FV Consumption and Risk of Hypertension
Sixteen studies from 14 articles13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 29, 33, 35 involving 541,792 patients (33,209 cases) evaluated the relationship between FV consumption and the risk of hypertension. Among the 16 studies, 11 revealed an inverse association and one showed a positive association, while four studies indicated no relationship. The pooled RR of hypertension for the highest vs the lowest consumption of FV was 0.812 (95% CI, 0.740–0.890; I 2=84.7%; REM; P heterogeneity=.000 [Figure 2]). Subgroup analysis by study design was conducted (Figure S1). The result suggested that there was a significant inverse association between FV consumption and risk of hypertension in the case‐control and cross‐sectional studies (RR, 0.80; 95% CI, 0.73–0.89; I 2=85.4%; REM), but the inverse association was not statistically significant in the cohort studies (RR, 0.83; 95% CI, 0.60–1.14; I 2=73.4%; REM).
Figure 2.
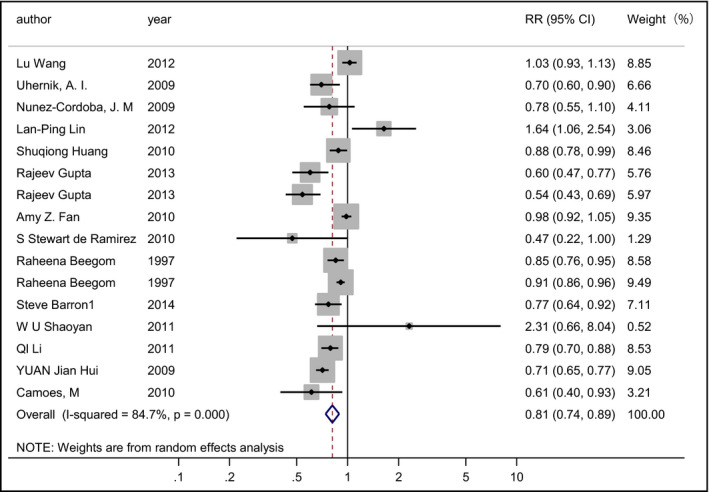
Forest plot of the relative risks (RRs) with corresponding 95% confidence intervals (CIs) of studies on fruit and vegetables consumption and risk of hypertension.
There were four studies in women and three studies in men. The pooled RRs were 0.83 (95% CI, 0.69–0.99; I 2=88.4%; REM [Figure S2]) for women and 0.75 (95% CI, 0.59–0.95; I 2=68.5%; REM [Figure S3]) for men. Nine studies were carried in Asia, and the pooled RR was 0.80 (95% CI, 0.71–0.91; I 2=85.9%; REM; P heterogeneity=.000 [Figure S4]).
Fruit Consumption and Risk of Hypertension
Eight studies7, 20, 22, 26, 27, 28, 31, 36 involving 52,358 patients (16,960 cases) evaluated the relationship between fruit consumption and the risk of hypertension. Among the eight studies, five revealed an inverse association while three showed no relationship. The pooled RR of hypertension for the highest vs the lowest consumption of fruit was 0.73 (95% CI, 0.62–0.86; I 2=68.9%; REM; P heterogeneity=.002 [Figure 3]). Subgroup analyses by continent and measurement of blood pressure (BP) were conducted (Figures S5 and S6). The results suggested that there was a statistically significant inverse association between fruit consumption and the risk of hypertension for studies carried out in Asia (RR, 0.70; 95% CI, 0.61–0.79; I 2=0%; REM) and in Africa (RR, 0.64; 95% CI, 0.47–0.86; I 2=0%; REM), but the inverse association was not statistically significant in America and Europe (RR, 0.94; 95% CI, 0.87–1.02; I 2=0%; REM). The pooled RRs were 0.69 (95% CI, 0.61–0.77; I 2=0%; REM) among studies in which BP was measured by the investigators and 0.94 (95% CI, 0.87–1.02; I 2=0%; REM) among studies in which BP was reported by the participants.
Figure 3.
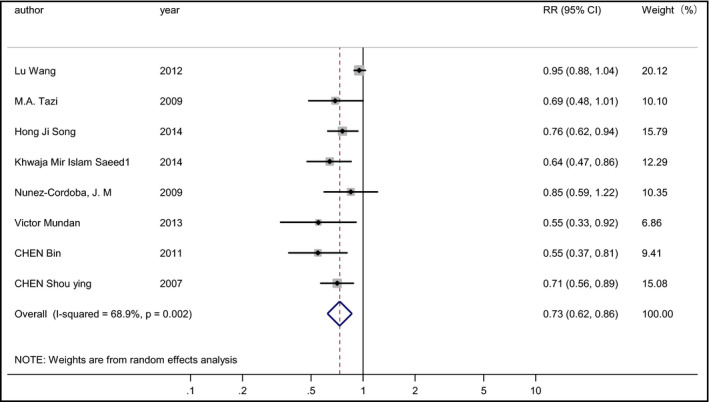
Forest plot of the relative risks (RRs) with corresponding 95% confidence intervals (CIs) of studies on fruit consumption and risk of hypertension.
Vegetables and Risk of Hypertension
Six studies20, 22, 25, 31, 32, 34 involving 84,906 patients (19,832 cases) evaluated the relationship between vegetable consumption and risk of hypertension. Among the six studies, one revealed an inverse association while five showed no relationship. The pooled RR of hypertension for the highest vs the lowest consumption of vegetables was 0.97 (95% CI, 0.92–1.02; I 2=49.5%; FEM; P heterogeneity=.078 [Figure S7]).
Meta‐Regression
To explore the sources of heterogeneity, meta‐regression with the covariates of year, continent, study design, status of adjusting for body mass index, and measurement of BP were performed. In the analysis of fruit consumption and risk of hypertension, study design (P=.017) and measurement of BP (P=.037) were found to contribute to heterogeneity. However, in the analyses of FV consumption and vegetable consumption and the risk of hypertension, no covariates were found to contribute to heterogeneity.
Influence Analysis and Small‐Study Effect
Influence analysis revealed that no individual study had an excessive influence on the above‐mentioned pooled RRs. The funnel plot and Egger test showed no evidence of significant small‐study effect in the analysis between hypertension risk and consumption of FV (P=.198; Figure 4) and vegetables (P=.216; Figure S8). For fruit consumption and hypertension risk, a small‐study effect was found (P=.002; Figure S9); however, after removing one study20 that had a strong effect on heterogeneity, no significant small‐study effect was found (P=.195; Figure S10) and the result remained significant (RR, 0.70; 95% CI, 0.63–0.78; I 2=0%; P heterogeneity=.625; FEM [Figure S11]).
Figure 4.
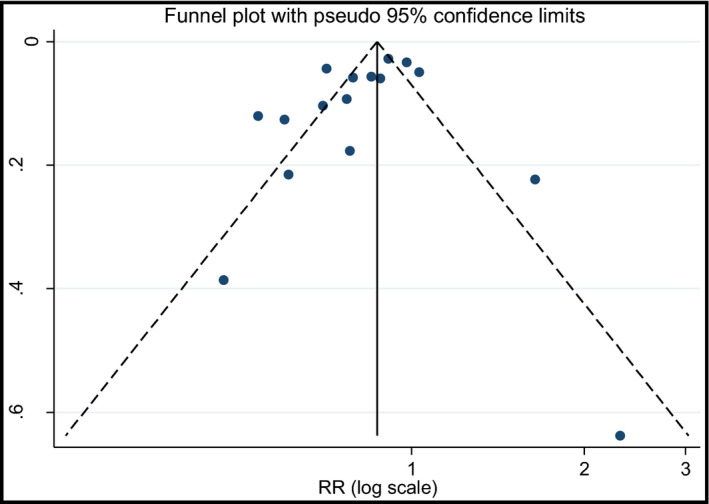
Funnel plot with pseudo 95% confidence limits for the analysis of fruit and vegetables consumption and risk of hypertension. s.e. indicates standard error; RR, relative risk.
Discussion
Our meta‐analysis included 25 studies from 23 available articles. To the best of our knowledge, this is the first meta‐analysis to quantitatively evaluate the relationship between FV consumption and hypertension risk. The findings were indicative of inverse associations between the consumption of FV and fruit and the risk of hypertension. In the subgroup analyses, there was a significant inverse association between fruit consumption and the risk of hypertension for studies carried out in Asia. The difference between continents may be influenced by the social and economic status of its populations. Most studies were carried out in Asia, which may also affect the difference. The association was also significant among studies in which BP was measured by investigators. The reason might be that the BP measured by investigators was more accurate than self‐reported BP.
There are several reasons behind the relationship between fruit and/or vegetables consumption and the risk of hypertension. First, FV are high in potassium, magnesium, vitamin C, folic acid, flavonoid, and carotenoid, which have been postulated to lower BP through improving endothelial function, modulating baroreflex sensitivity, causing vasodilation, and increasing antioxidant activity.10, 37, 38, 39, 40 Second, increased FV consumption may have an impact on diet structure, especially on increased dietary fiber consumption and reduced fat intake. High fat consumption has been shown to be significantly associated with increased risk of hypertension.7
Between‐study heterogeneity is common in meta‐analysis,41 and it is indispensable to explore the potential sources of heterogeneity among studies. Moderate to high heterogeneity was found in the present meta‐analysis. The results of meta‐regression and subgroup analyses revealed that the heterogeneity was associated with study design, continent, and measurement of BP. Several possible reasons may give rise to heterogeneity. First, differences in FV type, method of preservation, and cooking methods may contribute to the heterogeneity. Second, Fan and colleagues21 found that the relationship of FV consumption with hypertension was different between hypertensive patients who were on treatment and those who were not on treatment. However, the status of adjusting for therapy method was not provided in most of our studies. This may also contribute to the heterogeneity. Third, the difference between newly diagnosed hypertensive patients and hypertensive patients may influence heterogeneity. Finally, one study by Lin and colleagues19 included only adults with disabilities, which was different from other studies. Thus, the population characteristics may also potentially contribute to the between‐study heterogeneity.
Study Strengths
Our meta‐analysis has several strengths. First, the present meta‐analysis included a large number of participates, thus reducing sampling error to a great extent. Second, nearly all included studies had adjusted for potential confounders, including age, body mass index, alcohol intake, and family history of hypertension, increasing the credibility of the results. Third, we found a significantly inverse association among studies in which BP was measured by investigators, indicating that the results were stable and accurate.
Study Limitations
The present meta‐analysis also has several limitations. First, the researchers adjusted for some confounders but were diverse. Second, the measurement of BP was different in original studies, ie, reported by participants or measured by investigators. Third, the difference in diet assessment methods may influence the results in some degree. Some studies were measured with a food frequency questionnaire and others with a dietary history questionnaire. Last, we found an inverse association between FV consumption and the risk of hypertension in cohort studies, which was not statistically significant. This may be a result of the small number of cohort studies. Therefore, further prospective cohort studies are still needed to confirm these findings.
Conclusions
The present meta‐analysis revealed that the consumption of FV and fruit may reduce hypertension risk. Increased FV consumption should be advocated for the primary prevention of hypertension.
Supporting information
Figure S1. Forest plot of subgroup analysis by study design on FV consumption.
Figure S2. Forest plot of studies on FV consumption for females.
Figure S3. Forest plot of studies on FV consumption for males.
Figure S4. Forest plot of studies on FV consumption in Asia.
Figure S5. Forest plot of subgroup analysis by continent on fruit consumption.
Figure S6. Forest plot of subgroup analysis by blood pressure measurement on fruit consumption.
Figure S7. Forest plot of studies on vegetable consump‐tion and hypertension risk.
Figure S8. Funnel plot with pseudo 95% confidence limits for the analysis of vegetables consumption and risk of hypertension.
Figure S9. Funnel plot with pseudo 95% confidence limits for the analysis of fruit consumption and risk of hypertension.
Figure S10. Funnel plot with pseudo 95% confidence limits for the analysis of fruit consumption and risk of hypertension after remove one study.
Figure S11. Forest plot of studies on fruit consumption and hypertension risk after removing one study.
Data S1. Search strategy on PUBMED.
Acknowledgments and Disclosures
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
J Clin Hypertens (Greenwich). 2016;18:468–476. DOI: 10.1111/jch.12277. © 2016 Wiley Periodicals, Inc.
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 4. Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta‐analysis. J Clin Hypertens (Greenwich). 2012;14:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdulle A, Al‐Junaibi A, Nagelkerke N. High blood pressure and its association with body weight among children and adolescents in the United Arab Emirates. PLoS One. 2014;9:e85129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Padmanabhan S, Caulfield M, Dominiczak AF. Genetic and molecular aspects of hypertension. Circ Res. 2015;116:937–959. [DOI] [PubMed] [Google Scholar]
- 7. Shouying C, Zhimei XI, Qun ZUO, et al. Risk factors in rural residents with essential hypertension of different social economic levels. Chin J Hypertens. 2007;15:764–767. [Google Scholar]
- 8. Xiong XJ, Wang PQ, Li SJ, et al. Garlic for hypertension: a systematic review and meta‐analysis of randomized controlled trials. Phytomedicine. 2015;22:352–361. [DOI] [PubMed] [Google Scholar]
- 9. Dong JY, Tong X, Wu ZW, et al. Effect of soya protein on blood pressure: a meta‐analysis of randomised controlled trials. Br J Nutr. 2011;106:317–326. [DOI] [PubMed] [Google Scholar]
- 10. Tamai Y, Wada K, Tsuji M, et al. Dietary intake of vitamin B12 and folic acid is associated with lower blood pressure in Japanese preschool children. Am J Hypertens. 2011;24:1215–1221. [DOI] [PubMed] [Google Scholar]
- 11. May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000;28:1421–1429. [DOI] [PubMed] [Google Scholar]
- 12. Dinh QN, Drummond GR, Sobey CG, et al. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta R, Deedwania PC, Achari V, et al. Normotension, prehypertension, and hypertension in urban middle‐class subjects in India: prevalence, awareness, treatment, and control. Am J Hypertens. 2013;26:83–94. [DOI] [PubMed] [Google Scholar]
- 14. Uhernik AI, Erceg M, Milanovic SM. Association of BMI and nutritional habits with hypertension in the adult population of Croatia. Public Health Nutr. 2009;12:97–104. [DOI] [PubMed] [Google Scholar]
- 15. Huang S, Xu Y, Yue L, et al. Evaluating the risk of hypertension using an artificial neural network method in rural residents over the age of 35 years in a Chinese area. Hypertens Res. 2010;33:722–726. [DOI] [PubMed] [Google Scholar]
- 16. de Ramirez SS, Enquobahrie DA, Nyadzi G, et al. Prevalence and correlates of hypertension: a cross‐sectional study among rural populations in sub‐Saharan Africa. J Hum Hypertens. 2010;24:786–795. [DOI] [PubMed] [Google Scholar]
- 17. Beegom R, Singh RB. Association of higher saturated fat intake with higher risk of hypertension in an urban population of Trivandrum in south India. Int J Cardiol. 1997;58:63–70. [DOI] [PubMed] [Google Scholar]
- 18. Barron S, Balanda K, Hughes J, et al. National and subnational hypertension prevalence estimates for the Republic of Ireland: better outcome and risk factor data are needed to produce better prevalence estimates. BMC Public Health. 2014;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin LP, Liu CT, Liou SW, et al. High blood pressure in adults with disabilities: influence of gender, body weight and health behaviors. Res Dev Disabil. 2012;33:1508–1515. [DOI] [PubMed] [Google Scholar]
- 20. Wang L, Manson JE, Gaziano JM, et al. Fruit and vegetable intake and the risk of hypertension in middle‐aged and older women. Am J Hypertens. 2012;25:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan AZ, Mallawaarachchi DS, Gilbertz D, et al. Lifestyle behaviors and receipt of preventive health care services among hypertensive Americans aged 45 years or older in 2007. Prev Med. 2010;50:138–142. [DOI] [PubMed] [Google Scholar]
- 22. Nunez‐Cordoba JM, Alonso A, Beunza JJ, et al. Role of vegetables and fruits in Mediterranean diets to prevent hypertension. Eur J Clin Nutr. 2009;63:605–612. [DOI] [PubMed] [Google Scholar]
- 23. Shaoyan WU, Zhong ZUO, Xiaolin W, et al. Relationship between food classification and blood pressure. World Sci‐Tech R and D. 2011;33:667–669. [Google Scholar]
- 24. Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta‐regression. Stat Med. 2004;23:1663–1682. [DOI] [PubMed] [Google Scholar]
- 25. Awoke A, Awoke T, Alemu S, et al. Prevalence and associated factors of hypertension among adults in Gondar, Northwest Ethiopia: a community based cross‐sectional study. BMC Cardiovasc Disord. 2012;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tazi MA, Abir‐Khalil S, Lahmouz F, et al. Risk factors for hypertension among the adult Moroccan population. East Mediterr Health J. 2009;15:827–841. [PubMed] [Google Scholar]
- 27. Song HJ, Paek YJ, Choi MK, et al. Gender differences in the relationship between risk of hypertension and fruit intake. Prev Med. 2014;67:154–159. [DOI] [PubMed] [Google Scholar]
- 28. Saeed KM, Rasooly MH, Brown NJ. Prevalence and predictors of adult hypertension in Kabul, Afghanistan. BMC Public Health. 2014;14:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qi L, Ding X, Mao D, et al. Analysis on prevalence of hypertension and its associated factors among residents over 50 years old in Three Gorge Areas. Acta Univ Sci Med Chongqing. 2011;36:1513–1516. [Google Scholar]
- 30. Nuwaha F, Musinguzi G. Pre‐hypertension in Uganda: a cross‐sectional study. BMC Cardiovasc Disord. 2013;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mundan V, Muiva M, Kimani S. Physiological, behavioral, and dietary characteristics associated with hypertension among Kenyan defence forces. ISRN Prev Med. 2013;2013:740143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. La Vecchia C, Decarli A, Pagano R. Vegetable consumption and risk of chronic disease. Epidemiology. 1998;9:208–210. [PubMed] [Google Scholar]
- 33. Jian‐Hui Y, Jian‐Zhou Y, Chong‐Zheng GUO, et al. Relation between dietary intakes and hypertension among rural population in Shanxi Province. J Fourth Mil Med Univ. 2009;30:257–259. [Google Scholar]
- 34. Helelo TP, Gelaw YA, Adane AA. Prevalence and associated factors of hypertension among adults in Durame Town, Southern Ethiopia. PLoS One. 2014;9:e112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Camoes M, Oliveira A, Pereira M, et al. Role of physical activity and diet in incidence of hypertension: a population‐based study in Portuguese adults. Eur J Clin Nutr. 2010;64:1441–1449. [DOI] [PubMed] [Google Scholar]
- 36. Bin C, Deyun LI, Xiaodong L. Prevalence of hypertension and its influence factors among inhabitants aged 15–69 years in Zhuhai city. China Public Health. 2011;27:619–621. [Google Scholar]
- 37. Whelton PK, Klag MJ. Magnesium and blood pressure: review of the epidemiologic and clinical trial experience. Am J Cardiol. 1989;63:26G–30G. [DOI] [PubMed] [Google Scholar]
- 38. Toh JY, Tan VM, Lim PC, et al. Flavonoids from fruit and vegetables: a focus on cardiovascular risk factors. Curr Atheroscler Rep. 2013;15:368. [DOI] [PubMed] [Google Scholar]
- 39. Juraschek SP, Guallar E, Appel LJ, et al. Effects of vitamin C supplementation on blood pressure: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aburto NJ, Hanson S, Gutierrez H, et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee YH. Meta‐analysis of genetic association studies. Ann Lab Med. 2015;35:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Forest plot of subgroup analysis by study design on FV consumption.
Figure S2. Forest plot of studies on FV consumption for females.
Figure S3. Forest plot of studies on FV consumption for males.
Figure S4. Forest plot of studies on FV consumption in Asia.
Figure S5. Forest plot of subgroup analysis by continent on fruit consumption.
Figure S6. Forest plot of subgroup analysis by blood pressure measurement on fruit consumption.
Figure S7. Forest plot of studies on vegetable consump‐tion and hypertension risk.
Figure S8. Funnel plot with pseudo 95% confidence limits for the analysis of vegetables consumption and risk of hypertension.
Figure S9. Funnel plot with pseudo 95% confidence limits for the analysis of fruit consumption and risk of hypertension.
Figure S10. Funnel plot with pseudo 95% confidence limits for the analysis of fruit consumption and risk of hypertension after remove one study.
Figure S11. Forest plot of studies on fruit consumption and hypertension risk after removing one study.
Data S1. Search strategy on PUBMED.


