Abstract
Hypertension is common in renal transplant recipients (RTRs). Ambulatory blood pressure (BP) monitoring (ABPM) is important in diagnosing hypertension and diurnal BP variation. The authors set out to compare office BP and ABPM measurements to determine diurnal pattern and to evaluate echocardiographic findings in RTRs. ABPM and office BP measurements were compared in 87 RTRs. Echocardiographic evaluation was performed for each patient. The correlations between office and 24‐hour ABPM were 0.275 for mean systolic BP (P=.011) and 0.260 for mean diastolic BP (P=.017). Only 36.8% had concordant hypertension between office BP and ABPM, with a masked hypertension rate of 16.1% and white‐coat effect rate of 24.1%. Circadian BP patterns showed a higher proportion of nondippers (67.8%). Left ventricular mass index was increased in 21.8% of all recipients. There was a significant but weak correlation between office BP and ABPM.
Hypertension (HTN) is a prevalent disorder in renal transplant recipients (RTRs) and is considered one of the major risk factors for the development of cardiovascular disease (CVD).1 CVD represents the single most frequent cause of death in RTRs, accounting for approximately 40% of all‐cause mortality.2
Diagnosis of HTN has traditionally been based on measurements of blood pressure (BP) in the office or clinic. However, inadequate BP control may occur when office BP is used as the only method to monitor BP and drug adjustment.3 Ambulatory BP monitoring (ABPM) has recently gained popularity in more accurately diagnosing HTN and predicting outcome in hypertensive patients and those with chronic kidney disease.4 It has been shown that ABPM can predict left ventricular hypertrophy (LVH), mortality and morbidity, and progression toward end‐stage renal disease better than BP in patients with CKD.5, 6 In addition to accurate BP measurement, one of the variables measured by ABPM is diurnal BP variation. However, the optimal method for monitoring BP in RTRs remains unclear.
In our study, we compared office and ABPM measurements to determine the diurnal BP pattern and to assess the relationship between BP parameters and echocardiographic findings in RTRs.
Materials and Methods
Study Patients
We examined 498 consecutive adult RTRs (age ≥18 years) in whom the duration of transplantation was more than 1 year. The following exclusion criteria were used: history of diabetes mellitus, heart failure, ischemic heart disease, cardiomyopathy, or significant valvular heart disease; active infection; a serum creatinine level >1.5 mg/dL; or hemoglobin level <10 g/dL. Of the remaining 150 RTRs, 60 refused to participate in the study and three did not show up for the echocardiography appointment. Thus, 87 patients completed the study.
Study Protocol
Demographic (age and sex) and clinical (etiology of previous renal disease, date of transplant, donor type, height, and weight) data and previous renal replacement therapy (type and duration) records were retrieved from patient files. We recorded antihypertensive, antilipemic, and immunosuppressive medication use for each patient.
Blood samples were collected after an overnight fast for the measurement of urea, creatinine, fasting blood glucose, uric acid, hemoglobin, hematocrit, albumin, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, and total cholesterol. All laboratory parameters were measured using the Abbott Architect c8000 autoanalyzer (Abbott Diagnostics, Abbott Park, IL).
Glomerular filtration rate (GFR) was estimated using the abbreviated Modification of Diet in Renal Disease study equation.7 Body surface area (BSA) was estimated by a formula of DuBois.
BP Monitoring
Data for office BP were collected from patient files. Mean values of systolic BP (SBP) and diastolic BP (DBP) measurements at the last three visits prior to the study were used. These measurements were taken manually at morning clinics while the patient was seated. The measurements were not standardized as they were taken by different people.
ABPM was performed with the RZ250 model ABP recorder (Rozinn Electronics Inc, Glendale, NY) and the SE‐25S model BP monitor (Genexel‐Sein Inc, Kyunggi‐do, South Korea). An appropriately sized cuff for arm circumference was used with no arteriovenous fistula. Both devices were calibrated against a BP monitor calibrator (BP Pump 2; Fluke Biomedical, Everett, WA) at the beginning of the study. BP was measured every 30 minutes during daytime (8 am to 12 pm) and every 60 minutes during nighttime (12 pm to 8 am). A nocturnal BP fall of >10% of the daytime values were accepted as an arbitrary cutoff value to define patients as “dippers.”
Means of the office BP and ABPM values at 24 hours, daytime (awake), and nighttime (asleep) were recorded and compared. Thresholds for office BP were set at ≥130 mm Hg for SBP and/or ≥80 mm Hg for DBP in accordance with Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.8 Thresholds for ABPM were defined on the basis of average 24‐hour measurements of SBP ≥130 mm Hg and/or DBP ≥80 mm Hg, daytime measurements of SBP ≥135 mm Hg and/or DBP ≥85 mm Hg, and nighttime measurements of SBP ≥120 mm Hg and/or DBP ≥70 mm Hg according to the definition of HTN by out‐of‐office BP levels of the European Hypertension Society/European Cardiology Society.9 Ambulatory HTN was defined as a BP exceeding any of the above thresholds.
Echocardiography
Echocardiography was performed by standard methods by an experienced cardiologist blinded to patient characteristics using a Vivid 3 ultrasound system (General Electric, Milwaukee, WI) with a 1.7 MHz transducer. Left ventricular internal diameter at end‐diastole (LVIDd), interventricular septal thickness at end‐diastole (IVSTd), and posterior wall thickness at end‐diastole (PWTd) were measured from the parasternal long‐axis view according to the American Society of Echocardiography recommendations.10 Left ventricular mass (LVM) was calculated by the following formula: LVM(g)=1.04x ([LVIDd+PWTd+IVSTd]3–[LVIDd]3).11 LVM index (LVMI) was calculated as the ratio of LVM to BSA. The cutoff level for LVH was defined as LVMI ≥134 g/m2 in men and ≥110 g/m2 in women.12
Left ventricular volumes at end‐diastole (EDV) and end‐systole (ESV) were estimated using the “area‐length” method. Values were given for end‐diastolic diameter and end‐systolic diameter. From these measurements, left ventricular ejection fraction (EF=[EDV–ESV]/EDV) was calculated.
Measurements were made over three cardiac cycles and the mean values were calculated. Diastolic dysfunction was staged according to methods previously described.13
Statistical Analysis
Data were expressed as mean±standard deviation unless otherwise stated. Categorical variables were compared using chi‐square test. Continuous variables were compared using independent samples t test. Associations between continuous variables were evaluated by means of Pearson's correlation test. Bland‐Altman plots were used to visually assess the agreements between ABPM and office BP. All tests were performed using SPSS for Windows version 17.0 software (SPSS Inc, Chicago, IL). A P value <.05 was considered statistically significant.
Ethics
All patients gave informed consent and the study protocol was approved by the local medical ethics committee.
Results
Demographic features, clinical characteristics, biochemical parameters, use of immunosuppressive medications, and echocardiographic measurements of the study patients are shown in Table 1. Recipients were generally young and female. Most transplants were performed from living donors. Patients were followed up for 60.3±53 (median: 37, range: 13–254) months. Estimated GFR values were in the range between 43 mL/min/1.73 m2 and 130.4 mL/min/1.73 m2 (median: 85 mL/min/1.73 m2). Generally a calcineurin inhibitor–based triple immunosuppression protocol was used (steroids, 98.8%; tacrolimus, 79.3%; and mycophenolic acid derivatives, 78%). A total of 64.4% of all patients were taking antihypertensive medication consisting of mostly renin‐angiotensin‐aldosterone system blockers including angiotensin‐converting enzyme inhibitors (18.4%) and angiotensin receptor blockers, (28%) followed by β‐blockers (39.1%) and calcium channel blockers (14.9%). EF ranged between 45% and 80% (median: 61%). LVMI was increased in 21.8% of renal allograft recipients (female: 18.4%, male: 24.5%). Diastolic dysfunction was also present in 32% of renal transplantation patients, and 20.7% of recipients had stage 1 and 11.5% had stage 2 diastolic dysfunction.
Table 1.
Demographic Features, Clinical Characteristics, Biochemical Parameters, Immunosuppressive Medication Use, and Echocardiographic Measurements of Study Patients at Study Time
| Patients (N=87) | |
|---|---|
| Age, y | 37.8±11.6 |
| Male sex, % | 43.7 |
| Cause of chronic renal failure, % | |
| Unknown origin | 52.9 |
| Others | 18.4 |
| Chronic glomerulonephritis | 12.6 |
| Reflux | 9.2 |
| Hypertension | 6.9 |
| Cadaveric transplantation, % | 23 |
| Previous renal replacement therapy time, mo | 36.5±40.7, median 21 (0–252) |
| Working fistula rate, % | 42.5 |
| Urea, mg/dL | 33.9±10.5 |
| Creatinine, mg/dL | 0.95±0.2 |
| MDRD, mL/min/m2 | 84.9±19.7 |
| Uric acid, mg/dL | 5.0±1.5 |
| Fasting blood glucose, mg/dL | 86.2±9.7 |
| Albumin, g/dL | 4.0±0.2 |
| Hemoglobin, g/dL | 13.4±1.8 |
| Total cholesterol, mg/dL | 193.0±32.7 |
| Triglycerides, mg/dL | 148.3±78.8 |
| LDL cholesterol, mg/dL | 113.4±26.2 |
| HDL cholesterol, mg/dL | 52.6±15.7 |
| Steroid, % | 98.9 |
| Tacrolimus, % | 79.3 |
| Cyclosporine, % | 13.8 |
| Mycophenolate mofetil, % | 34.5 |
| Mycophenolate sodium, % | 42.5 |
| Azathioprine, % | 12.1 |
| Rapamycin, % | 1.1 |
| Ejection fraction, % | 64.3±5.8 |
| Left ventricular mass index, g/m2 | 101.6±25.2 |
| Presence of diastolic dysfunction, % | 32.2 |
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MDRD, Modification of Diet in Renal Disease.
Table 2 shows BP measurements obtained by the two methods––office BP and ABPM. The office BP method showed higher mean SBP and DBP values than that obtained by 24‐hour ABPM (P<.001). Pearson correlations between office and 24‐hour ABPM measurements were 0.275 for mean SBP (P=.011) and 0.260 for mean DBP (P=.017). According to Bland‐Altman analysis, we estimated with 95% confidence that the mean SBP value obtained by office BP was 8.3 mm Hg (38.1 [−21.5] mm Hg) higher than the mean SBP value obtained by 24‐hour ABPM (Figure) and the mean DBP value obtained by office BP monitoring was 6.4 mm Hg (27.2 [−14.2] mm Hg) higher than the mean DBP value obtained by 24‐hour ABPM.
Table 2.
BP Measurements Obtained With Office BP and ABPM
| Systolic | Diastolic | |
|---|---|---|
| Office BP, mm Hg | 123.8±13.1 | 79.6±8.7 |
| Average daytime ABPM, mm Hg | 117.8±12.3 | 75.1±9.3 |
| Average nighttime ABPM, mm Hg | 110.5±12.8 | 69.2±8.6 |
| Average 24‐h ABPM, mm Hg | 116.1±12.1 | 73.7±8.9 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure.
Figure 1.
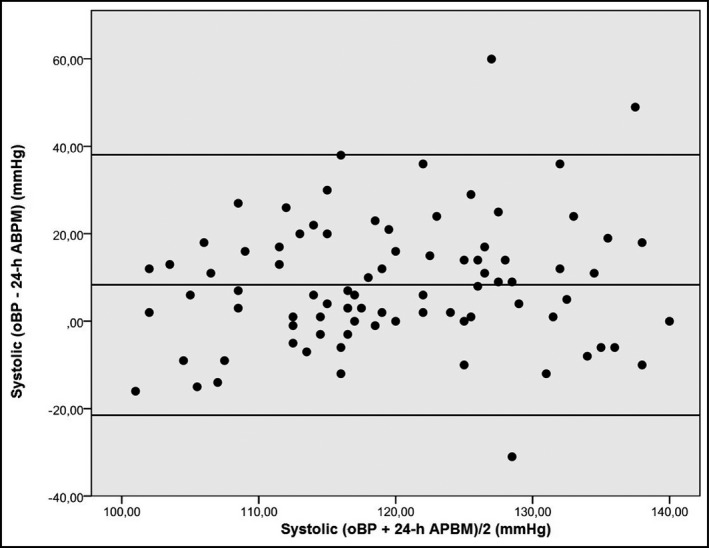
Bland‐Altman plots showing the agreement between systolic office blood pressure (oBP) and systolic 24‐hour ambulatory blood pressure monitoring (ABPM).
In our study, 20.7% of patients had concordant normotension and 36.8% had concordant HTN. Fifty‐three patients (60.9%) had office BP of at least 130/80 mm Hg, giving an overall white‐coat HTN rate of 24.1%. We found that 32 patients (36.8%) were normotensive according to office BP. However, after ABPM, we found that 43.8% of these patients were hypertensive. In Table 3, we compared day and night BP measurements between these two groups, with masked HTN and normotension defined as no HTN values obtained in office BP and ABPM. Circadian BP patterns showed a higher proportion of nondippers (67.8%). Demographic features, laboratory data, day and night BP measurements, and echocardiographic measurements of the dipper vs nondipper groups are shown in Table 4. Mean values of SBP and DBP depending on 24‐hour ABPM were not significantly different in the nondipper group compared with the dipper group. EF of the dipper group was significantly higher compared with that of the nondipper group. However, we did not find any significant correlation between dipping status and LVMI or between dipping status and diastolic dysfunction. There were no significant differences between the nondipper and dipper groups regarding age at transplantation, duration of follow‐up after transplantation, donor type, presence of functional fistula, and antihypertensive medication use (data not shown).
Table 3.
Day and Night BP Measurements in the Masked Hypertension vs Normotensiona Groups
| Masked Hypertension Group (n=14) | Normotension Group (n=18) | P Value | |
|---|---|---|---|
| Office SBP, mm Hg | 113.3±7.5 | 111.9±8.9 | .641 |
| Office DBP, mm Hg | 73.1±4.9 | 71.1±5.6 | .311 |
| Average daytime sABPM, mm Hg | 117.6±11.0 | 111.2±7.6 | .060 |
| Average daytime dABPM, mm Hg | 77.8±5.8 | 69.5±5.3 | .000 |
| Average nighttime sABPM, mm Hg | 114.1±8.4 | 101.6±9.1 | .000 |
| Average nighttime dABPM, mm Hg | 73.6±3.6 | 61.6±6.9 | .000 |
| Average 24‐h sABPM, mm Hg | 117.1±10.0 | 107.9±6.9 | .004 |
| Average 24‐h dABPM, mm Hg | 76.6±5.0 | 67.4±4.8 | .000 |
Abbreviations: BP, blood pressure; dABPM, diastolic ambulatory blood pressure monitoring; DBP, diastolic blood pressure; SBP, systolic blood pressure; sABPM, systolic ambulatory blood pressure monitoring. aNormotension group: no hypertensive values were obtained in both office BP and ambulatory blood pressure monitoring measurements.
Table 4.
Demographic Features, Laboratory Data, and Day and Night Blood Pressure Measurements of the Dipper vs Nondipper Groups
| Dipper (n=28) | Nondipper (n=59) | P Value | |
|---|---|---|---|
| Age, y | 36.1±9.7 | 38.8±12.4 | .308 |
| Male sex, % | 42.8 | 41.3 | .896 |
| Urea, mg/dL | 33.0±10.0 | 34.2±10.9 | .623 |
| Creatinine, mg/dL | 0.98±0.24 | 0.94±0.20 | .396 |
| Uric acid, mg/dL | 4.8±1.8 | 5.1±1.4 | .331 |
| MDRD, mL/min/1.73 m2 | 82.3±21.6 | 85.9±18.9 | .435 |
| Fasting blood glucose, mg/dL | 87.4±8.3 | 85.5±10.3 | .393 |
| Albumin, g/dL | 4.05±0.27 | 4.09±0.29 | .545 |
| Total cholesterol, mg/dL | 198.2±29.0 | 191.3±34.3 | .361 |
| Triglycerides, mg/dL | 150.8±75.1 | 147.5±81.8 | .857 |
| LDL cholesterol, mg/dL | 116.6±24.8 | 112.4±27.0 | .489 |
| HDL cholesterol, mg/dL | 54.4±16.5 | 51.9±15.5 | .501 |
| Office SBP, mm Hg | 125.5±13.3 | 123.3±13.1 | .476 |
| Office DBP, mm Hg | 80.3±9.5 | 79.4±8.5 | .643 |
| Average daytime sABPM, mm Hg | 122.0±14.5 | 115.6±10.7 | .023 |
| Average daytime dABPM, mm Hg | 78.9±11.8 | 73.3±7.3 | .027 |
| Average nighttime sABPM, mm Hg | 103.8±11.4 | 113.7±12.2 | .000 |
| Average nighttime dABPM, mm Hg | 65.3±9.9 | 71.1±7.3 | .003 |
| Average 24‐h sABPM, mm Hg | 117.7±14.6 | 115.3±10.8 | .400 |
| Average 24‐h dABPM, mm Hg | 75.8±11.8 | 72.7±7.1 | .202 |
| Ejection fraction, % | 66.5±4.5 | 63.3±6.1 | .016 |
| LVMI, g/m2 | 100.2±21.8 | 101.9±26.9 | .768 |
| Presence of diastolic dysfunction, % | 32.1 | 32.7 | .954 |
Abbreviations: dABPM, diastolic ambulatory blood pressure monitoring; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVMI, left ventricular mass index; MDRD, Modification of Diet in Renal Disease; sABPM, systolic ambulatory blood pressure monitoring; SBP, systolic blood pressure.
Table 5 shows the significant relationship between SBP parameters of ABPM and LVMI. However, there were no significant correlations between EF and ABPM parameters (data not shown).
Table 5.
The Relationship Between SBP Parameters and Left Ventricular Mass Index
| R | P Value | |
|---|---|---|
| Average daytime ABPM, mm Hg | 0.240 | .025 |
| 24‐h ABPM, mm Hg | 0.242 | .025 |
| Office SBP, mm Hg | 0.220 | .042 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; SBP, systolic blood pressure.
We also compared demographic, clinical, biochemical, and BP data among patients with and without diastolic dysfunction, we found that patients with diastolic dysfunction were older (P<.001), had received organs from mostly cadaveric donors (P=.027), and had higher triglyceride levels (P=.025).
Discussion
This study examined the relationship between BP measurements using ABPM and office BP in kidney transplant recipients with a good functioning graft. HTN is common following renal transplantation, affecting up to 80% of transplant recipients depending on the method of BP measurement.14 Posttransplant HTN is a multifactorial phenomenon and the known causes of HTN include immunosuppressive drugs, renal graft artery stenosis, recipient's native kidney, allograft dysfunction, obesity, recurrent or de novo renal disease, and genetic factors.15 However, optimal office and ambulatory BP targets have not been well‐defined in the renal transplant populatıon and there is variance depending on the cutoffs and series.16, 17
Some studies comparing both methods––office BP and ABPM––among nontransplant populations have shown BP values at home to be lower than those obtained in the office.18, 19 The difference between the two methods may be the result of the white‐coat effect, an elevation of BP in a clinic setting leading to possible overestimation of true BP. ABPM allows for the recognition of the white‐coat effect to be responsible for a proportion of resistant hypertensive patients.20 In our data, 24.1% of patients were considered to have white‐coat HTN. This is nearly in agreement with previous studies where white‐coat effect was present in 20% to 40%.16, 21 The concordance rates between office BP and ABPM change according to the studies. In a previous study, Stenehjem and colleagues21 showed a better correlation between these methods. The agreement between two methods as evaluated by means of Bland‐Altman analysis in our study was similar to previous larger‐scale studies.19, 22 However, we want to point out that we used the mean of three consecutive measurements reflecting a period of 6 to 9 months collected from patient files rather than the mean of a daily measurement.
A higher proportion of nondipping pattern among patients who had been transplanted for at least 1 year with a good functioning graft is another important finding in our study. In our study, 67.8% of patients were classified as nondippers. In many previous studies, nondipping pattern was reported to be between 25% and 90%.4, 16, 23, 24, 25 Loss of normal diurnal BP variation, which has been described to occur among patients with chronic kidney disease, has been associated with a greater rate of CVD events as well as progression of renal disease.26 Each 5‐mm Hg drop in nocturnal SBP was associated with a 14% reduction in risk of cardiovascular events at follow‐up.27 The status of dipper or nondipper was found independently from BP control and we did not find any parameter predicting this abnormality. However, in a previous study, Galiatsou and colleagues28 studied 46 renal transplant patients and concluded that cyclosporine significantly increased nighttime BP. In another study, Gatzka and colleagues29 found that normalization of the circadian blood profile with a marked decrease of BP during sleep was associated with longer transplantation duration independent from immunosuppressive agents used. Haydar and colleagues22 found that only age and GFR independently predicted diurnal BP variation (the ratio of the asleep‐to‐awake SBP).
Many factors affect the development of LVH among hemodialysis patients, including uremic toxins, anemia, hypoalbuminemia, hyperparathyroidism, arteriovenous fistula, HTN, and volume overload.30 Although renal transplantation improves some risk factors, LVH is still common in transplant recipients. Various studies31, 32, 33, 34, 35are in agreement with ours showing that LVMI is related to high SBP, but some authors36, 37 were unable to find any correlation between LVH and BP values.
We also examined the effect of dipping status on cardiac functions and we found that EF was lower in nondippers compared with dippers. However, it should be noted that EF was well within normal range in all study patients. We also did not find any association between dipping status and LVMI. In our study, BP was similar in both groups. Lipkin and colleagues38 studied 28 normotensive RTRs, of whom seven (25%) were nondippers who had significantly higher LVMI compared with dippers. Toprak and colleagues39 also demonstrated in 35 nondiabetic RTRs that nighttime SBP load was closely related to the increase in LVMI. On the other hand, similar to our findings, McGregor and colleagues40 and Seeman and colleagues37 did not find any relationship between dipping status and LVMI.
Study Limitations
Our study had some limitations. First, it was a cross‐sectional study and therefore it was hard to infer causality. Our office BP measurements were not standardized in any way. In addition, we examined a special group excluding patients with documented diabetes mellitus and decreased graft function; therefore, generalizability of our results may be limited. A longitudinal study would provide better data to assess BP control and cardiac functions.
Conclusions
Our study demonstrated that posttransplant HTN is poorly controlled and treated, particularly nocturnal HTN in RTRs. LVH is common among transplant patients and is likely related to hypertensive values obtained by ABPM. We showed that ABPM is a valuable tool in detecting dipping status, white‐coat HTN, and masked HTN, which are frequent problems among RTRs. We think that ABPM and office BP measurements should be regarded as complementary methods. However, longitudinal studies are warranted to prove the prognostic value of BP control based on ABPM to reduce target organ damage among RTRs.
J Clin Hypertens (Greenwich). 2016;18:766–771. DOI: 10.1111/jch.12755. © 2015 Wiley Periodicals, Inc.
References
- 1. Mange KC, Cizman B, Joffe M, et al. Arterial hypertension and renal allograft survival. JAMA. 2000;283:633–638. [DOI] [PubMed] [Google Scholar]
- 2. Ojo AO, Hanson JA, Wolfe RA, et al. Long‐term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307–313. [DOI] [PubMed] [Google Scholar]
- 3. Premasathian NC, Muehrer R, Brazy PC, et al. Blood pressure control in kidney transplantation: therapeutic implications. J Hum Hypertens. 2004;18:871–877. [DOI] [PubMed] [Google Scholar]
- 4. Covic AC, Segall L, Goldsmith DJA. Ambulatory blood pressure monitoring in renal transplantation: should ABPM be routinely performed in renal transplant patients? Transplantation. 2003;76:1640–1642. [DOI] [PubMed] [Google Scholar]
- 5. Peixoto AJ, White WB. Ambulatory blood pressure monitoring in chronic renal disease: technical aspects and clinical relevance. Curr Opin Nephrol Hypertens. 2002;11:507–516. [DOI] [PubMed] [Google Scholar]
- 6. White WB, Schulman P, McCabe EJ, et al. Average daily blood pressure, not office blood pressure, determines cardiac function in patients with hypertension. JAMA. 1989;261:873–877. [PubMed] [Google Scholar]
- 7. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 8. KDIGO . KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(suppl 3):S1–S155. [DOI] [PubMed] [Google Scholar]
- 9. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force of the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:2018–2024. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 11. Troy BL, Pombo J, Rackley CE. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation. 1972;45:602–611. [DOI] [PubMed] [Google Scholar]
- 12. Reichek N, Devereux RB. Reliable estimation of peak left ventricular systolic pressure by M‐mode echographic‐determined end‐diastolic relative wall thickness: identification of severe valvular aortic stenosis in adult patients. Am Heart J. 1982;103:202–203. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong WF. Evaluation of left ventricular diastolic function. In: Feigenbaum's Echocardiography. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:175. [Google Scholar]
- 14. Kasiske B, Anjum S, Shah R, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071–1081. [DOI] [PubMed] [Google Scholar]
- 15. Buescher R, Vester U, Wingen A‐M, et al. Pathomechanism and the diagnosis of arterial hypertension in pediatric renal allograft recipients. Pediatr Nephrol. 2004;19:1202–1211. [DOI] [PubMed] [Google Scholar]
- 16. Arias‐Rodríguez M, Fernández‐Fresnedo G, Campistol JM, et al. RETENAL Group (Control of Resistant Hypertension in Renal Transplant. Prevalence, Significance) . Prevalence and clinical characteristics of renal transplant patients with true resistant hypertension. J Hypertens. 2015;33:1074–1081. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed J, Ozorio V, Farrant M, Van Der Merwe W. Ambulatory vs office blood pressure monitoring in renal transplant recipients. J Clin Hypertens. 2015;17:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stergiou GS, Skeva II, Baibas NM, et al. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18:1745–1751. [DOI] [PubMed] [Google Scholar]
- 19. Fernandez Fresnedo G, Franco Esteve A, Gómez Huertas E, et al. Ambulatory blood pressure monitoring in kidney transplant patients: RETENAL study. Transplant Proc. 2012;44:2601–2602. [DOI] [PubMed] [Google Scholar]
- 20. Fernández‐Vega F, Tejada F, Baltar J, et al. Ambulatory blood pressure after renal transplantation. Nephrol Dial Transplant. 2001;16(suppl 1):110–113. [DOI] [PubMed] [Google Scholar]
- 21. Stenehjem AE, Gudmundsdottir H, Os I. Office blood pressure measurements overestimate blood pressure control in renal transplant patients. Blood Press Monit. 2006;11:125–133. [DOI] [PubMed] [Google Scholar]
- 22. Haydar AA, Covic A, Jayawardene S, et al. Insights from ambulatory blood pressure monitoring: diagnosis of hypertension and diurnal blood pressure in renal transplant recipients. Transplantation. 2004;77:849–853. [DOI] [PubMed] [Google Scholar]
- 23. Kooman JP, Christiaans MH, Boots JM, et al. A comparison between office and ambulatory blood pressure measurements in renal transplant patients with chronic transplant nephropathy. Am J Kidney Dis. 2001;37:1170–1176. [DOI] [PubMed] [Google Scholar]
- 24. Sorof JM, Poffenbarger T, Portman R. Abnormal 24‐hour blood pressure patterns in children after renal transplantation. Am J Kidney Dis. 2000;35:681–686. [DOI] [PubMed] [Google Scholar]
- 25. Farmer CK, Goldsmith DJ, Cox J, et al. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant. 1997;12:2301–2307. [DOI] [PubMed] [Google Scholar]
- 26. Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol. 2002;39:878–885. [DOI] [PubMed] [Google Scholar]
- 27. Hermida RC, Ayala DE, Mojón A, Fernández JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galiatsou E, Morris ST, Jardine AG, et al. Cardiac and vascular abnormalities in renal transplant patients: differential effects of cyclosporin and azathioprine. J Nephrol. 2000;13:185–192. [PubMed] [Google Scholar]
- 29. Gatzka CD, Schobel HP, Klingbeil AU, et al. Normalization of circadian blood pressure profiles after renal transplantation. Transplantation. 1995;59:1270–1274. [PubMed] [Google Scholar]
- 30. Parfrey PS, Harnett JD, Foley RN, et al. Impact of renal transplantation on uremic cardiomyopathy. Transplantation. 1995;60:908–914. [PubMed] [Google Scholar]
- 31. Matteucci MC, Giordano U, Calzolari A, et al. Left ventricular hypertrophy, treadmill tests, and 24‐hour blood pressure in pediatric transplant patients. Kidney Int. 1999;56:1566–1570. [DOI] [PubMed] [Google Scholar]
- 32. Ten Harkel AD, Cransberg K, Van Osch‐Gevers M, Nauta J. Diastolic dysfunction in paediatric patients on peritoneal dialysis and after renal transplantation. Nephrol Dial Transplant. 2009;24:1987–1991. [DOI] [PubMed] [Google Scholar]
- 33. Basiratnia M, Esteghamati M, Ajami GH, et al. Blood pressure profile in renal transplant recipients and its relation to diastolic function: tissue Doppler echocardiographic study. Pediatr Nephrol. 2011;26:449–457. [DOI] [PubMed] [Google Scholar]
- 34. Sezer S, Uyar ME, Colak T, et al. Left ventricular mass index and its relationship to ambulatory blood pressure and renal resistivity index in renal transplant recipients. Transplant Proc. 2013;45:1575–1578. [DOI] [PubMed] [Google Scholar]
- 35. Montanaro D, Gropuzzo M, Tulissi P, et al. Effects of successful renal transplantation on left ventricular mass. Transplant Proc. 2005;37:2485–2487. [DOI] [PubMed] [Google Scholar]
- 36. Morgan H, Khan I, Hashmi A, et al. Ambulatory blood pressure monitoring after renal transplantation in children. Pediatr Nephrol. 2001;16:843–847. [DOI] [PubMed] [Google Scholar]
- 37. Seeman T, Šimková E, Kreisinger J, et al. Control of hypertension in children after renal transplantation. Pediatr Transplant. 2006;10:316–322. [DOI] [PubMed] [Google Scholar]
- 38. Lipkin GW, Tucker B, Giles M, Raine AEG. Ambulatory blood pressure and left ventricular mass in cyclosporine‐ and non‐cyclosporin‐treated renal transplant recipients. J Hypertens. 1993;11:39–442. [DOI] [PubMed] [Google Scholar]
- 39. Toprak A, Koc M, Tezcan H, et al. Night‐time blood pressure load is associated with higher left ventricular mass index in renal transplant recipients. J Hum Hypertens. 2003;17:239–244. [DOI] [PubMed] [Google Scholar]
- 40. McGregor DO, Olsson C, Lynn KL. Autonomic dysfunction on ambulatory blood pressure in renal transplant recipients. Transplantation. 2001;71:1277–1281. [DOI] [PubMed] [Google Scholar]


