Abstract
Hypertension during pregnancy and preeclampsia are associated with increased arterial thrombotic risk in later life. Whether these complications are associated with risk of venous thromboembolism (VTE) on the short term after pregnancy and on the long term, that is, outside pregnancy, is largely unknown. We conducted a nationwide cohort study in women with at least 1 pregnancy and their first VTE risk by linking the Dutch perinatal registry (Perined) to anticoagulation clinics. We used Cox proportional hazard models to estimate hazard ratios (HRs) and corresponding 95% CI for VTE risk in women with hypertension during pregnancy, women with preeclampsia, compared with women with uncomplicated pregnancies (reference). A total of 1 919 918 women were followed for a median of 13.7 (interquartile range, 7.6–19.2) years for a total of 24 531 118 person-years in which 5759 first VTEs occurred; incidence rate: 2.3 (95% CI, 2.3–2.4) per 10 000 person-years. In the first pregnancy and 3-month postpartum period, VTE risk was higher in women with hypertension, HR, 2.0 (95% CI, 1.7–2.4), and highest among women with preeclampsia, HR, 7.8 (95% CI, 5.4–11.3), versus the reference group. On the long term, women with hypertension during pregnancy and preeclampsia had a higher VTE risk: HR, 1.5 (95% CI, 1.4–1.6) and HR, 2.1 (95% CI, 1.8–2.4), respectively, versus the reference group. When excluding events during pregnancy and postpartum, these HRs were 1.4 (95% CI, 1.3–1.5) and 1.6 (95% CI, 1.4–2.0), respectively. In conclusion, hypertension during pregnancy and preeclampsia are associated with an increased VTE risk during pregnancy and postpartum period and in the 13 years after.
Keywords: hypertension, preeclampsia, pregnancy, risk, venous thromboembolism
Approximately 10% of all pregnancies are complicated by high blood pressure, collectively called hypertensive disorders of pregnancy.1 One of the most severe hypertensive complications of pregnancy is preeclampsia. Preeclampsia is a disorder of pregnancy characterized by the onset of high blood pressure and often a significant amount of protein in the urine.2 When it arises, the condition begins after 20 weeks of pregnancy.2 Preeclampsia occurs in ≈2% of all and 4% of first pregnancies.3 The occurrence of hypertensive disorders is associated with higher risk of arterial cardiovascular diseases (ie, myocardial infarction and ischemic stroke) in later life, with the highest risk in women who have a history of preeclampsia.4,5
Venous thromboembolism (VTE) and arterial cardiovascular disease have been traditionally regarded as separate diseases with distinct causes and treatment. However, several studies in the past decade suggest some overlap in the pathophysiology of VTE and arterial cardiovascular disease.6–10 VTE is also a major contributor to maternal morbidity and mortality.11 In pregnancy, the risk of VTE is about 5-fold higher than during the nonpregnant situation, even when the pregnancy proceeds without apparent complications.11–13 Preeclampsia is associated with an even higher risk of VTE during the same pregnancy than uncomplicated pregnancies, although studies that looked into this were small numbered.14–18 Whether the occurrence of preeclampsia is also associated with a long-term increased risk of VTE, that is, also after pregnancy, is largely unknown. Moreover, for hypertension during pregnancy (ie, without the occurrence of preeclampsia), an association with VTE has not been clearly established, either during or after pregnancy.
In this large cohort study of pregnant women in the Netherlands (n=1 919 918 women with at least 1 pregnancy), we set out to determine whether hypertensive disorder of pregnancy is a risk factor for VTE both during pregnancy as in the following years.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
This is a nationwide cohort study that covers the entire area of the Netherlands, in which we obtained data between 1999 and 2012 from the Dutch Perinatal Registry (Perined). The Dutch Perinatal Registry is a linked database of medical registries from 4 professional groups that provide perinatal care in the Netherlands: general practitioner, midwives, gynecologists, and neonatologists/pediatricians. Perined captures information on 95% to 99% of the ≈180 000 annual deliveries (gestational age >22 weeks or fetal weight >500 g when duration is unknown) in the Netherlands.19 Data are collected at all stages and settings during pregnancy. Among the items collected are details concerning labor, birth and neonatal outcome, like the mode of delivery, maternal demographics, and medical conditions, like data on diastolic blood pressure during pregnancy and urinary protein levels. The data from the different professionals are joined so all information about the same child is combined in 1 record. In absence of an unique identifier from mother or child, the linkage is probabilistic and based on corresponding items, like date of birth and postal code. The probabilistic linkage is statistically founded and tests the hypothesis that 2 records describe the same individual.
Hypertensive Pregnancy Complications
In this study, hypertensive disorders of pregnancy were the exposures of interest. Hypertension of pregnancy was defined as the (at least on 1 occasion) highest diastolic blood pressure of 90 mm Hg or higher during the course of pregnancy, without proteinuria of ≥300 mg per 24 hours. Preeclampsia was defined as the (at least on 1 occasion) highest diastolic blood pressure of 90 mm Hg or higher during the course of pregnancy with the presence of proteinuria of ≥300 mg per 24 hours, as adapted from the official definition of the International Society for the Study of Hypertension in Pregnancy.20 The control group consisted of women with an uncomplicated pregnancy and who had a diastolic blood pressure <90 mm Hg during the course of pregnancy. The rationale for only using diastolic blood pressure to determine hypertension was based on the information available in the registry (ie, complete for diastolic blood pressure, incomplete for systolic blood pressure).
Linkage to Anticoagulation Clinics
To capture the outcome of interest, that is, the occurrence of VTE during follow-up, the data from the Dutch Perinatal Registry were linked to data from the anticoagulation clinics in the Netherlands, based on the participants’ full date of birth and postal code. anticoagulation clinics in the Netherlands monitor all patients managed with vitamin K antagonists (VKAs). Of all anticoagulation clinics in the Netherlands, n=48 of 49 (98%) participated in this study. The anticoagulation clinics that participated provided data on all women born after 1949 managed with VKAs for deep vein thrombosis, pulmonary embolism or both. The exact start dates of the management indication and the date of VTE diagnoses were provided. In addition, information on the type of event, that is, first or recurrent VTE, was specified. The Perined registry was linked to the data from the anticoagulation clinics including data up until August 1, 2017.
Deep Vein Thrombosis and Pulmonary Embolism Diagnoses
In the Netherlands, according to national guidelines, objective imaging techniques are required to establish a diagnosis of VTE. Typically, these consist of compression ultrasound of the legs for deep vein thrombosis and computed tomographic pulmonary angiography or ventilation perfusion scanning for pulmonary embolism.21 After a diagnosis of VTE, patients managed with VKAs are referred to an anticoagulation clinic for monitoring of international normalized ratio and adequate dosing.
Statistical Analyses
Observation time started at the start of the first pregnancy in the Perined registry and ended on the date of either a first VTE event, maternal death, or the date on which the Perined registry was linked to the data from the anticoagulation clinics, that is, August 1, 2017. The incidence rate with 95% CIs (based on a Poisson distribution) of first VTE events was calculated by dividing the number of VTEs by the total amount of observation time. We used Cox proportional hazard models to estimate hazard ratios (HRs) and corresponding 95% CIs for the risk of VTE in women with hypertensive disorders of pregnancy compared with women with uncomplicated pregnancies. The study design is shown in Figure 1. First, we assessed the risk of VTE during the first pregnancy and 3-month postpartum period included in the Perined registry (Figure 1A). Next, we assessed the risk of VTE during the complete follow-up period, where we included all VTE events regardless of whether they occurred during a pregnancy or 3-month postpartum period outside of pregnancy (Figure 1B). Last, we investigated whether hypertension during pregnancy and preeclampsia were associated with an increased risk of VTE exclusively in the period outside pregnancy. To do so, we studied the risk of VTE during the complete follow-up period but excluded VTE events that occurred during a pregnancy of 3-month postpartum period herewith only counting events that occurred outside of pregnancy (Figure 1C). We compared the rates of VTE among women with uncomplicated pregnancies during follow-up (reference group), women with hypertension during pregnancy in at least 1 pregnancy during follow-up, and women with preeclampsia in at least 1 pregnancy during follow-up. To further explore the nature of a potential association, analyses were adjusted for potential confounders on which information was available: number of pregnancies, age at start of follow-up, and self-reported ancestry.
Figure 1.
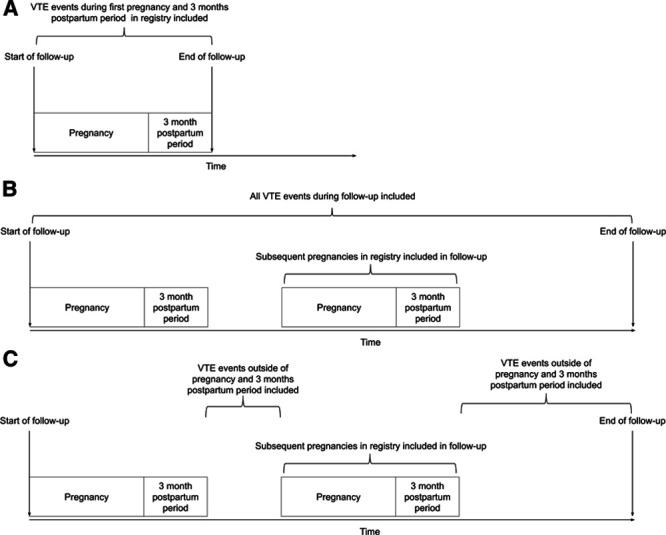
Overview of the study design and performed analyses. First, in analysis A, we assessed the risk of venous thromboembolism (VTE) during the first pregnancy and 3 mo postpartum period included in the study for women. Second, in analysis B, we assessed this risk of VTE during the complete follow-up including both events that occurred during pregnancy and 3 mo postpartum period, as well as events that occurred outside of pregnancy. Last, in analysis C, the complete follow-up time was used, yet events that occurred during pregnancy or 3 mo postpartum period were excluded.
Results
Selection of the Study Population
The flowchart of the study population is shown in Figure 2. There were 2 824 012 records of women with at least 1 pregnancy in the Perined registry that had a maternal date of birth and postal code available. Of these, there were 758 839 (27%) women with an identical date of birth and postal code, which were all excluded from the linkage. As a result, a total of 2 065 173 women with a first pregnancy had data available for linkage. In the dataset of the anticoagulant clinics, data on 38 580 women born after 1949 who received VKA for deep vein thrombosis or pulmonary embolism were available. Of these, 2405 (6%) women had an identical combination of postal code and date of birth, leaving 36 175 women for the linkage procedure. Linkage of these datasets based on date of birth and postal code yielded 7996 matches (ie, these women had at least 1 pregnancy in the Perined registry and had been treated for VTE at one of the anticoagulation clinics).
Figure 2.
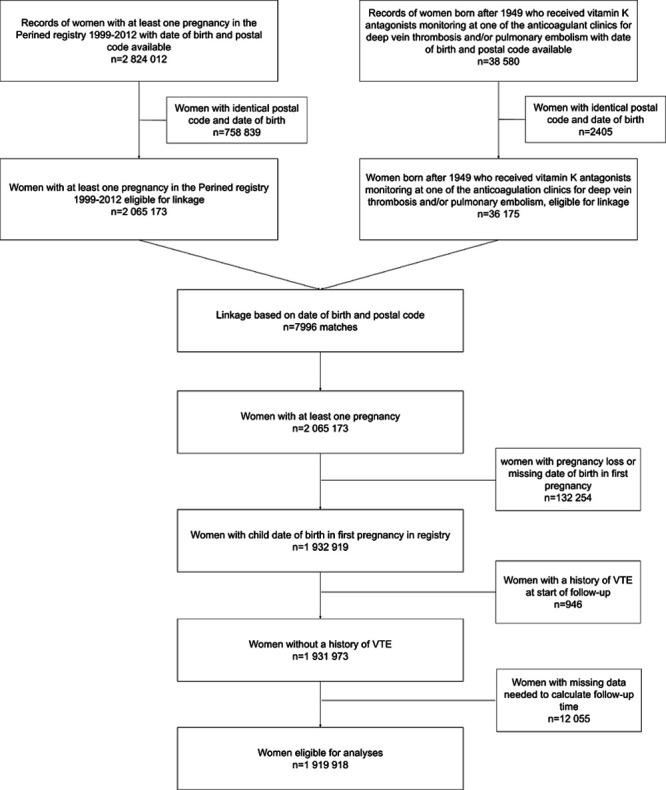
Flowchart of the study population and linkage procedure. There were 2 824 012 records of women with at least 1 pregnancy in the Perined registry that had a maternal date of birth and postal code available. Of these, there were 758 839 (27%) women with an identical date of birth and postal code, which were all excluded from the linkage, leaving 2 065 173 women. In the data of the anticoagulant clinics, data on 38 580 women born after 1949 who received vitamin K antagonists for deep vein thrombosis and pulmonary embolism were available. Of these, 2405 (6%) women had an identical combination of postal code and date of birth, leaving 36 175 women for the linkage. Of 2 065 173 women in the with at least 1 pregnancy, 1 932 919 had a date of birth of child in the first pregnancy in the registry available. Of these, 946 with a history of venous thromboembolism were excluded. Of the remaining, 1 919 918 had data available to calculate follow-up time and were included in the analyses.
Of the 2 065 173 women with a first pregnancy, 132 254 (6.4%) had a pregnancy loss or had a missing date of birth in the first pregnancy. As for these women the start of follow-up could not be determined, they were excluded from further analyses. Of the remaining 1 932 919 women, 946 (<0.1%) had a history of VTE at start of follow-up and were excluded. Last, of the 1 931 973 women who were left, 12 055 (0.6%) had missing data on values needed for calculation of follow-up, leaving a total of 1 919 918 eligible for the analyses.
Clinical Characteristics of the Study Population
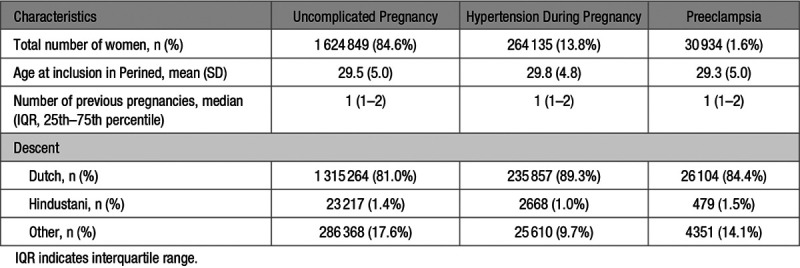
The 1 919 918 women included in the analyses were followed for a median of 13.7 years, that is, a total of 24 531 118 person-years in which 5759 first VTEs occurred, for an overall VTE incidence rate of 2.3 (95% CI, 2.3–2.4) per 10 000 person-years. The clinical characteristics of the study population are shown in Table 1. Of the 1 919 918 women in the analyses, 1 624 849 (84.6%) had uncomplicated pregnancies, 264 135 (13.8%) had ≥1 pregnancies complicated by hypertension and 30 934 (1.6%) had ≥1 pregnancies complicated by preeclampsia. The mean age at start of follow-up was similar among groups at about 29 years. The median number of previous pregnancies was 1 in all groups. There were differences in proportions of self-reported descent among the groups, with a slightly higher proportion of 89.3% Dutch in the hypertension during pregnancy group, followed by 84.4% in the preeclampsia group and 81.0% in the group with uncomplicated pregnancies.
Table 1.
Clinical Characteristics of the Study Population
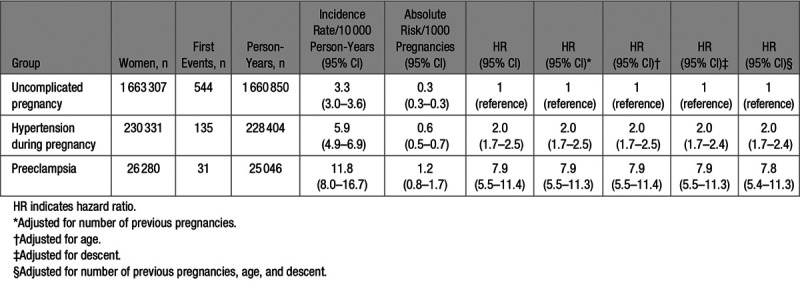
Risk of VTE in the First Pregnancy and Postpartum Period in the Registry
In the first analysis, follow-up ended after the 3-month postpartum period of the first pregnancy in the registry (Figure 1A). In the 1 919 918 pregnancies, 710 first VTE events occurred at an overall absolute risk of 0.4 (95% CI, 0.3–0.4) per 1000 pregnancies (Table 2). Women with uncomplicated pregnancies (reference group) had a risk of 0.3 (95% CI, 0.3–0.3) per 1000 pregnancies. This risk was higher in women with hypertensive disorder of pregnancy, that is, 0.6 (95% CI, 0.5–0.7) per 1000 pregnancies, adjusted HR, 2.0 (95% CI, 1.7–2.4), and highest among women with preeclampsia at 1.2 (95% CI, 0.8–1.7) per 1000 pregnancies, adjusted HR, 7.8 (95% CI, 5.4–11.3).
Table 2.
Risk of Venous Thromboembolism by Pregnancy Complication in the First Pregnancy of Follow-Up
Long-Term Risk of VTE
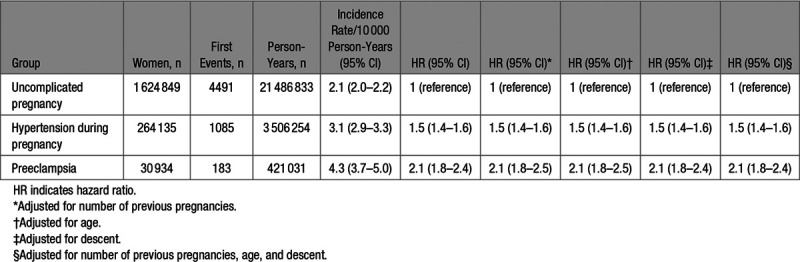
In the second analysis, we assessed the risk of VTE during the complete follow-up by pregnancy complications (Figure 1B). The absolute risk in the women with uncomplicated pregnancies was 2.1 (95% CI, 2.0–2.2) per 10 000 persons years (Table 3). This was 3.1 (95% CI, 2.9–3.3) per 10 000 persons years in the women with hypertension during pregnancy and highest in women with preeclampsia: 4.3 (95% CI, 3.7–5.0) per 10 000 person-years. Taking the women with uncomplicated pregnancies as reference group, the HR of a first VTE was 1.5 (95% CI, 1.4–1.6) in women with a hypertensive disorder of pregnancy and 2.1 (95% CI, 1.8–2.4) in women with preeclampsia. Adjustments for number of previous pregnancies, age, and self-reported descent did not change these estimates.
Table 3.
Long-Term Risk of Venous Thromboembolism During Follow-Up by Pregnancy Complications
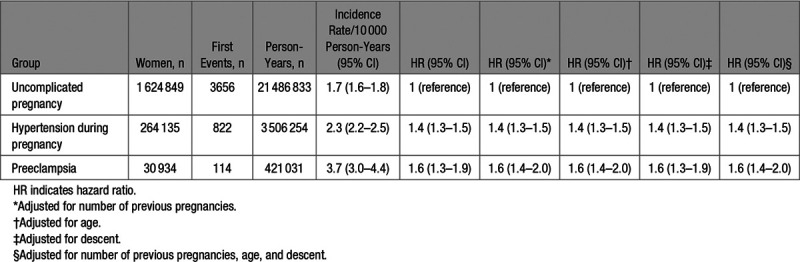
Last, we performed an analysis in which we excluded events that occurred during pregnancy or 3-month postpartum period (Figure 1C). This analysis yielded a similar pattern of the results, that is, an incidence of 1.7 (1.6–1.8) per 10 000 person-years in women with uncomplicated pregnancies, 2.3 (2.2–2.5) per 10 000 person-years in women with hypertension during pregnancy, and the largest risk in the preeclampsia group 3.7 (3.0–4.4) per 10 000 person-years (Table 4). Compared with the uncomplicated pregnancy group as reference, the adjusted HR was 1.4 (95% CI, 1.3–1.5) in the group with hypertensive disorders of pregnancy and 1.6 (95% CI, 1.4–2.0) in the women with preeclampsia.
Table 4.
Long-Term Risk of Venous Thromboembolism Outside of Pregnancy and Postpartum Period by Pregnancy Complications
Discussion
In this study, women with hypertension or preeclampsia during pregnancy had a higher risk of VTE, both during the corresponding pregnancy and postpartum period, as well as in the 13 following years, compared with women with uncomplicated pregnancies. The risk of VTE was higher in women with preeclampsia than in women with hypertension during pregnancy. These findings support the hypothesis that hypertensive complications of pregnancy entailing both hypertension during pregnancy and preeclampsia reflect an underlying predisposition to vascular disease, including VTE.
Our study has several strengths. First, we were able to conduct a nationwide study, where we included nearly all pregnancies in the Netherlands from 1999 up to 2012, strengthening the generalizability of the results and allowing accurate estimates. Second, because of the detailed nature of the registry we were able to classify the exposures based on clinical criteria and distinguish between hypertension during pregnancy and preeclampsia (ie, based on blood pressure and urinary protein loss), thereby reducing the risk of misclassification. As only patients from anticoagulation clinics are included in the study, the risk of misclassification, for example, superficial instead of deep vein thrombosis or misclassification by using diagnostic or hospital admission codes as outcome, is low.
Several limitations should also be considered. First, we only included patients who were monitored at the anticoagulation clinics in this study, which will have resulted in an underestimation of the incidence, since women who have VTE during pregnancy typically receive low-molecular-weight heparin and are often not switched to VKA in the postpartum period. In addition, patients who experienced a fatal pulmonary embolism or died before registering at the anticoagulation clinics are not in the study. However, although the incidences may have been underestimated, we expect our relative risk estimates to be accurate for the comparison during pregnancy and postpartum period as nearly all women with a VTE during pregnancy receive low-molecular-weight heparin independent of other pregnancy complications. Second, we only had information on the occurrence of venous events and not on outcomes such as death or other thrombotic events like myocardial infarction or ischemic stroke that would have led to initiation of anticoagulant therapy. Thus, the relative risk for hypertensive complications of pregnancy and risk of VTE might be slightly underestimated. Ideally, women with such outcomes would have been censored at time of these events. However, even though the risk of arterial cardiovascular disease is increased in women with a history of hypertensive pregnancy complications,4 the majority of our study population was <40 years of age at the end of follow-up. Among women of these ages, the absolute risk of CVD events is low: in the Netherlands among women aged 20 to 45 years in 2006 to 2010, 1.3 per 10 000 women were admitted for acute myocardial infarction and 3.5 per 10 000 were admitted for cerebrovascular diseases,22,23 so the effect of missing information on these conditions was probably negligible. Third, since the linkage of Perined and the anticoagulant clinics was not based on a unique identifier, there is a risk of misclassification. However, such misclassification will unlikely be influenced by the exposure (ie, hypertensive complication of pregnancy or uncomplicated pregnancy) and at most may have led again to some underestimation of the risks. Fourth, the definition of hypertension during pregnancy and preeclampsia used for the purpose of this study slightly deviates from the current clinical definition in guidelines. We used a diastolic blood pressure of ≥90 mm Hg on at least 1 occasion during any moment in the course of pregnancy. Several guidelines24,25 require a systolic or diastolic blood pressure of over 140 and 90 mm Hg, respectively, on 2 separate occasions, which may have led to a slight overestimation of the number of women with hypertension in the study which in turn may have resulted in an underestimation of the effect. Last, regarding causality, we only had information on a limited number of confounders. Potential remaining confounders such as thrombophilia and obesity could explain part of the observed association.2
Our observations on a VTE risk increase during pregnancy and postpartum period in women with preeclampsia are in line with previous studies.14–18 In addition, our study adds important information on a similar risk increase in women with hypertension during pregnancy and risk VTE. The underlying pathophysiology that explains this risk increase is not known.24 Mechanisms that have been proposed, specifically in context of preeclampsia, include endothelial dysfunction, platelet activation, a procoagulant and proinflammatory state, and release of neutrophil extracellular traps, all associated with preeclampsia,26 leading to a short-term increased risk. Our findings on a long-term increased VTE risk in women with hypertensive complications (ie, both hypertension during pregnancy and preeclampsia) are novel. In a study from the Danish National Patient Registry, women with a history of VTE (n=1419) were followed during their subsequent pregnancies.27 Compared with women without preconception VTE, women with preconception VTE had a higher risk of preeclampsia during their pregnancy: relative risk, 1.5 (95% CI, 1.3–1.8).27 Combined with the results from our study, these findings suggest that an underlying common predisposition is responsible for both the risk of preeclampsia in VTE.
Perspectives
In summary, hypertensive disorders of pregnancy and especially preeclampsia are associated with a short- and long-term increased risk of VTE. These findings imply that although the hypertensive disorders itself are transient and end with delivery of the placenta, the risk of VTE remains increased in the following years. This suggests that hypertensive disorders of pregnancy and preeclampsia in particular are associated with persistent underlying, currently unknown, risk factors. For this, 2 mechanisms are plausible: either an underlying predisposition increases the risk of both preeclampsia and VTE or preeclampsia causes permanent changes which then in turn lead to the observed long-term increased VTE risk. Potential underlying mechanisms are subject to speculation and could include a combination of acquired risk factors, genetic risk factors, or endothelial dysfunction. Mechanistic studies are needed to provide more information on the underlying mechanism and could give rise to preventive strategies, both for hypertensive pregnancy complications and VTE. Regardless of the underlying mechanism, our findings are of clinical relevance. Hypertensive disorders of pregnancy and preeclampsia in particular are predictors of the short- and long-term risk of VTE. Although the absolute risk of VTE remains low in this population, hypertensive disorders of pregnancy together with other important predictors can guide thromboprophylaxis decisions.13 A history of a hypertensive disorder of pregnancy is likely a valuable VTE predictor during new high-risk situations (eg, during surgery, plaster cast immobilization, or subsequent pregnancy) and should be considered in development of future risk assessment models.
Acknowledgments
We would like to express our gratitude to all participants and people involved in the Perined registry and anticoagulation clinics that participated in the current study. L.J.J. Scheres, S. Middeldorp, and S.C. Cannegieter designed the study and interpreted the data. L.J.J. Scheres performed the analyses and wrote the manuscript. N.F.M. Groenewegen coordinated the data collection of the anticoagulation clinics. S. Koole coordinated the collection of the Perined registry data. W.M. Lijfering, N.F.M. Groenewegen, S. Koole, C.J.M. de Groot, S. Middeldorp, and S.C. Cannegieter critically revised the manuscript. All authors take responsibility for the interpretation of the data and critical revision of the manuscript for important intellectual content.
Sources of Funding
L.J.J. Scheres was a PhD candidate of the cardiovascular healthy aging in women (CREW) project (2013T083) supported by the Dutch Heart Foundation.
Disclosures
L.J.J. Scheres received funding for the printing of his doctoral thesis from the Dutch Heart Foundation, Dutch Federation of Coagulation Clinics, Stichting tot Steun Promovendi Vasculaire Geneeskunde, Bayer, Daiichi Sankyo, LEO Pharma, and Pfizer. The other authors report no conflicts.
Novelty and Significance
What Is New?
In this study among 1 919 918 women with at least 1 pregnancy, we show that hypertension during pregnancy and preeclampsia are associated with an increased risk of venous thromboembolism (VTE) both during pregnancy and postpartum period and on the long term in the 13 years after.
What Is Relevant?
Hypertensive disorders of pregnancy together with other important predictors can guide physicians in deciding on thromboprophylaxis strategies in attempt to prevent VTE both on the short and long term.
Better understanding of the underlying predisposition that is responsible for our findings could give rise to new management options for both hypertensive disorders and VTE during pregnancy.
Summary
In this study, we demonstrate an association between hypertension during pregnancy and preeclampsia and an increased risk of VTE, both on the short term as on the long term. These findings may aid in the development of risk assessment strategies aimed to prevent VTE. In addition, further investigation of the underlying mechanism may give rise to research opportunities for management options for hypertensive disorders and VTE during pregnancy.
References
- 1.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008;21:521–526. doi: 10.1038/ajh.2008.20. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 2.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 3.Hernández-Díaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. doi: 10.1136/bmj.b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, Oudijk MA, Bots ML, van der Schouw YT. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension. 2015;66:1116–1122. doi: 10.1161/HYPERTENSIONAHA.115.06005. doi: 10.1161/HYPERTENSIONAHA.115.06005. [DOI] [PubMed] [Google Scholar]
- 5.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. doi: 10.1161/CIRCOUTCOMES.116.003497. doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 6.Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AW, Prins MH, Girolami A. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435–1441. doi: 10.1056/NEJMoa022157. doi: 10.1056/NEJMoa022157. [DOI] [PubMed] [Google Scholar]
- 7.Sørensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370:1773–1779. doi: 10.1016/S0140-6736(07)61745-0. doi: 10.1016/S0140-6736(07)61745-0. [DOI] [PubMed] [Google Scholar]
- 8.Klok FA, Mos IC, Broek L, Tamsma JT, Rosendaal FR, de Roos A, Huisman MV. Risk of arterial cardiovascular events in patients after pulmonary embolism. Blood. 2009;114:1484–1488. doi: 10.1182/blood-2009-05-220491. doi: 10.1182/blood-2009-05-220491. [DOI] [PubMed] [Google Scholar]
- 9.Roach RE, Lijfering WM, Flinterman LE, Rosendaal FR, Cannegieter SC. Increased risk of CVD after VT is determined by common etiologic factors. Blood. 2013;121:4948–4954. doi: 10.1182/blood-2013-01-479238. doi: 10.1182/blood-2013-01-479238. [DOI] [PubMed] [Google Scholar]
- 10.Lijfering WM, Flinterman LE, Vandenbroucke JP, Rosendaal FR, Cannegieter SC. Relationship between venous and arterial thrombosis: a review of the literature from a causal perspective. Semin Thromb Hemost. 2011;37:885–896. doi: 10.1055/s-0031-1297367. doi: 10.1055/s-0031-1297367. [DOI] [PubMed] [Google Scholar]
- 11.James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311–1315. doi: 10.1016/j.ajog.2005.11.008. doi: 10.1016/j.ajog.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6:632–637. doi: 10.1111/j.1538-7836.2008.02921.x. doi: 10.1111/j.1538-7836.2008.02921.x. [DOI] [PubMed] [Google Scholar]
- 13.Scheres LJJ, Bistervels IM, Middeldorp S. Everything the clinician needs to know about evidence-based anticoagulation in pregnancy. Blood Rev. 2019;33:82–97. doi: 10.1016/j.blre.2018.08.001. doi: 10.1016/j.blre.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 14.van Walraven C, Mamdani M, Cohn A, Katib Y, Walker M, Rodger MA. Risk of subsequent thromboembolism for patients with pre-eclampsia. BMJ. 2003;326:791–792. doi: 10.1136/bmj.326.7393.791. doi: 10.1136/bmj.326.7393.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol. 1999;94:595–599. doi: 10.1016/s0029-7844(99)00308-7. doi: 10.1016/s0029-7844(99)00308-7. [DOI] [PubMed] [Google Scholar]
- 16.Virkus RA, Løkkegaard E, Lidegaard Ø, Langhoff-Roos J, Nielsen AK, Rothman KJ, Bergholt T. Risk factors for venous thromboembolism in 1.3 million pregnancies: a nationwide prospective cohort. PLoS One. 2014;9:e96495. doi: 10.1371/journal.pone.0096495. doi: 10.1371/journal.pone.0096495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobsen AF, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium–a register-based case-control study. Am J Obstet Gynecol. 2008;198:233.e1–233.e7. doi: 10.1016/j.ajog.2007.08.041. doi: 10.1016/j.ajog.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Abdul Sultan A, Grainge MJ, West J, Fleming KM, Nelson-Piercy C, Tata LJ. Impact of risk factors on the timing of first postpartum venous thromboembolism: a population-based cohort study from England. Blood. 2014;124:2872–2880. doi: 10.1182/blood-2014-05-572834. doi: 10.1182/blood-2014-05-572834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perined. Available at: https://www.perined.nl/. Accessed September 23, 2019.
- 20.Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. doi: 10.1016/j.preghy.2014.02.001. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Dutch Internal Medicine Society. Guidance on antithrombotic management. 2015. Available at: https://internisten.nl/files/Richtlijn%20Antitrombotisch%20beleid_def.pdf.
- 22.Driver JA, Djoussé L, Logroscino G, Gaziano JM, Kurth T. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study. BMJ. 2008;337:a2467. doi: 10.1136/bmj.a2467. doi: 10.1136/bmj.a2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Statistics Netherlands (CBS) StatLine - Ziekenhuisopnamen; diagnose, herkomst, geslacht en leeftijd. Available at: http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=71540ned&D1=a&D2=1-2&D3=3&D4=a&D5=48,53&D6=l&D7=l&HDR=T,G5,G1,G2,G6&STB=G4,G3&VW=T. Accessed December 23, 2018.
- 24.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, et al. ESC Scientific Document Group. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. London: NICE; 2019. NG133] Available from: https://www.nice.org.uk/guidance/ng133. [PubMed] [Google Scholar]
- 26.Egan K, Kevane B, Ní Áinle F. Elevated venous thromboembolism risk in preeclampsia: molecular mechanisms and clinical impact. Biochem Soc Trans. 2015;43:696–701. doi: 10.1042/BST20140310. doi: 10.1042/BST20140310. [DOI] [PubMed] [Google Scholar]
- 27.Hansen AT, Schmidt M, Horváth-Puhó E, Pedersen L, Rothman KJ, Hvas AM, Sørensen HT. Preconception venous thromboembolism and placenta-mediated pregnancy complications. J Thromb Haemost. 2015;13:1635–1641. doi: 10.1111/jth.13046. doi: 10.1111/jth.13046. [DOI] [PubMed] [Google Scholar]


