Abstract
Our objective was to gain insight in the calculation and interpretation of population health metrics that inform disease prevention. Using as model environmental exposure to lead (ELE), a global pollutant, we assessed population health metrics derived from the Third National Health and Nutrition Examination Survey (1988 to 1994), the GBD (Global Burden of Disease Study 2010), and the Organization for Economic Co-operation and Development. In the National Health and Nutrition Examination Survey, the hazard ratio relating mortality over 19.3 years of follow-up to a blood lead increase at baseline from 1.0 to 6.7 µg/dL (10th–90th percentile interval) was 1.37 (95% CI, 1.17–1.60). The population-attributable fraction of blood lead was 18.0% (10.9%–26.1%). The number of preventable ELE-related deaths in the United States would be 412 000 per year (250 000–598 000). In GBD 2010, deaths and disability-adjusted life-years globally lost due to ELE were 0.67 million (0.58–0.78 million) and 0.56% (0.47%–0.66%), respectively. According to the 2017 Organization for Economic Co-operation and Development statistics, ELE-related welfare costs were $1 676 224 million worldwide. Extrapolations from the foregoing metrics assumed causality and reversibility of the association between mortality and blood lead, which at present-day ELE levels in developed nations is not established. Other issues limiting the interpretation of ELE-related population health metrics are the inflation of relative risk based on outdated blood lead levels, not differentiating relative from absolute risk, clustering of risk factors and exposures within individuals, residual confounding, and disregarding noncardiovascular disease and immigration in national ELE-associated welfare estimates. In conclusion, this review highlights the importance of critical thinking in translating population health metrics into cost-effective preventive strategies.
Keywords: environmental exposure, lead, mortality, risk factors, welfare costs
Unthinking respect for authority is the greatest enemy for truth.
—Albert Einstein (1879–1955)
The World Health Organization, the GBD (Global Burden of Disease) consortium, and the Organization for Economic Co-operation and Development (OECD) design population health metrics to inform strategies for the prevention of disease. However, most clinicians and health policy makers do not have the expertise to understand with the observational data, statistical approaches, and assumptions that underlie the computation of population health metrics. The objective of this review was to clarify for the unexperienced reader how population health metrics are generated and how they are extrapolated into potential health gains to be expected, if a risk factor is appropriately dealt with.
We chose environmental lead exposure as an exemplary case because of our record in hypertension research and cardiovascular epidemiology and because our studies searching for evidence that lead exposure might be the culprit leading to hypertension,1–3 renal dysfunction4–6 or cardiovascular disease,7,8 span close to 4 decades. Without any doubt, lead is an environmental toxicant, which at high exposure causes hypertension and renal failure.9,10 However, as documented in successive stages of the National Health and Nutrition Examination Survey (NHANES) in the United States,11–13 environmental lead exposure drastically fell over the past decades, that is, from 1999 to 2010. Nevertheless, the National Toxicology Program (2012)14 and the Environmental Protection Agency (2013)15 consider that blood lead levels below 5 µg/dL might cause hypertension and associated cardiovascular complications. Given our research track4–10 and the historical change in the exposure levels,11–13 we reviewed the evidence relating environmental lead exposure to mortality in adults, with assessment of the population-attributable risk fraction (PAF) and the welfare costs associated with lead exposure in adults.
Origins of a Dispute
An additional justification for using environmental lead exposure as an exemplary case was the publication in 2018 in The Lancet Public Health of the 20-year mortality of the NHANES III participants, whose baseline data, including blood lead, had been collected from 1988 until 1994.16–18 Lanphear et al18 made far-reaching extrapolations claiming that by reducing the blood lead concentration in Americans to the unrealistic level of <1 µg/dL, the annual death rate in the United States would fall by 18%. We expressed our reservations in a letter to the editor.19 Lanphear et al20 replied that lead is a causal risk factor for hypertension and coronary heart disease,20 referring to a 2013 document published by Environmental Protection Agency.15 They underpinned their argumentation by referring to how over the past 50 years the decline in the prevalence of hypertension and the incidence of cardiovascular mortality ran in parallel with the fall in environmental lead exposure.20 This argument completely disregarded the progress made over the same period in preventive medicine, lifestyle interventions, the growing awareness of patients affected by hypertension, the pharmacological management of high blood pressure and hyperlipidemia, and the invasive treatment of coronary heart disease. Over the same period, inhibitors of the renin-angiotensin-aldosterone system, statins, tissue plasminogen activators, and percutaneous coronary angioplasty became routine treatment modalities. The Environmental Protection Agency document did state that the epidemiological studies associating hypertension and atherosclerosis with lead exposure were consistent and that they were supported by experimental data.15 However, the Environmental Protection Agency also referred to the uncertainty concerning the intensity, timing, frequency, and duration of the lead exposure levels required to cause these adverse health outcomes.15
Sources of Information
The extrapolations in Lanphear’s 2018 article contrasted starkly with our own research experience from 19841 until today.3 To investigate whether our point of view might be challenged by the literature, we ran a PubMed search with keywords “mortality” and “blood lead”. It identified 103 studies published from 1975 until 2019. We excluded 10 studies dealing with mortality from noncardiovascular disease, including 7 focusing on cancer mortality; 18 studies reporting on nonfatal health outcomes in relation to lead exposure, 6 with cardiovascular and 12 with noncardiovascular health outcomes; 16 articles dealing with sources or routes of lead exposure; 6 studies describing determinants of the biomarkers of internal lead exposure; 5 studies conducted in patients with end-stage renal disease; 11 reviews or commentaries; 17 studies describing lead toxicity in exposed animals, and 3 articles without any relevance. This left 17 studies with relevance to the association between total or cardiovascular mortality in relation to environmental lead exposure,16–18,21–34 of which 6 were conducted in lead-exposed workers.21,22,24,28,29,34 Further references were identified via the reference list of 3 articles based on the follow-up of the NHANES III cohort.16–18 Mortality statistics and demographic information were retrieved from the World Health Organization website and national websites in Belgium.
Mortality in NHANES III Participants
The NHANES III survey (1988–1994) involved the collection of clinical variables, questionnaire data, and biochemical measurements, including blood lead, among a representative sample of the adult population of the United States. Blood lead was measured by graphite furnace atomic absorption spectrophotometry.16–18 The detection limit was 1.0 µg/dL. For participants (8%) with blood lead levels below the detection limit, a level of 0.7 µg/dL was imputed.16–18 These NHANES III baseline data were linked with the National Death Index, using probabilistic matching based on 12 identifiers for each participant to ascertain vital status and the cause of death. Follow-up was the time between the baseline examination date, the date of death, a participant’s 90th birthday, or the censoring date, whichever came first. The censoring date was December 31, 2000,16,17 or December 31, 2011.18
From the initial report (Table 1)16 to the most recent article (Table 2),18 the 12.0-year follow-up (maximum) was extended by 19.3 years (interquartile range, 17.6–21.0 years). The geometric mean blood lead concentrations were 2.6 µg/dL (interquartile range, 1.9–3.6 µg/dL)16 and 2.7 µg/dL (interquartile range, 2.0–3.8 µg/dL),18 respectively. In the first report, multivariable-adjusted hazard ratios were expressed for an increment in the blood lead concentration from the 20th to 80th percentile (Table 1)16 and in the primary analyses of the most recent report for a blood lead increase from the 10th to the 90th percentile (Table 2).18
Table 1.
Mortality in 13 946 NHANES III Participants Followed Up Until December 31, 2000
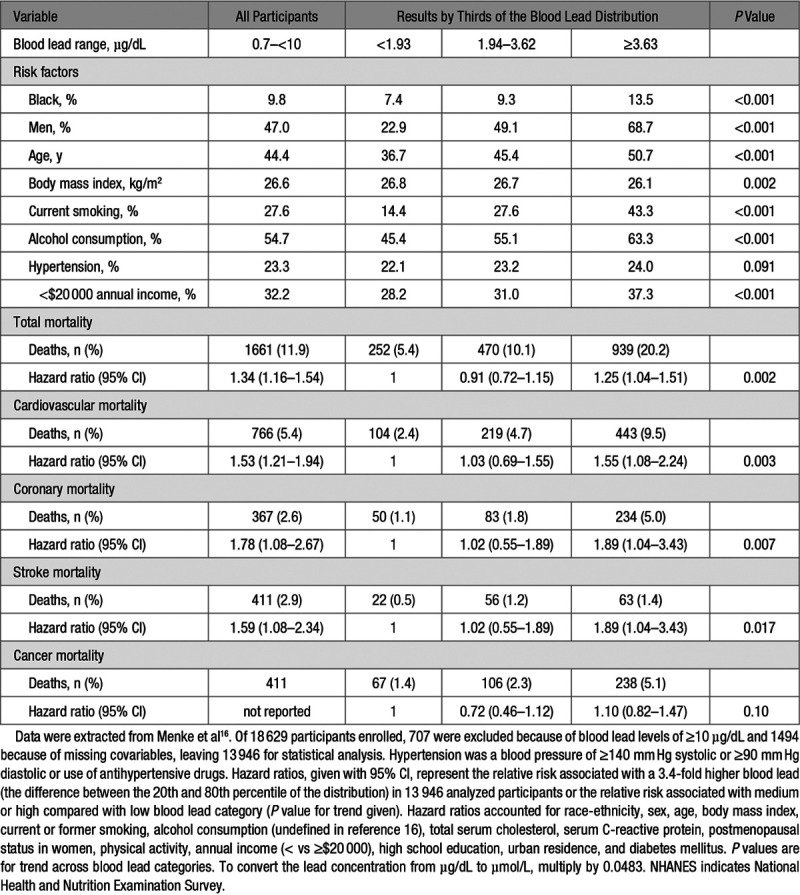
Table 2.
Mortality in 14 289 NHANES III Participants Followed Up Until December 31, 2011
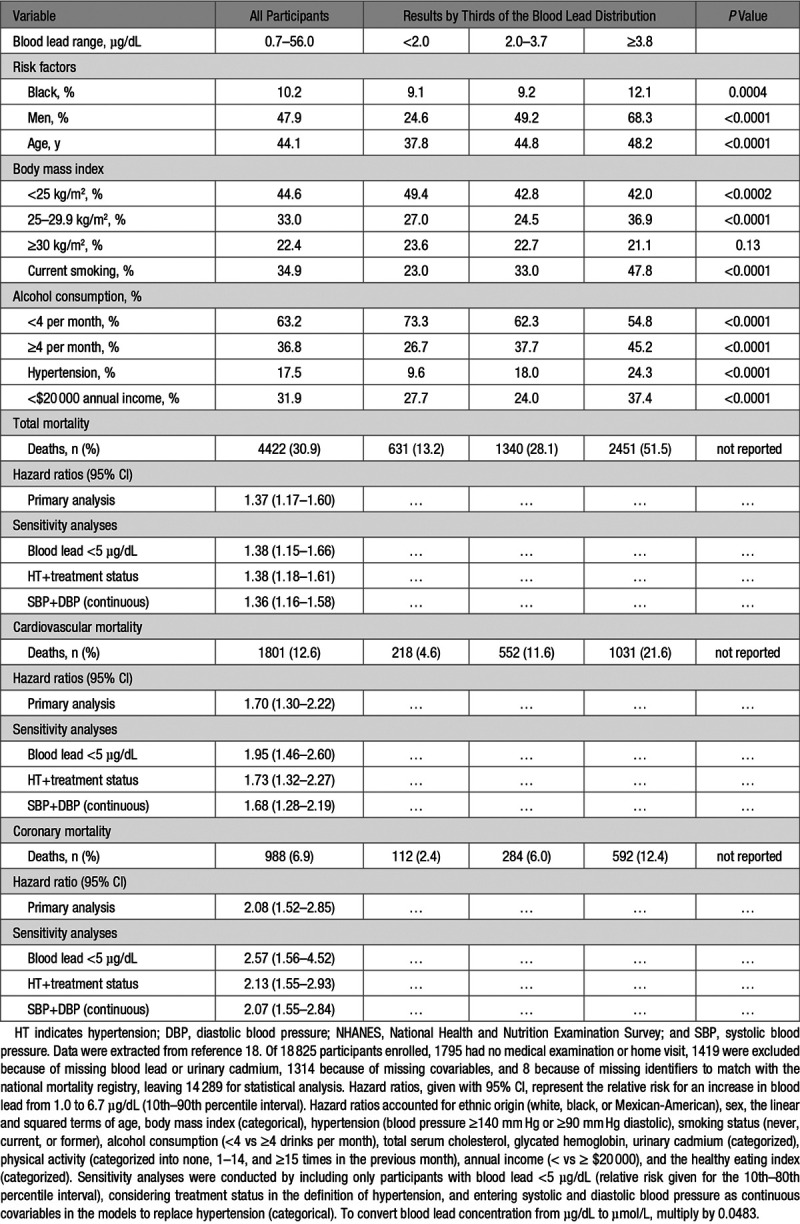
In 13 946 participants followed up until 31 December 2000 (Table 1),16 the multivariable-adjusted hazard ratios were 1.34 (95% CI, 1.16–1.54) for total mortality, 1.53 (CI, 1.21–1.94) for cardiovascular mortality, and 1.78 (CI, 1.08–2.67) for coronary mortality.16 In 14 289 individuals followed up until 31 December 2011 (Table 2),18 the corresponding estimates of relative risk were 1.37 (CI, 1.17–1.60), 1.70 (CI, 1.30–2.22), and 2.08 (CI, 1.52–2.85), respectively. From individual measures of blood lead and their associated hazard ratios, in the 2018 report,18 PAF (also known as the population impact factor)35,36 was computed as the integral of hazard ratios at each blood lead level weighted by the logarithmically transformed population distribution of blood leads over the total range from 0.70 to 56.0 µg/dL. The PAFs amounted to 18.0% (CI, 10.9%–26.1%) for total mortality, 28.7% (CI, 15.5%–39.5%) for cardiovascular mortality, and 37.4% (CI, 23.4%–48.6%) for coronary mortality. Given the annual number of deaths in the United States for total, cardiovascular, and coronary mortality (2 288 888; 891 896; and 494 652, respectively), the estimates of preventable deaths, assuming that blood lead concentrations were all reduced to 1.0 µg/dL or less, amounted to 412 000 (CI, 250 000–598 000) for total mortality, 256 000 (CI, 138 000–352 000) for cardiovascular mortality, and 185 000 (CI, 116 000–241 000) for coronary mortality.
Generalizability of NHANES III Mortality Results
In particular, the 2018 report on the long-term association between mortality and blood lead over a median follow-up of 19.3 years18 has little relevance for public health policies in the second decade of the 21st century. The justification for this assessment includes (1) the nonrepresentativeness of the NHANES III blood lead levels for contemporary exposure; (2) the way in which PAF was computed, including the extremes of the blood lead concentration; (3) absence of an established causal pathway linking mortality to lead at present-day environmental exposure levels; (4) the neglect to consider competing risks and absolute as opposed to relative risk, especially at the high end of the age distribution; (5) residual confounding; and (6) the drastic reduction by application of modern pharmacological and invasive therapies of the case-fatality rates associated with coronary, cerebrovascular, and other vascular accidents.
Outdated Level of Environmental Lead Exposure
In calculating PAF, Lanphear et al18 took into account the hazard ratio estimates over the whole distribution of blood lead, ranging from the imputed level of 0.7 μg/dL in 1150 participants (8.0%) up to 56.0 μg/dL. The blood lead concentration was 5 µg/dL or higher in 3632 participants (25.4%; misreported as 20.0%18). In analyses of 12 725 NHANES IV participants examined from 2003 until 2010,13 the geometric mean blood concentration in all participants was 1.41 µg/dL with lower levels in women than in men (1.25 versus 1.80 µg/dL) and in whites compared with blacks and Hispanics (1.46 versus 1.57 µg/dL). All blood lead levels were below 30 µg/dL.13 Thus, the associations of mortality with blood lead in NHANES III are no longer representative for current exposure of the adult population in the United States. The same applies to reports associating death with blood lead in earlier NHANES surveys.23
Lead is a cumulative toxicant, which is for 90% to 95% stored in bone, from where it is recirculated with a half-life of 20 to 25 years.37,38 Blood lead, for 99% carried by red blood cells, reflects recent exposure over the past 1 to 2 months and the amount of lead released and recirculated from bone.37 Both bone38,39 and blood4,38,39 lead increase with advancing age. Bone lead correlates with blood lead38,39 and explains around 20% of the variance in blood lead, depending on seasonality38 and hormonal and other endogenous and environmental stimuli influencing the balance between bone formation and resorption.39 Recirculation of lead from bone explains why there is a lag time when environmental2 or occupational37 lead exposure drops. These lead toxicokinetics are relevant to the mortality results of the HNANES III cohort studies.16–18 Indeed, the blood lead concentrations measured at the baseline examination16–18 did not so much reflect environmental lead exposure from 1988 until 1994, but with older baseline age of NHANES III participants, they were increasingly representative for the preexisting body burden originating from the historical environmental lead contamination. In the United States (https://scienceprogress.org/2008/10/a-brief-history-of-lead-regulation), lead-containing paint was effectively banned in 1976, and leaded gasoline was completely phased out only in 1995.40
The Population-Attributable Risk Fraction
To address the anticipated criticism that NHANES III blood lead levels by far exceeded those associated with contemporary environmental lead exposure, the primary analysis of the 10-year follow-up (Table 1)16 excluded participants with blood leads of 10 µg/dL or higher and a sensitivity analysis of the 20-year follow-up (Table 2)18 only included individuals with a blood lead concentration of <5 µg/dL.
PAF was calculated as the proportional decline in mortality that would occur if the blood lead concentrations of all participants would be reduced to a reference level of 1.0 µg/dL.18 The lower the desired null-effect blood lead concentration, the higher the relative risks and PAF must be. Obtaining a blood lead level <1.0 µg/dL in all Americans is an illusionary goal. Thus, the reported PAF of 18%18 is irrelevant for public health policies.
The association of the relative risk of death with blood lead was respectively computed for a 3.4-fold increment in the exposure biomarker (difference between the 20th and 80th percentiles of blood lead, 1.46 to 4.92 µg/dL)16 or for a 6.7-fold increase (difference between 10th and 90th percentiles, 1.0–6.7 µg/dL).18 A more credible approach would have been to compute relative risk for smaller increments in blood lead, such as a doubling. In an ongoing study of newly hired workers,41,42 employed at North American lead recycling plants, blood lead increased over 2 years about 3-fold from 4.1 to 13.7 µg/dL, making a 2-fold change in a nonoccupational setting a more reasonable quantity, given the current environmental lead exposure levels in the United States.13 Assuming a log-linear association between mortality and the logarithmically transformed blood lead concentration, the multivariable-adjusted hazard ratios given in Table 2 recalculated for 2.0-fold instead of a 6.7-fold increase in blood lead were 1.10 (CI, 1.05–1.15), 1.17 (CI, 1.08–1.27), and 1.24 (1.13–1.37), for total, cardiovascular and coronary mortality, respectively. This illustrates the enormous impact of desirable changes in the exposure variable on estimates of relative risk. Selecting an attainable blood lead target higher than 1 µg/dL would also have substantially attenuated the PAF and the number of preventable deaths, as unrealistically reported by Lanphear et al.18
In this context, one should note that blood lead levels ranging from 1.94 to 3.62 µg/dL did not confer a significantly elevated 10-year risk of all-cause or cause-specific mortality compared with a blood lead concentration below 1.93 µg/dL (Table 1).16 In Figure 1 in the article by Lanphear, the 95% CIs of the hazard ratios for total, cardiovascular, and coronary mortality all indicated that relative risk was significantly greater than unity, even at blood lead levels ranging from 0.7 to 2.5 µg/dL, including 1150 participants with undetectable blood lead.18
Association Between Mortality and Environmental Lead Exposure
That hypertension is the causal pathway linking mortality to environmental or occupational lead exposure is a deeply rooted paradigm, even if it originated from experimental and human studies at extremes of the exposure spectrum, based on research dating back more than half a century ago.43,44 Indisputably, lead intoxication causes hypertension, in part secondary to lead-induced nephropathy.45 Lead-related hypertension also involves various other mechanisms, mainly explored in experimental studies at exposures not achievable in humans.46,47 The pathogenic pathways proposed included—but by no means were limited to—impairment of nitric oxide signaling, heightened oxidative stress, stimulation of the adrenergic nervous system, imbalance between vasodilating and vasoconstricting autocoids and circulating hormones, and interference with vascular smooth muscle Ca2+ signaling resulting in an increase in peripheral arterial resistance.48 However, the dose-effect and dose-response curves linking hypertension and blood pressure to environmental lead exposure, if such associations still exist at current-day exposure levels in developed countries, remain to be elucidated. Telomere length is a marker of cellular and biological aging and the physiological oldness of the cardiovascular system.49,50 If there is a causal association between the risk of death and lead exposure, telomere length should be inversely correlated with blood lead. At variance with this idea, in the 1999 to 2002 NHANES survey,51 the multivariable-adjusted telomere length in circulating leukocytes was shorter with higher blood cadmium (association size for a doubling of the exposure marker, −2.46% [CI, −3.74 to −1.17]; P<0.001), but not with a doubling of blood lead (−0.07% [CI, −1.38 to 1.26]; P=0.90). These findings highlight issues of competing risks, residual confounding, and co-exposure to environmental contaminants, which will be discussed later in this review.
Based on a comprehensive assessment of the literature published until 2007, Navas-Acien et al52 concluded that there was sufficient evidence to infer a causal association of high blood pressure with lead exposure, but that the evidence was inconclusive to deduce a causal relation of cardiovascular outcomes with such exposure. In a meta-analysis of summary statistics extracted from 31 studies involving 58 518 participants, all published before February 2001,53 doubling of blood lead was associated with a marginally higher blood pressure. The pooled estimates averaged 1.0 mm Hg (CI, 0.5–1.4 mm Hg) systolic and 0.6 mm Hg (CI, 0.4–0.8 mm Hg) diastolic. In a prospective population study of 728 individuals (50.7% women; age range, 20–82 years), blood pressure was measured conventionally at baseline (1985–1989) and at follow-up (1991–1995) and by 24-hour ambulatory monitoring at follow-up.2 Over a median of 5.2 years (range, 3.5–8.4 years), the geometric mean blood lead concentration dropped by 32% from the baseline level of 8.7 µg/dL (range, 1.7–72.5 µg/dL). The small changes in the systolic/diastolic blood pressure on conventional measurement (−1.5/+1.7 mm Hg) were unrelated to the blood lead concentration at baseline or to the changes in this exposure marker over follow-up. Similarly, the 24-hour ambulatory blood pressure was not associated with blood lead at baseline or follow-up.2
An analysis of NHANES IV data (2003–2010) demonstrated weak and inconsistent associations of blood pressure with blood lead.13 These observations based on environmental lead exposure levels in the United States more representative of the contemporary situation practically eliminated high blood pressure as the mechanism driving the association between cardiovascular or coronary mortality and blood lead in the United States.13 Of note, the 2006 NHANES III article (Table 1)16 analyzed blood pressure as the average of all available readings in each participant (3 in most participants). Hypertension was a blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic or use of antihypertensive medications.16 At baseline, across thirds of the blood lead distribution, hypertension was not associated with blood lead (Table 116; P value for trend, 0.091). Hypertension was therefore not carried through as a covariable in the association of mortality with blood lead (Table 1).16 In the article by Lanphear (Table 2),18 the first blood pressure reading was excluded and the average of all remaining blood pressure measurements (2 in most participants) was applied to classify participants according to hypertension status. In this analysis (Table 2),18 hypertension was a blood pressure of ≥140 mm Hg systolic or ≥90 mm Hg diastolic. Being on antihypertensive medications is associated with a substantially higher cardiovascular risk, often modeled by adding 10 mm Hg to the recorded systolic blood pressure.54 The primary analyses reported by Lanphear et al18 did not account for this higher risk. However, sensitivity analyses, in which the definition of hypertension did include antihypertensive drug treatment or in which systolic and diastolic blood pressure were entered as continuously distributed covariables to replace hypertension as a categorical variable, were confirmatory (Table 2).18 Thus, adjustment for blood pressure or hypertension, irrespective of its definition, did not remove the association of all-cause, cardiovascular, or coronary mortality with blood lead (Table 2).18 This observation strongly argues against the hypothesis that lead-induced hypertension is causing cardiovascular and coronary disease and thereby explains the association between total mortality and environmental lead exposure. Stroke, the complication of hypertension most closely related to the blood pressure level,55 was not reported in the long-term follow-up of the NHANES III cohort (Table 2)18 and was only weakly associated with blood lead in the 10-year follow-up (Table 1).16
Competing Risks
The number of deaths in the top third of the NHANES III blood lead distribution amounted to 2451 (55.4% of all-cause mortality; Table 2).18 The 2011 National Vital Statistics Report56 listed cause-specific mortality corresponding in time with the end of the 20-year follow-up of the NHANES III participants.18 Standardized per 100 000 deaths, from 45 up to 84 years, malignancies contributed 434 more deaths to all-cause mortality, whereas only from age 85 years onwards heart disease overtook malignant disease contributing 2435 extra deaths. Lanphear et al18 did not model the competing risks of fatal cardiovascular and noncardiovascular diseases, both contributing to all-cause mortality.57,58
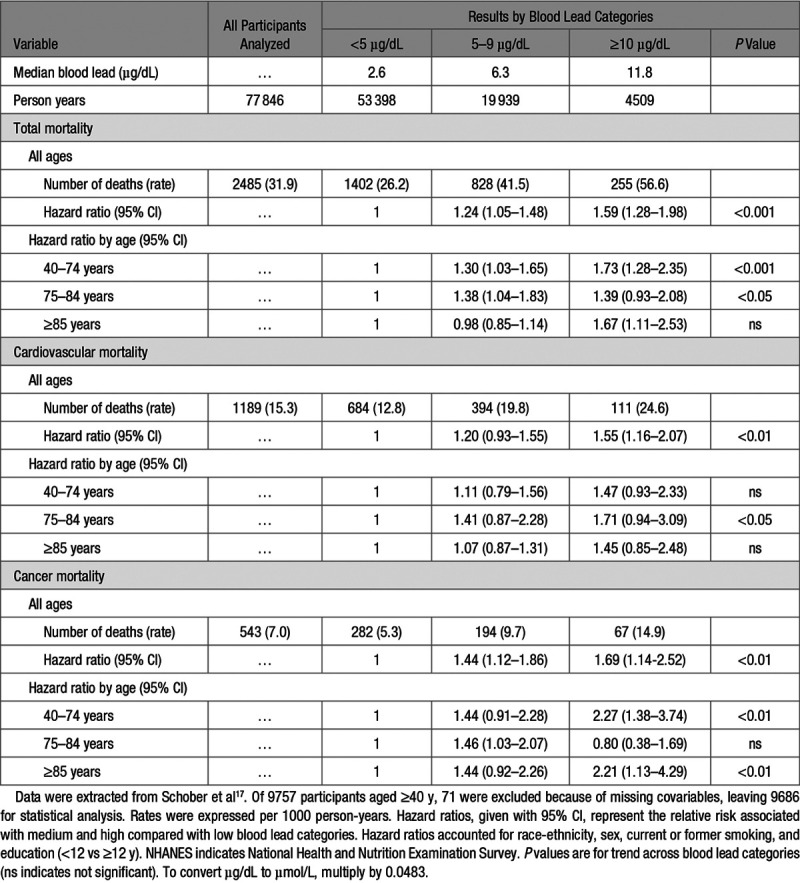
The multivariable-adjusted hazard ratios for cancer mortality in the 10-year follow-up of the NHANES III cohort (Table 1)16 were 0.72 (CI, 0.46–1.12) and 1.10 (CI, 0.82–1.47) in the middle and top third of the blood lead distribution, compared with the low exposure group, with no significant trend across blood lead categories (P=0.10). In an analysis of the same data (Table 317), NHANES III participants aged 40 years and older were stratified by arbitrarily defined blood lead categories (<5, 5–9, and ≥10 µg/dL) and age groups (40–74, 75–84, and ≥85 years). In all age groups combined, the risk of cancer mortality was elevated in the middle and high blood lead categories compared with the low exposure group (hazard ratios 1.44 and 1.69, respectively; Table 3).17 The trend P value for cancer mortality was significant in the age band from 40 to 74 years and from 85 years onward. For cardiovascular mortality, the trend P value was significant in all age groups combined. However, analyzed by age strata, none of the hazard ratios expressing the risk of cardiovascular mortality in the middle or high blood lead categories reached significance; the trend P value reached significance (P<0.05), but only in the age band from 75 to 84 years (Table 317). The analyses summarized in Table 317 demonstrate that absolute risk, as captured by mortality rates, exponentially rise with age and that competing risks do exists and should be accounted for.
Table 3.
Mortality in 9686 NHANES III Participants Aged ≥40 y Followed Up Until December 31, 2000
Relative Versus Absolute Risk
Human life is finite. When it comes to absolute risk, age beats all other risk indicators by far. In all analyses of the NHANES III cohort summarized in the tables of this review, death rates were highest in the top third of the blood lead distribution, in which age was significantly higher than in the middle and low thirds. Absolute risk only starts rising from middle age onwards with competing contributions of cardiovascular and noncardiovascular disease to all-cause mortality. Indeed death rates decline with higher age for noncardiovascular disease with an opposite lifetime course for cardiovascular disease.59,60
In the 20-year follow-up on the NHANES III cohort (Table 2),18 hazard ratios were significantly larger in participants younger than 50 years compared with those in older individuals: 2.24 (CI, 1.50–3.34) vs 1.53 (CI, 1.18–1.98) for total mortality (P=0.003), 2.93 (CI, 1.60–5.36) vs 2.08 (CI, 1.35–3.19) for cardiovascular mortality (P=0.01), and 4.68 (CI, 2.42–9.05) vs 2.46 (CI, 1.51–4.01) for coronary mortality (P=0.02). Lanphear et al18 failed to distinguish relative risk (as captured by hazard ratios) from absolute risk (as captured by incidence rates). For a lifetime course approach in prevention, this distinction is essential. Using hypertension as a representative risk factor, relative risk is high and absolute risk is low at younger age, whereas at old age, relative risk is low but absolute risk is high.59,60 Assuming reversibility, addressing a risk factor at young age will decrease relative risk with little effect on absolute risk, whereas in the elderly, relative risk will be barely affected, whereas absolute risk will diminish, thereby enhancing life in calendar years and in quality.59,60
Residual Confounding
As highlighted by NHANES III investigators61 and experts familiar with population health metrics,35 risk factors cluster within individuals and cannot be addressed in isolation. This oversight in the 20-year NHANES III follow-up18 might explain why the hazard ratio for cardiovascular mortality was greater (P=0.03) in nonsmokers than in smokers: 2.19 (CI, 1.47–3.26) vs 1.32 (CI, 0.86–2.05). Lanphear et al18 adjusted for household income, but this adjustment is unlikely to address in a comprehensive manner the socioeconomic gradients and differences in access to health care, which in the United States run in parallel with racial and ethnic disparities. None of the NHANES III analyses reported in the tables of this review showed that estimates of relative risk were similar across various ethnic, social, and income groups, for instance by reporting the appropriate interaction terms of socioeconomic status with the blood lead levels.
Imprecision of Mortality as Metric of Cardiovascular Health
A major limitation of the NHANES III studies16–18 is their selective focus on mortality. The introduction of stroke units and the wide availability of invasive coronary care and thrombolysis reduced the case-fatality rate of most cardiovascular complications of hypertension. Not accounting for nonfatal events, therefore, limits the generalizability of the NHANES III reports.16–18
The GBD Study 2012
A disability-adjusted life year is a summary metric that reflects the sum of years lived with a disability and the years of life lost. It, therefore, reflects both quality of life and premature mortality.59
Methods
In the 2012 GBD report, estimation of the disease burden of a risk factor involved 5 steps: (1) definition of outcome-risk factor pairs; (2) estimation of the distributions of exposure to a risk factor in populations; (3) estimation of the assumed etiological effect size, most often expressed as relative risk per unit of exposure; (4) choice of an alternative (counterfactual) exposure distribution with which the current exposure distribution was compared; (5) and computation of the disease burden attributable to a risk factor, including uncertainty from various sources. Evidence from epidemiological studies had to show a consistent association between disease and exposure, including prospective observational studies, with little or no evidence to the contrary. The association had to be biologically plausible. The PAF for environmental lead exposure was extrapolated from the bone lead level to be expected from the age-specific cumulative exposure to a blood lead concentration of 2.0 µg/dL62 (Section 3 in Table 159). Effect (or association) sizes were adjusted for measured confounders but not for factors assumed to be along the causal pathway.
The GBD consortium assumed a causal association of lead with intellectual disability (below 15 years of age)63 and with systolic blood pressure (from 25 years onwards).52,64 Mediated via blood pressure, lead exposure was assumed to cause right heart disease; ischemic heart disease; ischemic, hemorrhagic, and other nonischemic stroke; hypertensive heart disease; aortic aneurysm; the aggregate of cardiomyopathy, myocarditis, and endocarditis; the aggregate of atrial fibrillation and flutter; pulmonary vascular disease; other cardiovascular disease; and chronic kidney disease.65 If evidence was only available for the relative risk of either morbidity or mortality, the assumption made was that estimates of relative risk would equally apply to both fatal and nonfatal outcomes.
Results
In 2010, high blood pressure was the leading single risk factor globally, accounting for 9.4 million deaths (95% uncertainty interval [UI], 8.6–10.1 million) and 7.0% (UI, 6.2%–7.7%) of global disability-adjusted life years lost.59 For environmental lead exposure, these estimates were 0.67 million deaths (UI, 0.58–0.78 million) and 0.56% of disability-adjusted life years lost (UI, 0.47%–0.66%), respectively.59 Worldwide, for both sexes and all ages combined, high blood pressure moved up in the global risk factor ranks from rank 4 in 1990 to rank 1 (UI, 1–2) in 2010 and environmental lead exposure from rank 30 to rank 25 (UI, 23–29).59 In 2010, in high-income North America, high blood pressure occupied rank 3 and lead exposure rank 24.59
Interpretation
The GBD consortium listed among possible limitations of their result: (1) residual confounding; (2) uncertainty as to the extent to which effect sizes were generalizable; and (3) the impossibility to account for temporal changes in the exposure to risk factors. Our take on the GBD statistics was that the fall over time in environmental lead exposure was not sufficiently accounted for. This might explain why globally, in spite of declining environmental exposure,2,11–13 environmental lead exposure moved up from risk factor rank 30 in 1990 to rank 25 in 2010.59 Data from the older literature might have contributed to this counterintuitive observation. Publication bias and patching up nonsignificant hazard ratios at lower exposure levels by significant P values for trend across quantiles of blood lead (Table 116 and Table 317) were biases perhaps not sufficiently addressed in the literature review by the GBD consortium. Furthermore, the issue of residual confounding requires calculating PAF for clusters of risk factors, rather than for a single risk indicator. Indeed, cardiovascular risk factors66–68 and exposures to various environmental pollutants1,5,69 cluster within individuals. The GBD estimates did not account for co-exposures to risk factors and contaminants.
According to the World Health Organization demographic data, in 2010, the population of the United States (309 million) represented ≈4.5% of the world’s population (6.9 billion). If the statistics of the GBD 2012 report are truly generalizable (PAF, 0.67 deaths worldwide),59 preventable deaths related to environmental lead exposure in the United States would amount to ≈30 150 per year, an estimate >10-fold smaller than that proposed by Lanphear.18
OECD Welfare Cost Estimates
In 2019, OECD published a document estimating the welfare costs associated with environmental lead exposure.70
Methods
The key metric in computing welfare costs was premature mortality. This metric is simply a measure of unfulfilled life expectancy. Because deaths of younger people are often more easily preventable, the premature mortality rate gives greater weight to the death of younger than older people. The premature death rate is calculated by multiplying the number of deaths occurring at each age by the number or remaining years of life up to a selected limit (70 years). The assumption is that in high-income countries, people will live to the age of 70 years (the selected limit). If a person dies at 20 years, therefore that person is considered to have lost 50 years of life. The value of a statistical life is generally derived from aggregating individuals’ willingness-to-pay to secure a marginal reduction in the risk of premature death. Therefore, in the context of this review, the welfare cost was evaluated in terms of what the population at large would be willing to pay to avoid premature deaths due to exposure to environmental risks. Computation of the value of a statistical life rest on (1) a constant ($3 million in 2005); (2) the calculation of purchasing power parity to adjust estimates in United States dollars to the per capita gross domestic product in each country, relative to the OECD average; and (3) an income elasticity index ε to account for differences in income levels and elasticities across countries from 2005 onwards (ε=0.8, 0.9, and 1.0 for high-, middle-, and low-income countries).
Interpretation From a Belgian National Perspective
The estimated welfare costs related to environmental lead exposure in 2017 were $1 676 224 million worldwide and $6805 million in Belgium. In 2017, premature mortality in Belgium (read from Table A.1 in Environment Directorate, Organisation for Economic Co-operation and Development70) ran at 150 deaths per million (1725 deaths in 11.34 million Belgian residents) and came at a cost of 1.4% of the Belgian gross domestic product ($492.7 billion), that is, $6.9 billion.
Apart from the value of a statistical life definition, several confounders should be considered in the interpretation of the OECD lead-related welfare cost estimates for Belgium. First, until the mid-1990s, Belgium was the second largest cadmium producer in the world, which resulted in a historical contamination of the soil with toxic metals, including lead,4 cadmium,71 and arsenic72 in the surroundings of industrial sites. Closure of the zinc and cadmium smelters and remediation of heavily contaminated soils removed part of the exposure but did not abolish it. Both first- and second-hand tobacco smoking are sources of exposure to cadmium.73 The OECD statistics did not account for these co-exposures. Second, 2015 statistics downloaded from the World Health Organization mortality database (https://www.who.int/healthinfo/mortality_data/en/) showed that in Belgium, malignancies (International Classification of Diseases Tenth Revision, C00–D48) caused 10 074 premature deaths within the age range from 20 to 70 years (4270 and 5804 deaths in women and men, respectively); the corresponding death toll for cardiovascular combined with chronic kidney disease (International Classification of Diseases Tenth Revision, I00-I99 and N00-N27) was 4433 (1361 deaths in women and 3072 in men). In other words, premature mortality in Belgium occurring from age 20 to 70 years was only for 30.6% attributable to cardiovascular and renal illnesses and for 69.4% to cancer. The GBD 2010 Study assumed a causal link of cardiovascular disease and chronic kidney disease with environmental lead exposure, which was mediated via high blood pressure but not a relation of lead exposure with cancer.59 According to the International Agency for Research on Cancer, there is in humans only limited evidence for the carcinogenicity of inorganic lead (Group 2A) and no evidence for organic lead compounds (Group 3).74 Finally, on January 1, 2018, Belgium had a total population of 11.43 million people, including 1 357 556 registered foreign residents (11.9%), who contributed to the premature mortality statistics. Western Europeans accounted for 46.2% of the immigrant population, Eastern Europeans for 11.7%, Turks and Moroccans for 8.7%, and a wide range of other countries for the remaining 33.4% (Figure). Lead exposure varies widely globally.75,76 Overall, 98% of adults affected by exposure to lead now live in low- and middle-income countries.76 To what extent premature mortality and welfare costs in Belgium were overestimated by deaths related to environmental or occupational lead exposure of foreign residents in their countries of origin, in particular, third-world countries, remains unassessed.
Figure.
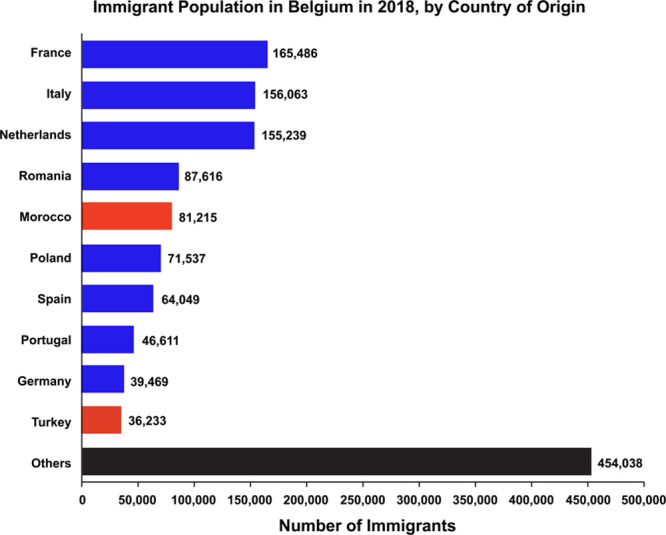
Immigrant population in Belgium in 2018, by country of origin.
Conclusions
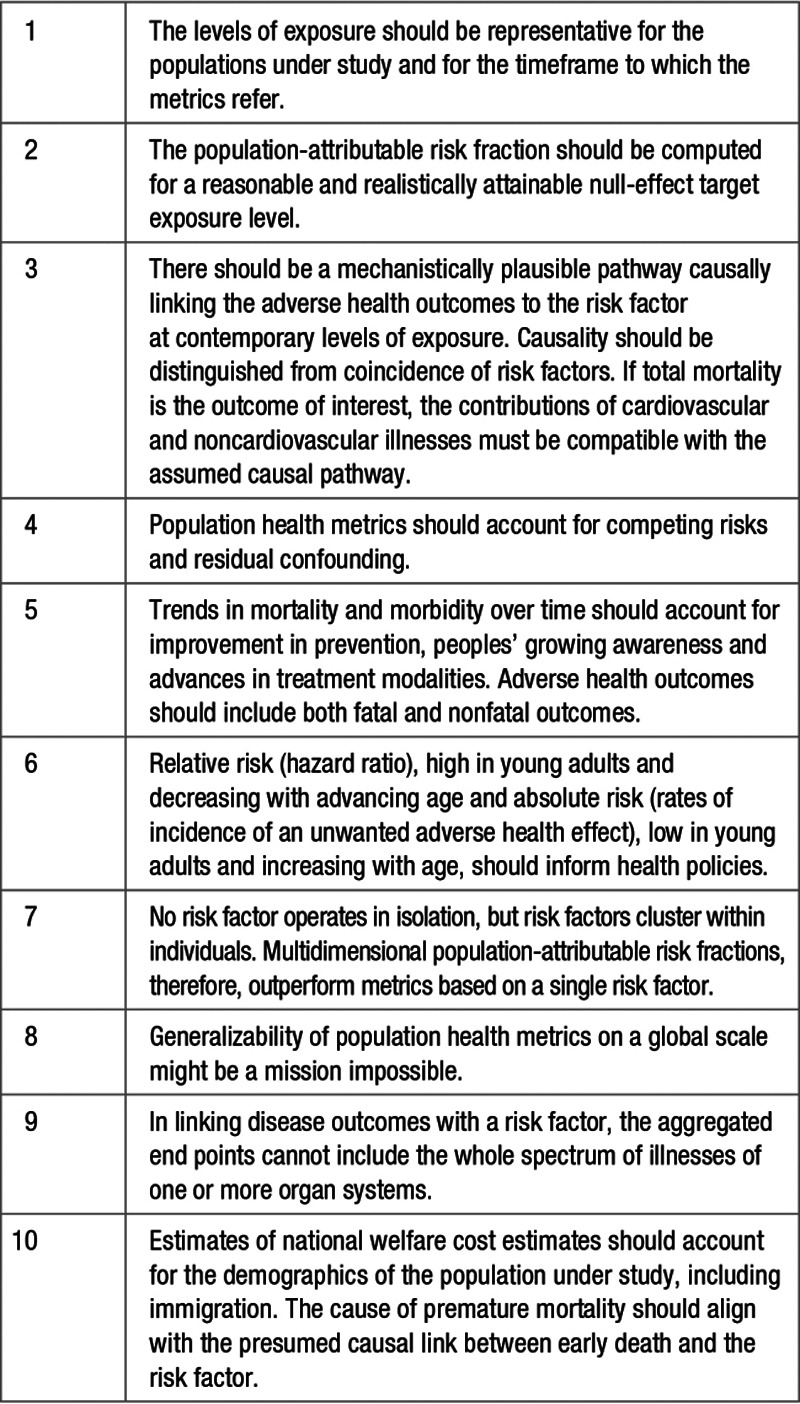
Lead is an environmental hazard that should be addressed worldwide. However, not accounting for the assumptions underlying population health metrics leads to an overestimation of the health gains that can be reasonably expected. In the case of environmental exposure to lead, extrapolations from population health metrics might gain in credibility, if the causality of the association between blood pressure and contemporary lead exposure levels, much lower than in the previous century, were to be reassessed (Table 4). Other areas for improvement include the development of multidimensional population health metrics and estimates of welfare costs that account for clustering of cardiovascular risk factors, social deprivation, and co-exposures within individuals (Table 4). Such an approach would substantially reduce residual confounding. Furthermore, population scientists need to redefine the cardiovascular and renal outcomes likely to be associated with present-day environmental lead exposure. To be representative, population health metrics should account for nonfatal adverse health outcomes. This also involves setting the no-risk threshold at a level that is scientifically sound and can be reasonably achieved within the current global and national economic context. Finally, it might not be possible to provide globally achievable risk estimates in view of the large differences in environmental exposure to a risk factor, for example, lead, between the developed and developing world. Many of the improvements to be proposed would require the labor-intensive analysis of individual-level meta-analytic resources to avoid the pitfalls of using summary statistics,77 pooled from studies conducted over a large time interval with widely different methods in diverse geographic and sociocultural settings.
Table 4.
Minimal Checklist for Evaluating Population Health Metrics
In this review, environmental lead exposure was used as an exemplary model. However, mutatis mutandis, similar approaches might be applied to other risk factors, which via hypertension might increase cardiovascular complications. If a risk factor is to be addressed, the extrapolated benefits should always be balanced against potential unwanted effects, such as economic sustainability, willingness to pay, or adverse health outcomes, such as those potentially associated with excessive lowering of sodium intake.78,79
Acknowledgments
The authors gratefully acknowledge the expert clerical assistance of Vera De Leebeeck and Renilde Wolfs at the Studies Coordinating Centre in Leuven, Belgium.
Sources of Funding
The European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT) currently supports the Research Unit Hypertension and Cardiovascular Research. An unrestricted grant from the International Lead Association (www.ila-lead.org) partially supports the data collection and analysis of the ongoing Study for Promotion of Health in Recycling Lead (SPHERL; NCT02243904). The sponsors had no role in the preparation of this report.
Disclosures
None.
Footnotes
This article was sent to Daniel W. Jones, Guest Editor, for review by expert referees, editorial decision, and final disposition.
References
- 1.Staessen J, Bulpitt CJ, Roels H, Bernard A, Fagard R, Joossens JV, Lauwerys R, Lijnen P, Amery A. Urinary cadmium and lead concentrations and their relation to blood pressure in a population with low exposure. Br J Ind Med. 1984;41:241–248. doi: 10.1136/oem.41.2.241. doi: 10.1136/oem.41.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staessen JA, Roels H, Fagard R for the PheeCad Investigators. Lead exposure and conventional and ambulatory blood pressure. A prospective population study. J Am Med Ass. 1996;275:1563–1570. [PubMed] [Google Scholar]
- 3.Yang WY, Efremov L, Mujaj B, Zhang ZY, Wei FF, Huang QF, Thijs L, Vanassche T, Nawrot TS, Staessen JA. Association of office and ambulatory blood pressure with blood lead in workers before occupational exposure. J Am Soc Hypertens. 2018;12:14–24. doi: 10.1016/j.jash.2017.10.010. doi: 10.1016/j.jash.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Staessen JA, Lauwerys RR, Buchet JP, Bulpitt CJ, Rondia D, Vanrenterghem Y, Amery A. Impairment of renal function with increasing blood lead concentrations in the general population. The Cadmibel Study Group. N Engl J Med. 1992;327:151–156. doi: 10.1056/NEJM199207163270303. doi: 10.1056/NEJM199207163270303. [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Nawrot T, Hond ED, Thijs L, Fagard R, Hoppenbrouwers K, Koppen G, Nelen V, Schoeters G, Vanderschueren D, et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet. 2001;357:1660–1669. doi: 10.1016/s0140-6736(00)04822-4. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]
- 6.Mujaj B, Yang WY, Zhang ZY, Wei FF, Thijs L, Verhamme P, Staessen JA. Renal function in relation to low-level environmental lead exposure. Nephrol Dial Transplant. 2019;34:941–946. doi: 10.1093/ndt/gfy279. doi: 10.1093/ndt/gfy279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WY, Zhang ZY, Thijs L, Cauwenberghs N, Wei FF, Jacobs L, Luttun A, Verhamme P, Kuznetsova T, Nawrot TS, et al. Left ventricular structure and function in relation to environmental exposure to lead and cadmium. J Am Heart Assoc. 2017;6:e004692. doi: 10.1161/JAHA.116.004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu CG, Wei FF, Yang WY, Zhang ZY, Mujaj B, Thijs L, Feng YM, Boggia J, Nawrot TS, Struijker-Boudier HAJ, et al. Central hemodynamics in relation to blood lead in young men prior to chronic occupational exposure. Blood Press. 2019;28:279–290. doi: 10.1080/08037051.2019.1610654. doi: 10.1080/08037051.2019.1610654. [DOI] [PubMed] [Google Scholar]
- 9.Batuman V, Landy E, Maesaka JK, Wedeen RP. Contribution of lead to hypertension with renal impairment. N Engl J Med. 1983;309:17–21. doi: 10.1056/NEJM198307073090104. doi: 10.1056/NEJM198307073090104. [DOI] [PubMed] [Google Scholar]
- 10.Wedeen RP. Bone lead, hypertension, and lead nephropathy. Environ Health Perspect. 1988;78:57–60. doi: 10.1289/ehp.887857. doi: 10.1289/ehp.887857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). J Am Med Ass. 1994;272:284–291. [PubMed] [Google Scholar]
- 12.Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–2161. doi: 10.1001/archinte.165.18.2155. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- 13.Hara A, Thijs L, Asayama K, Gu YM, Jacobs L, Zhang ZY, Liu YP, Nawrot TS, Staessen JA. Blood pressure in relation to environmental lead exposure in the National Health and Nutrition Examination survey 2003 to 2010. Hypertension. 2015;65:62–69. doi: 10.1161/HYPERTENSIONAHA.114.04023. doi: 10.1161/HYPERTENSIONAHA.114.04023. [DOI] [PubMed] [Google Scholar]
- 14.National Toxicology Program. In: NTP Monograph on Health Effects of Low-Level Lead. Research Triangle Park, NC: National Toxicology Program, National Institute of Environmental Health Sciences; 2012. Renal effects. pp. 77–87. [Google Scholar]
- 15.United States Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment. Research Triangle Park, NC: 2013. Integrated Science Assessment for Lead. Available at: http://www.epa.gov/ncea/isa/lead.htm. [Google Scholar]
- 16.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 μmol/L (10 μg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 17.Schober SE, Mirel LB, Graubard BI, Brody DJ, Flegal KM. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III mortality study. Environ Health Perspect. 2006;114:1538–1541. doi: 10.1289/ehp.9123. doi: 10.1289/ehp.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health. 2018;3:e177–e184. doi: 10.1016/S2468-2667(18)30025-2. doi: 10.1016/S2468-2667(18)30025-2. [DOI] [PubMed] [Google Scholar]
- 19.Yang WY, Zhang ZY, Mujaj B, Thijs L, Staessen JA. Environmental exposure to lead: old myths never die. Lancet Public Health. 2018;3:e362. doi: 10.1016/S2468-2667(18)30131-2. doi: 10.1016/S2468-2667(18)30131-2. [DOI] [PubMed] [Google Scholar]
- 20.Lanphear BP, Hornung RW, Auinger P, Allen R. Author’s reply. Lancet Public Health. 2018;3:e363. doi: 10.1016/S2468-2667(18)30128-2. [DOI] [PubMed] [Google Scholar]
- 21.Malcolm D, Barnett HA. A mortality study of lead workers 1925-76. Br J Ind Med. 1982;39:404–410. doi: 10.1136/oem.39.4.404. doi: 10.1136/oem.39.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhardsson L, Lundström NG, Nordberg G, Wall S. Mortality and lead exposure: a retrospective cohort study of Swedish smelter workers. Br J Ind Med. 1986;43:707–712. doi: 10.1136/oem.43.10.707. doi: 10.1136/oem.43.10.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443–2449. doi: 10.1001/archinte.162.21.2443. doi: 10.1001/archinte.162.21.2443. [DOI] [PubMed] [Google Scholar]
- 24.Carta P, Aru G, Nurchis P, Cadeddu C, Polizzi M, Nieddu V, Sali G, Gaviano L, Flore C, Sanna Randaccio F. [Study on mortality by specific cause among workers at a lead and zinc foundry in Sardinia]. G Ital Med Lav Ergon. 2005;27(suppl 1):43–45. [PubMed] [Google Scholar]
- 25.Khalil N, Wilson JW, Talbott EO, Morrow LA, Hochberg MC, Hillier TA, Muldoon SB, Cummings SR, Cauley JA. Association of blood lead concentrations with mortality in older women: a prospective cohort study. Environ Health. 2009;8:15. doi: 10.1186/1476-069X-8-15. doi: 10.1186/1476-069X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, Hu H. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the Department of Veterans Affairs Normative Aging Study. Circulation. 2009;120:1056–1064. doi: 10.1161/CIRCULATIONAHA.108.827121. doi: 10.1161/CIRCULATIONAHA.108.827121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhury R, Sarnat SE, Darrow L, McClellan W, Steenland K. Mortality among participants in a lead surveillance program. Environ Res. 2014;132:100–104. doi: 10.1016/j.envres.2014.03.008. doi: 10.1016/j.envres.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 28.McElvenny DM, Miller BG, MacCalman LA, Sleeuwenhoek A, van Tongeren M, Shepherd K, Darnton AJ, Cherrie JW. Mortality of a cohort of workers in Great Britain with blood lead measurements. Occup Environ Med. 2015;72:625–632. doi: 10.1136/oemed-2014-102637. doi: 10.1136/oemed-2014-102637. [DOI] [PubMed] [Google Scholar]
- 29.Kim MG, Ryoo JH, Chang SJ, Kim CB, Park JK, Koh SB, Ahn YS. Blood lead levels and cause-specific mortality of inorganic lead-exposed workers in South Korea. PLoS One. 2015;10:e0140360. doi: 10.1371/journal.pone.0140360. doi: 10.1371/journal.pone.0140360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoki Y, Brody DJ, Flegal KM, Fakhouri TH, Axelrad DA, Parker JD. Blood lead and other metal biomarkers as risk factors for cardiovascular disease mortality. Medicine (Baltimore) 2016;95:e2223. doi: 10.1097/MD.0000000000002223. doi: 10.1097/MD.0000000000002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Z, Zhen S, Orsini N, Zhou Y, Zhou Y, Liu J, Taylor AW. Association between dietary lead intake and 10-year mortality among Chinese adults. Environ Sci Pollut Res Int. 2017;24:12273–12280. doi: 10.1007/s11356-017-8871-2. doi: 10.1007/s11356-017-8871-2. [DOI] [PubMed] [Google Scholar]
- 32.Steenland K, Barry V, Anttila A, Sallmén M, McElvenny D, Todd AC, Straif K. A cohort mortality study of lead-exposed workers in the USA, Finland and the UK. Occup Environ Med. 2017;74:785–791. doi: 10.1136/oemed-2017-104311. doi: 10.1136/oemed-2017-104311. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Hernandez A, Navas-Acien A, Pastor-Barriuso R, Crainiceanu CM, Redon J, Guallar E, Tellez-Plaza M. Declining exposures to lead and cadmium contribute to explaining the reduction of cardiovascular mortality in the US population, 1988-2004. Int J Epidemiol. 2017;46:1903–1912. doi: 10.1093/ije/dyx176. doi: 10.1093/ije/dyx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barry V, Steenland K. Lead exposure and mortality among U.S. workers in a surveillance program: results from 10 additional years of follow-up. Environ Res. 2019;177:108625. doi: 10.1016/j.envres.2019.108625. doi: 10.1016/j.envres.2019.108625. [DOI] [PubMed] [Google Scholar]
- 35.Murray CJ, Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S. Comparative quantification of health risks conceptual framework and methodological issues. Popul Health Metr. 2003;1:1. doi: 10.1186/1478-7954-1-1. doi: 10.1186/1478-7954-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heeringa SG, Berglund PA, West BT, Mellipilán ER, Portier K. Attributable fraction estimation from complex sample survey data. Ann Epidemiol. 2015;25:174–178. doi: 10.1016/j.annepidem.2014.11.007. doi: 10.1016/j.annepidem.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Rabinowitz MB. Toxicokinetics of bone lead. Environ Health Perspect. 1991;91:33–37. doi: 10.1289/ehp.919133. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira S, Aro A, Sparrow D, Hu H. Season modifies the relationship between bone and blood lead levels: the Normative Aging Study. Arch Environ Health. 2002;57:466–472. doi: 10.1080/00039890209601439. doi: 10.1080/00039890209601439. [DOI] [PubMed] [Google Scholar]
- 39.Korrick SA, Schwartz J, Tsaih SW, Hunter DJ, Aro A, Rosner B, Speizer FE, Hu H. Correlates of bone and blood lead levels among middle-aged and elderly women. Am J Epidemiol. 2002;156:335–343. doi: 10.1093/aje/kwf042. doi: 10.1093/aje/kwf042. [DOI] [PubMed] [Google Scholar]
- 40.Needleman HL. The removal of lead from gasoline: historical and personal reflections. Environ Res. 2000;84:20–35. doi: 10.1006/enrs.2000.4069. doi: 10.1006/enrs.2000.4069. [DOI] [PubMed] [Google Scholar]
- 41.Hara A, Gu YM, Petit T, Liu YP, Jacobs L, Zhang ZY, Yang WY, Jin Y, Thijs L, Wei FF, et al. Study for promotion of health in recycling lead - rationale and design. Blood Press. 2015;24:147–157. doi: 10.3109/08037051.2014.996409. doi: 10.3109/08037051.2014.996409. [DOI] [PubMed] [Google Scholar]
- 42.Yu CG, Yang WY, Saenen N, Wei FF, Zhang ZY, Mujaj B, Thijs L, Feng YM, Nawrot TS, Staessen JA. Neurocognitive function in relation to blood lead among young men prior to chronic occupational exposure. Scand J Work Environ Health. 2019;45:298–307. doi: 10.5271/sjweh.3798. doi: 10.5271/sjweh.3798. [DOI] [PubMed] [Google Scholar]
- 43.Cramér K, Dahlberg L. Incidence of hypertension among lead workers. A follow-up study based on regular control over 20 years. Br J Ind Med. 1966;23:101–104. doi: 10.1136/oem.23.2.101. doi: 10.1136/oem.23.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beevers DG, Erskine E, Robertson M, Beattie AD, Campbell BC, Goldberg A, Moore MR, Hawthorne VM. Blood-lead and hypertension. Lancet. 1976;2:1–3. [PubMed] [Google Scholar]
- 45.Wedeen RP, Maesaka JK, Weiner B, Lipat GA, Lyons MM, Vitale LF, Joselow MM. Occupational lead nephropathy. Am J Med. 1975;59:630–641. doi: 10.1016/0002-9343(75)90224-7. doi: 10.1016/0002-9343(75)90224-7. [DOI] [PubMed] [Google Scholar]
- 46.Iannaccone A, Carmignani M, Boscolo P. Neurogenic and humoral mechanisms in arterial hypertension of chronically lead-exposed rats. Med Lav. 1981;72:13–21. [PubMed] [Google Scholar]
- 47.Evis MJ, Kane KA, Moore MR, Parratt JR. The effects of chronic low lead treatment and hypertension on the severity of cardiac arrhythmias induced by coronary artery ligation in anesthetized rats. Toxicol Appl Pharmacol. 1985;80:235–242. doi: 10.1016/0041-008x(85)90080-8. doi: 10.1016/0041-008x(85)90080-8. [DOI] [PubMed] [Google Scholar]
- 48.Vaziri ND. Mechanism of lead-induced hypertension and cardiovascular disease. Am J Physiol. 2008;295:H454–H465. doi: 10.1152/ajpheart.00158.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDonnell BJ, Yasmin, Butcher L, Cockcroft JR, Wilkinson IB, Erusalimsky JD, McEniery CM. The age-dependent association between aortic pulse wave velocity and telomere length. J Physiol. 2017;595:1627–1635. doi: 10.1113/JP273689. doi: 10.1113/JP273689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martens DS, Wei FF, Cox B, Plusquin M, Thijs L, Winckelmans E, Zhang ZY, Nawrot TS, Staessen JA. Retinal microcirculation and leukocyte telomere length in the general population. Sci Rep. 2018;8:7095. doi: 10.1038/s41598-018-25165-6. doi: 10.1038/s41598-018-25165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zota AR, Needham BL, Blackburn EH, Lin J, Park SK, Rehkopf DH, Epel ES. Associations of cadmium and lead exposure with leukocyte telomere length: findings from National Health and Nutrition Examination Survey, 1999-2002. Am J Epidemiol. 2015;181:127–136. doi: 10.1093/aje/kwu293. doi: 10.1093/aje/kwu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological re-appraisal of the association between blood pressure and blood lead: a meta-analysis. J Hum Hypertens. 2002;16:123–131. doi: 10.1038/sj.jhh.1001300. doi: 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- 54.Niiranen TJ, Henglin M, Claggett B, Muggeo VMR, McCabe E, Jain M, Vasan RS, Larson MG, Cheng S. Trajectories of blood pressure elevation preceding hypertension onset: an analysis of the Framingham Heart Study Original Cohort. JAMA Cardiol. 2018;3:427–431. doi: 10.1001/jamacardio.2018.0250. doi: 10.1001/jamacardio.2018.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Staessen JA, Kuznetsova T, Stolarz K. Hypertension prevalence and stroke mortality across populations. JAMA. 2003;289:2420–2422. doi: 10.1001/jama.289.18.2420. doi: 10.1001/jama.289.18.2420. [DOI] [PubMed] [Google Scholar]
- 56.Kochanek KD, Murphy SL, Xu J. Deaths: final data for 2011. Natl Vital Stat Rep. 2015;63:1–120. [PubMed] [Google Scholar]
- 57.Schoop R, Beyersmann J, Schumacher M, Binder H. Quantifying the predictive accuracy of time-to-event models in the presence of competing risks. Biom J. 2011;53:88–112. doi: 10.1002/bimj.201000073. doi: 10.1002/bimj.201000073. [DOI] [PubMed] [Google Scholar]
- 58.Wolbers M, Blanche P, Koller MT, Witteman JC, Gerds TA. Concordance for prognostic models with competing risks. Biostatistics. 2014;15:526–539. doi: 10.1093/biostatistics/kxt059. doi: 10.1093/biostatistics/kxt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Thijs L, Zhang ZY, Asayama K, Hansen TW, Boggia J, Björklund-Bodegård K, Yang WY, Niiranen TJ, Ntineri A, et al. Opposing age-related trends in absolute and relative risk of adverse health outcomes associated with out-of-office blood pressure. Hypertension. 2019;74:1333–1342. doi: 10.1161/HYPERTENSIONAHA.119.12958. doi: 10.1161/HYPERTENSIONAHA.119.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis JS, Banfield E, Lee HY, Peng HL, Chang S, Wood AC. Lifestyle behavior patterns and mortality among adults in the NHANES 1988-1994 population: a latent profile analysis. Prev Med. 2019;120:131–139. doi: 10.1016/j.ypmed.2019.01.012. doi: 10.1016/j.ypmed.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 62.US Department of Health and Human Services PHSCoDC. Centers for Disease Control; 1991. Preventing lead poisoning in young children. Available at: http://wonder.cdc.gov/wonder/prevguid/p0000029/p0000029.asp. Accessed September 23, 2019. [Google Scholar]
- 63.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Navas-Acien A, Schwartz BS, Rothenberg SJ, Hu H, Silbergeld EK, Guallar E. Bone lead levels and blood pressure endpoints: a meta-analysis. Epidemiology. 2008;19:496–504. doi: 10.1097/EDE.0b013e31816a2400. doi: 10.1097/EDE.0b013e31816a2400. [DOI] [PubMed] [Google Scholar]
- 65.Muntner P, He J, Vupputuri S, Coresh J, Batuman V. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003;63:1044–1050. doi: 10.1046/j.1523-1755.2003.00812.x. doi: 10.1046/j.1523-1755.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 66.Renard D, Molenberghs G, Van Oyen H, Tafforeau J. Investigation of the clustering effect in the Belgian Health Interview Survey 1997. Arch Public Health. 1998;56:345–361. [Google Scholar]
- 67.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 68.Birch J, Petty R, Hooper L, Bauld L, Rosenberg G, Vohra J. Clustering of behavioural risk factors for health in UK adults in 2016: a cross-setional survey. J Public Health. 2019;41:e226–e236. doi: 10.1093/pubmed/fdy144. doi: 10.1093/pubmed/fdy144:dfy144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Staessen J, Bruaux P, Claeys-Thoreau F, DePlaen P, Ducoffre G, Lauwerys R, Roels H, Rondia D, Sartor F, Amery A. The relationship between blood pressure and environmental exposure to lead and cadmium in Belgium. Environ Health Perspect. 1988;78:127–129. doi: 10.1289/ehp.8878127. doi: 10.1289/ehp.8878127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Environment Directorate, Organisation for Economic Co-operation and Development. Estimation of welfare costs from exposure to lead. 59th joint meeting of the Chemicals Committee and the Working Party in Chemicals, Pesticides and Biotechnology. OECD. 2019. p. JT03446545.
- 71.Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L, Vangronsveld J, Van Hecke E, Staessen JA. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol. 2006;7:119–126. doi: 10.1016/S1470-2045(06)70545-9. doi: 10.1016/S1470-2045(06)70545-9. [DOI] [PubMed] [Google Scholar]
- 72.Buchet JP, Staessen J, Roels H, Lauwerys R, Fagard R. Geographical and temporal differences in the urinary excretion of inorganic arsenic: a Belgian population study. Occup Environ Med. 1996;53:320–327. doi: 10.1136/oem.53.5.320. doi: 10.1136/oem.53.5.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Apostolou A, Garcia-Esquinas E, Fadrowski JJ, McLain P, Weaver VM, Navas-Acien A. Secondhand tobacco smoke: a source of lead exposure in US children and adolescents. Am J Public Health. 2012;102:714–722. doi: 10.2105/AJPH.2011.300161. doi: 10.2105/AJPH.2011.300161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volume 84. Inorganic and Organic Lead Compounds. Lyon, France: World Health Organization; International Agency for Research on Cancer; 2006. pp. 377–378. [Google Scholar]
- 75.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 76.Mathers C, Stevens G, Mascarenhas M. Global Health Risks. Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 77.Nawrot TS, Roels HA, Vangronsveld J, Staessen JA. Cadmium from zinc smelter emission and variation in cancer incidence: the hierarchy of evidence. Eur J Cancer Prev. 2012;21:497–498. doi: 10.1097/CEJ.0b013e32834ef1ce. doi: 10.1097/CEJ.0b013e32834ef1ce. [DOI] [PubMed] [Google Scholar]
- 78.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, et al. PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 79.Asayama K, Stolarz-Skrzypek K, Persu A, Staessen JA. Systematic review of health outcomes in relation to salt intake highlights the widening divide between guidelines and the evidence. Am J Hypertens. 2014;27:1138–1142. doi: 10.1093/ajh/hpu126. doi: 10.1093/ajh/hpu126. [DOI] [PubMed] [Google Scholar]


