Supplemental digital content is available in the text.
Key Words: Concussion, Traumatic Brain Injury, Magnetic Resonance Imaging, Neuroimaging
Abstract
Objective
The aims of this study were to investigate changes in regional brain volume after concussion (mild traumatic brain injury) and to examine the relationship between change in brain volume and cognitive deficits.
Design
Twenty-eight patients with mild traumatic brain injury and 27 age-matched controls were included in this study. Magnetic resonance imaging (3 T) data were obtained from the participants. Structural brain volume changes were examined using tensor-based morphometry, which identifies regional structural differences in the whole brain, including cerebrospinal fluid, gray matter, and white matter. Volume contraction and expansion were compared between groups using a two-sample t test. The association between time post-injury or neurocognitive function and volumetric changes was examined using regression analysis.
Results
Individuals with mild traumatic brain injury exhibited volume reduction in the brainstem, including the pontine reticular formation. Regional cerebral volume changes were not associated with time post-injury but were significantly associated with neurocognitive function, especially with executive card sorting test, forward digit span test, and performance on verbal learning test. The greater regional cerebral volume was associated with better cognitive performance after mild traumatic brain injury.
Conclusion
Decreased brainstem volume may indicate its vulnerability to traumatic injury, and cerebral volume in specific regions was positively associated with patients’ cognitive function after injury.
What Is Known
There have been inconsistent reports on whether concussion causes structural brain changes and whether these changes correlate with the severity of postconcussion symptoms.
What Is New
Individuals with concussion had reduced brainstem volume, particularly in the pontine reticular formation. Time post-injury was not significantly associated with regional volume changes. Neurocognitive functions, such as memory and executive function, were positively correlated with regional cerebral volume changes, which means that the smaller regional volume is related to worse cognitive performance after injury.
Mild traumatic brain injury (mTBI), also known as concussion, is still underrecognized despite its high incidence rate and clinical significance.1 Many individuals with mTBI experience cognitive deficits,2 which can last for more than a month. However, computed tomography and magnetic resonance imaging (MRI) of the brain do not reveal conspicuous abnormal findings, such as contusion or hemorrhage, after mTBI, even in patients with severe symptoms. Technical advances in neuroimaging have aided in the detection of subtle structural brain changes and its association with cognitive deficits after mTBI. It has been reported that patients with mTBI exhibit significant changes in brain volume, particularly reduced volume of the gray and white matter.3 Neurocognitive measures of memory, attention, and various postconcussive symptoms are significantly associated with regional changes in brain volume after mTBI.3 It has also been postulated that structural brain changes after mTBI should be considered in the context of time post-injury, which may reflect compensatory remodeling of cortical regions correlated with cognitive performance after the injury.4 Notwithstanding these clinical findings, there is one study that reported no structural volume changes after mTBI.5 Further studies are still needed to identify useful imaging biomarkers for diagnosing and predicting mTBI outcomes.
In previous studies, structural volume changes after traumatic brain injury (TBI) have been examined using several approaches: region-of-interest (ROI) analysis,6 whole-brain voxel-based morphometry (VBM) analysis,3,4 and tensor-based morphometry analysis (TBM).5 The first approach ignores other brain areas except the ROI. The second approach analyzes the brain based on different tissue types, such as gray and white matter. TBM captures regional structural changes in the whole brain without analyzing brain tissue subtypes independently. Because of this advantage, TBM has been successfully used for the evaluation of structural changes in the injured brain, which makes it harder to separate brain tissue subtypes.7 In addition, TBM offers good registration results with highly deformable transformation models over VBM.8 It can avoid the complicated process of the independent analysis of brain tissue subtypes, which can increase the possibility of false-positive ratio. Therefore, TBM has the advantage of assessing structural changes in the whole brain of mTBI patients. Among the seven observational (cross-sectional and longitudinal) studies that examined brain changes after TBI, only one focused on mild injury.5,7,9–13 The study found no difference between the mTBI group and controls and between the initial and follow-up scans.5
The aims of this study, therefore, were to examine changes in brain volume after mTBI and to determine the relationship between changes in brain volume and time post-injury and between brain volume changes and cognitive deficits, using TBM. Based on previous volumetric studies on mTBI, the following hypotheses were considered: (1) reduced brain volumes would be observed in patients with mTBI compared with controls, (2) regional brain volume may be significantly associated with time post-injury, reflecting vulnerability of the brain to injury or cortical plasticity,14 (3) regional brain volume changes would be significantly associated with neurocognitive measures in mTBI patients, and (4) volume changes in white matter would be greater than in gray matter, reflecting diffuse axonal injury due to mTBI.15
METHODS
Participants
This retrospective study was approved by the institutional review board of the hospital and has been written in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (see Checklist, Supplemental Digital Content 1, http://links.lww.com/PHM/B117). Of the 120 patients who visited the concussion clinic between October 2016 and February 2018, 28 patients with mTBI (9 men; mean ± SD age, 47.4 ± 10.4 yrs) were included (Fig. 1). In this study, mTBI was defined by the following criteria: loss of consciousness (<30 mins), posttraumatic amnesia (<24 hrs), mental status change, or focal neurological deficits. Most of the patients visited the hospital several days after the accident because of persisting symptoms; therefore, Glasgow Coma Scale score within 24 hrs after injury was unavailable in many cases. Because this study was designed to retrospectively analyze electronic medical records and information on the clinical, behavioral, and cognitive symptoms, written informed consent was not obtained from the patients, as the institutional review board waived this requirement.
FIGURE 1.
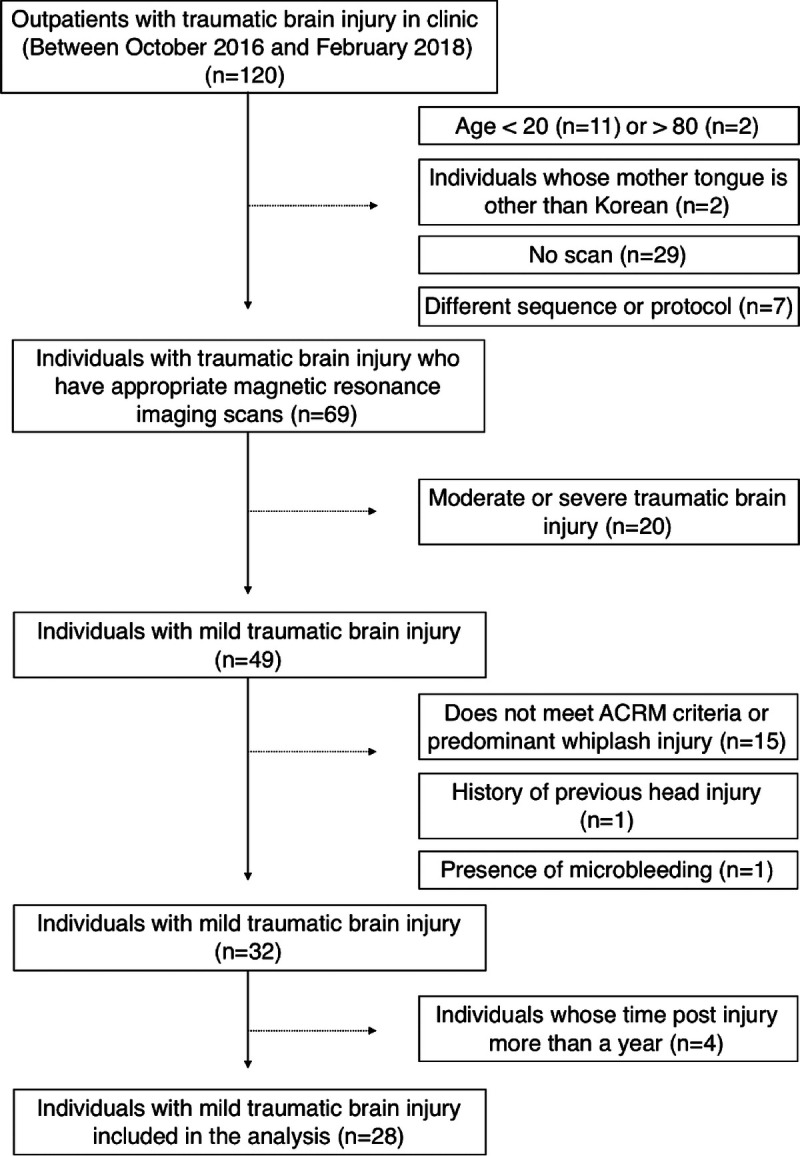
Flowchart of selecting individuals with mTBI in the study.
Control patients were selected from the radiology report database.16 The patients had visited the outpatient clinic of the neurology department for various neurologic symptoms, particularly for mild headache or migraine, and had undergone MRI between October 2016 and June 2017. In total, 27 age- and sex-matched subjects (8 men; 48.3 ± 11.6 yrs old) were included in this study as controls. Age matching was achieved through 10-yr age stratification. One mTBI patient had no matched control. The control group showed no abnormalities or had few nonspecific mild T2 high-intensity signals on MRI scans and had no previous history of trauma or other serious illnesses outside the brain that might potentially cause neurologic dysfunction.16 Data are available upon reasonable request.
Clinical Assessment
The patients’ symptoms and status were routinely assessed using several clinical assessment tools and questionnaires, including the Rivermead Post-Concussion Symptom Questionnaire (RPCSQ),17 Extended Glasgow Outcome Scale,18 Patient Health Questionnaire-9 (PHQ-9),19 and Computerized NeuroCognitive Function Test (CNT40; MaxMedica, Seoul, Korea).20–22 The RPCSQ is devised to measure symptom severity by asking patients to rate the degree of symptoms on a scale of 0 to 4 on 16 items. The Extended Glasgow Outcome Scale is a measure of disability and recovery after TBI comprising 19 items. The PHQ-9 measures depression with nine items. The CNT incorporates an auditory continuous performance test (attention), a verbal learning test (memory), a digit span test (memory), and a card sorting test (executive function).
Outcome measures included the following: the number of correct responses and commission errors in the auditory continuous performance test; the number of words recalled in the first trial (A1), in the fifth trials (A5), and after 20 mins (delayed recall) in the verbal learning test; the number of digit span of the subject in the forward and backward digit span tests; and the perseverative rate of set-shifting in the card sorting test. The t scores, which have a mean of 50 and a standard deviation of 10, based on age-matched normative samples, were provided.20–22 The response validity of the test has been previously assessed.20–22
Not all participants underwent all tests, and data were obtained retrospectively. Detailed characteristics of the patients are summarized in Table 1 and Supplemental Digital Content Table 1 (Supplemental Digital Content 2, http://links.lww.com/PHM/B118). Cognitive test scores could not be obtained from the control subjects because they were selected retrospectively.
TABLE 1.
Characteristics of patients
| ID | Sex | Age, years | GCS | LOC | PTA | Duration, days | RPCSQ | GOSE | PHQ-9 | Mode of Injury | Previous Mental Health Information | Age of the Matched Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | F | 69 | 15 | – | <5 mins | 50 | NA | NA | NA | Car accident | – | 67 |
| 02 | F | 60 | 15 | – | – | 60 | 32 | 6 | 8 | Struck by object | – | 60 |
| 03 | F | 46 | 15 | <30 mins | <30 mins | 56 | NA | NA | NA | Pedestrian car accident | – | 45 |
| 04 | F | 53 | 15 | <5 mins | 1 hr | 201 | 37 | 6 | 9 | Pedestrian car accident | – | 52 |
| 05 | M | 47 | 15 | – | – | 130 | NA | NA | NA | Struck by object | – | 55 |
| 06 | M | 48 | 15 | <1 min | <5 mins | 65 | NA | NA | NA | Struck by object | – | 58 |
| 07 | F | 41 | NA | – | – | 34 | 45 | 5 | 18 | Car accident | – | 41 |
| 08 | M | 53 | 15 | – | <10 mins | 36 | 30 | 5 | 7 | Car accident | – | 60 |
| 09 | F | 54 | 15 | <5 mins | <24 hrs | 108 | 41 | 6 | 19 | Struck by object | – | 53 |
| 10 | F | 60 | 15 | <30 mins | <10 mins | 150 | 39 | 6 | 7 | Fell down | – | 63 |
| 11 | F | 54 | 15 | <30 mins | <24 hrs | 107 | 56 | 5 | 18 | Struck by object | – | 56 |
| 12 | F | 56 | 15 | – | 1 hr | 31 | 25 | 7 | 5 | Fell down | – | 56 |
| 13 | F | 49 | NA | 5–10 mins | 3–5 mins | 46 | NA | NA | NA | Struck by object | – | 49 |
| 14 | F | 56 | 15 | – | – | 35 | NA | NA | NA | Struck by object | NA | 56 |
| 15 | M | 44 | NA | <30 mins | <2 hrs | 126 | 43 | 5 | 14 | Pedestrian car accident | – | 45 |
| 16 | M | 35 | 15 | <10 mins | <24 hrs | 162 | 28 | 5 | 12 | Car accident | NA | 33 |
| 17 | F | 50 | NA | <30 mins | <1 hr | 66 | 26 | 6 | 4 | Pedestrian car accident | – | 50 |
| 18 | M | 37 | 15 | – | – | 31 | 36 | NA | 11 | Struck by object | – | 36 |
| 19 | M | 45 | 15 | <5 mins | – | 75 | 46 | 5 | 23 | Car accident | – | 46 |
| 20 | F | 57 | 15 | <1 min | – | 51 | 59 | 5 | 24 | Car accident | – | 58 |
| 21 | F | 30 | 15 | – | <10 mins | 26 | NA | NA | NA | Car accident | – | 28 |
| 22 | F | 46 | NA | <30 mins | <3 hrs | 57 | NA | NA | NA | Bicycle accident | NA | 45 |
| 23 | F | 31 | 15 | <1 min | – | 45 | 34 | 5 | 12 | Car accident | – | 34 |
| 24 | F | 24 | 15 | – | <1 min | 25 | 36 | 6 | 10 | Car accident | – | 23 |
| 25 | M | 47 | NA | 20 mins | – | 287 | NA | NA | NA | Struck by object | – | – |
| 26 | F | 58 | NA | – | – | 51 | 25 | 5 | 10 | Fell down | Depression | 58 |
| 27 | F | 40 | 15 | <1 min | – | 33 | 33 | 5 | 13 | Pedestrian car accident | – | 38 |
| 28 | M | 36 | NA | 5 mins | – | 18 | 25 | 5 | 6 | Fell down | – | 34 |
NA for the GCS indicates “not available” because the patients visited outpatient clinics in postacute phases. NA for previous mental health information indicates “not available” because there was no information in the electronic medical records. Only scores measured within a month before or after brain scanning are presented here.
GCS indicates Glasgow coma scale; LOC, loss of consciousness; PTA, posttraumatic amnesia; GOSE, Extended Glasgow Outcome Scale; F, female; M, male; NA, not available.
Image Acquisition
Brain imaging data were obtained using a Discovery MR750w 3.0 T scanner (General Electric Healthcare, Milwaukee, WI) equipped with a 32-channel head coil. Structural T1 images were acquired using a sagittal three-dimensional fast spoiled gradient-recalled-echo acquisition protocol with the following parameters: image matrix, 256 × 230 mm; voxel size, 1 mm3; field of view, 256 mm; flip angle, 12 degrees; repetition time, 8.5 msecs for 18 patients and 16 controls, 8.6 msecs for nine patients and six controls, 8.4 msecs for one patient and two controls, and 8.7 msecs for one control; and echo time, 3.2 msecs for all patients and controls. Two controls had the same parameters with repetition time of 8.5 and 8.6 msecs and image matrix of 256 × 256 mm. The numbers of sagittal slices spanning the whole brain differed slightly among the subjects, depending on brain size (from 146 to 182 slices).
Data Preprocessing
All structural T1 images were manually inspected to detect high signal noise or motion artifacts. After realigning the anterior and posterior commissure line using the Statistical Parametric Mapping tool (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), the intensity inhomogeneity of the images was N3-corrected using c3d (http://www.itksnap.org). The structural images were skull-stripped and linearly registered to the International Consortium for Brain Mapping template (ICBM-53), with the nine-parameter transformation using mutual information as the similarity function. The linearly registered subject images were used to construct a study-specific template using symmetric normalization (SyN) through Advanced Normalization Tools.23 Only the images of control subjects were used to create the study-specific template. The linearly registered structural T1 images were nonlinearly aligned to the study-specific template using SyN algorithm.23 The deformation and Jacobian determinant maps, representing local shape changes from the individual T1 images to the stereotaxic template image, were then estimated. Jacobian determinant values lower than 1 indicate volume contraction, whereas values higher than 1 indicate volume expansion. For statistical analyses, the Jacobian determinant maps of the two groups were log-transformed.
Statistical Analyses
Statistical analyses were performed using the SPM12 software. Statistical significance was set at an uncorrected P < 0.001, with a cluster-based family-wise error rate correction P < 0.05. Age and total intracranial volume were used as nuisance variables in all statistical tests.
Comparing Volumes Between the Groups
Volume contraction and expansion were compared between the groups using the log-transformed Jacobian determinant maps and a voxel-wise two-sample t test.
Brain Volume Correlation with Time Post-injury or Neurocognitive Performance
The effect of time post-injury on structural brain volume was examined using voxel-wise regression analysis. Two-sample t test was also performed after dividing the individuals with mTBI into two groups: early (<60 days) and late (≥60 days) phases after injury. The 60 days cut-off was chosen to represent the optimal value for early and late phases after injury in this study, which was similar to the median value of time post-injury (53.5 days in 28 mTBI patients). The association between neurocognitive function measured using the CNT in patients with mTBI and brain volume was examined using voxel-wise regression analysis. To investigate the volume difference between the mTBI and control groups, especially the brain regions that showed a significant correlation between regional volume and cognitive scores in the mTBI patients, the averaged log-transformed Jacobian determinant values of the given regions were calculated and compared between the groups using an in-house MATLAB (Mathworks, Natick, MA) script. Attempts were also made to identify the most probable anatomical localization of white matter tracts to examine what areas the observed white matter volume belonged to and to compare the results with those in previous diffusion tensor imaging studies. Using the atlasquery tool of the FMRIB Software Library (https://fsl.fmrib.ox.ac.uk), the “JHU ICBM-DTI-81 white matter labels” was used as an ROI atlas. Among the 48 white matter tract labels, the tracts with clusters at greater than 1% probability were reported in this study.
Correlation Among Other Clinical Assessment
The relationship between the outcomes of clinical assessment of mTBI patients and the time post-injury using Pearson correlation analysis was additionally analyzed. The number of inputs in this correlation analysis was different in each case because not all individuals with mTBI underwent all tests. Statistical significance was set at P < 0.05.
RESULTS
Subject Characteristics
The period between injury and brain MRI (i.e., time post-injury) ranged from 18 to 287 days (mean, 77.2 ± 62.3 days). No abnormal findings were observed on MRI with T1-weighted and susceptibility weighted imaging, except for one patient who had a tiny old lacunae infarction of the left thalamus, which was not associated with the symptom after mTBI. The means and standard deviations of the RPCSQ, Extended Glasgow Outcome Scale, and PHQ-9 of the patients were 36.6 ± 9.9, 5.4 ± 0.6, and 12.1 ± 5.9, respectively.
Volume Contractions Observed in the mTBI Group
The ratio of the mean volume in the mTBI group compared with the control group is visualized in Figure 2. Brainstem volume was significantly reduced in the mTBI group compared with the control group (Fig. 3). Volume contraction was prominent mostly around the pontine reticular formation. No volume expansion was observed. Using the atlasquery tool, the white matter tracts with volume contraction included the left superior and bilateral middle cerebellar peduncles, pontine crossing tract, bilateral corticospinal tracts, and left cerebral peduncle.
FIGURE 2.
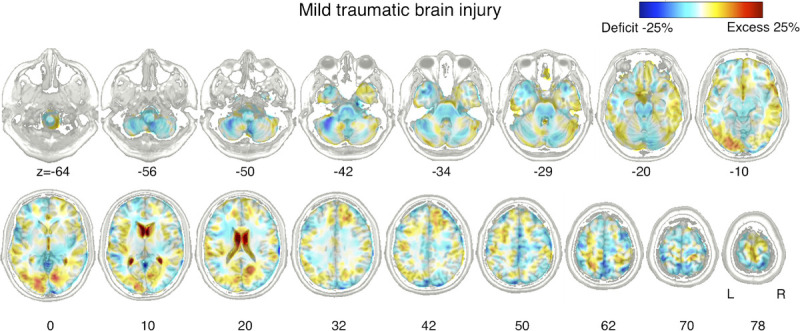
Ratio of the mean volume in the individuals with mTBI relative to that of controls. Percentage difference between the average volumes of the two groups were visualized in blue-red representing volume contraction and expansion compared with the controls.
FIGURE 3.
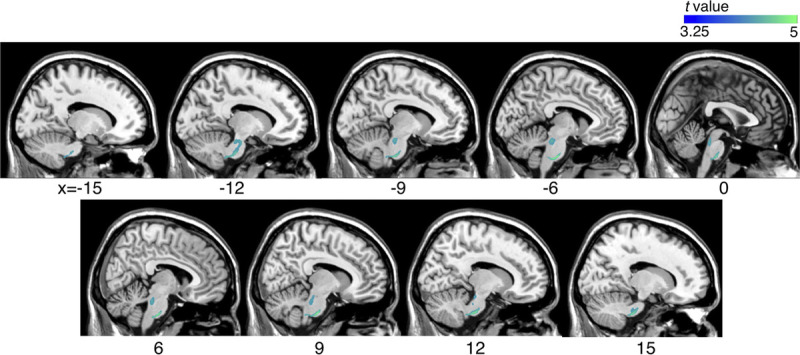
Comparison of volume changes between the mTBI and the control groups using tensor-based morphometry. Volume contraction in individuals with mTBI was compared with controls (uncorrected P < 0.001; with cluster-based family-wise ratio correction, P < 0.05). Blue represents the areas where volume contraction was observed in the mTBI group.
Effects of Time Post-injury on Regional Brain Volumes
Overall, there was no significant association between the time post-injury and structural brain changes in the mTBI group. No significant difference was observed between mTBI patients in the early (<60 days) and late (≥60 days) phases after injury.
Association of Regional Brain Volumes with Neurocognitive Function
Neurocognitive function, assessed using the CNT test, revealed a significant association between regional brain volume and CNT subtests’ results, including the card sorting test, forward digit span test, and verbal learning performance (A5 and delayed recall). Brainstem volume reduction in patients with mTBI did not correlate with neurocognitive function scores.
The executive function assessed by the card sorting test demonstrated a positive correlation with volume in the right superior and middle temporal areas (Table 2, Fig. 4A). No white matter tracts related to these areas were identified.
TABLE 2.
Association of regional brain volumes with neurocognitive performance measured by the CNT
| Contrast | Cluster | Peak T | MNI Coordinates | Region | Tissue Type | Lobe | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Card sorting test | ||||||||
| 1195 | 6.86 | 58 | 2 | −10 | Superior temporal gyrus | White matter | Temporal lobe | |
| 6.11 | 47 | 0 | −12 | Insula | * | Sublobar | ||
| 4.70 | 54 | 14 | −5 | * | * | Fronto-temporal space | ||
| 4.04 | 58 | −7 | −13 | Middle temporal gyrus | White matter | Temporal lobe | ||
| 3.92 | 56 | 4 | −2 | Superior temporal gyrus | Gray matter | Temporal lobe | ||
| Forward digit span test | ||||||||
| 1671 | 7.72 | −13 | 5 | −11 | Lentiform nucleus | Gray matter | Sublobar | |
| 7.61 | −4 | 15 | −13 | Extra-nuclear | White matter | Sublobar | ||
| 4.17 | −24 | 8 | −12 | Lentiform nucleus | Gray matter | Sublobar | ||
| 1199 | 7.44 | 31 | −16 | 13 | Extra-nuclear | White matter | Sublobar | |
| 4.18 | 31 | −28 | 9 | Extra-nuclear | White matter | Sublobar | ||
| 1323 | 6.98 | −39 | 32 | 37 | Middle frontal gyrus | Gray matter | Frontal lobe | |
| 5.12 | −26 | 32 | 44 | Middle frontal gyrus | Gray matter | Frontal lobe | ||
| 4.71 | −44 | 34 | 28 | Middle frontal gyrus | White matter | Frontal lobe | ||
| Verbal learning test; A5 | ||||||||
| 3214 | 7.37 | 35 | −31 | 27 | Extra-nuclear | White matter | Sublobar | |
| 4.51 | 31 | −20 | 35 | Subgyral | White matter | Frontal lobe | ||
| 1092 | 7.33 | −17 | −30 | 36 | Cingulate gyrus | White matter | Limbic lobe | |
| 4.06 | −28 | −21 | 33 | Subgyral | White matter | Frontal lobe | ||
| Verbal learning test; delayed recall | ||||||||
| 5307 | 7.54 | −18 | −33 | 37 | Cingulate gyrus | White matter | Limbic lobe | |
| 7.01 | −7 | −35 | 29 | Cingulate gyrus | White matter | Limbic lobe | ||
| 6.91 | −23 | −48 | 29 | Subgyral | White matter | Parietal lobe | ||
| 5.80 | −23 | −55 | 20 | Subgyral | White matter | Temporal lobe | ||
| 5.63 | 3 | −33 | 27 | * | * | * | ||
| 5.01 | −32 | −54 | 14 | Subgyral | White matter | Temporal lobe | ||
| 1712 | 6.89 | 26 | 14 | 6 | Extra-nuclear | White matter | Sublobar | |
| 5.11 | 34 | 3 | 8 | Extra-nuclear | White matter | Sublobar | ||
| 4.73 | 26 | 2 | −8 | Lentiform nucleus | Gray matter | Sublobar | ||
| 11211 | 6.71 | 20 | 21 | 18 | Extra-nuclear | White matter | Sublobar | |
| 6.57 | 21 | 8 | 21 | Extra-nuclear | White matter | Sublobar | ||
| 6.31 | 19 | −28 | 37 | Cingulate gyrus | White matter | Limbic lobe | ||
| 6.08 | 17 | 33 | −2 | Extra-nuclear | White matter | Sublobar | ||
| 5.76 | 9 | 33 | 2 | Extra-nuclear | White matter | Sublobar | ||
| 5.66 | 30 | −28 | 31 | Subgyral | White matter | Frontal lobe | ||
| 1671 | 4.93 | −17 | 24 | 32 | Cingulate gyrus | White matter | Limbic lobe | |
| 4.66 | −22 | 11 | 29 | Subgyral | White matter | Frontal lobe | ||
| 4.56 | −18 | 30 | 24 | Cingulate gyrus | White matter | Limbic lobe | ||
*No relevant region, tissue type, or lobe corresponds to the MNI coordinates.
MNI indicates Montreal Neurological Institute.
FIGURE 4.
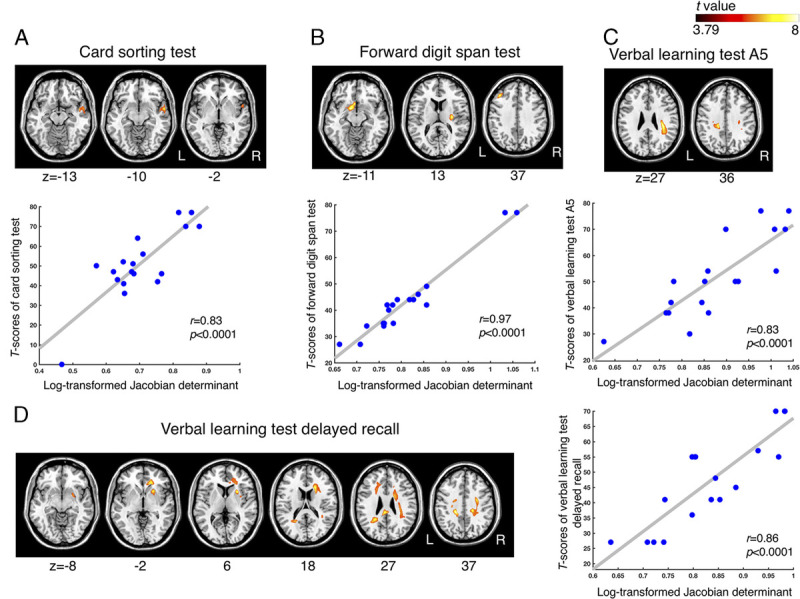
Regression analysis of the relationship between regional brain volume and neurocognitive performance. The brain areas show a significant positive correlation with (A) executive function assessed using the card sorting test, (B) memory function assessed using the forward digit span test, (C) A5 verbal learning test, and (D) delayed recall verbal learning test (uncorrected P < 0.001; with cluster-based family-wise ratio correction, P < 0.05). The hot color map represents the brain region where its volume is positively correlated with cognitive scores. The scatterplots display the correlation between the averaged log-transformed Jacobian determinant values of the brain areas in the individuals with mTBI and the T scores of (A) the card sorting test, (B) forward digit span test, (C) A5 verbal learning test, and (D) delayed recall verbal learning test.
Forward working memory performance demonstrated a positive correlation with volume in the left globus pallidus, putamen, and middle frontal areas (Table 2, Fig. 4B). The right posterior limb and the retrolenticular part of the internal capsule, and the external capsule were identified as the white matter tracts relevant to the above-mentioned structures.
Verbal learning performance measured using the A5 test demonstrated a significant positive correlation with the volume of the right frontal subgyral and left cingulate regions (Table 2, Fig. 4C). The atlasquery tool revealed that the white matter tracts contained in these areas included the body of corpus callosum, bilateral superior and posterior corona radiata, and right superior longitudinal fasciculus.
The delayed recall performance demonstrated a positive correlation with the volume of the right frontal, bilateral limbic, and left temporoparietal lobes and right sublobar areas. It included bilateral cingulate and right putamen areas (Table 2, Fig. 4D). The body, genu, and splenium of the corpus callosum, bilateral superior, anterior and posterior corona radiata, right external capsule, left cingulum, right superior longitudinal fasciculus, and superior fronto-occipital fasciculus were the relevant white matter tracts of the observed structures.
The averaged Jacobian determinant value of the brain areas that were significantly correlated with the CNT subscores was not different between groups in each test (P > 0.05).
Association Between the Outcomes of Clinical Assessment and Time Post-injury After mTBI
The time post-injury did not show a significant correlation with the neurocognitive function assessed by the CNT test, the post-injury psychiatric status assessed by the PHQ-9, and the subjective complaints after injury assessed by the RPCSQ (P > 0.05). The post-injury psychiatric status after mTBI showed a significant association with the outcomes of RPCSQ (r = 0.83, P < 0.00001) and the correct response of the auditory continuous performance test from the CNT test (r = −0.56, P = 0.03). There was no significant relationship between the outcome of RPCSQ and neurocognitive performance (P > 0.05).
DISCUSSION
Approximately 80% of the estimated 69 million people who sustain a TBI every year have mTBI.1 In many countries, a considerable number of mTBI patients are treated by physiatrists and receive rehabilitation services for variable physical and cognitive complaints. This highlights the importance of concussion management in practice by physiatrists. As a basis for understanding and searching for an optimal rehabilitation strategy for physiatrists, this study aimed to investigate the structural brain changes after mTBI using TBM. To the authors’ knowledge, this present study is the first to demonstrate that brainstem volume is reduced in mTBI patients compared with controls. A previous study showed that brainstem volume in mTBI patients was reduced over time but did not reveal significant differences when compared with controls.24 The result from the present study is consistent with previous findings observed in more severe TBI patients.10 It is interesting to note that cerebral volume was not significantly different in individuals with mTBI and controls despite the previous evidence.3 The methodologic difference between the two studies could account for the observed differences. Previous studies showing reduced cerebral volume after mTBI used VBM or ROI analyses, which did not include the entire brainstem.3,4 The present results suggest that the brainstem, rather than other cerebral regions, is more vulnerable after mTBI, given that the primary axis of the brainstem crosses at the rostro-caudal axis of the brain.25 The brainstem has been recognized as the primary site of damage and dysfunction related to TBI.26 The excessive shear stress around the brainstem caused by the trauma27 may lead to extensive loss of brainstem volume without significant contraction of cerebral volume. Brainstem white matter degeneration after a traumatic event has been observed in animal studies.28,29
In addition, loss of consciousness—a cardinal sign of mTBI—is believed to be related to the transient disruption of brainstem function.30 It has been reported that the Glasgow Coma Scale, which measures the state of consciousness, is associated with the location of traumatic axonal injury lesions in the brainstem (i.e., unilateral and bilateral side) in patients with moderate to severe TBI.31 In a previous study, volume contraction of the brainstem was related to symptoms after TBI, such as processing speed on a working memory task32 and poor postural controls.33 However, in the present study, changes in brainstem volume after mTBI did not correlate with cognitive performance. In addition, the relationship between structural brain volume and RPCSQ scores was analyzed. The additional analysis showed that there was no significant relationship between brain volume changes and subjective TBI complaints. This study did not assess postural control in these patients. The lack of significant correlation between brainstem volume and cognitive performance or subjective complaints after mTBI might be a result of the analytical method used in this study. TBM considers individual voxel as a unique statistical measurement, and the small association measure may easily disappear when multiple comparison correction is applied. In contrast, the ROI analysis could be useful in detecting the association between such compact compartments and the cognitive performance, which was already reported in the previous study.32 The brainstem has been known to be involved in tonic attention,34 but a specifically designed test for assessing tonic attentional performance was not used in this study. The brainstem may also be associated with executive function, with its many connections with the cerebellum and the frontal/subcortical system. A study of healthy participants demonstrated high connectivity between the brainstem and executive control network.35 However, no significant associations between cognitive functions and brainstem volume changes were found in this study. Further studies are needed to examine the relationship between cognitive performance, subjective symptom complaints, autonomic dysfunctions, and poor postural control and volumetric changes in the brainstem, by using specific cognitive tests relevant to brainstem function in a diverse methodological framework.
Investigating the effect of time post-injury on regional volume was one of the goals in the present study; there was no association between time post-injury and brain volume in the mTBI group. The absence of association was similarly observed when the mTBI group was divided into early and late phases of injury. The rigorous statistical criterion of multiple comparison, i.e., the cluster-based family-wise ratio correction applied in the relatively small sample size data in the present study might account for the findings. There was increased volume in the white matter area near the right hippocampal gyrus in the mTBI group with the late phase of injury (≥60 days) compared with the early phase (<60 days) (uncorrected P < 0.001) within a cluster of an extent threshold of k > 100 (see Supplemental Digital Content Fig. 1, Supplemental Digital Content 3, http://links.lww.com/PHM/B119). In the additional analysis, the mTBI group with early phase of injury did not show increased volume in the brain compared with those with late phase of injury. The result was consistent with previous studies showing time-dependent plastic changes of the brain, represented by increased brain volume in mTBI patients.4,24,36
The present results showed a significant positive correlation between regional volume and cognitive performance in specific brain areas, many in the white matter regions, including the corpus callosum, corona radiata, and superior longitudinal fasciculus. These brain areas, previously reported to experience brain volume reduction, are notoriously vulnerable to injury.37 Nevertheless, in the present study, there was no significant volume contraction or enlargement in these brain areas in the mTBI group compared with the control group. The results indicate the possibility that regional brain volume progressively recovers to normal after mTBI. This finding is similar to the one from a previous study that reported an association of increased volume with better cognitive performance.36 In another study, moderate-severe TBI group was divided into TBI-slow and TBI-normal subgroups, depending on the evidence of functional recovery. Longitudinally, the TBI-slow group exhibited volume contraction, whereas the TBI-normal group exhibited volume expansion in several white matter areas.9
Executive function assessed using the card sorting test was positively correlated with the volume of the right superior and middle temporal areas. These results were similar to those in a previous study reporting a positive correlation between the volume of the superior temporal area and the results of the Wisconsin Card Sorting test.38 In the forward digit span test, the observed regions were the left globus pallidus, putamen, and middle frontal area, which were positively correlated with working memory capacity in the previous functional MRI study.39 The brain areas in which volumes are correlated with cognitive test scores are relevant to that function.
Likewise, in the delayed recall test, the observed regions were mostly in the fronto-limbic-temporal areas, encompassing both gray and white matter tissues, and the sublobar areas, including the corpus callosum and putamen. The fronto-limbic-temporal areas are the brain regions in which the greatest stress-strain actions occur after TBI.40 Cognitive impairment is frequently observed in TBI patients with reduced volume in the frontotemporal areas.4
The results of this study showed that a significant correlation between specific brain regional volume and neurocognitive performance was observed mostly in the white matter areas rather than gray. In the case of the A5 and delayed recall tests, several white matter tracts belonged to the observed areas, such as the corpus callosum, the bilateral corona radiata, right external capsule, left cingulum, right superior longitudinal fasciculus, and superior fronto-occipital fasciculus. These were consistently observed in white matter tracts in the mTBI group with altered white matter integrity.13 In a case series investigating severe TBI, the fractional anisotropy value in the corpus callosum was positively correlated with neurocognitive function test performance, such as reading fluency, math scores, and verbal working memory scores.41 Fractional anisotropy in the left anterior corona radiata is positively correlated with attentional control in the patients with mTBI.42 Although changes in volume and tissue integrity are different phenomena, they may be related to one another. For example, the reduced compression of axonal tracts could be due to heavy atrophy in the surrounding areas.10 However, the exact nature of the relationship remains unclear; therefore, it can only be speculated whether white matter tracts with altered integrity also have altered volumetric changes after TBI.
In the present study, time post-injury was not correlated with cognitive scores, depression, and post-injury complaints after mTBI. Because this study was a cross-sectional study that analyzed retrospectively obtained data, initial symptom severity might have affected the results of analysis of time post-injury. Longitudinal studies are necessary to investigate this relationship. It seems that RPCSQ scores, which encompassed the question about depression, were positively correlated with depressive mood after mTBI assessed by PHQ-9 test. The result suggests that the individuals with mTBI who have depressive mood also experience more severe subjective symptoms caused by the injury. In addition, the individuals with mTBI who have higher PHQ-9 scores showed lower cognitive performance, assessed by the correct response of the auditory continuous performance test. This highlights the importance of psychiatric symptoms, which influence or are associated with subjective complaints and cognitive function after mTBI, regardless of the duration of time post-injury. The results indicate that depression in mTBI patients can impair improvement of affected domains.
There were several limitations to the present study. First, because of the high heterogeneity of TBI in terms of type and severity, the results of this study may not necessarily be generalizable. The present results showed no significant association between time post-injury and structural brain changes, and there was no significant difference between mTBI patients in early (<60 days) and late (≥60 days) phases after injury. Without multiple comparison correction, there was a trend toward having increased brain volume (white matter areas near the right hippocampal gyrus) in the mTBI group with late phase of injury. In addition, the association between the response to the neurocognitive test after mTBI and time post-injury is important for investigating the structural brain changes after mTBI. The relationship was analyzed using the Pearson correlation analysis as an additional analysis, but no significant association between them was found. Nevertheless, time post-injury is indeed a significant factor, which causes brain morphological changes after mTBI.36 Future studies should include larger and more homogeneous samples to be representative. However, the present results were consistent with previous studies demonstrating volume expansion reflecting plastic changes and brainstem damage.10,13 Although volume contraction was not detected in the cerebral cortices, the discrepant results may have been caused by differences in methodology and/or sample size. Individual variation may also explain the discrepant results. Second, neurocognitive function scores were not assessed in the control group, making it difficult to conclusively determine whether the correlation between brain volume and memory scores was a result of plastic changes in the brain. The relevance of the association would have been clearer if the neurocognitive function scores of the control group were obtained and the relationship between the brain volume and memory scores of the control group was analyzed. It is also possible that individuals with mTBI, who had a larger brain volume, may have had good cognitive performance before the injury. This might have led to better performance on cognitive tasks after injury. A longitudinal study would be necessary to clarify the association between regional brain volume changes and cognitive performance. Third, previous mental health information of three mTBI patients was unavailable, and this might have influenced the TBI outcome. Prospective studies should control for this variable to avoid its potential influence on mTBI outcome.
CONCLUSIONS
The results of this study emphasized that specific brain regions—preferentially the brainstem—are vulnerable to mTBI, represented by reduced volume. The significant positive correlation between regional cerebral volume and cognitive functions suggests that larger brain volume is associated with better cognitive performance, such as memory and executive function.
Supplementary Material
Footnotes
Grants: B.-M. Oh and E. Kim were supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2017R1A2B3005912 and NRF-2018R1C1B6002554, respectively).
Author contributions—conceptualization: E. Kim, H.G. Seo, and B.-M. Oh; methodology: E. Kim; formal analysis: E. Kim; investigation: E. Kim; resources: E. Kim; data curation: H.H. Lee, S.H. Lee, S.H. Choi, R.-E. Yoo, W.-S. Cho, S.J. Yun, and M.-G. Kang; writing–original draft preparation: E. Kim; writing–review and editing: H.G. Seo and B.-M. Oh; visualization: E. Kim; supervision: H.G. Seo and B.-M. Oh; project administration: B.-M. Oh; funding acquisition: E. Kim and B.-M. Oh.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
Contributor Information
Eunkyung Kim, Email: eunkyung@snu.ac.kr.
Han Gil Seo, Email: hangilseo@snu.ac.kr.
Hyun Haeng Lee, Email: happyhaeng@gmail.com.
Seung Hak Lee, Email: seunghak@gmail.com.
Seung Hong Choi, Email: verocay1@snu.ac.kr.
Roh-Eul Yoo, Email: jakeunkong@naver.com.
Won-Sang Cho, Email: nsdrcho@gmail.com.
Seo Jung Yun, Email: sjselena0611@gmail.com.
Min-Gu Kang, Email: kangmingu.ryan@gmail.com.
Byung-Mo Oh, Email: keepwiz@gmail.com.
REFERENCES
- 1.Dewan MC Rattani A Gupta S, et al. : Estimating the global incidence of traumatic brain injury. J Neurosurg 2018. doi: 10.3171/2017.10.JNS17352 [DOI] [PubMed] [Google Scholar]
- 2.McInnes K Friesen CL MacKenzie DE, et al. : Mild traumatic brain injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS One 2017;12:e0174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean PJA Sato JR Vieira G, et al. : Long-term structural changes after mTBI and their relation to post-concussion symptoms. Brain Inj 2015;29:1211–8 [DOI] [PubMed] [Google Scholar]
- 4.Killgore WDS Singh P Kipman M, et al. : Gray matter volume and executive functioning correlate with time since injury following mild traumatic brain injury. Neurosci Lett 2016;612:238–44 [DOI] [PubMed] [Google Scholar]
- 5.Narayana PA Yu X Hasan KM, et al. : Multi-modal MRI of mild traumatic brain injury. Neuroimage Clin 2015;7:87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu TC Wilde EA Bigler ED, et al. : Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev Neurosci 2010;32:361–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis EL Hua X Villalon-Reina J, et al. : Tensor-based morphometry reveals volumetric deficits in moderate/severe pediatric traumatic brain injury. J Neurotrauma 2016;33:840–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan AR, Wang L, Beg MF: Unified voxel- and tensor-based morphometry (UVTBM) using registration confidence. Neurobiol Aging 2015;36(S1):S60–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis EL Faskowitz J Rashid F, et al. : Diverging volumetric trajectories following pediatric traumatic brain injury. Neuroimage Clin 2017;15:125–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farbota KD Sodhi A Bendlin BB, et al. : Longitudinal volumetric changes following traumatic brain injury: A tensor-based morphometry study. J Int Neuropsychol Soc 2012;18:1006–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J Avants B Patel S, et al. : Structural consequences of diffuse traumatic brain injury: A large deformation tensor-based morphometry study. Neuroimage 2008;39:1014–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutkenhoff ES McArthur DL Hua X, et al. : Thalamic atrophy in antero-medial and dorsal nuclei correlates with six-month outcome after severe brain injury. Neuroimage Clin 2013;3:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidaros A Skimminge A Liptrot MG, et al. : Long-term global and regional brain volume changes following severe traumatic brain injury: A longitudinal study with clinical correlates. Neuroimage 2009;44:1–8 [DOI] [PubMed] [Google Scholar]
- 14.Eierud C Craddock RC Fletcher S, et al. : Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage Clin 2014;4:283–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E Seo HG Lee HH, et al. : Altered white matter integrity after mild to moderate traumatic brain injury. J Clin Med 2019;8:1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo RE Choi SH Oh BM, et al. : Quantitative dynamic contrast-enhanced MR imaging shows widespread blood-brain barrier disruption in mild traumatic brain injury patients with post-concussion syndrome. Eur Radiol 2019;29:1308–17 [DOI] [PubMed] [Google Scholar]
- 17.King N Crawford S Wenden F, et al. : The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995;242:587–92 [DOI] [PubMed] [Google Scholar]
- 18.Jennett B Snoek J Bond M, et al. : Disability after severe head injury: Observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 1981;44:285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha KS, Kwon JS, Lyoo IK: Development and standardization of the computerized attention assessment for Korean adults. J Korean Neuropsychiatr Assoc 2002;41:335–46 [Google Scholar]
- 21.Kwon JS Lyoo IK Hong KS, et al. : Development and standardization of the computerized memory assessment for Korean adults. J Korean Neuropsychiatr Assoc 2002;41:347–62 [Google Scholar]
- 22.Lyoo IK, Kwon JS, Ha KS: Development and standardization of the computerized higher cortical function assessment for Korean adults. J Korean Neuropsychiatr Assoc 2002;41:538–50 [Google Scholar]
- 23.Avants BB Epstein CL Grossman M, et al. : Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zagorchev L Meyer C Stehle T, et al. : Differences in regional brain volumes two months and one year after mild traumatic brain injury. J Neurotrauma 2016;33:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delano-Wood L Bangen K Sorg S, et al. : Brainstem white matter integrity is related to loss of consciousness and postconcussive symptomatology in veterans with chronic mild to moderate traumatic brain injury. Brain Imaging Behav 2015;9:500–12 [DOI] [PubMed] [Google Scholar]
- 26.Gaetz M: The neurophysiology of brain injury. Clin Neurophysiol 2004;115:4–18 [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Yang KH, King AI: A proposed injury threshold for mild traumatic brain injury. J Biomech Eng 2004;126:226–36 [DOI] [PubMed] [Google Scholar]
- 28.Browne KD Chen XH Meaney DF, et al. : Mild traumatic brain injury and diffuse axonal injury in swine. J Neurotrauma 2011;28:1747–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garman RH Jenkins LW Switzer RC III, et al. : Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J Neurotrauma 2011;28:947–59 [DOI] [PubMed] [Google Scholar]
- 30.Parvizi J, Damasio AR: Neuroanatomical correlates of brainstem coma. Brain 2003;126(pt 7):1524–36 [DOI] [PubMed] [Google Scholar]
- 31.Moe HK Moen KG Skandsen T, et al. : The influence of traumatic axonal injury in thalamus and brainstem on level of consciousness at scene or admission: A clinical magnetic resonance imaging study. J Neurotrauma 2018;35:975–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fearing MA Bigler ED Wilde EA, et al. : Morphometric MRI findings in the thalamus and brainstem in children after moderate to severe traumatic brain injury. J Child Neurol 2008;23:729–37 [DOI] [PubMed] [Google Scholar]
- 33.Boisgontier MP Cheval B Chalavi S, et al. : Individual differences in brainstem and basal ganglia structure predict postural control and balance loss in young and older adults. Neurobiol Aging 2017;50:47–59 [DOI] [PubMed] [Google Scholar]
- 34.Witte EA, Marrocc RT: Alteration of brain noradrenergic activity in rhesus monkeys affects the alerting component of covert orienting. Psychopharmacology (Berl) 1997;132:315–23 [DOI] [PubMed] [Google Scholar]
- 35.Bär KJ de la Cruz F Schumann A, et al. : Functional connectivity and network analysis of midbrain and brainstem nuclei. Neuroimage 2016;134:53–63 [DOI] [PubMed] [Google Scholar]
- 36.Singh P, Killgore WD: Time dependent differences in gray matter volume post mild traumatic brain injury. Neural Regen Res 2016;11:920–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayana PA: White matter changes in patients with mild traumatic brain injury: MRI perspective. Concussion 2017;2:CNC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nestor PG Shenton ME Mccarley RW, et al. : Neuropsychological correlates of MRI temporal-lobe abnormalities in schizophrenia. Am J Psychiatry 1993;150:1849–55 [DOI] [PubMed] [Google Scholar]
- 39.McNab F, Klingberg T: Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 2008;11:103–7 [DOI] [PubMed] [Google Scholar]
- 40.Bigler ED, Maxwell WL: Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging Behav 2012;6:108–36 [DOI] [PubMed] [Google Scholar]
- 41.Ewing-Cobbs L Hasan KM Prasad MR, et al. : Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. AJNR Am J Neuroradiol 2006;27:879–81 [PMC free article] [PubMed] [Google Scholar]
- 42.Niogi SN Mukherjee P Ghajar J, et al. : Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 2008;131:3209–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


