ABSTRACT
Cell migration is a highly dynamic and energy-intensive process that ensures the correct targeting of cells during embryonic and postnatal development. In recent work, we highlighted the importance of macroautophagy/autophagy in regulating the dynamics of cell migration under baseline conditions and in response to a diverse set of molecular factors. Genetic suppression of autophagy-related genes induced longer stationary phases in migrating cells and cell stalling at the beginning of the migratory stream. We also showed that autophagy is required for recycling of the focal adhesion molecule PXN (paxillin), and is induced by energy levels of cells via AMPK activation. This recent study revealed the importance of autophagy in the maintenance of cell migration, and showed that the dynamic interplay between autophagy and energy levels is required to sustain neuronal migration and to cope with diverse micro-environmental factors.
KEYWORDS: AMPK, ATG5, ATG12, ATP/ADP, autophagy, cell migration, paxillin, time-lapse imaging
Cell migration is a dynamic process composed of stationary and migratory phases. In recent work, we highlighted the importance of macroautophagy (hereafter autophagy) in controlling cell migration. We used mouse olfactory bulb neurogenesis as a model system to study the involvement of autophagy in neuronal migration in the early postnatal and adult brains. In the postnatal mouse brain, neural stem cells are retained in the subventricular zone, and give rise to neuroblasts that migrate long distances along the rostral migratory stream (RMS) to become interneurons in the olfactory bulb. Immunostaining against ATG5 and LC3 in the RMS as well as an electron microscopy analysis revealed that migratory neuroblasts express high levels of autophagy-related proteins and display numerous autophagosomes [1]. Combined live imaging of cell migration and RFP-GFP-LC3 puncta has shown that the dynamics and density of autophagosomes vary markedly during the migratory and stationary phases of cell migration. This differential biogenesis of autophagosomes and their dynamics during the migratory and stationary phases suggest that the autophagic process is temporally regulated during cell migration. The suppression of autophagy-related genes such as Atg5 and Atg12 in neuroblasts, and the impairment of autophagic flux by bafilomycin A1 leads to a deficit in neuroblast migration due to an increase in the duration of the stationary phases. These results revealed the importance of autophagy in maintaining normal migratory dynamics.
It is worth mentioning that the stationary phases are not a synonym of cell immobility given that, during these phases, the cell body is stationary but the leading process remains very motile and senses the micro-environment in search of migration-affecting cues. During the stationary phases, membrane proteins and/or damaged organelles are recycled, allowing neuroblasts to reenter the migratory phase. Disruption of autophagy impairs these processes as shown by the accumulation of the focal adhesion molecule PXN (paxillin) in the leading process of neuroblasts. Interestingly, however, while the suppression of autophagy impairs neuronal migration it does not block it completely. This indicates that other pathways may compensate for the impairment of autophagy, and allow cells to sustain their migration at some level. The nature and role of these compensatory mechanisms remain to be investigated, but they may involve other vesicular pathways that are known to be involved in cell migration. This raises the possibility that the interplay and convergent nature of different pathways for regulating cell migration may be used to design new strategies to maintain neuronal migration when it is affected by mutations in specific genes and/or pathways.
The temporal regulation of autophagy depends on energy levels, which are also dynamically modulated in migrating neuroblasts. Live imaging of intracellular ATP/ADP levels during different migration phases showed that there is a gradual decrease in energy levels during the migratory phases leading to cell pausing, which is used by cells to restore their energy levels and reenter the migratory phase (Figure 1). This dynamic regulation of ATP/ADP levels during cell migration led us to explore the impact of AMPK, a major sensor of energy in cells and an autophagy activator. The suppression of AMPK activation by compound C or a ULK1/ULK2 deficiency both lead to the same migratory deficit as an ATG5 or ATG12 deficiency in wild-type cells. This effect is lacking, however, in ATG5-deficient neuroblasts, indicating that these two major homeostatic pathways dynamically interact to sustain the periodicity of neuronal migration.
Figure 1.
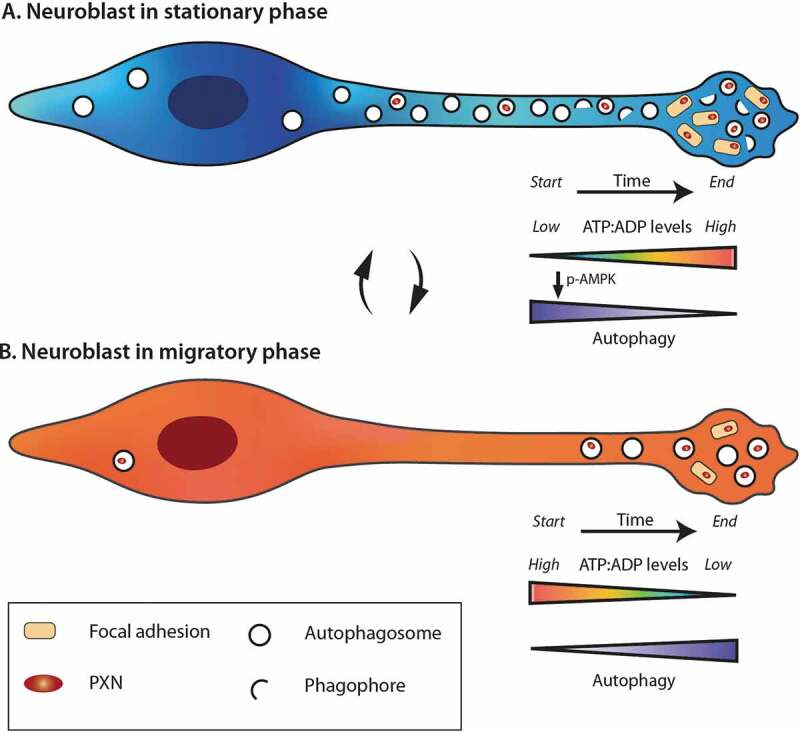
Dynamic changes in the energy levels and autophagy during the different phases of cell migration. The stationary (A) and migratory (B) phases are accompanied by different dynamics in the ATP/ADP levels and autophagy. During the migratory phases, neuroblasts consume energy and the decrease in ATP:ADP ratio leads to the entry of cells into stationary phase, and activation of AMPK, which in turn induces autophagy. During the stationary phases, neuroblasts restore their ATP pool and have high autophagy flux. The turnover of focal adhesion molecules is also taking place during these periods by a direct autophagy-dependent recycling of PXN. When the energetic level is high enough, neuroblasts reenter into the migratory phase
Our work also showed that autophagy-dependent and energy-induced regulation of the dynamics of neuronal migration occurs not only under homeostatic conditions but is also engaged in response to different molecular cues that either maintain neuronal migration or decrease it [1]. Interestingly, this bi-directional regulation of autophagy in response to migration-promoting or -suppressing cues is linked to the recycling of focal adhesions. As the suppression of autophagy also leads to the accumulation of PXN in the leading process of neuroblasts, these results suggested that migratory cells target specific autophagic cargoes, and that the induction of autophagy is also spatially controlled. It is conceivable that autophagic recycling is not restricted to PXN. As mitochondria dynamics are crucial for cell migration, the role played by mitophagy in neuronal migration would be interesting to explore. Another cellular component which is associated with the cell migration process is the cytoplasmic membrane, as neuroblasts change their shape markedly during the different phases of cell migration. Autophagy might thus be also involved in membrane dynamics and turnover during migration.
Our results revealed that autophagy is spatially and temporally regulated during the different phases of cell migration, and is induced by energy requirements (Figure 1). While further work is needed to understand how autophagy and energy consumption interact with other intracellular pathways during cell migration and in other developmental processes such as cell maturation, our results underscored the roles played by these two major homeostatic processes in coping with different micro-environmental signals in order to sustain neuronal migration in vivo.
Acknowledgments
We thank Mireille Massouh at MassouhBioMedia for the schematic drawing.
Funding Statement
This work was supported by Canadian Institute of Health Research (CIHR) grant PJT-159733 to AS.
Disclosure statement
All authors declare no conflict of interest.
Reference
- [1].Bressan C, Pecora A, Gagnon D, et al. The dynamic interplay between ATP/ADP levels and autophagy sustain neuronal migration in vivo. eLife. 2020;9:e56006. [DOI] [PMC free article] [PubMed] [Google Scholar]


