Abstract
Objective
To investigate the effect of local injection of platelet-rich plasma (PRP) on the rate of orthodontic tooth movement.
Materials and Methods
Sixteen female patients were randomly allocated in a split-mouth study design to receive PRP injections with CaCl2 activating solution on one side (intervention side) while the other side received CaCl2 injection only (control side). Canine retraction was performed on 0.017 × 0.025-inch stainless steel archwire applying 1.5 N retraction force. PRP and CaCl2 injections were done at 0, 3, and 6 weeks. The duration of the study was 4 months. Data were collected from digitized models. Assessment of pain accompanying the procedure was done using a visual analogue scale.
Results
The rate of canine retraction was faster on the intervention side in the first 2 months, with a statistically significant difference in the first month (P = .049). On the other hand, the rate was statistically significantly slower on the intervention side in the third month following cessation of PRP injections (P = .02). Pain increased following injections on both sides.
Conclusions
PRP showed a positive potential to accelerate the rate of tooth movement when injected in the first 2 months. Repeated injections of PRP to maintain a steady rate of accelerated tooth movement warrant further investigation.
Keywords: Platelet-rich plasma, Acceleration, Tooth movement, Pharmacological approaches, Canine retraction, Digital models
INTRODUCTION
Orthodontic tooth movement is the product of a biological response to an interference in the physiological equilibrium in the dentofacial complex by an externally applied force.1 Various approaches have been attempted to accelerate the rate of orthodontic tooth movement. Although each approach has claimed to be superior to the others in different studies, conflicting evidence concerning each technique still exists.2,3 Pharmacologic approaches to accelerate the rate of orthodontic tooth movement have been investigated in humans since the 1980s.4 If proven clinically efficient, pharmacologic approaches might surpass other approaches as they are less invasive, less costly, and more controlled. The problem that remains is their concomitant side effects that might occur especially in association with systemic administration.
One of the recently used local agents to accelerate the rate of orthodontic tooth movement is platelet-rich plasma (PRP).5 PRP is defined as an autologous concentration of platelets in a minute volume of plasma.6 The alpha granules in the platelets are the most abundant secretory granules. They contain numerous proteins, including growth factors and chemokines, which are crucial for primary hemostasis and wound healing.7,8 Research concerning PRP has focused on its applications in regenerative medicine.9 The relationship between PRP and orthodontic tooth movement has drawn some attention lately. In two recently published animal studies, Rashid et al.5 and Gülec et al.10 evaluated the effects of PRP on tooth movement. Both studies showed a positive correlation between local injection of PRP and acceleration of orthodontic tooth movement. On the other hand, Akbulut et al.11 reported no beneficial effects of PRP injections on tooth movement, yet they recommended that further studies be conducted.
The aim of the current study was to investigate the effect of local injection of PRP on the rate of orthodontic tooth movement clinically and also to report any associated pain.
MATERIALS AND METHODS
Subjects
The current study was a split-mouth randomized controlled clinical trial with a 1:1 allocation following the CONSORT statement reporting guidelines. The study was performed at Cairo University and approved by the Faculty of Dentistry Ethical Committee (No. 1522015). No changes were reported after commencement of the study.
The sample requirement for this study was calculated to be 10 female patients using Minitab software based on the results of Abou Ela et al.,12 who implemented a comparable split-mouth study design. Six additional patients were included to consider sample attrition. A computer-generated random list was created (https://www.random.org/), and allocation concealment was achieved with opaque sealed envelopes. Patient eligibility criteria are presented in Table 1. Consent was obtained from the patients and/or their legal guardian before recruitment.
Table 1. .
Eligibility Criteria for Patients Included in the Study
| Inclusion Criteria |
Exclusion Criteria |
| Female patients | Systemic disease or syndrome |
| Age 18 ± 3 y | Abnormalities in teeth size and/or shape |
| Full permanent dentition | Vertical, transverse, or anteroposterior skeletal discrepancies |
| Good general and oral health | History of previous orthodontic treatment |
| Severe crowding or protrusion requiring first premolars extractions | Anti-inflammatory medication |
METHODS
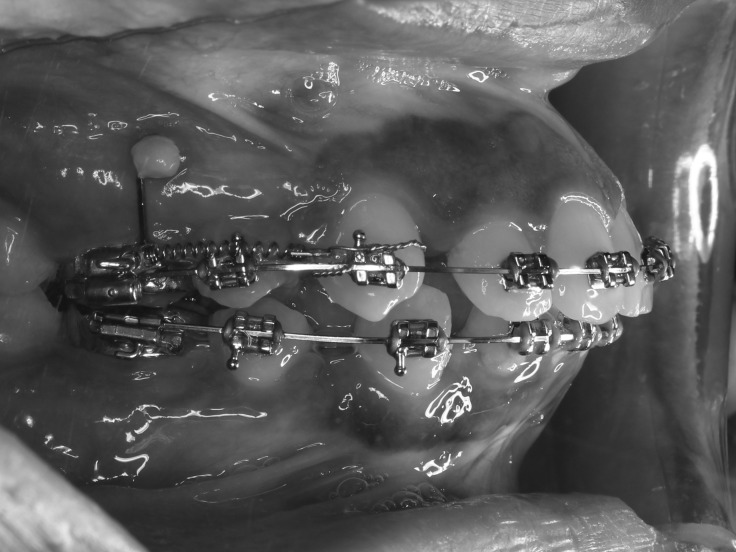
Full preoperative records were obtained, and Roth 0.022-inch brackets (American Orthodontics, Sheboygan, Wis) were bonded. Leveling and alignment were accomplished until 0.017 × 0.025-inch stainless steel archwire was reached. Two mini-screws (1.6 × 8 mm, bracket head design; Dual Top Anchor System, Jeil Medical Corporation, Seoul, Korea) were inserted in the interradicular region between the upper second premolars and upper first molars on each side at the level of the junction between the attached and nonkeratinized gingiva. First molars were anchored to the miniscrew using a 0.019 × 0.025-inch stainless steel wire. NiTi closed-coil springs delivering a retraction force of 1.5 N12 per side were attached to the right and left canine hooks following the injection of the PRP on the intervention side (Figure 1).
Figure 1.
Fixed appliance setup during canine retraction.
PRP Preparation and Injection
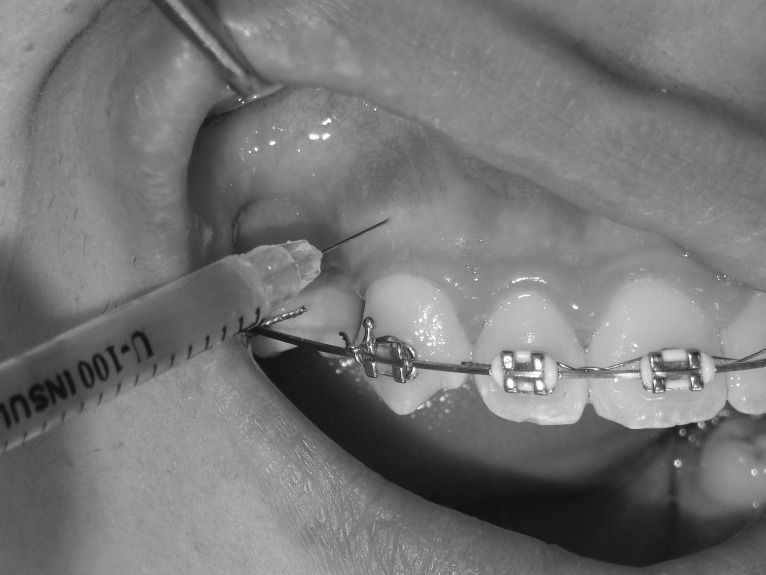
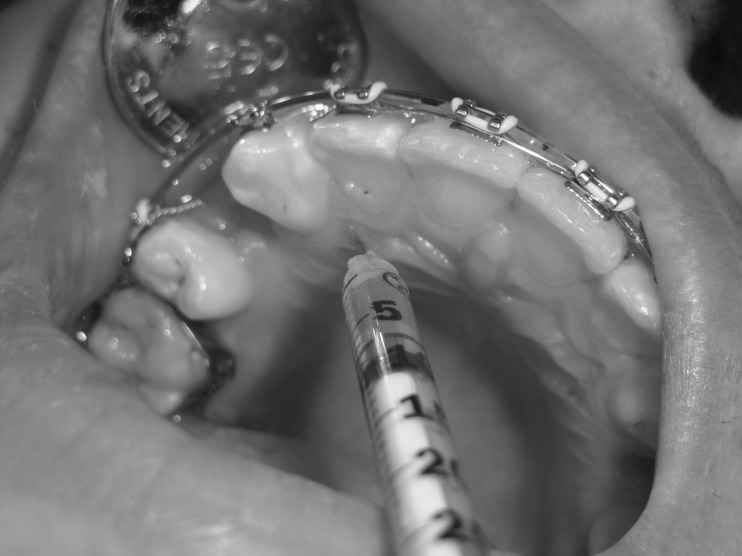
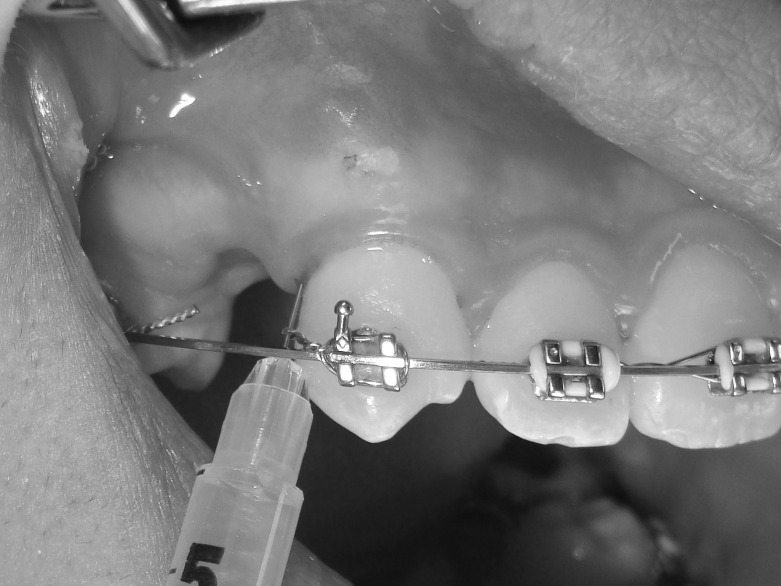
PRP was prepared by the double-spin technique as described by Marx and Garg13 and Rashid et al.5 The intervention side was anesthetized with 2% mepivacaine with vasoconstrictor and left for 15 minutes. Then, 25 units (0.25 mL) of PRP was injected intraligamentally in the middle, distobuccal, and distopalatal areas of the distal surface of the canines (5 units each area) together with submucosal injections buccally and palatally (5 units each area). Immediately following PRP injection, the same volume of 10% CaCl2 solution was injected for activation of PRP. The intervention side was injected at the following intervals: 0, 21, and 42 days. The control side was anesthetized and injected with 25 units of 10% CaCl2 following the same protocol and frequency as the intervention side (Figures 2–4).
Figure 2.
Submucosal injection of 5 units of platelet-rich plasma at the buccal surface on the intervention side.
Figure 4.
Submucosal injection of 5 units of platelet-rich plasma on the palatal aspect of the intervention side.
Figure 3.
Intraligamental injection of 5 units of platelet-rich plasma in the distobuccal area of the distal surface of the canine on the intervention side.
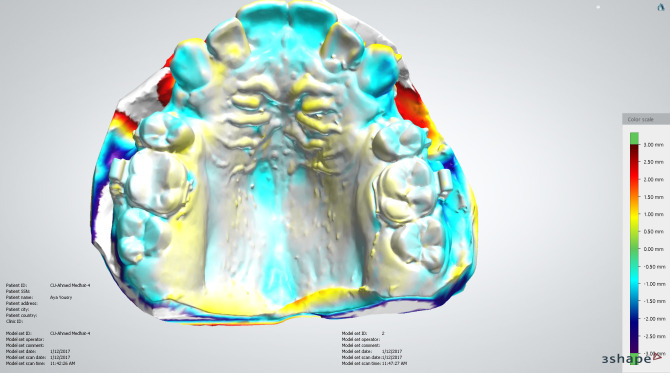
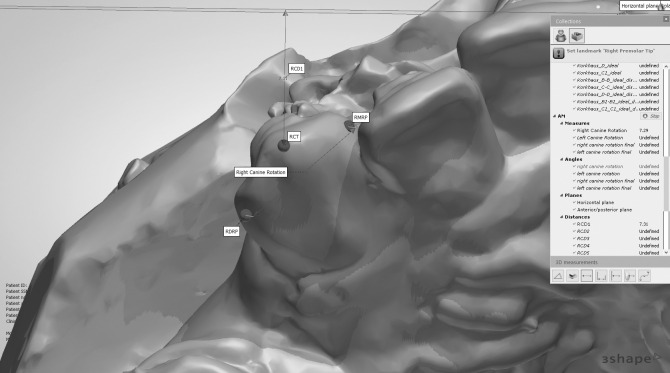
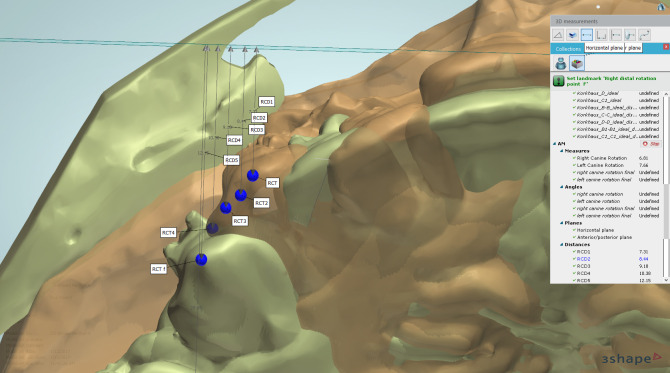
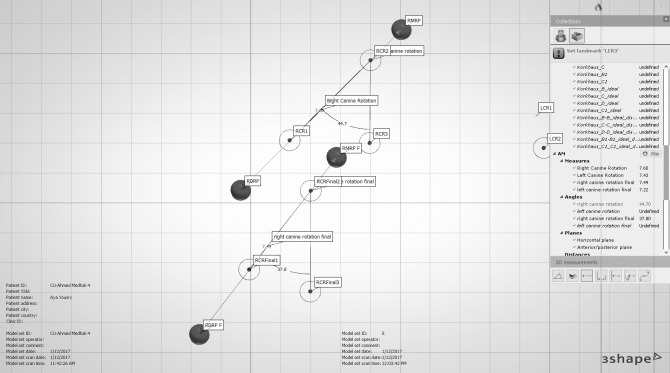
Alginate impressions for the upper arches were made before canine retraction and monthly until the fourth month (T0–T4). Stone models were scanned using a 3-Shape scanner (R500, 3shape, Copenhagen, Denmark). The five consecutive digital models were superimposed using 3-Shape analyzer software14 (3shape, Copenhagen, Denmark). Color-coded superimposition was done to verify the accuracy of the superimposed models15 (Figure 5). The change in the canine position in the superimposed models was measured to detect the rate of canine retraction per month (Figure 6). The rate of canine retraction was calculated from the difference in the canine position in the consecutive models (Figure 7). In addition, the mesial and distal contact points of the canines were used to form a horizontal line that formed an angle with the median palatine raphe (Figure 8). The change in that angle between the initial and final models indicated the amount of canine rotation. Blinding of measurements was performed only during outcome assessment, which was carried out by two assessors not involved in the clinical procedures.
Figure 5.
Color-coded verification of the superimposition of the digital models.
Figure 6.
Point localization of the canine. RCT indicates the most incisal point on the right canine tip; RMRP, mesial contact point of the maxillary right canine; LDMRP, distal contact point of the maxillary right canine; RCD1, perpendicular distance from RCT to the median palatine raphe.
Figure 7.
The canine tip localized on the five consecutively superimposed digital models.
Figure 8.
Measurements of canine rotation after removing images of the digital models for clarity. RMRP and RMRP f indicate the mesial contact point of the maxillary right canine at T0 and T4, respectively; LDMRP and LDMRP f, distal contact point of the maxillary right canine at T0 and T4, respectively; RMRP 1, RMRP 2, and RMRP 3, angle between the right canine and the median palatine raphe at T0; RMRP final 1, RMRP final 2, and RMRP final 3, the same angle at T4.
Pain was assessed using a visual analogue scale questionnaire that was completed by the patient starting from the day following PRP injection and repeated every week until the seventh week. The format of the questionnaire was a 10-cm line, and the patients were requested to mark a location on the line corresponding to the amount of pain they experienced, with 0 indicating no pain and 10 indicating unbearable pain.16 The patients were instructed not to use any additional analgesics.
Statistical Analysis
Analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, Ill) for Windows. Shapiro-Wilk tests of normality were used to test normality of all quantitative variable distributions. A paired t-test was used to determine the statistical significance of differences between the experimental and control sides for preoperative and postoperative measurements.
The significance level was set at P ≤ .05. For inter- and intraobserver reliability, concordance correlation coefficients including 95% confidence limits were used.
RESULTS
Numbers Analyzed for Each Outcome
Of the 16 recruited patients, one patient discontinued the trial due to traveling. For the remaining 15 patients, the rate of canine retraction and rotation together with concomitant pain assessment were evaluated.
The rate of canine retraction showed a statistically significant difference between the two sides in the first month (P = .049), with a mean value of 1.35 ± 0.62 mm/mo for the control side compared with 1.55 ± 0.63 mm/mo for the intervention side, reflecting acceleration of tooth movement with PRP injection. Surprisingly, at the third month, a statistically significant difference between the two sides (P = .020) was reported, but this time it was greater on the control side, with a mean value of 1.01 ± 0.63 mm/mo compared with 0.59 ± 0.96 mm/mo for the intervention side, reflecting a deceleration in the rate of tooth movement on the intervention side following cessation of PRP injections. The total distance traveled by the canine following retraction was similar in both groups during the 4 months of the study period, with a mean of 4.53 ± 1.12 mm for the control side and 4.57 ± 1.32 mm for the intervention side (Tables 2 and 3; Figure 9). Canine distal-in rotation was comparable in both groups, with a mean difference of 1.036° (Table 4).
Table 2. .
Mean Values for the Rate of Canine Retraction in Both Groups (mm)
| Measurement |
Group |
Min |
Max |
Mean |
SD |
| First month (T0–T1) | Control | 0.57 | 2.60 | 1.35 | 0.62 |
| Intervention | 0.39 | 2.92 | 1.55 | 0.63 | |
| Second month (T1–T2) | Control | 0.76 | 1.99 | 1.27 | 0.40 |
| Intervention | 0.07 | 2.73 | 1.33 | 0.87 | |
| Third month (T2–T3) | Control | −0.41 | 1.96 | 1.01 | 0.63 |
| Intervention | −1.39 | 2.08 | 0.59 | 0.96 | |
| Fourth month (T3–T4) | Control | 0.09 | 2.08 | 0.90 | 0.50 |
| Intervention | 0.26 | 2.29 | 1.10 | 0.58 |
Table 3. .
Comparison of the Mean Differences in the Amount of Canine Retraction Between Groups for Each Month
| Measurement |
Group |
Mean Difference |
SD |
SEM |
95% Confidence Interval of Mean Difference |
t |
df |
P Value |
|
| Lower |
Upper |
||||||||
| First month (T0–T1) | Control | −0.20133 | 0.44694 | 0.11540 | −0.44884 | 0.04617 | −1.75 | 14 | .049* |
| Intervention | |||||||||
| Second month (T1–T2) | Control | −0.06067 | 0.79570 | 0.20545 | −0.50131 | 0.37998 | −0.295 | 14 | .772 |
| Intervention | |||||||||
| Third month (T2–T3) | Control | 0.41600 | 0.61618 | 0.15910 | 0.07477 | 0.75723 | 2.62 | 14 | .020* |
| Intervention | |||||||||
| Fourth month (T3–T4) | Control | −0.20133 | 0.67889 | 0.17529 | −0.57729 | 0.17463 | −1.15 | 14 | .270 |
| Intervention | |||||||||
Significant at P ≤ .05.
Figure 9.
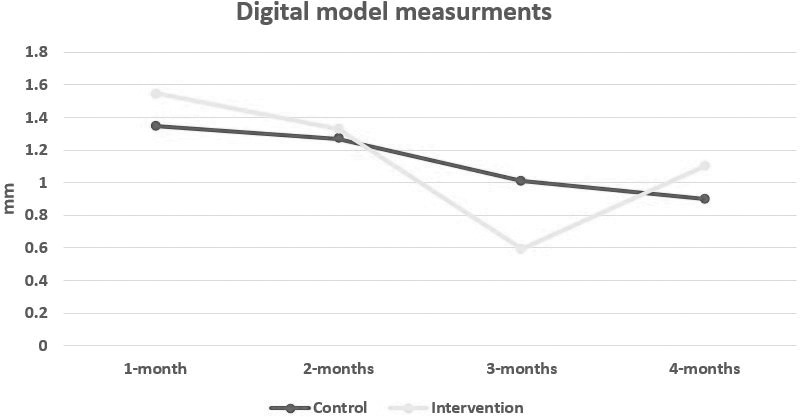
Line chart showing the rate of canine retraction on the intervention vs control sides.
Table 4. .
Comparison of the Mean Differences in the Total Amount of Canine Rotation and Retraction Between Groups
| Measurement |
Group |
Mean |
SD |
Mean Difference |
SD |
SEM |
95% Confidence Interval of Mean Difference |
t |
df |
P Value |
|
| Lower |
Upper |
||||||||||
| Canine rotation | Control | 15.46 | 11.39 | 1.03600 | 10.56011 | 2.72661 | −4.8120 | 6.88400 | 0.380 | 14 | .710 |
| Intervention | 14.42 | 9.70 | |||||||||
| Total retraction (T0–T4) | Control | 4.53 | 1.12 | −0.04733 | 1.3703 | 0.35382 | −0.80619 | 0.7115 | −0.13 | 14 | .895 |
| Intervention | 4.57 | 1.32 | |||||||||
Significant at P ≤ .05.
All patients returned their pain questionnaires, and none reported using any analgesics. An increase in pain scores was reported in the first, fourth, and seventh weeks following each injection on both the intervention and control sides.
DISCUSSION
PRP has been used extensively in various fields of dentistry, showing vast advantages and applications.13,17 Previous literature revealed that methods of PRP preparation have evolved in many techniques,18 all aiming at standardization of the procedure for PRP preparation. First attempts of PRP preparation reported the use of a mixture of 10 mL of 10% calcium chloride combined with 10,000 units of bovine thrombin for PRP activation.19 Activation with bovine thrombin is no longer recommended, as this was reported to cause coagulopathy resulting from cross-reactivity of anti–bovine factor V antibodies with human factor V.20 Hence, suggestions to use autologous thrombin21 or calcium chloride alone were recommended.20 Textor and Tablin21 showed that calcium chloride activation might be the most inexpensive and effective method, although it might need 20 minutes for activation of PRP.20
The injection technique in five different areas around the retracted canine followed the same protocol reported by Rashid et al.5 This may be compared with the findings of Gülec et al.,10 who injected the PRP only at the buccal vestibular mucosa adjacent to the mesial root of the first molar. Intraligamentary injection at the distobuccal and distopalatal surfaces of the canine root was advocated in this study based on the work by Von Böhl et al.,22,23 who reported that, as a consequence of local stress and shear concentrations, most hyalinized areas were not found in the area of the central plane but rather lingually and buccally from it. Rapid bone metabolism was required in these areas to accelerate orthodontic tooth movement.24 Conversely, previous studies evaluating the effects of local pharmacologic agent injection to accelerate tooth movement reported only one or two submucosal or gingival injection sites.4,10,25–27
Injections were delivered every 3 weeks and repeated until the sixth week following the protocol reported by Rashid et al.5 Although the study continued for 4 months, injections were not continued through the whole duration of the study in order to determine whether PRP injections were enough in the initial phases of tooth movement only, as compared with corticotomy12 or low-level laser therapy applications.3
The results of the current study showed a faster rate of canine retraction on the intervention side in the first month by 15% and in the second month by 5%. Despite this increase being statistically significant in the first month (P = .049) and nonsignificant in the second month (P = .772), the results reflected a positive correlation between PRP injection and acceleration of orthodontic tooth movement. Unexpectedly, in the third month and following cessation of PRP injections, the rate of canine retraction on the intervention side was significantly (P = .020) slower than the control side by 40%. This might be related to a negative feedback mechanism in the growth factors' release, similar to hormonal negative feedback that occurs in association with elevated blood and/or tissue concentration. Hence, increasing the tissue concentration of growth factors incidental to local injection of PRP could have affected the normal production of growth factors during orthodontic tooth movement.28–30 In the last month of the study period, results showed a nonsignificant difference in the rate of canine retraction between the two sides. The rate of canine retraction on the control side was slightly more than that reported by Aboul-Ela et al.12 and less than that reported by Hayashi et al.31 On the other hand, Gülec et al.10 reported that PRP induced acceleration of orthodontic tooth movement by 1.4 to 1.7 times. Similarly, Rashid et al.5 reported a higher value of acceleration (PRP:control rate = 2.13:1) compared with the present study. This could have been due to the difference in the nature of their studies being animal studies with a more controlled environment together with a possible difference in the PRP composition.
Pain scores showed no difference between the intervention and control sides. This could be related to the forceful intraligamental injection procedure itself rather than an effect related to the pharmacologic agent.
CONCLUSIONS
Despite the statistically significant increase in the rate of canine retraction during the early stages of tooth movement concomitant with PRP injections, PRP did not exhibit long-term acceleration effects.
The effect of repeated injections of PRP throughout the course of canine retraction to maintain a steady rate of accelerated tooth movement needs to be further investigated.
ACKNOWLEDGMENT
The authors would like to thank Dr. Noha Al-ashmawi and Dr. Mohamed Abd Elghafour for their participation in the measurements.
REFERENCES
- 1. .Proffit WR. Biologic basis of orthdontic therapy. In: Proffit WR, Fields HW, editors. Contemporary Orthodontics. St Louis, Mo: Mosby; 2000. [Google Scholar]
- 2. .Long H, Pyakurel U, Wang Y, Liao L, Zhou Y, Lai W. Interventions for accelerating orthodontic tooth movement: a systematic review. Angle Orthod. 2013;83:164–171. doi: 10.2319/031512-224.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. .Yi J, Xiao J, Li H, Li Y, Li X, Zhao Z. Effectiveness of adjunctive interventions for accelerating orthodontic tooth movement: a systematic review of systematic reviews. J Oral Rehabil. 2017;44:636–654. doi: 10.1111/joor.12509. [DOI] [PubMed] [Google Scholar]
- 4. .Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod. 1984;85:508–518. doi: 10.1016/0002-9416(84)90091-5. [DOI] [PubMed] [Google Scholar]
- 5. .Rashid A, ElSharaby FA, Nassef EM, Mehanni S, Mostafa YA. Effect of platelet-rich plasma on orthodontic tooth movement in dogs. Orthod Craniofac Res. 2017;20:102–110. doi: 10.1111/ocr.12146. [DOI] [PubMed] [Google Scholar]
- 6. .Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 7. .Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 8. .Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 9. .Del Corso M, Vervelle A, Simonpieri A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: periodontal and dentoalveolar surgery. Curr Pharm Biotechnol. 2012;13:1207–1230. doi: 10.2174/138920112800624391. [DOI] [PubMed] [Google Scholar]
- 10. .Güleç A, Bakkalbaşı BÇ, Cumbul A, Uslu Ü, Alev B, Yarat A. Effects of local platelet-rich plasma injection on the rate of orthodontic tooth movement in a rat model: a histomorphometric study. Am J Orthod Dentofac Orthop. 2017;151:92–104. doi: 10.1016/j.ajodo.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 11. .Akbulut S, Yagci A, Yay AH, Yalcin B. Experimental investigation of effects of platelet-rich plasma on early phases of orthodontic tooth movement. Am J Orthod Dentofac Orthop. 2019;155:71–79. doi: 10.1016/j.ajodo.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 12. .Aboul-Ela SMBE-D, El-Beialy AR, El-Sayed KMF, Selim EMN, El-Mangoury NH, Mostafa YA. Miniscrew implant-supported maxillary canine retraction with and without corticotomy-facilitated orthodontics. Am J Orthod Dentofacial Orthop. 2011;139:252–259. doi: 10.1016/j.ajodo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 13. .Marx RE, Garg AK. Dental and Craniofacial Applications of PlateletRich Plasma. City, ST: Quintessence Pub; CO, USA: 2005. pp. 37–49. [Google Scholar]
- 14. .Abdi AH, Nouri M. Registration of serial maxillary models via the weighted rugae superimposition method. Orthod Craniofacial Res. 2017;20:79–84. doi: 10.1111/ocr.12142. [DOI] [PubMed] [Google Scholar]
- 15. .Nichols DA, Gardner G, Carballeyra AD, Marsh CM. Reproducibility of bracket positioning in the indirect bonding technique. Am J Orthod Dentofac Orthop. 2013;144:770–776. doi: 10.1016/j.ajodo.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 16. .Farzanegan F, Zebarjad SM, Alizadeh S, Ahrari F. Pain reduction after initial archwire placement in orthodontic patients: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 2012;141:169–173. doi: 10.1016/j.ajodo.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 17. .Marx RE. A source of multiple autologous growth factors for bone grafts. In: Lynch SE, Genco RJ, Marx RE, editors. Applications in Maxillofacial Surgery and Periodontics. Quintessence Pub. CO; Chicago, IL, USA: 1999. pp. 71–82. In Tissue Engineering: In. [Google Scholar]
- 18. .Chahla J, Cinque ME, Piuzzi NS, et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. Bone Joint Surg Am. 2017;99:1769–1779. doi: 10.2106/JBJS.16.01374. [DOI] [PubMed] [Google Scholar]
- 19. .Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–1299. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 20. .Alsousou J, Ali A, Willett K, Harrison P. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24:173–182. doi: 10.3109/09537104.2012.684730. [DOI] [PubMed] [Google Scholar]
- 21. .Textor JA, Tablin F. Activation of equine platelet-rich plasma: comparison of methods and characterization of equine autologous thrombin. Vet Surg. 2012;41:784–794. doi: 10.1111/j.1532-950X.2012.01016.x. [DOI] [PubMed] [Google Scholar]
- 22. .Von Böhl M, Maltha J, Von den Hoff H, Kuijpers-Jagtman AM. Changes in the periodontal ligament after experimental tooth movement using high and low continuous forces in beagle dogs. Angle Orthod. 2004;74:16–25. doi: 10.1043/0003-3219(2004)074<0016:CITPLA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23. .von Böhl M, Maltha JC, Von Den Hoff JW, Kuijpers-Jagtman AM. Focal hyalinization during experimental tooth movement in beagle dogs. Am J Orthod Dentofacial Orthop. 2004;125:615–623. doi: 10.1016/j.ajodo.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 24. .Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofac Orthop. 2006;129:1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 25. .Kobayashi Y, Takagi H, Sakai H, Hashimoto F, Mataki S, Kobayashi K, Kato Y. Effects of local administration of osteocalcin on experimental tooth movement. Angle Orthod. 1998;68:259–266. doi: 10.1043/0003-3219(1998)068<0259:EOLAOO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 26. .Leiker BJ, Nanda RS, Currier GF, Howes RI, Sinha PK. The effects of exogenous prostaglandins on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 1995;108:380–388. doi: 10.1016/s0889-5406(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 27. .McGorray SP, Dolce C, Kramer S, Stewart D, Wheeler TT. A randomized, placebo-controlled clinical trial on the effects of recombinant human relaxin on tooth movement and short-term stability. Am J Orthod Dentofacial Orthop. 2012;141:196–203. doi: 10.1016/j.ajodo.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 28. .Vassbotn FS, Havnen OK, Heldin C-H, Holmsen H. Negative feedback regulation of human platelets via autocrine activation of the platelet-derived growth factor α-receptor. J Biol Chem. 1994;269:13874–13879. [PubMed] [Google Scholar]
- 29. .Shah P, Keppler L, Rutkowski J. A review of platelet derived growth factor playing pivotal role in bone regeneration. J Oral Implantol. 2012;XL:120419070552000. doi: 10.1563/AAID-JOI-D-11-00173. [DOI] [PubMed] [Google Scholar]
- 30. .Miyazono K. Positive and negative regulation of TGF-beta signaling. J Cell Sci. 2000;113(pt 7):1101–1109. doi: 10.1242/jcs.113.7.1101. [DOI] [PubMed] [Google Scholar]
- 31. .Hayashi K, Uechi J, Murata M, Mizoguchi I. Comparison of maxillary canine retraction with sliding mechanics and a retraction spring: a three-dimensional analysis based on a midpalatal orthodontic implant. Eur J Orthod. 2004;26:585–589. doi: 10.1093/ejo/26.6.585. [DOI] [PubMed] [Google Scholar]