Abstract
Objectives
To compare the effect of use of laser, casein phosphopeptide–amorphous calcium phosphate (CPP–ACP), and their combination on prevention of enamel demineralization using polarized light microscopy to assess lesion depth.
Materials and Methods
Eighty premolars were randomly allocated to four equal groups (n = 20): Group I: Control group, no preventive measures. Group II: CPP–ACP. Group III: Er,Cr:YSGG laser. Group IV: Er,Cr:YSGG laser followed by CPP–ACP. Specimens were subjected to thermocycling and brushing protocols equivalent to 1 year intraorally. Then, all teeth were subjected to acid challenge. Teeth were then sectioned longitudinally and examined under a polarized light microscope and lesion depth was measured.
Results
Group IV resulted in the least lesion depth with a significant difference between it and all other groups. CPP–ACP alone and laser alone also showed a significant difference in white spot lesion (WSL) depth compared to the control group; however, no significant difference was found between them.
Conclusions
The combined use of laser and CPP–ACP showed the best prevention against WSL development. The use of CPP–ACP or laser alone also resulted in a significant reduction in lesion depth but was significantly less than their combined use, with no significant difference between them.
Keywords: Er,Cr:YSGG Laser; CPP-ACP; Prevention; WSL
INTRODUCTION
Patients usually seek orthodontic treatment to improve esthetics.1 However, fixed orthodontic appliances can result in white spot lesions (WSL), creating another esthetic concern for the patient and causing disappointment for both the patient and orthodontist at the time of debonding.2 Demineralized enamel is more porous than sound enamel with a difference in refractive indices resulting in more light scattering in demineralized enamel. Therefore, it appears whiter in color than sound enamel.3
The prevention of plaque accumulation by good oral hygiene measures is crucial for controlling the development of WSLs.4 However, despite the use of prophylactic measures, Hadler-Olsen et al.5 reported that 60% of orthodontic patients developed one or more new WSLs at the end of orthodontic treatment.
Many novel materials aid in the prevention of WSLs, such as those containing casein phosphopeptide–amorphous calcium phosphate (CPP–ACP).6 CPP forms nanoclusters with ACP, providing a pool of calcium and phosphate, which can maintain the supersaturation of saliva. Since CPP–ACP can stabilize calcium and phosphate in the solution, it can also help in the buffering of plaque pH so calcium and phosphate levels in plaque are increased.6 When CPP–ACP was compared with fluoride compounds, which are the gold standard for prevention of demineralization, a systematic review in 2018 pointed out a high level evidence of remineralizing potential of CPP–ACP on WSLs compared with fluoridated toothpaste and fluoride varnish without a statistically significant difference.7 A systematic review in 2019 that reviewed effectiveness of CPP–ACP products in prevention of WSLs in orthodontic patients, showed that, although CPP–ACP-containing products did not differ from other fluoride products, they were able to reduce WSLs and neutralize the pH around orthodontic brackets.8
Recently, it was shown that treatment with lasers reduced subsurface demineralization. A variety of explanations have been given including enamel surface melting, recrystallization and changing the enamel organic matrix.9 Nevertheless, the actual mechanisms remain unclear. The most common lasers used for caries prevention are CO2; Er, Cr:YSGG.10 To achieve additional prevention of enamel demineralization, special attention focused on laser irradiation with fluorides showing promising synergistic results.11,12 The purpose of the current study was to evaluate the effect of the combined use of Er,Cr:YSGG laser and CPP–ACP on prevention of demineralization of permanent teeth.
MATERIALS AND METHODS
A randomized controlled in vitro study design was used. The sample size estimate was calculated using Power and Sample Size Calculation computer software (Epi-Info 7 software, Atlanta, GA, USA). At α = 0.05 and a power of 0.80, a sample size of 20 teeth per group, a total of 80 premolars, was needed.13
After ethical committee approval, human premolars were collected from subjects treated in the Orthodontic Department, Faculty of Dentistry, Alexandria University, Alexandria, Egypt. All subjects were born and resided where the average level of fluoride in the drinking water was 0.36 mg/L and no additional fluoride was added to the drinking water.14 The selected teeth were required to have an intact buccal enamel surface, with no obvious decalcifications, cracks, or stains. Also, teeth were excluded if they had been previously bonded or received any chemical treatment.
Tooth Preparation
After extraction, all remnants were removed and the teeth were cleaned with water and stored in artificial saliva solution at 37°C for the study duration.15 The solution contained artificial saliva (20 mmol/L NaHCO3, 3 mmol/L NaH2 PO4, 1 mmol/L CaCl2) at neutral pH 7 to simulate the oral environment. The solution was changed every day.16 Each tooth was assigned a number from 1 to 80 for identification purposes and these numbers were used to randomly assign the samples to the four study groups.
Grouping
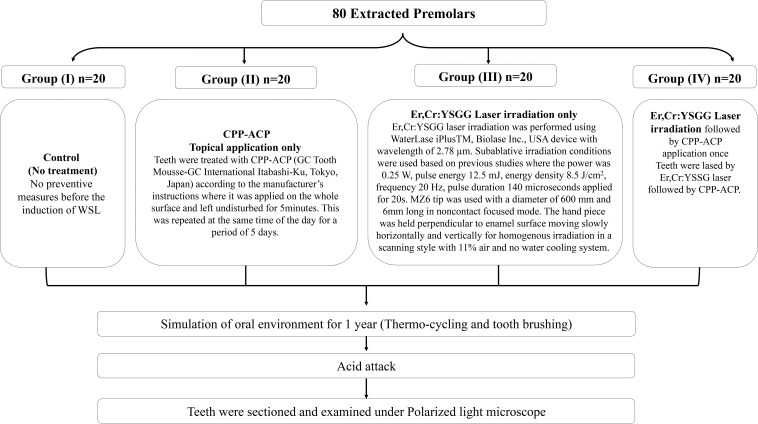
The teeth were randomly assigned to four groups of 20 each using a random number generator and each group was stored in a separate beaker labeled with the group name and containing 200 mL of artificial saliva solution at room temperature. Procedures for each group are shown in the flowchart (Figure 1).
Figure 1.
Summary of the methodology.
Simulation of Oral Environment
Teeth in all groups underwent 10 separate rounds of brushing and thermocycling with a total of 15,000 brushing cycles under a vertical load of 200 g (Equilabor Brushing machine),17 and 10,000 thermocycles in water between 5°C ± 2°C and 55°C ± 2°C with a dwell time of 30 seconds and transfer time of 5 seconds (SD Mechatronik, Feldkirchen-Westerham, Germany).18 This simulated a period of approximately 1 year in the oral environment.17,18
Acid Challenge
Teeth were subjected to an artificial caries challenge. Teeth were cycled between standard Ten Cate demineralizing solution19 (pH 5) consisting of 2.20 mM calcium, 2.20 mM phosphate, 50 mM buffer (acetic acid/ K acetate) and artificial saliva at 37°C for 6 hours of demineralization followed by 18 hours of remineralization over a period of 10 days to perform the acid challenge.20 The solutions were changed every other day during the experiment.
Specimen Preparation for Polarized Light Microscope Examination
Teeth were sectioned longitudinally in a buccolingual direction using a diamond disk under water cooling, and then reduced by hand grinding on polishing boards using progressively finer grades of aluminum oxide powder until a thickness of 150 μm was reached.20 Sections were photographed using a polarized light microscope under maximum illumination. Photomicrographs were made at 50X magnification. The polarized light microscope was connected to a computer with an imaging analysis software program having a digital ruler that was used to measure the lesion all over, and recorded the largest and the smallest lesion depths; the average lesion depth was calculated accordingly. The lesion depth was calculated in millimeters using a 1:50 scale and then converted into μm by multiplying it by 103.
Statistical Analysis
Data were analyzed using IBM SPSS software package version 20.0 (IBM Corp., Armonk, NY). Qualitative data were described using number and percent. The Kolmogorov-Smirnov test was used to verify the normality of the distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation, and median. Significance of the obtained results was judged at the 5% level. The tests used were: Chi-square test, Fisher's Exact or Monte Carlo correction, and Kruskal-Wallis test.
RESULTS
Polarized Light Microscope Examination
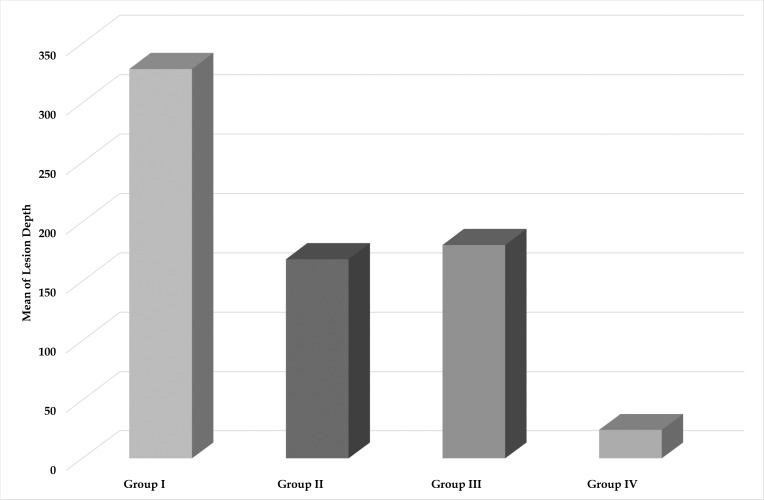
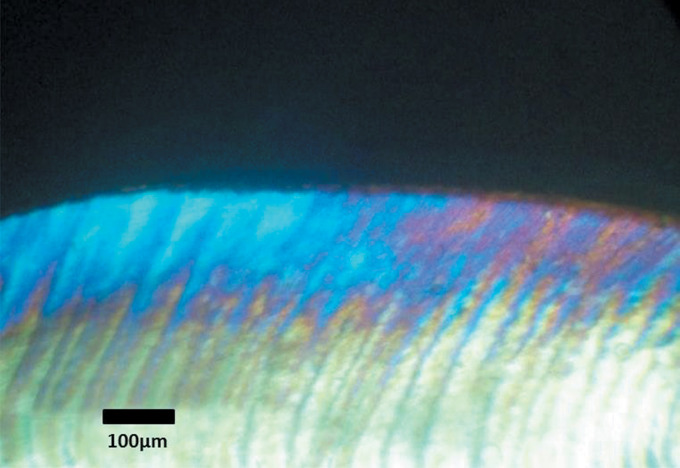
Polarized light microscopy was used to quantify the demineralized lesion depth and qualitatively evaluate the lesions histologically. A comparison of the mean depth of demineralization in μm among the study groups is shown in Table 1 and Figure 2. Group I showed the deepest lesion depth followed by Group III, then Group II, and the least lesion depth was found in Group IV (328.56 μm ± 127.99, 180.45 μm ± 24.27, 168.48 μm ± 28.84, and 24.5 μm ± 8.6, respectively). These findings were confirmed by histological evaluation of the lesions where all four groups displayed increased birefringence of the enamel. In Group I, the positively birefringent zone was covered by a thin negatively birefringent surface layer. The lesions extended deep into the enamel layer before merging with the normal birefringence of intact enamel (Figure 3).
Table 1. .
Comparison Among the Four Study Groups According to Lesion Deptha
|
Group I
(n = 20) |
Group II
(n = 20) |
Group III
(n = 20) |
Group IV
(n = 20) |
H |
P |
|
| Lesion Depth | ||||||
| Min.–Max. | 98.8–492.9 | 122.5–199.7 | 131.1–206.8 | 11.4–33.1 | ||
| Mean ± SD. | 328.56 ± 127.99 | 168.48 ± 28.84 | 180.45 ± 24.27 | 24.5 ± 8.6 | 53.243* | <.001* |
| Median | 360.2 | 174.65 | 186.15 | 28.75 | ||
| Sig. Bet. Grps | p1 = .004*, p2 = .044*, p3 < .001*, p4 = .384, p5 < .001*, p6 < .001* | |||||
H, p: H and P values for Kruskal-Wallis test, Sig. bet. grps was done using Post Hoc Test (Dunn's multiple comparisons test).
p1: P value for comparing between group I and group II. p2: P value for comparing between group I and group III. p3: P value for comparing between group I and group IV. p4: P value for comparing between group II and group III. p5: P value for comparing between group II and group IV. p6: P value for comparing between group III and group IV.
Statistically significant at P ≤ .05.
Figure 2.
Bar graph showing comparison of lesion depth in μm among the four groups.
Figure 3.
Polarized light microscope picture of a section from group I.
Group II and Group III resulted in 48.7% and 45% less lesion depth, respectively, compared to Group I. A significant difference was found when comparing each of those groups to Group I; however, no significant difference was found between Group II and Group III. Upon histological evaluation, both Groups II and III showed areas of increased birefringence and Group II showed less deep lesions than Group I. However, Group III also showed less deep lesion than Group I but deeper than Group II (Figure 4 and 5).
Figure 4.
Polarized light microscope picture of a section from group II.
Figure 5.
Polarized light microscope picture of a section from group III.
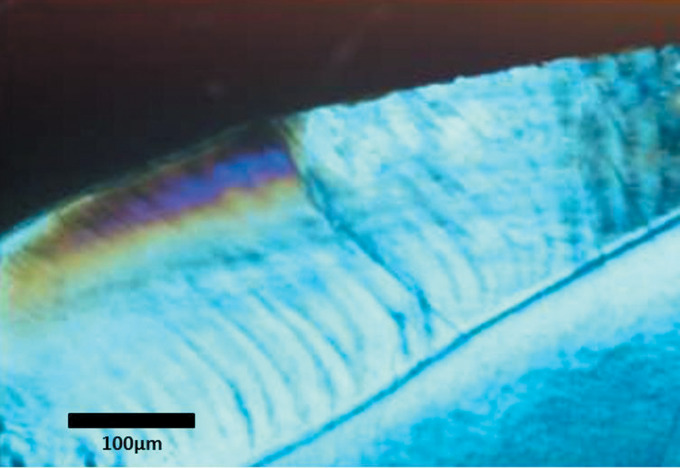
Group IV resulted in 92.5% less lesion depth compared to Group I with a significant difference between Group IV and all other groups. This was confirmed by histological examination, which showed the most superficial merge of the subsurface lesions with normal enamel compared to the other groups, showing the least lesion depth (Figure 6).
Figure 6.
Polarized light microscope picture of a section from group IV.
DISCUSSION
Fixed orthodontic appliances were previously reported to increase the prevalence of WSLs despite the use of prophylactic measures.5 Many preventive measures were described in the literature to increase enamel resistance to demineralization such as fluoride compounds, CPP–ACP and laser irradiation.21
Simulation of the oral environment was accomplished carefully in this study. Human teeth were used and stored in an artificial saliva solution at neutral pH immediately after their extraction and throughout the research period to simulate the salivary composition intraorally.15 Featherstone and Mellberg22 showed that bovine tooth enamel dissolved two or three times faster than human enamel; therefore, human premolar teeth were used in this study. Also, teeth were subjected to thermocycling and brushing cycles equivalent to one year in the oral environment.17,18
Group I, the control group with no preventive measures, showed the highest lesion depth (328.56 μm) upon comparison with the other three groups, which received various preventive measures. This emphasized the beneficial effect of applying preventive measures to reduce the incidence of WSLs.21
The use of CPP–ACP in the current study was based upon the positive results demonstrated in previous studies that investigated the effect of different CPP–ACP formulations on the prevention of WSLs,23,24 with no significant difference compared to fluoride compounds.7,8. Use of CPP–ACP resulted in significantly less lesion depth (48.7%) compared with the control group.
Examination of the enamel morphology using scanning electron microscopy (SEM) conducted by Oshiro et al.25 on bovine teeth showed that CPP–ACP had the ability to prevent demineralization despite the use of different timing protocols to induce artificial WSLs. Additionally, a meta-analysis concluded that in vivo randomized control trials suggested a caries preventive effect for CPP–ACP following long-standing clinical use.26
Another preventive measure tested in this study was hard tissue Laser irradiation using a Er,Cr:YSGG laser. For caries preventive effects, laser irradiation is not supposed to ablate the surface as done when cutting enamel but, rather to modify the morphological or chemical composition or solubility of enamel instead.27 Subablative parameters were used based on previous studies,17,26,27 since there was no published specific protocol for laser irradiation for caries prevention. No water spray was used even though some authors recommended its use to prevent any side effect of laser irradiation. Hossain and colleagues28 found that depths of the lesions produced after Er,Cr:YSGG irradiation with water spray were significantly greater than those without water spray concluding that water played an important role in the ablation of tissues. In this study, Er,Cr,YSGG laser using subablative parameters resulted in significantly less lesion depth (45%) compared with the control group. This percentage was comparable to the conclusion of a review article published in 200610 regarding the laser effects on caries inhibition showing that the percentage inhibition of caries by laser varied from 30% to 97.2%. In addition, the current results were similar to those found by Hossain et al.29 with a significant caries preventive role of the Er,Cr:YSGG laser at 6W and 5W pulse energies, using spectrophotometry and SEM assessment.
Although statistically insignificant, the laser group showed more demineralization compared to the CPP–ACP group. This was in agreement with the results of Apel et al.,30 who concluded that even with the best energy density used for caries prevention (8 J/cm2), fine cracks in enamel could be produced and act as a starting point for acid attack. This may explain why the laser group in the current study showed more demineralization than the CPP–ACP group.
The combination of laser and fluoride treatment was previously shown to have a significant synergistic effect to enhance the resistance of enamel to demineralization.9 In the present study, the model was reconstructed using laser irradiation with CPP–ACP to evaluate if the combination had a significant synergistic effect on the prevention of enamel demineralization or not. The results of the present study proved that their combined use resulted in significantly lesser lesion depth compared to the other groups (24.5 μm).
Different mechanisms may explain the synergistic effect of laser with CPP–ACP in prevention of demineralization. Firstly, recrystallization of hydroxyapatite crystals may result in new crystalline phases larger than the initial ones as was observed in an X-ray diffraction study by Zezell et al.31 after using Er,Cr:YSGG. These new crystals, in the presence of CPP–ACP, could lead to enhancement of subsurface calcium and phosphate content due to increased uptake of these ions by laser.32
Additionally, the increased resistance to demineralization could be attributed to better retention of calcium and phosphate ions for a longer time after laser irradiation. This is probably due to better penetration of these ions deep into the micro spaces formed by the recrystallization of the enamel crystals by laser as well as the heat generated in depth by laser.32 Similarly, Zezell et al.31 observed a significant increment of CaF2 formation and retention in the irradiated samples explaining the increased enamel resistance to demineralization.
Also, the synergistic effect observed in the laser-CPP–ACP combination could be related to the purification of enamel hydroxyapatite. Aminabadi et al.32 observed fine cracks in the laser alone group under SEM resulting in a decrease in surface microhardness in this group. However, these cracks were not seen in the CPP–ACP group and were coated with amorphous crystals and globular deposits in the CPP–ACP plus laser group. It was concluded that when laser was combined with ACP, the fluoride ions came in contact with the free calcium and phosphate ions, forming new hydroxyapatite and fluorapatite crystals. These amorphous crystals coated the cracks produced by the laser, forming a smoother, more homogenous glazed surface, resulting in the purification of the enamel surface during their combined use.
CONCLUSIONS
The use of CPP–ACP alone or laser alone resulted in a significant reduction in lesion depth compared to the control group; however, it was significantly less than their combined use.
No significant difference was found between CPP–ACP alone or laser alone in reduction of lesion depth.
The combined use of Er,Cr:YSGG Laser and CPP–ACP was the best preventive measure against WSL formation evidenced by a significantly lesser lesion depth compared to all other groups.
Recommendation: In vivo studies should be conducted to test the combined effect of Er,Cr:YSGG Laser and CPP–ACP on prevention of demineralization.
REFERENCES
- 1. .Samsonyanová L, Broukal ZA. Systematic review of individual motivational factors in orthodontic treatment: facial attractiveness as the main motivational factor in orthodontic treatment. Int J Dent. 2014;2014:938274. doi: 10.1155/2014/938274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. .Øgaard B. Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofacial Orthop. 1989;96:423–427. doi: 10.1016/0889-5406(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 3. .Kidd E, Fejerskov O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res. 2004;83:C35–C38. doi: 10.1177/154405910408301s07. [DOI] [PubMed] [Google Scholar]
- 4. .Lovrov S, Hertrich K, Hirschfelder U. Enamel demineralization during fixed orthodontic treatment-incidence and correlation to various oral-hygiene parameters. J Orofac Orthop. 2007;68:353–363. doi: 10.1007/s00056-007-0714-1. [DOI] [PubMed] [Google Scholar]
- 5. .Hadler-Olsen S, Sandvik K, El-Agroudi MA, et al. The incidence of caries and white spot lesions in orthodontically treated adolescents with a comprehensive caries prophylactic regimen-a prospective study. Eur J Orthod. 2012;34:633–639. doi: 10.1093/ejo/cjr068. [DOI] [PubMed] [Google Scholar]
- 6. .Wilson N. Minimally invasive dentistry The management of dental caries 1st ed. London: Quintessence; 2007. pp. 69–70. [Google Scholar]
- 7. .Indrapriyadharshini K, Madan Kumar PD, Sharma K, et al. Remineralizing potential of CPP-ACP in white spot lesions - A systematic review. Indian J Dent Res. 2018;29:487–496. doi: 10.4103/ijdr.IJDR_364_17. [DOI] [PubMed] [Google Scholar]
- 8. .Pithon MM, Baião FS, Sant'Anna LID, et al. Effectiveness of casein phosphopeptide-amorphous calcium phosphate-containing products in the prevention and treatment of white spot lesions in orthodontic patients: A systematic review. J Investig Clin Dent. 2019;10:e12391. doi: 10.1111/jicd.12391. [DOI] [PubMed] [Google Scholar]
- 9. .Moslemi M, Fekrazad R, Tadayon N, et al. Effects of ER, Cr:YSGG laser irradiation and fluoride treatment on acid resistance of the enamel. Pediatr Dent. 2009;31:409–413. [PubMed] [Google Scholar]
- 10. .Ana PA, Bachmann L, Zezell DM. Lasers effects on enamel for caries prevention. Laser Phys. 2006;16:865–875. [Google Scholar]
- 11. .Westerman GH, Flaitz CM, Powell GL, et al. In vitro caries formation in primary tooth enamel: Role of argon laser irradiation and remineralizing solution treatment. J Am Dent Assoc. 2006;137:638–644. doi: 10.14219/jada.archive.2006.0260. [DOI] [PubMed] [Google Scholar]
- 12. .Tagliaferro EPS, Rodrigues LKA, dos Santos MN, et al. Combined effects of carbon dioxide laser and fluoride on demineralized primary enamel: an in vitro study. Caries Res. 2007;41:74–76. doi: 10.1159/000096109. [DOI] [PubMed] [Google Scholar]
- 13. .Daniel W. Biostatistics A Foundation for Analysis in the Health Science 6th ed. NY: John Wiley and Sons, Inc; 1995. [Google Scholar]
- 14. .Hassan SA, El-Awamry ZK, Omer TM. Rate of consumption and recommendations of fluoride intake in Egypt from drinking water and the effect on the health of children and adults. Ann Agric Sci. 2004;49:191–207. [Google Scholar]
- 15. .Leung VWH, Darvell BW. Artificial salivas for in vitro studies of dental materials. J Dent. 1997;25:475–484. doi: 10.1016/s0300-5712(96)00068-1. [DOI] [PubMed] [Google Scholar]
- 16. .Ou XY, Zhao YH, Ci XK, et al. Masking white spots of enamel in caries lesions with a non-invasive infiltration technique in vitro. Genet Mol Res. 2014;13:6912–6919. doi: 10.4238/2014.August.29.14. [DOI] [PubMed] [Google Scholar]
- 17. .van Dijken JW, Ruyter IE. Surface characteristics of posterior composites after polishing and toothbrushing. Acta Odontol Scand. 1987;45:337–346. doi: 10.3109/00016358709096356. [DOI] [PubMed] [Google Scholar]
- 18. .Gale M, Darvell BW. Thermal cycling procedure for laboratory testing of dental restorations. J Dent. 1999;27:89–99. doi: 10.1016/s0300-5712(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 19. .Ten Cate J, Dujisters P. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16:201–210. doi: 10.1159/000260599. [DOI] [PubMed] [Google Scholar]
- 20. .Santaella MR, Braun A, Matson E, et al. Effect of diode laser and fluoride varnish on initial surface deminralization of primary dentition enamel: an in vitro study. Int J Pediatr Dent. 2004;14:199–203. doi: 10.1111/j.1365-263X.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 21. .Roopa KB, Pathak S, Poornima P, et al. White spot lesions: a literature review. J Pediatr Dent. 2015;3:1–7. [Google Scholar]
- 22. .Featherstone J, Melberg JR. Relative rates of progress of artificial carious lesions in bovine, ovine and human enamel. Caries Res. 1981;15:109–114. doi: 10.1159/000260508. [DOI] [PubMed] [Google Scholar]
- 23. .Reynolds EC, Cai F, Shen P, et al. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugarfree chewing gum. J Dent Res. 2003;82:206–211. doi: 10.1177/154405910308200311. [DOI] [PubMed] [Google Scholar]
- 24. .Shen P, Cai F, Nowicki A, et al. Remineralization of enamel subsurface lesions by sugar free chewing gum containing casein phosphopeptide amorphous calcium phosphate. J Dent Res. 2001;80:2066–2070. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- 25. .Oshiro M, Yamaguchi K, Takamizawa T, et al. Effect of CPP-ACP paste on tooth mineralization: an FE-SEM study. J Oral Sci. 2007;49:115–120. doi: 10.2334/josnusd.49.115. [DOI] [PubMed] [Google Scholar]
- 26. .Yengopal V, Mickenautsch S. Caries preventive effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP): a meta-analysis. Acta Odontol Scand. 2009;67:321–332. doi: 10.1080/00016350903160563. [DOI] [PubMed] [Google Scholar]
- 27. .Featherstone JD, Nelson DG. Laser effects on dental hard tissues. Adv Dent Res. 1987;1:21–26. doi: 10.1177/08959374870010010701. [DOI] [PubMed] [Google Scholar]
- 28. .Hossain M, Nakamura Y, Kimura Y, et al. Caries preventive effect of Er:YAG laser irradiation with or without water mist. J Clin Laser Med Surg. 2000;18:61–65. doi: 10.1089/clm.2000.18.61. [DOI] [PubMed] [Google Scholar]
- 29. .Hossain M, Kimura Y, Nakamura Y, et al. A study on acquired acid resistance of enamel and dentin irradiated by Er,Cr: YSGG laser. J Clin Laser Med Surg. 2001;19:159–163. doi: 10.1089/10445470152927991. [DOI] [PubMed] [Google Scholar]
- 30. .Apel C, Meister J, Götz H, et al. Structural changes in human dental enamel after subablative erbium laser irradiation and its potential use for caries prevention. Caries Res. 2005;39:65–70. doi: 10.1159/000081659. [DOI] [PubMed] [Google Scholar]
- 31. .Zezell DM, Ana PA, Benetti C, et al. Compositional and crystallographic changes on enamel when irradiated by ND:YAG or Er, Cr:YSGG lasers and its resistance to demineralization when associated with fluoride. Laser Dent. 2010;7509:1–12. [Google Scholar]
- 32. .Aminabadi NA, Najafpour E, Samiei M, et al. Laser-Casein phosphopeptide effect on remineralization of early enamel lesions in primary teeth. J Clin Exp Dent. 2015;7:e261–267. doi: 10.4317/jced.52165. [DOI] [PMC free article] [PubMed] [Google Scholar]