ABSTRACT
Phage therapy is recognized as a promising alternative to antibiotics in treating pulmonary bacterial infections, however, its use has not been reported for treating secondary bacterial infections during virus pandemics such as coronavirus disease 2019 (COVID-19). We enrolled 4 patients hospitalized with critical COVID-19 and pulmonary carbapenem-resistant Acinetobacter baumannii (CRAB) infections to compassionate phage therapy (at 2 successive doses of 109 plaque-forming unit phages). All patients in our COVID-19-specific intensive care unit (ICU) with CRAB positive in bronchoalveolar lavage fluid or sputum samples were eligible for study inclusion if antibiotic treatment failed to eradicate their CRAB infections. While phage susceptibility testing revealed an identical profile of CRAB strains from these patients, treatment with a pre-optimized 2-phage cocktail was associated with reduced CRAB burdens. Our results suggest the potential of phages on rapid responses to secondary CRAB outbreak in COVID-19 patients.
KEYWORDS: Phage therapy, carbapenem-resistant Acinetobacter baumannii, nosocomial infections, COVID-19
Introduction
Bacteriophage therapy is recognized as a promising alternative to antibiotics in treating pulmonary bacterial infections [1–3], however, its use has not been reported for treating secondary bacterial infections during virus pandemics such as coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Severely ill patients with COVID-19 are under the threat of hospital-acquired multidrug-resistant (MDR) bacterial infections, which typically cause longer ventilator occupancy and poorer clinical outcomes than viral infections alone [4–6]. Carbapenem-resistant Acinetobacter baumannii (CRAB) is an ubiquitous nosocomial pathogen which ranked in the top critical priority list of MDR bacteria guiding new antibiotics development [7]. Alternative therapeutic modalities, as well as novel methods for rapid surveillance of MDR bacterial infections, are in demand. Here, we report findings from a case series study, that assessed the efficacy and safety of compassionate phage therapy on secondary CRAB infections in patients hospitalized with critical COVID-19. While both phage susceptibility testing and multilocus-sequence typing (MLST) determined the same genotype of the CRAB strains from these patients, treatment with 2 successive doses of pre-optimized 2-phage cocktail was associated with reduced CRAB burdens and may have contributed to the clinical improvement of the patients.
Materials and methods
Study design
This study was conducted in an ICU specialized for COVID-19 patients in Shanghai Public Health Clinical Center. The study protocol was modified for critical COVID-19 patients for emergency use from an ongoing case-series study (ChiCTR1900020989, an amendment of the study protocol to shed excess exclusion criteria and make possible the enrolment of critically ill patients, was approved by the Ethics Committee of Shanghai Public Health Clinical Center on 28 March, 2019, Approval No. 2017-S027-08). All patients in the ICU with CRAB positive in bronchoalveolar lavage fluid or sputum samples were eligible for study inclusion if antibiotic treatment failed to eradicate their CRAB infections. Over the study period from 1 March to 28 April 2020, four critically ill men aged 62–81 years were enrolled after their guardians signed to give informed consent. The administered route, dose, and frequency of antibiotic treatment are determined by the clinical need, and phage therapy does not alter antibiotic treatment or other treatments. Assessments of clinical and laboratory data were collected directly from the medical reports. Phage susceptibility tests of isolated CRAB strains were performed in the designated laboratory by professional staff wearing biosafety level III personal protective equipment. The endpoint of the study is the elimination of target bacteria and/or patient discharge from ICU or death.
Phage susceptibility assay (phage-typing)
The susceptibilities of the target bacteria to 124 phages in our library were measured by spot assays [8]. All potential CRAB strains from a single sample distinguished in size, colour, and viscosity of the colonies were analysed. Random selection of 3 bacterial colonies was an alternative if no apparent distinction was observed. The lytic efficacy was assessed by the clarity of plaques, scored as transparent, opaque, or no plaque. Phage ɸAb124 (Podoviridae, GenBank ID: MT633129), distinguished by producing a large transparent plaque against all the baseline CRAB isolates from the 4 patients, was selected as the “first-line” phage for therapeutic use.
Next evolution phage-typing (NEPT)
NEPT was introduced to preview the potential phage resistance of target bacteria and screen additional phage(s) for its prevention. Briefly, a 3-mL logarithmic phase culture of the original CRAB isolate in LB medium was infected with 107 plaque-forming unit (PFU) of ɸAb124 and cultured for 8 h at 37 °C with aeration. The emergent ɸAb124-resistant CRAB strain(s) was subjected to another phage-typing. Among phages producing transparent plaques against the emergent strain, ɸAb121 (Myoviridae, GenBank ID: MT623546) was selected as the “second-line” phage for therapeutic use because of its marked differences from ɸAb124 (phage-typing profile, morphology, and genetic background) and its in vitro synergism with ɸAb124.
Phage preparation and administration
Phages were grown on their original host using solid media and recovered by diffusion into SM buffer (5.8 g/L NaCl, 20 mM Tris HCl pH 7.5, 2 g/L MgSO4.7H2O), yielding lysates with titres of >1 × 109 pfu/mL. Phage particles were purified using CIM® Anion-exchange column QA (BIA Separations, Slovenia) according to the manual. The concentrate was dialysed against 0.9% sodium chloride physiological solution (Shandong Qidu Pharmaceutical) 3 times for a minimum of 3 h each [9]. The resulting phage-containing solution was sterilized through a 0.22 µm filter, aliquoted and packaged at the Good Manufacturing Practice (GMP) facility of Zhongshan Hospital of Fudan University, Shanghai, China. ɸAb124 was titrated using the CRAB strain isolated from Patient 1 at 3 days before treatment, while ɸAb121 was titrated using the ɸAb124-resistant strain isolated from Patient 1 the day after ɸAb124 treatment. Single ɸAb124 (1ɸ) or the cocktail (2ɸ) was diluted in saline to 10 mL with a concentration of 108 PFU/mL of each phage, and administered via the respiratory or topical route within 20 min, by using a vibrating-mesh nebulizer (Aerogen Solo) connected to the dry side of the humidifier attached to the ventilator (Evita V300, Dräger) or through a gauze pad. For each course of treatment, 2 doses of lytic phages were administered successively with an interval of 1 h.
Primary and secondary indicators
The primary indicators were changes in CRAB load and in bacterial anti-phage resistance from baseline through the first 24 h after treatment, measured by semi-quantitative streak-plate method and phage susceptibility assay, respectively. The secondary indicators include clinical status on a 7-point ordinal scale [10] (1. dead, 2. hospitalized, on extracorporeal membrane oxygenation or invasive mechanical ventilation, 3. hospitalized, on noninvasive ventilation or high flow oxygen devices, 4. hospitalized, requiring supplemental oxygen, 5. hospitalized, not requiring supplemental oxygen, 6. not hospitalized, but unable to resume normal activities, 7. not hospitalized, with resumption of normal activities) at baseline, 7, 14 and 30 days after treatment, changes in inflammatory indicators including chest radiographies, white blood cells, neutrophils, procalcitonin and hypersensitive C-reactive protein before and after phage therapy.
Multilocus sequence typing (MLST)
MLST was performed to analyse the clonal relatedness of CRAB strains from the studied patients. The fragments of housekeeping genes used by the Oxford and the Pasteur MLST schemes were both amplified, sequenced and analysed following the protocol on the MLST website (http://pubmlst.org/abaumannii/).
Safety
All patients were treated in the ICU setting and safety was assessed using clinical and laboratory parameters, including fever, vital sign monitoring, comprehensive metabolic panels and serial blood examinations (ie, leukocytosis, cytokine storm). Each patient was followed closely by the Shanghai COVID-19 Clinical Treatment Expert Group to assess pathogenic agent, clinical resolution and improvement of infection (i.e. bacterial burden, radiological findings, sputum production), as well as the development of adverse event.
Results
Patient characteristics
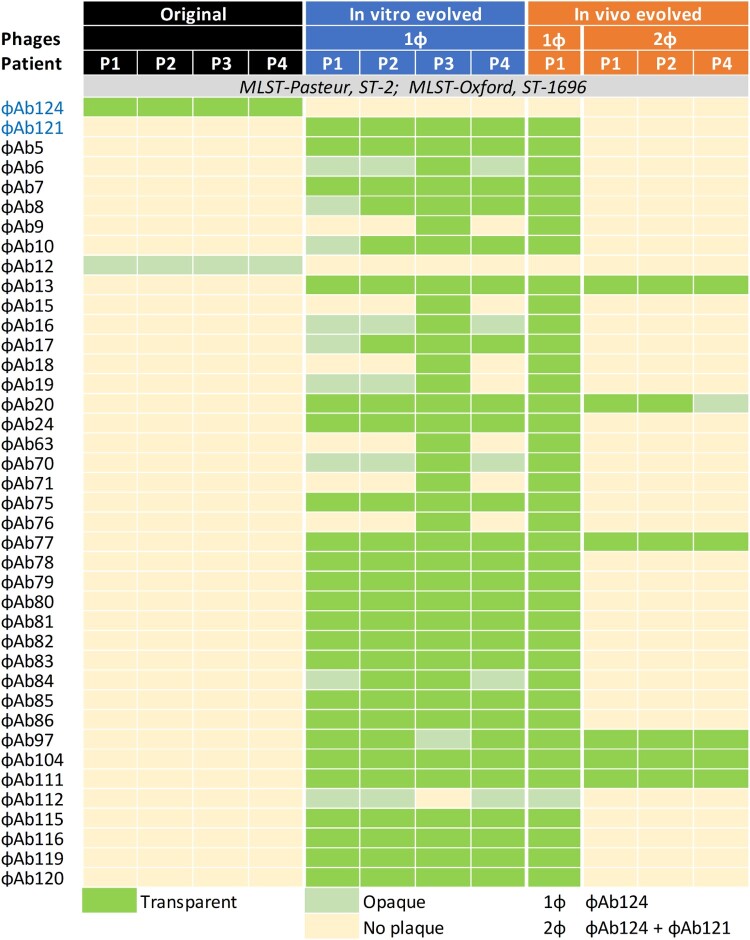
From 1 March 2020, through 28 April 2020, four critically ill men aged 62–81 years were enrolled to compassionate phage therapy after their guardians signed the informed consent (Figure 1). Details regarding baseline characteristics before phage therapy can be found in Table 1. Notably, all patients were indicated to be SARS-CoV-2 free by the absence of detectable viral RNA in throat swabs and the positive antiviral IgG/IgM detections in sera. While opportunistic bacterial and fungal infections were sporadically detected, CRAB infections that emerged consecutively in Patient 4, Patient 1, Patient 2, and Patient 3, were the longest existed and the most serious antibiotic-resistance pathogen. CRABs were continuously detected in the sputum and bronchoalveolar lavage fluid, and can be found also in the urine of Patient 4, Patient 2 also acquired a topical infection at the jugular incision of extracorporeal membrane oxygenation (ECMO) intubation. Several high-grade antibiotics had been applied to these patients since the detection of CRAB (6–50 days before phage therapy) but had failed to eliminate it. The latest CRAB isolates from all patients showed an identical profile of phage susceptibility that podoviral ɸAb124 was the sole phage with effective lytic activity (Figure 2).
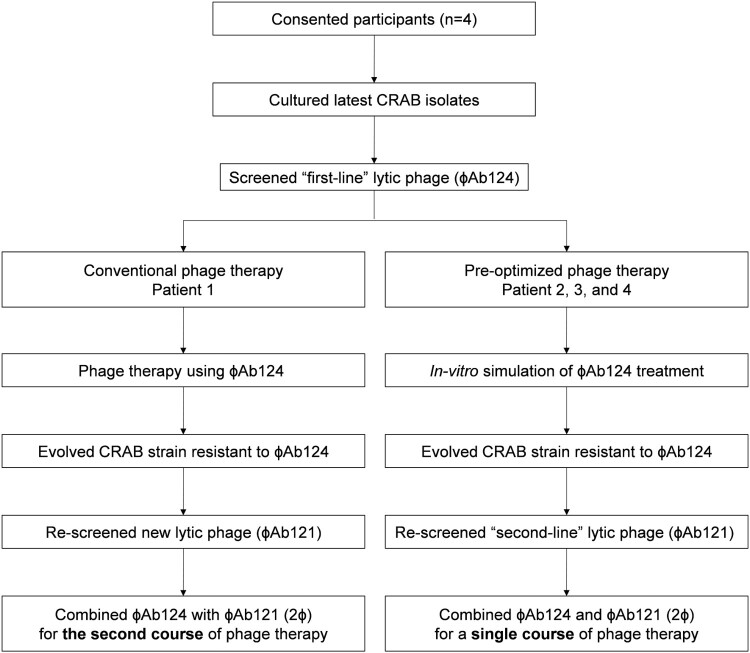
Figure 1.
Study diagram. Flow diagram of the conventional phage therapy procedure for Patient 1 and the pre-optimized procedure (via next evolution phage-typing, NEPT) for the rest 3 patients.
Table 1.
Baseline characteristics, treatments, and outcomes of the patients*.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |||
|---|---|---|---|---|---|---|
| Characteristics at baseline | ||||||
| Age-year | 62 | 64 | 81 | 78 | ||
| SARS-CoV-2 status (RNA, Antibodies)a | (Negative, Positive) | (Negative, Positive) | (Negative, Positive) | (Negative, Positive) | ||
| CRAB infections (days after admission to ICU) | Respiratory tract (18) | Respiratory tract (21) | Wound (34)b | Respiratory tract (40) | Respiratory tract (18) | |
| Days before phage therapy | 11 | 38 | 1 | 6 | 50 | |
| CRAB detected samples | Sputum, BALF | Sputum, BALF | Secretion | Sputum, BALF | Sputum, BALF, Urine | |
| Coexisting infections | Candida albicans | Candida albicans, Ralstonia mannitolilytica | ND | Candida albicans, Candida glabrata | Candida albicans, CSKP, Sphingomonas paucimobilis | |
| Antibiotic and life-support treatment | ||||||
| Principal antibiotics (Susceptibility) | CFP/SUL (S/I) | CFP/SUL (S/I), TGC (S), LEV (S/I) |
MPM (R), CFP/SUL (S/I) | TGC (S), IPM (R), CFP/SUL (S/I) | ||
| Life-support status | MV, ECMO | MV, ECMO | MV, ECMO, CRRT | MV | ||
| Phage therapy | ||||||
| Therapeutic phages | 1ɸ | 2ɸ | 2ɸ | 2ɸ | 2ɸ | 2ɸ |
| Administrated route | Inhalation | Inhalation | Inhalation | Wet compress | Inhalation | Inhalation |
| Primary indicators | ||||||
| Semi-quantitative CRAB (24 h before, after)c | (4+, 2+) | (4+, 2+) | (3+, 2+) | (4+, Negative) | (4+, Negative) | (4+, 2+) |
| Phage resistance of evolved CRAB | Yes | Yes | Yes | NA | NA | Yes |
| Adverse event | fever, IL-6&IL-8 storm | ND | ND | ND | ND | ND |
| Outcome | Discharged from hospital Day 30 |
Discharged from hospital Day 9 |
Died Day 10 | Discharged from ICU Day 7, Died Day 40 | ||
*SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; CRAB, Carbapenem-resistant Acinetobacter baumanni; ICU, Intensive care unit; BALF, Bronchoalveolar lavage fluid; ND, Not detectable; CSKP, Carbapenem-resistant Klebsiella Pneumoniae; CFP/SUL, Cefoperazone-sulbactam; TGC, Tigecycline; LEV, Levofloxacin; MPM, Meropenem; IPM, Imipenem; S, Susceptible; R, Resistant; I, Intermediate; MV, Mechanical ventilation; ECMO, Extracorporeal membrane oxygenation; CRRT, Continuous Renal Replacement Therapy; 1ɸ, ɸ124; 2ɸ, ɸ124+ɸ121; NA, Not applicable.
SARS-CoV-2 RNA status in throat swab and IgG/IgM status in serum.
Wound infection on the jugular intubation site of ECMO.
Semi-quantitative burden of bacteria was measured by streak-plate method.
Figure 2.
Changes of bacterial phage susceptibility profiles upon in-vivo and in-vitro phage challenges. Representative CRAB isolates and their phage-resistant derivates induced by phage therapy or in-vitro next evolution phage-typing (NEPT) were analysed by Phage-typing and multilocus-sequence typing (MLST) using both the Pasteur and the Oxford schemes.
Conventional phage therapy
Patient 1 was the first participant and he firstly received a course of ɸAb124 via nebulization. A decline in semi-quantitative CRAB burden in suctioned sputum was observed, however, a resurgence was soon emerged, which was resistant to ɸAb124 but was susceptible to dozens of other phages in our phage library, among which we selected myoviral ɸAb121 to incorporate ɸAb124 for the second course of treatment (Figure 2).
Pre-optimized phage therapy
We adopted the next evolution phage-typing (NEPT) strategy for in vitro simulation of bacterial anti-ɸAb124 resistance. We discovered that randomly selected ɸAb124-resistant colonies derived from the original CRAB isolates of the patients displayed similar phage-lysis profiles highly matched with the resistant clone that emerged in Patient 1 after ɸAb124 treatment (Figure 2). Thus, a phage cocktail (2ɸ) that contained ɸAb124 to target the original strain and ɸAb121 tailored to kill the evolved ɸAb124-resistant bacteria, was used for the ensuing phage therapies (Figure S1). The in vitro killing curve assay indicated a synergistic effect between ɸAb124 and ɸAb121 in efficiently suppressing the recurrence of target bacteria within 8 h (Figure S3).
Clinical outcomes
Phage therapy was followed by a decline in semi-quantitative CRAB burden in all treatments (Table 1), however, bacterial anti-phage resistance was observed in 4 out of 6 treatments. After a single course of phage inhalations, a minute improvement of the chest radiographs was observed in Patient 1 and 2, while Patient 3, and 4’s pulmonary condition was unchanged (Figure S2A). Patient 2’s CRAB was also detected at the jugular incision of ECMO intubation and this was categorized as a potentially serious complication because the secretion might approach and invade the jugular vein, thus, 2ɸ was emergently applied via wet compress, in the following days the secretion was absorbing without CRAB detection and the skin converted to normal and dry (Figure S2B).
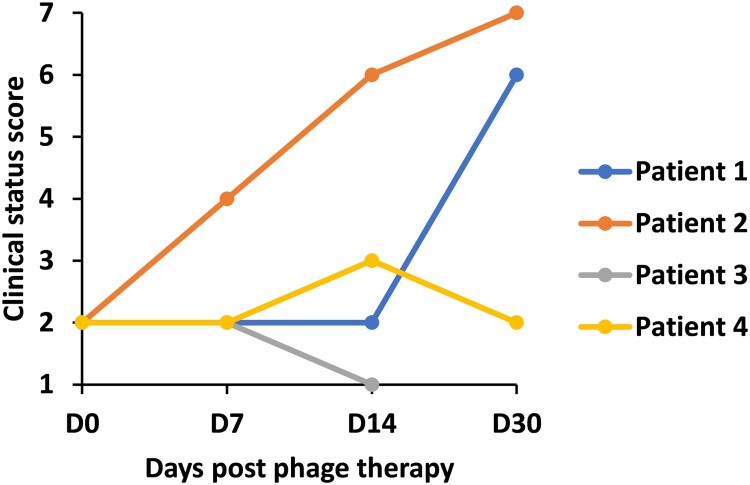
At the end of this study, Patient 1 and Patient 2 were weaned from ECMO and subsequently discharged from the hospital; Patient 4’s illness severity was improved and he was discharged from ICU on day 7 post phage inhalation, however, he died of respiratory failure a month later; Patient 3’s CRAB was eliminated but an un-subdued Carbapenem-resistant Klebsiella Pneumoniae (CRKP) infection was followed and he died of respiratory failure 10 days after phage therapy (Table 1, Figure 3).
Figure 3.
Changes of clinical status within 30 days post phage therapy. Clinical status on a 7-point ordinal scale (1. dead, 2. hospitalized, on extracorporeal membrane oxygenation or invasive mechanical ventilation, 3. hospitalized, on noninvasive ventilation or high flow oxygen devices, 4. hospitalized, requiring supplemental oxygen, 5. hospitalized, not requiring supplemental oxygen, 6. not hospitalized, but unable to resume normal activities, 7. not hospitalized, with resumption of normal activities) was measured at baseline, 7, 14 and 30 days after treatment.
Advert events
Patient 1 had experienced an atypical cytokine storm at 4 h post ɸAb124 administration. This was manifested as a transient fever along with a dramatic increase in interleukins 6 and 8 (IL-6 up to 596.32 pg/mL and IL-8 up to 112.05 pg/ mL), but other cytokines have stayed in the reference scope. A day later IL-6 and IL-8 returned to the normal levels (Table S1).
Discussion
To our knowledge, this study describes the first therapeutic use of phages for the secondary bacterial infections in COVID-19 patients and the first clinical application of NEPT strategy to preview bacterial anti-phage resistance.
Bacterial anti-phage resistance is one of the major obstacles for phage therapy [3,11]. In this study, despite the in vitro proof of synergistic effect between ɸAb124 and ɸAb121, phage resistance was observed in 4 out of 6 treatments. Nevertheless, bacterial gain of anti-phage resistance might be accompanied by losing advantages in other respects such as virulence [12]. Patient 1 and Patient 2 who saw clinical improvement in chest radiographs were finally discharged from hospital. In contrast, conventional antibiotic treatment had been tried previously and failed to suppress CRAB infection or improve the condition of these patients. Suggesting that phage therapy may have contributed to the recovery of the patients.
Other factors affecting clinical outcome may include phage penetration into the infected site and the host immune state. Phages and the immune system may work synergistically to get rid of bacterial infection [13]. Results from animal model revealed the importance of host immune system in facilitating phage-mediated bacterial elimination [14]. In critically ill COVID-19 patients, the injured respiratory system and impaired immune responses can dramatically reveal the inadequacy of available antibiotic treatments [15,16], may also impair the efficiency of phage delivery and bacterial elimination.
Identifying the source of infection and the route of transmission is crucial for effective disease containment. Conventional bacterial typing methods such as Pulse field gel electrophoresis (PFGE), MLST and whole-genome sequencing are costly and time-consuming [17]. In this report, a single phage-typing can be achieved within 8 h and the NEPT protocol only takes twice the time. Our results also indicate that, on the basis of the epidemiological connections and available phage collections, phage susceptibility assay may serve as a rapid and cost-effective method for bacterial typing, as well as a routine database to monitor nosocomial infections and as a prelude to preparing ready-to-use phages. However, the frequent shift in phage susceptibility should be counted and may be overcome by pre-performed NEPT database. Additional whole-genome alignment can be helpful to validate the bacterial phage-typing but is expensive.
Previous studies have demonstrated that, compared to antibiotic treatment, the rapid lysis of bacteria by phages leads to less endotoxin release from gram-negative organisms and does not increase the innate inflammatory response [18,19]. However, Patient 1 experienced an incident resembling a dramatic wave in IL-6 and IL-8 at four hours post ɸAb124 inhalation, which is distinguished from the previously described patterns of a cytokine storm [20,21]. Risk prevention measures against cytokine storm should be considered during phage therapy.
Our exploratory study is of small size because of the containment of the COVID-19 outbreak in Shanghai as well as the control of CRAB outbreak in our center. Nevertheless, we report here a rewarding application of phages in treating and tracing MDR bacterial infections in ventilator-supported patients. These results may open new opportunities for the prevention and treatment of bacterial secondary infection, a should be an indispensable part of pandemic planning and management.
Supplementary Material
Acknowledgments
We thank Dr Douglas B. Lowrie and Dr Chen Zhao from Shanghai Public Health Clinical Center for critically proofreading the manuscript. We thank all members of the Shanghai COVID-19 Clinical Treatment Expert Group for thoughtful discussions. We also thank our patients, and the health professionals for providing outstanding patient care at considerable personal risk. This work was funded by the Shanghai Public Health Clinical Center (grant SJTNY to T.Z., grant RCJJ2019-06 to L.K.C.), National Major Science and Technology Projects of China (grant 2020ZX09201001-005-003 to N.W.), and Shanghai Commission of Science and Technology (grant 20Y11900300 to N.W.).
Funding Statement
This work was supported by National Major Science and Technology Projects of China [grant number 2020ZX09201001-005-003]; Shanghai Municipal Science and Technology Commission [grant number 20Y11900300]; Shanghai Public Health Clinical Center [grant number RCJJ2019-06,SJTNY].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Aslam S, Courtwright AM, Koval C, et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. AJTMBR. 2019 Sep;19(9):2631–2639. DOI: 10.1111/ajt.15503. PubMed PMID: 31207123; PubMed Central PMCID: PMCPMC6711787. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maddocks S, Fabijan AP, Ho J, et al. Bacteriophage therapy of ventilator-associated pneumonia and empyema caused by pseudomonas aeruginosa. Am J Respir Crit Care Med. 2019 Nov 1;200(9):1179–1181. DOI: 10.1164/rccm.201904-0839LE. PubMed PMID: 31437402; eng. [DOI] [PubMed] [Google Scholar]
- 3.Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant mycobacterium abscessus. Nat Med. 2019 May;25(5):730–733. DOI: 10.1038/s41591-019-0437-z. PubMed PMID: 31068712; PubMed Central PMCID: PMCPMC6557439. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verroken A, Scohy A, Gerard L, et al. Co-infections in COVID-19 critically ill and antibiotic management: a prospective cohort analysis. Crit Care. 2020 Jul 9;24(1):410), DOI: 10.1186/s13054-020-03135-7. PubMed PMID: 32646494; PubMed Central PMCID: PMCPMC7347259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Zhang Y, Wu J, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020 Dec;9(1):1958–1964. DOI: 10.1080/22221751.2020.1812437. PubMed PMID: 32815458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bengoechea JA, Bamford CGG.. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020 May 26. DOI: 10.15252/emmm.202012560. PubMed PMID: 32453917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics, 2017 Feb 27. Available from: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/.
- 8.Tan D, Zhang Y, Qin J, et al. A frameshift mutation in wcaJ associated with phage resistance in Klebsiella pneumoniae. Microorganisms. 2020 Mar 7;8(3). DOI: 10.3390/microorganisms8030378. PubMed PMID: 32156053; PubMed Central PMCID: PMCPMC7142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J, Wu N, Bao J, et al. Heterogeneous Klebsiella pneumoniae co-infections complicate personalized bacteriophage therapy. Front Cell Infect Microbiol. 2020;10(608402). DOI: 10.3389/fcimb.2020.608402. PubMed PMID: 33569355; PubMed Central PMCID: PMCPMC7868542. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Physicians RCo. National Early Warning Score (NEWS) 2: standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. London: RCP 2017. Available from: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2.
- 11.Schooley RT, Biswas B, Gill JJ, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017 Oct;61(10). DOI: 10.1128/aac.00954-17. PubMed PMID: 28807909; PubMed Central PMCID: PMCPMC5610518. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burmeister AR, Fortier A, Roush C, et al. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc Natl Acad Sci U S A. 2020 May 26;117(21):11207–11216. DOI: 10.1073/pnas.1919888117. PubMed PMID: 32424102; PubMed Central PMCID: PMCPMC7260982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung CYJ, Weitz JS.. Modeling the synergistic elimination of bacteria by phage and the innate immune system. J Theor Biol. 2017 Sep 21;429:241–252. DOI: 10.1016/j.jtbi.2017.06.037. PubMed PMID: 28668337; eng. [DOI] [PubMed] [Google Scholar]
- 14.Lin YW, Chang RY, Rao GG, et al. Pharmacokinetics/pharmacodynamics of antipseudomonal bacteriophage therapy in rats: a proof-of-concept study. Clin Microbiol Infect. 2020 Sep;26(9):1229–1235. DOI: 10.1016/j.cmi.2020.04.039. PubMed PMID: 32387436; eng. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. 2020;8(4):420-422. DOI: 10.1016/s2213-2600(20)30076-x. PubMed PMID: 32085846. [DOI] [PMC free article] [PubMed]
- 16.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. 2020;180(7):934–943. DOI: 10.1001/jamainternmed.2020.0994. PubMed PMID: 32167524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang S, Orsi RH, Luo H, et al. Assessment and comparison of molecular subtyping and characterization methods for salmonella. Front Microbiol. 2019;10:1591), DOI: 10.3389/fmicb.2019.01591. PubMed PMID: 31354679; PubMed Central PMCID: PMCPMC6639432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dufour N, Delattre R, Chevallereau A, et al. Phage therapy of pneumonia is not associated with an overstimulation of the inflammatory response compared to antibiotic treatment in mice. Antimicrob Agents Chemother. 2019 Aug;63(8). DOI: 10.1128/AAC.00379-19. PubMed PMID: 31182526; PubMed Central PMCID: PMCPMC6658787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dufour N, Delattre R, Ricard JD, et al. The lysis of pathogenic Escherichia coli by bacteriophages releases less Endotoxin than by beta-Lactams. Clin Infect Dis. 2017 Jun 1;64(11):1582–1588. DOI: 10.1093/cid/cix184. PubMed PMID: 28329379; PubMed Central PMCID: PMCPMC5434335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. DOI: 10.1016/S0140-6736(20)30566-3. PubMed PMID: 32171076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tisoncik JR, Korth MJ, Simmons CP, et al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012 Mar;76(1):16–32. DOI: 10.1128/MMBR.05015-11. PubMed PMID: 22390970; PubMed Central PMCID: PMCPMC3294426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.