Abstract
Objective
To determine whether severe perivascular space (PVS) dilation is associated with longitudinal cognitive decline and incident dementia over 4 and 8 years, respectively, we analyzed data from a prospective cohort study.
Methods
A total of 414 community-dwelling older adults aged 72–92 years were assessed at baseline and biennially for up to 8 years, with cognitive assessments, consensus dementia diagnoses, and 3T MRI. The numbers of PVS in 2 representative slices in the basal ganglia (BG) and centrum semiovale (CSO) were counted and severe PVS pathology defined as the top quartile. The effects of severe PVS pathology in either region or both regions and those with severe BG PVS and severe CSO PVS were examined. White matter hyperintensity volume, cerebral microbleed number, and lacune number were calculated.
Results
Participants with severe PVS pathology in both regions or in the CSO alone had greater decline in global cognition over 4 years, even after adjustment for the presence of other small vessel disease neuroimaging markers. The presence of severe PVS pathology in both regions was an independent predictor of dementia across 8 years (odds ratio 2.91, 95% confidence interval 1.43–5.95, p = 0.003). The presence of severe PVS pathology in all groups examined was associated with greater dementia risk at either year 4 or 6.
Conclusions
Severe PVS pathology is a marker for increased risk of cognitive decline and dementia, independent of other small vessel disease markers. The differential cognitive associations for BG and CSO PVS may represent differences in their underlying pathology.
Perivascular spaces (PVS) are fluid-filled spaces that surround small blood vessels within the brain parenchyma. There is increasing evidence for the association of MRI-visible dilated PVS with small vessel disease (SVD)1–3 and neurodegenerative4,5 pathology.
Few longitudinal studies have examined the association of PVS with incident dementia5,6 and a recent review reported ambiguous findings for the association with Alzheimer disease (AD) or all-cause dementia.7 There are also mixed findings regarding the association of PVS with cognitive impairment; specifically, our data8 and 2 recent meta-analyses failed to find an association with cognitive impairment cross-sectionally.9,10 There is stronger evidence for an association between PVS and longitudinal cognitive impairment.5,6,11
PVS are commonly seen in the basal ganglia (BG) and centrum semiovale (CSO). This may represent at least partially different pathophysiology, with BG PVS being associated with more hypertension-related pathology and CSO PVS associated with amyloid pathology and ultimately, higher incidence of AD.4,12 These regions may in turn be associated with impairments in different cognitive domains, resulting from damage to specific cognitive networks or due to underlying pathology.
Our primary aim was to determine whether total PVS severity (severe PVS pathology in either region) was associated with decline in global cognition and incident dementia. We also explored whether severity in both regions, or regional BG or CSO PVS severity, was associated with these same outcomes. Our secondary aim was to determine whether PVS severity, either totally or specifically in the BG or CSO, was associated with impairments in specific cognitive domains. We aimed to determine whether any impairments seen were independent of other neuroimaging markers of SVD, which might suggest an independent pathway for the contribution of PVS to cognitive impairment.
Methods
Participants
Participants were part of the Sydney Memory and Ageing Study (MAS).13 This is a longitudinal, population-based study, which started in 2005 and investigates cognitive decline in the elderly. Participants were aged 70–90 years at entry to the MAS, living in the community, and able to complete their assessments in English. Exclusion criteria were major psychiatric or CNS disorders including schizophrenia, bipolar disorder, motor neuron disease, multiple sclerosis, CNS inflammation, developmental disability, or any other condition that may have interfered with assessment completion.13
At each wave, 2 years apart, participants had an MRI scan, comprehensive neuropsychological assessment, medical examination, and blood collection (including APOE genotyping in wave 1). For this study, we used data from wave 2, when a more comprehensive MRI protocol was introduced, and this was considered baseline for the current study. Our sample of 414 comprised all participants at wave 2 who consented and were eligible to have an MRI scan (figure 1). Comprehensive neuropsychological assessment was available for waves 2 to 4 (baseline to year 4 of this PVS study) and dementia diagnosis for waves 2 to 6 (baseline to year 8).
Figure 1. Participant Flow Chart.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all participants. Ethical approval was received from the University of New South Wales Human Research Ethics Committee.13 This study is compliant with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.14
Neuropsychological Assessment
Trained research psychologists administered a battery of tests, grouped into cognitive domains: attention and processing speed (Digit Symbol Coding,15 Trail-Making Test A16), executive function (Controlled Oral Word Association Test,16 Trail-Making Test B,16 Benton Visual Retention Test17), language (Boston Naming Test: 30 items,18 Semantic Fluency [Animals]16), visuospatial (Block Design19), and memory (Logical Memory,20 Rey Auditory Verbal Learning Test16). At each wave of the study, raw test scores were transformed to z scores using the wave 1 mean and SD values of a healthy reference group: 723 MAS participants. Domain scores were calculated by averaging the z scores of component tests. These composite scores were then standardized as z scores, calculated using the means and SDs for the wave 1 healthy reference sample. A global cognition score was calculated by averaging all domain scores and again, transforming to a z score using the healthy reference sample. Diagnosis of dementia was made using DSM-IV21 criteria at each wave by a multidisciplinary consensus panel consisting of old age psychiatrists, neuropsychiatrists, and neuropsychologists.13
Radiologic Examination
All MRI scans were performed at a single wave, on the same machine, a Philips 3T Achieva Quasar Dual scanner. A 3D T1-weighted sequence (1 × 1 × 1 mm3, repetition time [TR]/echo time [TE] 6.39/2.9 ms), a T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence (TR/TE/inversion time 10,000/110/2,800 ms; thickness 3.5 mm; 0.898 × 0.898 mm2), and a susceptibility-weighted imaging sequence (measures previously described22) were performed. All images were coregistered using Statistical Parametric Mapping version 5 (SPM5). Total white matter hyperintensities (WMHs) volume quantification was automated, using FLAIR and T1-weighted images,23 and adjusted for total intracranial volume.24 All images were analyzed using MRIcron version 15.25 Lacunes and cerebral microbleeds (CMBs) were defined using STRIVE (Standards for Reporting Vascular Changes on Neuroimaging) criteria.26
Perivascular Space Rating Scale
The number of PVS were counted in a single axial slice in the BG and CSO, respectively, according to a recently published scale.8 For full details of the scale development including psychometric properties, see the original article.8 In brief, PVS were counted on predefined axial slices, 2 mm and 37 mm superior to the anterior commissure. PVS were defined according to STRIVE26 criteria, i.e., they have CSF signal intensity and “follow the course of penetrating vessels, appearing linear when viewed parallel to the course of a penetrating vessels or round or ovoid when imaged perpendicular to the course of the vessel.”26 They were differentiated from lacunes by their lack of a hyperintense rim on FLAIR sequences and by size, lacunes usually being greater than 3 mm in diameter. We included PVS of any diameter if they fulfilled other diagnostic criteria.6 Figure 2 shows examples of severe BG and severe CSO PVS pathology.
Figure 2. Severe Basal Ganglia and Severe Centrum Semiovale Perivascular Space (PVS) Pathology.
(A) Severe basal ganglia and (B) severe centrum semiovale PVS pathology. Arrow points to an example of a PVS in each region.
Covariates
Hypertension was defined as a blood pressure of ≥140/90 mm/Hg (mean of 2 seated readings) or if the participant received a prior medical diagnosis of hypertension. Diabetic status was determined by a prior medical diagnosis or a fasting blood glucose value ≥7 mmol/L.
Analysis
Based on the number of PVS in the BG slice, individuals were dichotomized at the top quartile, with those in the top quartile (≥7) considered to have severe pathology. This was repeated for the CSO slice, with individuals with ≥2 PVS considered as having severe pathology. Four nonexclusive binary PVS, predictor variables were then created for analysis; (1) an “any severe PVS” group, representing those with severe PVS in either (or both) brain regions (severe BG or severe CSO PVS) vs those with mild/absent (lower 3 quartiles) PVS in both regions; (2) a subsample of this group, the “both severe PVS,” representing those with severe PVS in both regions of interest (severe BG and severe CSO PVS) vs those with mild/absent (lower 3 quartiles) PVS in either region; and 2 regional subgroups: (3) a severe BG PVS group, with participants dichotomized into individuals with severe BG PVS pathology vs those with mild/absent (lower 3 quartiles) BG PVS; and (4) a severe CSO PVS pathology group, with participants dichotomized into individuals with severe CSO PVS pathology vs those with mild/absent (lower 3 quartiles) CSO PVS.
The decision to examine those with the upper quartile of severity was made a priori based on evidence suggesting there may only be cognitive sequelae for those with the most severe PVS pathology, measured by size or frequency.5,6
To examine differences in baseline characteristics between groups defined by the 4 PVS binary predictors described above, t-test, Mann-Whitney U tests, and χ2 tests were used.
WMH volume was log-transformed to better approximate the normal distribution. Distributions of measures of cognition were inspected, and outliers (defined as being more than 3 SDs from the mean) were winsorised to values at that distance from mean values.
Linear mixed models (LMMs), with random intercepts and slopes, were used to examine relationships between PVS pathology in either region (any severe PVS group) vs mild/absent PVS in both regions and cognitive decline over 3 waves. Wave, PVS, and the PVS*wave product interaction term were included in each of the equations along with the covariates listed below. Because we were interested in the effects of PVS on longitudinal cognitive decline, we report only measure estimates (B-coefficients) with standard errors for the PVS*wave product interaction terms.
The following covariates were in the equation. Model 1 included age, sex, education, APOE ε4 carrier status, body mass index, smoking status, hypertension, and diabetes. Model 2 included the covariates in model 1 with the addition of the neuroimaging measures, WMH volume (log-transformed), presence of lacunes, and presence of multiple (≥1) microbleeds. These analyses were then repeated for the other predictor variables: the both severe PVS, severe BG PVS, and severe CSO PVS groups. Results from the LMM individual cognitive domain analyses were Bonferroni corrected for multiple testing (α set at 0.01). This considered each set of repeated analyses with the cognitive domains as outcomes as a family of 5 independent tests.
The associations of any severe PVS with incident dementia were examined using logistic regression to implement discrete-time survival analysis.27–29 This approach better handles events occurring in discrete time periods and provides flexible solutions to model data that violate the proportional hazard assumption.27 We were interested in examining incident dementia from wave 2 onwards and so only those without dementia at wave 2 (baseline) were used in the analyses. Data were structured into a person–period format. Each participant could have up to 4 rows of data, corresponding to the 4 assessment waves (wave 3 to wave 6). First, a dummy variable was created to indicate whether a participant developed dementia at a particular wave (0 = no dementia, 1 = dementia). Second, 4 discrete time indicators were computed by coding each of the 4 measurement waves, with a value of 1 being set for the time period it represents and all other values being set to 0. Finally, at a particular wave, if a participant was diagnosed with dementia, or if he or she dropped out (i.e., were censored), subsequent rows for that participant were removed. The 4 time indicators were included as predictors of dementia status in the logistic regression model while the intercept was excluded. These variables were used as multiple intercepts to estimate the hazard function, or probability of the onset of dementia occurring in the period since the previous wave.27 The discrete time hazard is a conditional probability that denotes the event (incident dementia) will occur in a particular period, given it has not occurred earlier, and it is modeled as (log) odds in logistic regression. Regression equations are presented in the supplementary appendix (data available from Dryad, doi.org/10.5061/dryad.931zcrjj9).
To determine whether severe BG or CSO PVS was associated with higher overall odds of dementia over all time periods relative to no severe PVS, PVS was included as a predictor in the analysis (proportional odds model). The analysis was repeated for the other PVS subtypes (i.e., both severe PVS, severe BG PVS, severe CSO PVS). The regression coefficients of PVS were converted to odds ratios (ORs), and thus reflected the ratio of the odds of developing dementia for those with severe PVS (and subtypes) compared to those with a less severe pathology at every time period. ORs above and below 1 indicated that being in the PVS group was associated with higher or lower odds of developing dementia over time, respectively.
The above analysis was repeated to test whether the effect of PVS on dementia risk was moderated by time, including interaction terms between PVS and each of the 4 dummy variables and excluding the PVS term from the analysis (nonproportional odds model). Each interaction term represented the effect of PVS on dementia risk in each 2-year time period among those who did not have dementia at the previous wave. Hereafter, at year 4 corresponds to the 2-year period between year 2 and year 4, for example. In other words, each OR corresponding to the interaction term indicated whether being in the severe PVS group, relative to the no severe PVS group, was associated with higher or lower odds of developing dementia over a 2-year period. Both sets of analyses adjusted for the same set of covariates as the LMM above.
All analyses were performed using SPSS statistical package (IBM SPSS Statistics for Windows, version 25).
Missing Data
Both LMM and the discrete-time survival approach better allow analysis of missing dependent variable data for a particular wave, compared with using the more traditional case-wise deletion method, as they minimized loss of information, loss of power, and biased estimates.30,31 Chained equations were used to impute missing covariate data—25 imputations were chosen with parameter estimates based on the pooled estimates across all imputations.
To explore our patterns of missing data, we analyzed potential differences in baseline characteristics between completers and noncompleters. We also performed sensitivity analysis, repeating the main analyses for only those individuals with complete global cognition data over 4 years and complete dementia data over 8 years. We also tested the association between PVS and incident dementia by simulating data that assume higher rates of developing dementia among noncompleters. This was done to increase confidence in our main results that assume data missing-at-random, given that it is possible that attrition is higher for those more cognitively impaired (missing-not-at-random).
Finally, we conducted supplemental analyses, examining (1) the association between PVS and decline in global cognition for the subset of 400 participants who were dementia-free at baseline and (2) the association of PVS with individual cognitive test results.
Data Availability
Anonymized data from the MAS are available on request.
Results
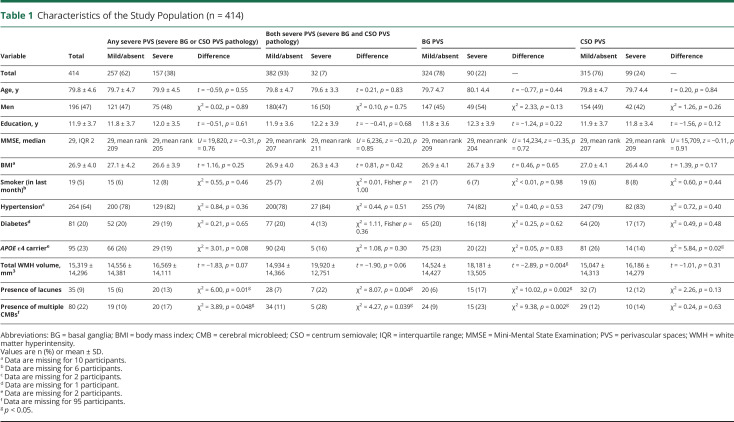
Participant characteristics are presented in table 1 (n = 414; mean age 79.8 [SD 4.6]; 196 [47%] men). There were 157 (38%) individuals with severe PVS pathology in either region, 32 (7%) with severe pathology in both regions, 90 (22%) with severe BG PVS pathology, and 99 (24%) with severe CSO PVS pathology.
Table 1.
Characteristics of the Study Population (n = 414)
The groups did not differ by age, sex, or vascular risk factors, with the exception of those with severe CSO PVS, who were less likely to be an APOE ε4 carrier (14% compared to 26% with absent/mild CSO PVS, χ2 = 5.84, df[1], p = 0.02). Individuals with severe BG PVS had greater WMH volume (log transformed; t = −2.89, p = 0.004), and were more likely to have lacunes (17% compared to 6% with absent/mild BG PVS, χ2 = 10.02, df[1], p = 0.002) and multiple CMBs (23% compared to 9% with absent/mild BG PVS, χ2 = 9.38, df[1], p = 0.002). In contrast, individuals with severe CSO PVS did not have greater amounts of other SVD imaging pathology.
Participants in the any severe PVS group did not have a more rapid decline in global cognition compared to those with less severe disease (table 2). Participants with severe disease in both locations, however, had a more rapid decline in global cognition compared to those with less severe disease. This remained significant after adjusting for all demographic and vascular risk factors and other neuroimaging measures: model 2 (unstandardized B −0.175, SE 0.076, p = 0.020). When regional subgroups were examined, those with severe CSO PVS had a more rapid decline in global cognition compared to those with less severe disease, even after adjusting for all covariates: model 2 (unstandardized B −0.111, SE 0.048, p = 0.020).
Table 2.
Longitudinal Relationship of Basal Ganglia (BG) and Centrum Semiovale (CSO) Perivascular Spaces (PVS) With Cognition Over 4 Years (n = 414)
Results of the association of PVS with individual cognitive domains are presented in table 2. The presence of severe total or regional BG or CSO PVS pathology was not associated with longitudinal decline in any of the 5 cognitive domains assessed after Bonferroni correction for multiple testing.
Ten participants were diagnosed with dementia at study baseline and 4 participants had missing dementia information from all waves. These 14 participants were excluded from the dementia analyses. Of 400 participants at baseline, 97 (24%) were diagnosed with dementia over the 8 years of follow-up. Of those with severe PVS in either region, 40 of 152 participants (26%) developed dementia and in individuals with severe PVS in both regions, 12 of 31 participants (39%) developed dementia. A total of 27 of 89 (30%) participants with severe BG PVS at baseline developed dementia, as did 25 of 94 participants (27%) with severe CSO PVS (data available from Dryad, table e-1, doi.org/10.5061/dryad.931zcrjj9).
Table 3 displays the results of the discrete-time survival analysis. If a participant was in the any severe PVS group or had just severe BG or CSO PVS pathology, he or she was no more likely to develop dementia, compared to an individual with mild/absent PVS pathology, respectively. However, if he or she had pathology in both areas, he or she had nearly triple the odds of developing dementia, at every time period, over 8 years (OR 2.91, 95% confidence interval [CI] 1.43–5.95, p = 0.003), independent of other vascular and neuroimaging covariates.
Table 3.
Longitudinal Relationship of Basal Ganglia (BG) and Centrum Semiovale (CSO) Perivascular Spaces (PVS) With Incident Dementia Over 8 Years (n = 400)
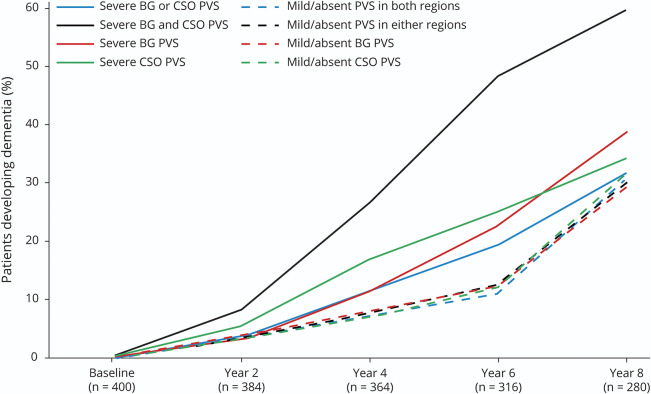
There was a strong interaction with time, such that for all 4 predictor variable groups, severe PVS was associated with an increased risk of dementia at either year 4 or 6. This effect remained after adjusting for all other covariates. The strongest effect was seen in those with severe pathology in both areas, with an OR of 4.75, 95% CI 1.51–14.95, p = 0.008 of developing dementia at year 4 compared to those with absent/mild PVS in either region. The association of severe PVS with dementia was not seen at year 8. Figure 3 shows, for each wave, the predicted probability of developing dementia in each PVS group, based on the discrete-time survival analysis results. The full results (model 2 only) are presented in table e-2 (data available from Dryad, doi.org/10.5061/dryad.931zcrjj9) so the OR of the other covariates in the model can be seen, with age and APOE ε4 carrier status having the strongest relationship with dementia status.
Figure 3. Predicted Probability at Each Wave of Developing Dementia Since Baseline in Each Perivascular Space (PVS) Group, Based on Discrete-Time Survival Analysis.
Predicted values adjusted for age, sex, education, APOE ε4 carrier status, body mass index, smoking status, hypertension, diabetes, white matter hyperintensity volume (log-transformed), presence of lacunes, and presence of multiple (≥1) microbleeds. BG = basal ganglia; CSO = centrum semiovale.
A comparison of the baseline characteristics for completers vs noncompleters is shown in table e-3 (data available from Dryad, doi.org/10.5061/dryad.931zcrjj9). Noncompleters were significantly older (80.6 years vs 79.3 years, t = 2.75, p = 0.006) and more likely to be male (60% vs 43%, χ2 = 9.89, p = 0.002) and to have lacunes (13% vs 6%, χ2 = 5.15, p = 0.02).
Results from the sensitivity analyses examining only those 331 participants who had full global cognition data for all 4 years are presented in table e-4 (data available from Dryad, doi.org/10.5061/dryad.931zcrjj9). Similar to the original analysis, greater decline in global cognition is seen for those with severe CSO PVS and those with severe PVS in both regions. Life tables for the dementia analysis are presented in table e-1 (data available from Dryad, doi.org/10.5061/dryad.931zcrjj9) with complete data available on 280 participants (70% completers) by year 8. The results of the completers-only dementia analysis (table e-5, data available from Dryad, doi.org/10.5061/dryad.931zcrjj9) resemble the main analysis, with participants with severe PVS pathology in both regions demonstrating an overall increase in dementia incidence. When time interactions were examined, the association between PVS pathology in either location and midstudy dementia incidence (year 4–6) remained. To investigate whether the nonsignificant time interaction effects at year 8 were partly due to attrition bias driven by dementia status, we simulated incident dementia data for noncompleters that assumed higher rates of developing dementia (table e-6, data available from Dryad, doi.org/10.5061/dryad.931zcrjj9). This did not significantly change the overall results. Table e-7 (data available from Dryad, doi.org/10.5061/dryad.931zcrjj9) displays the results of the LMM analysis for the subset of 400 participants who were dementia-free at the start of this study, with similar results to the original cohort of 414 participants. Table e-8 (data available from Dryad, doi.org/10.5061/dryad.931zcrjj9) presents the associations of PVS with individual cognitive test scores; participants with severe pathology in both regions show impairments on tests associated with verbal learning, attention and processing speed, and language.
Discussion
We found that having severe PVS pathology in both regions examined predicted incident dementia. When regions were assessed individually, severe BG PVS and CSO PVS pathology predicted early dementia at years 6 and 4, respectively. There were also regional differences when assessing longitudinal cognitive change. It was also only participants with severe PVS pathology in both regions, or those with severe CSO PVS pathology, who had an increased rate of cognitive decline over 4 years. Severe BG PVS pathology was not associated with cognitive decline.
These results remained significant after adjusting for the other neuroimaging measures of SVD, suggesting there is an independent mechanism for PVS as a biomarker of cognitive impairment and dementia apart from being a general marker of SVD pathology. Dilated PVS may be a biomarker of impaired waste clearance,32–34 for example.
The effect of PVS may differ by region. For CSO PVS specifically, several nonexclusive mechanisms may affect cognition. Enlargement of PVS in the white matter is reportedly associated with cerebral amyloid-β pathologies,12 and CSO PVS pathology might indicate the presence of cerebral amyloid angiopathy (CAA) or a mixed hypertensive/CAA4,35–37 pathophysiology. It may be a more diffuse CAA–Alzheimer association driving cognitive sequelae, with a cycle of impaired clearance of amyloid and other toxins leading to greater damage of the neurovascular unit and cerebral parenchyma. Alternatively, it may be damage to particular white matter tracts in the CSO, including through CAA pathology that leads to cognitive impairment.
In contrast, BG PVS are a marker for hypertensive arteriopathy, as are other traditional markers such as lacunes, WMHs, and multiple CMBs, albeit with some regional variation.22 This would explain the associations between BG PVS and other neuroimaging measures, which did not occur with CSO PVS.
The strongest findings were for those 32 (7%) participants with severe PVS in both regions. This could be due to 2 nonexclusive mechanisms: first, that this group has the most severe and widespread PVS pathology. Post hoc analysis found this group did not have a significant increase in PVS frequency, with a median of 10 BG PVS and 4 CSO PVS, compared to 9 BG PVS in those with just severe BG PVS pathology and 4 CSO PVS in those with just severe CSO PVS pathology. Alternatively, or in addition, there may be an additive effect of the 2 different pathologies present in the different regions—hypertensive arteriopathy and CAA—which together produce a larger association with cognitive decline and dementia.
The weak association between PVS and vascular risk factors is unsurprising given the literature, which reports variable results for the association of PVS with both vascular and demographic risk factors.2,6,8,37–40 This may be partially explained by the wide variety of definitions and rating scales used to quantify PVS.
We found associations with decline in global cognition but not in any of the constituent cognitive domains examined. Some of these domains had a trend toward significance and we can speculate there may be an additive effect that when combined into a global cognition composite produced a significant association. The majority of studies of PVS and cognition are cross-sectional, with 2 recent meta-analyses reporting no association of PVS severity with impaired cognition.9,10 We could find only 3 prospective studies examining cognitive change. Ding et al.6 reported associations of large PVS with declines in processing speed independent of other SVD markers, but not of verbal memory or executive function. Zhu et al.5 reported that BG PVS were associated with declines in Trails A and B, but WM PVS were not. They did not adjust for the presence of other SVD neuroimaging measures. After adjusting for WMHs and CMBs, Benjamin et al.41 did not find an association between PVS and decline in total cognition, executive function, or processing speed. The mixed results suggest insufficient evidence linking either total or regional PVS and declines in specific cognitive domains.
For incident dementia, our results are somewhat consistent with the 2 longitudinal studies of PVS and dementia in the general population. Ding et al.6 reported an association between large PVS and vascular but not all-type dementia or AD. Their mean follow-up of 5.2 years is similar to the period in which we found the strongest effect of PVS. Similarly, Zhu et al.5 had a median follow-up of 3.5 years in their study, which found an association between WM PVS and dementia, after adjusting for WMH.
When examining regions individually, the lack of association between BG and CSO PVS and dementia across 8 years may reflect the sharp increase in the numbers developing dementia without severe PVS pathology at the year 8 follow-up, when participants were, on average, in their late 80s. New dementia cases peaked at year 4 in those with severe CSO PVS pathology and then declined at subsequent waves, but for those with mild/absent PVS pathology, new cases continued to rise thorough the 8 years. Those with severe PVS pathology may have dropped out earlier and the signal from PVS was lost due to the stronger effect of age over time. The sensitivity analysis showed noncompleters were more likely to be older and male, both groups that have increased morbidity and mortality, supporting this argument.
Unexpectedly, individuals with severe CSO PVS pathology were less likely to be an APOE ε4 carrier. The association between APOE genotype and PVS has been little studied, with 1 recent article finding no association.42 Our seemingly paradoxical finding is not easily explained and may be a type 1 error and affected by sample size (i.e., only 14 individuals had severe CSO PVS pathology and were APOE ε4 carriers).
There were limitations to our study. Although 8 years is a longer follow-up period than most comparable studies, even longer prospective periods may be more informative for dementia analysis. We also only had detailed cognitive data for 4 years. The scale used to rate PVS had good psychometric properties and was easy to use,8 but the selection of 2 predetermined slices may have missed PVS in other areas, particularly for those with an atypical pattern of PVS distribution. The scale rated PVS primarily from T1-weighted images, but PVS are more easily visible on T2-weighted imaging.8,43 The scale may therefore have underestimated PVS frequency. There was attrition, which increased over the study period, and by year 8, dementia status could not be determined in 30% of participants. Using LMM and discrete-time survival analysis logistic regression minimized data loss and maximized power as we could still include participants who did not have complete data across all waves. Results were unaffected when sensitivity analysis was used to examine only study completers. These analytic strategies assume that missing is at random or completely at random. Indeed, our attrition data are consistent with other aging and dementia longitudinal studies,44 and noncompleters are generally more likely to be unwell or cognitively impaired.44,45 Our analysis confirmed that noncompleters had characteristics at baseline (age, male sex, presence of lacunes) that predisposed them to greater morbidity. The potential informative attrition may have biased our results, especially the surprising finding on dementia risk at year 8. Our sensitivity analysis suggested that informative attribution could not fully explain the current results, which requires further investigation.
We found differential associations between the region of PVS pathology and cognitive decline and incident dementia. This suggests differences in their underlying pathology or differential effects due to the location of PVS and effect on white matter tracts. The associations remained significant in the presence of other SVD neuroimaging markers, suggesting at least a partially independent pathway of PVS-related injury. Further research is needed into the etiology and sequelae of PVS pathology since PVS may be an important potential biomarker to help with early dementia diagnosis, prognosis, and subtyping. Importantly, future studies should divide PVS analyses by region and attempt to standardize PVS visual rating. Study duration needs to be carefully considered when examining incident dementia due to the interaction of time with PVS.
Glossary
- AD
Alzheimer disease
- BG
basal ganglia
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- CMB
cerebral microbleed
- CSO
centrum semiovale
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FLAIR
fluid-attenuated inversion recovery
- LMM
linear mixed model
- MAS
Memory and Ageing Study
- OR
odds ratio
- PVS
perivascular spaces
- SVD
small vessel disease
- TE
echo time
- TR
repetition time
- WMH
white matter hyperintensity
Appendix. Authors

Footnotes
CME Course: NPub.org/cmelist
Study Funding
M. Paradise was supported by the Australian National Health and Medical Research Council (NHMRC) NNIDR–DCRC Early Diagnosis and Prevention Shared Grant and the Josh Woolfson Memorial Scholarship. The Sydney Memory and Ageing Study was supported by NHMRC grants 350833 and 568940.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011537 for full disclosures.
References
- 1.Zhu YC, Dufouil C, Mazoyer B, et al. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. AJNR Am J Neuroradiol 2011;32:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010;41:450–454. [DOI] [PubMed] [Google Scholar]
- 3.Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol 2008;255:692–696. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer's disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimers Dis 2015;43:415–424. [DOI] [PubMed] [Google Scholar]
- 5.Zhu YC, Dufouil C, Soumare A, Mazoyer B, Chabriat H, Tzourio C. High degree of dilated Virchow-Robin spaces on MRI is associated with increased risk of dementia. J Alzheimers Dis 2010;22:663–672. [DOI] [PubMed] [Google Scholar]
- 6.Ding J, Sigurethsson S, Jonsson PV, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol 2017;74:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smeijer D, Ikram MK, Hilal S. Enlarged perivascular spaces and dementia: a systematic review. J Alzheimers Dis 2019;72:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paradise M, Beaudoin M, Dawes L, et al. Development and validation of a rating scale for dilated perivascular spaces on MRI. J Neurol Sci 2019;409:116621. [DOI] [PubMed] [Google Scholar]
- 9.Hilal S, Tan CS, Adams HHH, et al. Enlarged perivascular spaces and cognition: a meta-analysis of 5 population-based studies. Neurology 2018;91:e832–e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis F, Ballerini L, Wardlaw JM. Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: a systematic review and meta-analysis. Int J Stroke 2019;14:359–371. [DOI] [PubMed] [Google Scholar]
- 11.Arba F, Quinn TJ, Hankey GJ, et al. Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. Int J Stroke 2018;13:47–56. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee G, Kim HJ, Fox Z, et al. MRI-visible perivascular space location is associated with Alzheimer's disease independently of amyloid burden. Brain 2017;140:1107–1116. [DOI] [PubMed] [Google Scholar]
- 13.Sachdev PS, Brodaty H, Reppermund S, et al. The Sydney Memory and Ageing Study (MAS): methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non-demented cohort of Australians aged 70-90 years. Int Psychogeriatr 2010;22:1248–1264. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III). 3rd ed. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 16.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 17.Benton AL, Sivan AB, Spreen O. Der Benton Test. 7th ed. Bern: Huber; 1996. [Google Scholar]
- 18.Kaplan E. The Boston Naming Test. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 19.Wechsler D. WAIS-R Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 20.Wechsler D. Wechsler Memory Scale. 3rd ed. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Washington, DC: American Psychiatric Association; 2011. [Google Scholar]
- 22.Paradise M, Seruga A, Crawford JD, et al. The relationship of cerebral microbleeds to cognition and incident dementia in non-demented older individuals. Brain Imaging Behav 2019;13:750–761. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Liu T, Zhu W, et al. UBO Detector: a cluster-based, fully automated pipeline for extracting white matter hyperintensities. Neuroimage 2018;174:539–549. [DOI] [PubMed] [Google Scholar]
- 24.Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage 2004;22:144–154. [DOI] [PubMed] [Google Scholar]
- 25.Rorden C. MRIcron Version 15 [online]. Available at: nitrc.org/projects/mricron. Accessed November 06, 2020. [Google Scholar]
- 26.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer JB, Willet JB. Applied Longitudinal Data Analysis. New York: Oxford University Press; 2003. [Google Scholar]
- 28.Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodological) 1972;34:187–220. [Google Scholar]
- 29.Singer JD, Willett JB. It's about time: using discrete-time survival analysis to study duration and the timing of events. J Stat Educ 1993;18:155–195. [Google Scholar]
- 30.Molenberghs G, Kenwood MG. Missing Data in Clinical Studies. Hoboken: Wiley; 2007. [Google Scholar]
- 31.Diggle PJ, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. 2nd ed. Oxford: Oxford University Press; 2013. [Google Scholar]
- 32.Brown R, Benveniste H, Black SE, et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 2018;114:1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the CNS: is there a 'glymphatic' system? Acta Neuropathol 2018;135:387–407. [DOI] [PubMed] [Google Scholar]
- 34.Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci 2017;131:2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yakushiji Y, Charidimou A, Hara M, et al. Topography and associations of perivascular spaces in healthy adults: the Kashima scan study. Neurology 2014;83:2116–2123. [DOI] [PubMed] [Google Scholar]
- 36.Charidimou A, Jaunmuktane Z, Baron JC, et al. White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology 2014;82:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology 2013;80:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MD. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am J Neuroradiol 1989;10:929–936. [PMC free article] [PubMed] [Google Scholar]
- 39.Hurford R, Charidimou A, Fox Z, Cipolotti L, Jager R, Werring DJ. MRI-visible perivascular spaces: relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J Neurol Neurosurg Psychiatry 2014;85:522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010;41:2483–2490. [DOI] [PubMed] [Google Scholar]
- 41.Benjamin P, Trippier S, Lawrence AJ, et al. Lacunar infarcts, but not perivascular spaces, are predictors of cognitive decline in cerebral small-vessel disease. Stroke 2018;49:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shams S, Martola J, Charidimou A, et al. Topography and determinants of magnetic resonance imaging (MRI)-visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc 2017;6:e006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis 2015;39:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol 2005;58:13–19. [DOI] [PubMed] [Google Scholar]
- 45.Burke SL, Hu T, Naseh M, et al. Factors influencing attrition in 35 Alzheimer's disease centers across the USA: a longitudinal examination of the National Alzheimer's Coordinating Center's Uniform data set. Aging Clin Exp Res 2019;31:1283–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from the MAS are available on request.