Abstract
Objective
Hemidecortication is a therapeutic option in patients with drug-resistant structural epilepsy. If surgery is performed early enough in left-hemispheric pathology, the plasticity of the developing brain may enable the right hemisphere to take over language—if this has not occurred before surgery. A systematic overview of potential predictors of language outcome after left hemidecortication in children is warranted.
Methods
In a systematic literature review, we analyzed 58 studies on language lateralization after congenital or postneonatally acquired left-hemispheric pathology, and on language outcome after left-sided hemidisconnection, such as hemispherotomy. Single-subject data were pooled to determine the distribution of lateralization across etiologies in congenital lesions and across age groups in acute postneonatal lesions. A hierarchical linear regression assessed the influence of age at surgery, lesion type, age at seizure onset, and presurgery language function on language outcome after left hemidecortication.
Results
In acute postneonatal lesions, younger age at injury was significantly associated with right-sided language lateralization (Cramér V = 0.458; p = 0.039). In patients with hemidecortication, age at surgery was not significantly associated with language outcome (Cramér V = −0.056; p = 0.584). Presurgical language function was the most powerful predictor for postsurgical language outcome (F4,47 = 7.35, p < 0.0001), with good presurgical language bearing the risk of postsurgical deterioration. In congenital pathology, right-sided language lateralization was most frequent in pre-/perinatal stroke (Cramér V = 0.357; p < 0.0001).
Conclusions
We propose a presurgical decision algorithm with age, presurgical language function, language lateralization, and left-hemispheric structural pathology as decision points regarding surgery.
Right-hemispheric reorganization is common after left-sided pre-/perinatal brain lesions.1–6 But how long will the plastic window be open, preventing a child from retaining aphasia after left-sided hemispherotomy?
We insufficiently understand the factors predicting language outcome after hemidecortication. Rasmussen's and Milner's series of Wada tests in epileptic patients demonstrated that the developing brain can represent language in the right hemisphere after a left-hemispheric lesion.7 The neurosurgical literature often assumes as common knowledge that recovery from extensive damage to left-hemispheric language areas is possible until school age.7,8 However, studies trying to identify a timeframe conclude that regarding hemidecortication, age seems to be less important than etiology.9 In their “Review of cognitive outcome after hemidecortication in humans,” Vargha-Khadem and Polkey10 demonstrated that even after late left hemispherectomies, language outcome could be surprisingly good. The interactions between age at insult, lesion type, epilepsy onset/severity, and postsurgical seizure freedom may determine whether language can be retained or recovered after left hemidecortication.
We analyzed publications addressing language in patients with pre-/peri- and postnatal left-hemispheric brain pathology (acute and progressive), with and without hemispherotomy. To inform a clinical decision algorithm regarding possibility for surgical treatment, we asked the following questions:
(a) What are the age limits for successful right-hemispheric language reorganization and (b) which factors can modify these limits? Literature suggests 3 modifying factors: epilepsy, language delay, and progressive pathology.9,11,12
How does, in congenital pathology, the type of brain lesion influence language reorganization?
Methods
For this review, we followed the PRISMA guidelines as completely as possible within our research question. Although the review was not registered, we make all data used available in the supplementary tables.
Literature Search and Article Selection
We conducted a systematic hierarchical search of PubMed (pubmed.org) and PsycINFO (via ebscohost.com) for studies published after the review of Vargha-Khadem and Polkey.10 Inclusion criteria were left-hemispheric pathology, age at injury <18 years, information on language lateralization (language fMRI/MEG/PET and hemidecortication), and publication date (January 1992–June 2018). We included studies with mixed adult/pediatric samples when they presented individual pediatric data. For language lateralization, we accepted fMRI, MEG, and PET. Wada tests were not accepted because in many recent publications, they were conducted unilaterally (or not further specified), leaving bilateral language lateralization possible. The exclusion criterion was potentially bilateral brain damage (e.g., traumatic brain injury or tuberous sclerosis complex).
Search terms were (“stroke” OR “brain infarction” OR “lesion” OR “malformation” OR “hemiparesis” OR “hemiplegia” OR “cerebral palsy”) AND (“prenatal” OR “perinatal” OR “congenital” OR “neonatal” OR “infant” OR “child” OR “adolescent”) AND (“language” AND (“lateralization” OR “lateralisation” OR “representation” OR “organization” OR “organisation” OR “reorganization” OR “reorganisation” OR “hemispherectomy” OR “hemispherotomy” OR “hemidecortication” OR “epilepsy surgery”)) AND (“1992/01/01”[MDAT]: “2018/06/30”[MDAT]).
Using self-developed electronic data extraction forms, we screened titles and abstracts for inclusion criteria, assessed all resulting full texts for eligibility, and categorized them according to one of the research questions (done by Karen Lidzba, Martin Staudt, Sarah Bürki, and Eva Franz): (1a) Age limits: Acute, non-progressive left-hemispheric lesions acquired postneonatally; (1b) Age limits: Hemi-decortication; (2) Underlying brain lesion: congenital pathology.
After the automatic search, we searched resulting articles for additional references and processed them in the same way. In cases of missing data in the publications, we contacted the authors.
Quality Assessment and Assessment of Bias
The Newcastle-Ottawa Scale13 provides quality assessment of case-control and cohort studies for systematic reviews. Although 5 of the 9 points are designed for the use in treatment studies, 4 of the items score population selection. For the sake of uniformity throughout our review, we used these 4 items to assess the quality of all studies included in this review, being comparative or not. In noncomparative studies, we assessed case definition and representativeness; in studies with control group, we also scored selection and definition of controls. We excluded studies if case definition was unsatisfactory. As yet, no validation has been published for the Newcastle-Ottawa Scale,13 and there is 1 critical evaluation pointing out some caveats regarding the rating of, e.g., outcome measures.14 However, because of the lack of better instruments, we opted for the truncated version that has been used before.15
Regarding assessment of bias, we adopted 5 categories of the reporting bias as assessed in Cochrane reviews,16 although we could not review intervention studies for which these categories were originally created. Table e-1 (links.lww.com/CPJ/A175) lists our (in part arbitrary) definitions for risk levels in the bias types.16 We approached the duplicate publication bias by including only the last of several studies from the same group with potentially the same patients.
Data Analyses
Age Limits for Successful Right-hemispheric Language Reorganization
We categorized language function as normal, mildly impaired, and severely impaired, based on the information given in the articles. If standardized language measures were reported, we used SDs as thresholds: less than 1 SD below mean (normal); 1 to 1.5 SD below mean (mildly impaired); and more than 1.5 SD below mean (severely impaired). If the authors did not conduct standardized tests (due to inclusion of very young or cognitively very impaired patients), we adopted their clinical impression as reported in the article. For all analyses, we set p < 0.05 as threshold of significance.
Acute Postneonatal Lesions
We included all patients with acute, postneonatally acquired focal brain lesions with documented aphasia (as defined in the original article) and language lateralization at follow-up (principal outcome measures). We included patients who fell ill before age 3 years if the lesion affected left-sided language areas on neuroimaging.
We categorized language lateralization, either adopting the authors' categorization or based on laterality indices (LIs) provided in the publication. If the authors provided both regional and hemispheric LI, we used the more specific regional LI. We categorized values between −0.2 and +0.2 as bilateral and values ≥0.2 or ≤−0.2 as right or left lateralized (direction varies between groups). We assumed successful right-hemispheric language reorganization if normal/mildly impaired language was associated with right-hemispheric language lateralization. We categorized age at injury as infancy (≤2 years) and childhood/adolescence (≥3 years).
Modifiers of Age Limits: Epilepsy, Language Function, and Progressive Pathology
We included patients with left hemidecortication, when the publication included data on pre- and post-surgery language performance. The principal summary measure was the difference between post- and presurgical language function. We categorized pre-/post-surgery difference in language function as follows: improvement/deterioration for gain/loss ≥1 SD in standardized language assessment (or verbal IQ) or shift to better/lower category and no change for the remainder. We assumed successful right-hemispheric language reorganization if language did not deteriorate after surgery.
To further characterize the potential modifiers of age limits for successful right-hemispheric language reorganization, we performed a hierarchical linear regression on the variable pre-/post-surgery language change (deterioration and no deterioration), with the following predictors entered stepwise: (1) age at hemidecortication (months), (2) lesion type (malformation; pre-/perinatal stroke; Rasmussen encephalitis; Sturge-Weber syndrome), (3) age at seizure onset (months), and 4) presurgery language function (severely impaired, mildly impaired, and normal). We excluded postneonatal stroke due to the negligible sample size (n = 3).
Influence of the Underlying Brain Lesion
To answer the second research question, we analyzed the single-subject language lateralization data available of all patients with congenital pathology, i.e., brain malformations, developmental tumors, and pre-/perinatally acquired focal lesions. These analyses were independent of language performance data.
Results
Search Output
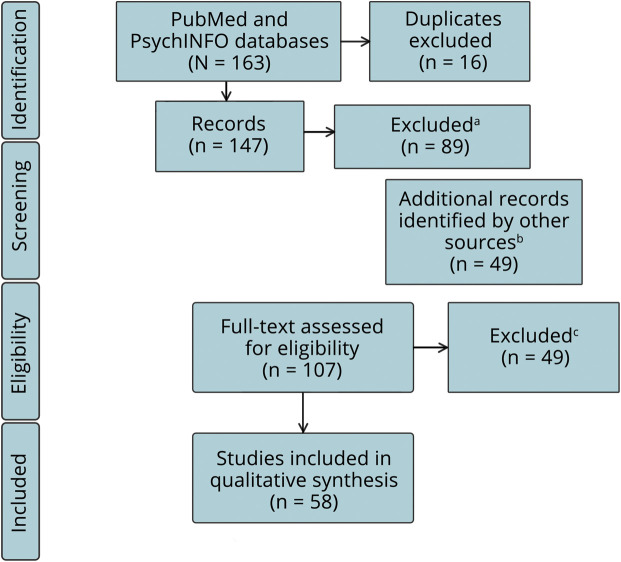
Figure 1 illustrates the search output, and table e-2 (links.lww.com/CPJ/A175) lists all 58 resulting articles grouped by category. We found mostly single-case studies and case series (postneonatal lesions: 7/12; hemidecortication: 20/25; congenital lesions: 8/26). Five studies on postneonatal lesions, 10 on hemidecortication, and 7 on congenital lesions included a control group. For case definition (according to the Newcastle-Ottawa Scale), most studies relied on MRI plus other clinical data. Around half of the studies had adequate case representativeness (postneonatal lesions: 6/12; hemidecortication: 15/25; congenital lesions: 10/26).
Figure 1. Search Output and PRISMA Steps.
aExclusion criteria: no or wrong pathology/surgery (n = 36); no data on language/language representation (n = 22); no (individual) child data (n = 3); pure methods article (n = 10); no study (n = 12); and article not in English (n = 6). bAdditional references from articles and reviews in search output. cExclusion criteria: no relation made between side/age at injury and language outcome (n = 18); insufficient or missing lesion characterization (n = 8); same patients reported in more than 1 article (n = 5); no language outcome or (fMRI or PET) lateralization reported (n = 13); and pure methods article (n = 5).
Risk of reporting bias was low to medium for data on congenital and postneonatal lesions and medium to high for data on hemidecortication (figure e-1, links.lww.com/CPJ/A174).
Age Limits for Successful Right-hemispheric Language Reorganization
Acute Postneonatal Lesions
Statistical Analysis
Individual data on language lateralization were available in 31 patients fulfilling all inclusion criteria (tables e-2 and e-3, links.lww.com/CPJ/A175). Lesions acquired until age 2 years showed a higher proportion of subsequent right-hemispheric language representation than those acquired after age 2 years (Cramér V = 0.458; p = 0.039; figure 2).
Figure 2. Successful Right-hemispheric Language Lateralization After Acute Postneonatally Acquired Left-hemispheric Lesions to a Previously Healthy Brain.
Figures in chart are absolute numbers.
Narrative Literature Review
Right-hemispheric language lateralization is rare following postneonatally acquired left-hemispheric stroke.17–22 Most patients with successful right-hemispheric language reorganization were younger than 3 years.22,23 In patients with lesions acquired between 3 and 5 years of age, fMRI lateralization was bilateral or left.17,18,21 In children aged 5 years and older at the time of injury, language recovery has been associated with left-lateralized language on the short term,17 but with right lateralization on the long term.19
Patient groups who had recovered from aphasia after postneonatally acquired left-hemispheric cerebral vascular insult demonstrated right-lateralized, bilateral, or left-lateralized language in fMRI, whereas the language of healthy controls and that of recovered patients with adult aphasia were left lateralized.4,17,18 Lesion characteristics did not seem to be related to language lateralization.4,18
Modifiers of Age Limits: Epilepsy, Language Function, and Progressive Pathology
Statistical Analyses
We identified 59 patients with sufficient language data before and after left-sided hemidecortication (tables e-2 and e-4, links.lww.com/CPJ/A175). Younger age at surgery correlated at trend level with positive postsurgical language outcome (Spearman ρ = −0.248, p = 0.058, 2 tailed).
In the hierarchical linear regression analysis (table 1), age at surgery did not predict postsurgical language outcome. Lesion type added 9% to the variance explained by the first model, age at seizure onset added 17% to the second model, and presurgical language function added 21% to the third model. The final model with all 4 predictors was most meaningful (F4,47 = 7.35, p < 0.0001). Presurgical language function was the only relevant coefficient in this model. Patients with initially impaired language function had a good chance to remain stable or improve postsurgically, whereas patients with initially good language function had a risk of deterioration (figure 3).
Table 1.
Hierarchical Stepwise Regression Analysis on the Predictors of Postsurgical Language Outcome Compared With Presurgical Language Function (Deterioration vs No Deterioration)
Figure 3. Change in Language Function After Left-sided Hemidecortication.
Presurgical language function, etiology, and age at surgery. (A) Patients with impaired presurgical language. (B) Patients with normal presurgical language.
Narrative Literature Review
Across patients with drug-resistant epilepsy, etiology predicts postsurgical language outcome9,24–28 much better than age at surgery.9,25,29 Patients with large malformations of cortical development demonstrate the least linguistic progress after surgery, whereas patients with Rasmussen encephalitis or infarctions fare better. Etiology determines age at seizure onset and age at surgery.26 Irrespective of etiology or hemisphere, patients improved in language function for at least 12 months postsurgery,25 especially those with better presurgical cognitive development.28 Shorter epilepsy duration and successful withdrawal of antiepileptic medication correlated with better postsurgery language function.25
Patients with brain malformations are young at seizure onset and surgery is often performed at an early age. Compared with other groups, their intelligence and language performance is lowest before and after surgery.26,30–32 Children with malformations often continue to have seizures postoperatively,8,28 and seizure control predicts postoperative language development.9
Patients with Rasmussen encephalitis often have a better preoperative cognitive and linguistic level than patients with other etiologies.26 Left-hemispheric perinatal infarctions leading to drug-resistant epilepsy in infancy often entail severe mental retardation presurgically, but accelerated development postsurgically.33 In contrast to malformations, acquired and progressive pathology is associated with laterality effects in language function, both pre- and postsurgically.9,26,29,34 Left-sided surgery may lead to postsurgical aphasia (as defined by the authors of the original articles) or severe language deficits in patients between 6 and 17 years initially35–38 and to subnormal language function at longer follow-up.27,37,38 In Rasmussen encephalitis, language often deteriorates presurgically and may shift to the right hemisphere.38–40 Published data on postsurgical language recovery are largely based on case reports and draw a heterogeneous picture (table e-2, links.lww.com/CPJ/A175).
Influence of An Underlying Congenital Brain Lesion
Statistical Analyses
We included 134 patients with congenital left-hemispheric lesions and language lateralization data (tables e-2 and e-5, links.lww.com/CPJ/A175). Patients had left-hemispheric malformations of cortical development (n = 41; focal cortical dysplasia, heterotopia, and polymicrogyria), developmental tumors (n = 25; dysembryoplastic neuroepithelial tumor and ganglioglioma), and pre- or perinatal stroke (n = 68; venous infarctions and arterial ischemic stroke). Most studies determined language lateralization by fMRI word generation tasks (N = 19). One study each reported MEG and PET data.
Lateralization differed between lesion types (Cramér V = 0.357; p < 0.0001; figure 4). Right-lateralized language was frequent in pre-/perinatal stroke (60% right/25% bilateral/15% left), whereas it was rare in brain malformations (18% right/15% bilateral/68% left). The group of developmental tumors showed an intermediate pattern (35% right/12% bilateral/54% left).
Figure 4. Language Lateralization After Congenital Left-sided Brain Pathology.

Absolute patient numbers in the graph.
Narrative Literature Review
Age should be a first switch in our algorithm: any brain younger than 3 years seems to be able to represent language in the right hemisphere without obvious deficits.
Pre- or perinatally acquired left-hemispheric stroke was often associated with right-lateralized or bilateral activation for a range of language tasks, especially concerning expressive language.1–5,41 Right-hemispheric regions involved in language processing typically mirrored the left-hemispheric ones in healthy controls.1–3,41 Compared with normal controls regarding variability of brain activation, patients involved additional supramodal brain regions in language tasks, leading the authors to infer the usage of alternative strategies.2,42 Lesion size did not correlate with language lateralization.2,5,41 In patients with pre-/perinatal infarctions, damage to the Broca area,3 the facial motor tract,1 or the insular cortex and the supramarginal gyrus6 might be predictors for right-hemispheric language representation.
Brain malformations and developmental tumors rarely induced right-hemispheric language representation,43–45 unless the lesion involved the left inferior or middle frontal gyri.24,46 Malformed brain regions usually did not harbor language; however, it tended to be localized in close vicinity.47 Age at epilepsy onset inconsistently correlated with language lateralization.12,43,44 Only few studies assessed language performance in detail, and the results are inconsistent, with better verbal functions being correlated both with typical37 and with atypical language lateralization.48
Discussion
Based on this literature review, we developed an algorithm aiding the decision-making process before left-sided surgery, with respect to the preservation of language (figure 5).
Figure 5. Flowchart of the Proposed Decision Algorithm Based on the Results of the Literature Review.
Admittedly, we have inferred this algorithm from a low-level evidence base. Because of ethical reasons, randomized controlled trials do not—and probably will not ever—exist on left-sided hemispherotomy in children with language as primary outcome. Thus, our suggestions must stay tentative, and decisions must always be based on the individual case.
Age should be a first switch in our algorithm: any brain younger than 3 years seems to be able to represent language in the right hemisphere without obvious deficits. During the first 3 years of life, surgery can therefore be assumed low risk regarding language outcome. This is in line with recent data suggesting that, during the first 3 years of life, surgery outcome on general cognitive development is favorable.49,50 Obviously, the third birthday is an arbitrary cutoff derived from the little data available in the literature—a broad landmark rather than a definite milestone.
In children older than 3 years, the presence or absence of language function is the next checkpoint. In a preverbal child, there are 2 options: either the child remains nonspeaking or it develops language after surgery—maybe facilitated by the cessation of seizures. This assumption is based on the hierarchical regression results, where presurgical language function dominated all other predictors (age at surgery, age at seizure onset, and pathology) with respect to postsurgical language outcome. Children with impaired presurgical language function had a good chance to improve or remain stable, whereas children with good language function had a risk of deterioration.
The role of the underlying brain lesion becomes relevant when language mapping is not possible or inconclusive.
The role of the underlying brain lesion becomes relevant when language mapping is not possible or inconclusive. In brain malformations and developmental tumors, language is usually represented in the left hemisphere.24,47 Pre- or perinatally acquired infarctions have a high probability of right-hemispheric language representation; however, 40% of these patients still have left-hemispheric contribution. Thus, in speaking patients older than 3 years, without language lateralization data, left-sided surgery can always produce a language loss.
For these individual considerations, literature on patients who underwent left-sided hemidecortication is informative. It illustrates that language outcome depends on a complex interplay of type and location of underlying pathology, presurgical language function, age at seizure onset, and age at surgery. If a brain is to be protected from destruction by a progressive disease such as Rasmussen encephalitis, the risk of aphasia is often accepted. The published cases demonstrate that even adolescents can recover considerably from postsurgical aphasia. Importantly, postsurgery seizure control determined, in part, linguistic development.27
Our study has several limitations. We used databases to retrieve the studies analyzed. The fact that we excluded most studies identified and that we retrieved a large part from other sources illustrates the lack of consent on definitions and diagnoses in the field of language representation, language reorganization, and early brain lesions or childhood epilepsy. Compared with the randomized controlled trials in common diseases, study quality is relatively low with respect to case representativeness or—if included at all—the selection of controls, but all studies reliably characterized the underlying disease. With respect to outcome variables, we had to work with broad categorization, based on standardized testing in some cases, but relying on clinical impression in others. By including case reports, we must acknowledge publication bias as a relevant danger: unexpected individual courses of disease are published more often than cases with a less spectacular outcome. However, the large proportion of case studies reflects the low prevalence of hemidecortication, and thus, a case report on this condition in a child is still worthwhile whatever the outcome. In addition, in all 3 analyses, we also included data from larger studies, and these data do match the case report data.
Acknowledgment
The authors thank Eva Franz for her help with article screen and quality check and Prof. Ilves and Dr. Elkana for their willingness to provide additional data.
Appendix. Authors

Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, Krageloh-Mann I. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology 2001;57:122–125. [DOI] [PubMed] [Google Scholar]
- 2.Tillema JM, Byars AW, Jacola LM, et al. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang 2008;105:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzzetta A, Pecini C, Biagi L, et al. Language organisation in left perinatal stroke. Neuropediatrics 2008;39:157–163. [DOI] [PubMed] [Google Scholar]
- 4.Ilves P, Tomberg T, Kepler J, et al. Different plasticity patterns of language function in children with perinatal and childhood stroke. J Child Neurol 2014;29:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szaflarski JP, Allendorfer JB, Byars AW, et al. Age at stroke determines post-stroke language lateralization. Restor Neurol Neurosci 2014;32:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lidzba K, de Haan B, Wilke M, Krageloh-Mann I, Staudt M. Lesion characteristics driving right-hemispheric language reorganization in congenital left-hemispheric brain damage. Brain Lang 2017;173:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann NY Acad Sci 1977;299:355–369. [DOI] [PubMed] [Google Scholar]
- 8.Vargha-Khadem F, O'Gorman AM, Watters GV. Aphasia and handedness in relation to hemispheric side, age at injury and severity of cerebral lesion during childhood. Brain 1985;108:677–696. [DOI] [PubMed] [Google Scholar]
- 9.Curtiss S, de Bode S, Mathern GW. Spoken language outcomes after hemispherectomy: factoring in etiology. Brain Lang 2001;79:379–396. [DOI] [PubMed] [Google Scholar]
- 10.Vargha-Khadem F, Polkey CE. A review of cognitive outcome after hemidecortication in humans. Adv Exp Med Biol 1992;325:137–151. [DOI] [PubMed] [Google Scholar]
- 11.Vargha-Khadem F, Carr LJ, Isaacs E, Brett E, Adams C, Mishkin M. Onset of speech after left hemispherectomy in a nine-year-old boy. Brain 1997;120:159–182. [DOI] [PubMed] [Google Scholar]
- 12.Sabbah P, Chassoux F, Leveque C, et al. Functional MR imaging in assessment of language dominance in epileptic patients. Neuroimage 2003;18:460–467. [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [online]. Available at: ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 10, 2019.
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 15.Ego A, Lidzba K, Brovedani P, et al. Visual-perceptual impairment in children with cerebral palsy: a systematic review. Dev Med Child Neurol 2015;57(suppl 2):46–51. [DOI] [PubMed] [Google Scholar]
- 16.Sterne JAC, Egger M, Moher D. Chapter 10: addressing reporting biases. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Intervention. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 17.Elkana O, Frost R, Kramer U, et al. Cerebral reorganization as a function of linguistic recovery in children: an fMRI study. Cortex 2011;47:202–216. [DOI] [PubMed] [Google Scholar]
- 18.Westmacott R, Askalan R, Macgregor D, Anderson P, Deveber G. Cognitive outcome following unilateral arterial ischaemic stroke in childhood: effects of age at stroke and lesion location. Dev Med Child Neurol 2010;52:386–393. [DOI] [PubMed] [Google Scholar]
- 19.Elkana O, Frost R, Kramer U, Ben-Bashat D, Schweiger A. Cerebral language reorganization in the chronic stage of recovery: a longitudinal fMRI study. Cortex 2013;49:71–81. [DOI] [PubMed] [Google Scholar]
- 20.Lauterbach M, da Costa RG, Leal G, Willmes K, Martins IP. Recovering from acquired childhood aphasia (ACA): 20 years later, learning about the neuroplasticity of language. Behav Neurol 2010;23:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everts R, Lidzba K, Wilke M, et al. Lateralization of cognitive functions after stroke in childhood. Brain Inj 2010;24:859–870. [DOI] [PubMed] [Google Scholar]
- 22.Lidzba K, Kupper H, Kluger G, Staudt M. The time window for successful right-hemispheric language reorganization in children. Eur J Paediatr Neurol 2017;21:715–721. [DOI] [PubMed] [Google Scholar]
- 23.Tierney MC, Varga M, Hosey L, Grafman J, Braun A. PET evaluation of bilingual language compensation following early childhood brain damage. Neuropsychologia 2001;39:114–121. [DOI] [PubMed] [Google Scholar]
- 24.Anderson DP, Harvey AS, Saling MM, et al. FMRI lateralization of expressive language in children with cerebral lesions. Epilepsia 2006;47:998–1008. [DOI] [PubMed] [Google Scholar]
- 25.Gröppel G, Dorfer C, Mühlebner-Fahrngruber A, et al. Improvement of language development after successful hemispherotomy. Seizure 2015;30:70–75. [DOI] [PubMed] [Google Scholar]
- 26.Pulsifer MB, Brandt J, Salorio CF, Vining EP, Carson BS, Freeman JM. The cognitive outcome of hemispherectomy in 71 children. Epilepsia 2004;45:243–254. [DOI] [PubMed] [Google Scholar]
- 27.de Bode S, Smets L, Mathern GW, Dubinsky S. Complex syntax in the isolated right hemisphere: receptive grammatical abilities after cerebral hemispherectomy. Epilepsy Behav 2015;51:33–39. [DOI] [PubMed] [Google Scholar]
- 28.Lettori D, Battaglia D, Sacco A, et al. Early hemispherectomy in catastrophic epilepsy: a neuro-cognitive and epileptic long-term follow-up. Seizure 2008;17:49–63. [DOI] [PubMed] [Google Scholar]
- 29.Liégeois F, Cross JH, Polkey C, Harkness W, Vargha-Khadem F. Language after hemispherectomy in childhood: contributions from memory and intelligence. Neuropsychologia 2008;46:3101–3107. [DOI] [PubMed] [Google Scholar]
- 30.Iwatani Y, Kagitani-Shimono K, Tominaga K, et al. Long-term developmental outcome in patients with West syndrome after epilepsy surgery. Brain Dev 2012;34:731–738. [DOI] [PubMed] [Google Scholar]
- 31.Schropp C, Sörensen N, Krauss J. Early periinsular hemispherotomy in children with Sturge-Weber syndrome and intractable epilepsy-outcome in eight patients. Neuropediatrics 2006;37:26–31. [DOI] [PubMed] [Google Scholar]
- 32.Mariotti P, Iuvone L, Torrioli MG, Silveri MC. Linguistic and non-linguistic abilities in a patient with early left hemispherectomy. Neuropsychologia 1998;36:1303–1312. [DOI] [PubMed] [Google Scholar]
- 33.Bittar RG, Rosenfeld JV, Klug GL, Hopkins IJ, Harvey AS. Resective surgery in infants and young children with intractable epilepsy. J Clin Neurosci 2002;9:142–146. [DOI] [PubMed] [Google Scholar]
- 34.Stark RE, Bleile K, Brandt J, Freeman J, Vining EP. Speech-language outcomes of hemispherectomy in children and young adults. Brain Lang 1995;51:406–421. [DOI] [PubMed] [Google Scholar]
- 35.Hertz-Pannier L, Chiron C, Jambaqué I, et al. Late plasticity for language in a child's non-dominant hemisphere. Brain 2002;125:361–372. [DOI] [PubMed] [Google Scholar]
- 36.Boatman D, Freeman J, Vining E, et al. Language recovery after left hemispherectomy in children with late-onset seizures. Ann Neurol 1999;46:579–586. [DOI] [PubMed] [Google Scholar]
- 37.Bulteau C, Grosmaitre C, Save-Pedebos J, et al. Language recovery after left hemispherotomy for Rasmussen encephalitis. Epilepsy Behav 2015;53:51–57. [DOI] [PubMed] [Google Scholar]
- 38.Trudeau N, Colozzo P, Sylvestre V, Ska B. Language following functional left hemispherectomy in a bilingual teenager. Brain Cogn 2003;53:384–388. [DOI] [PubMed] [Google Scholar]
- 39.Telfeian AE, Berqvist C, Danielak C, Simon SL, Duhaime AC. Recovery of language after left hemispherectomy in a sixteen-year-old girl with late-onset seizures. Pediatr Neurosurg 2002;37:19–21. [DOI] [PubMed] [Google Scholar]
- 40.Loddenkemper T, Wyllie E, Lardizabal D, Stanford LD, Bingaman W. Late language transfer in patients with Rasmussen encephalitis. Epilepsia 2003;44:870–871. [DOI] [PubMed] [Google Scholar]
- 41.Raja Beharelle A, Dick AS, Josse G, et al. Left hemisphere regions are critical for language in the face of early left focal brain injury. Brain 2010;133:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fair DA, Brown TT, Petersen SE, Schlaggar BL. fMRI reveals novel functional neuroanatomy in a child with perinatal stroke. Neurology 2006;67:2246–2249. [DOI] [PubMed] [Google Scholar]
- 43.Pataraia E, Simos PG, Castillo EM, et al. Reorganization of language-specific cortex in patients with lesions or mesial temporal epilepsy. Neurology 2004;63:1825–1832. [DOI] [PubMed] [Google Scholar]
- 44.Hadac J, Brozova K, Tintera J, Krsek P. Language lateralization in children with pre- and postnatal epileptogenic lesions of the left hemisphere: an fMRI study. Epileptic Disord 2007;9(suppl 1):S19–S27. [DOI] [PubMed] [Google Scholar]
- 45.Benson RR, Logan WJ, Cosgrove GR, et al. Functional MRI localization of language in a 9-year-old child. Can J Neurol Sci 1996;23:213–219. [DOI] [PubMed] [Google Scholar]
- 46.Liegeois F, Connelly A, Cross JH, et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain 2004;127:1229–1236. [DOI] [PubMed] [Google Scholar]
- 47.Vitali P, Minati L, D'Incerti L, et al. Functional MRI in malformations of cortical development: activation of dysplastic tissue and functional reorganization. J Neuroimaging 2008;18:296–305. [DOI] [PubMed] [Google Scholar]
- 48.Everts R, Harvey AS, Lillywhite L, et al. Language lateralization correlates with verbal memory performance in children with focal epilepsy. Epilepsia 2010;51:627–638. [DOI] [PubMed] [Google Scholar]
- 49.Ramantani G, Kadish NE, Brandt A, et al. Seizure control and developmental trajectories after hemispherotomy for refractory epilepsy in childhood and adolescence. Epilepsia 2013;54:1046–1055. [DOI] [PubMed] [Google Scholar]
- 50.Kadish NE, Bast T, Reuner G, et al. Epilepsy surgery in the first 3 years of life: predictors of seizure freedom and cognitive development. Neurosurgery 2019;84:E368–E377. [DOI] [PubMed] [Google Scholar]