Abstract
Aging physicians are at a higher risk of cognitive impairment, undermining patient safety and unraveling physicians' careers. Neurologists, occupational health physicians, and psychiatrists will participate in both health system policy decisions and individual patient evaluations. We address cognitive impairment in aging physicians and attendant risks and benefits. If significant cognitive impairment is found after an appropriate evaluation, precautions to confidentially support physicians' practicing safely for as long as possible should be instituted. Understanding that there is heterogeneity and variability in the course of cognitive disorders is crucial to supporting cognitively impaired, practicing physicians. Physicians who are no longer able to practice clinically have other meaningful options.
As more physicians worldwide work into later years,1,2 driven by changing economies, retirement practices, and shifting attitudes regarding aging, there has been a tandem rise in those practicing with cognitive impairment, potentially placing both the health of patients and the careers of such physicians at risk3,4 (table 1). Safeguards to protect patients and physicians will allow more of us to practice later in life, enriching patient care with wisdom borne of experience.5,6
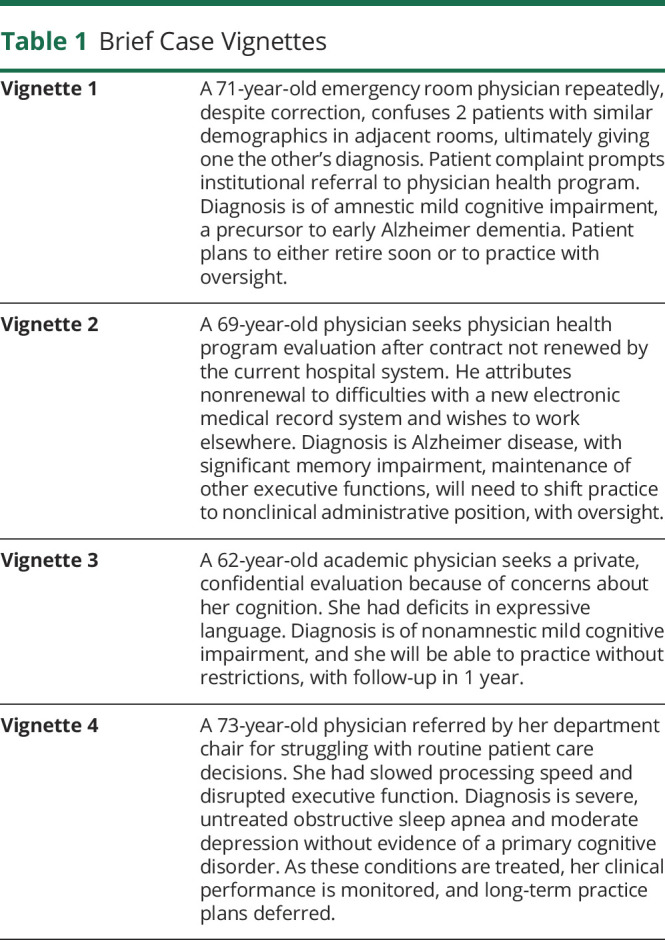
Table 1.
Brief Case Vignettes
Age remains the single biggest risk factor for cognitive impairment in physicians.1,3,7–9 Specific data on the numbers of cognitively impaired physicians are limited. However, extrapolating from population data, the estimated prevalence of mild cognitive impairment (20,400; 21%) and dementia (6,460; 7%) may be as high as 28% in physicians aged 70 years and older.3 This would represent 27,000 of the 95,000 American physicians in this age group with active licenses, although the number in active practice is unknown.3
The actual numbers may be lower, if impaired physicians stop practicing earlier than unimpaired peers, or if physicians' greater cognitive reserve ameliorates the effects of age-associated neuropathologic decline.3 Conversely, if impairment is defined as deterioration in high-level clinical decision-making, or a substantial decline from a superior baseline, despite normal performance on cognitive testing, the actual numbers may be higher.
An early study of computerized testing of 356 American physicians aged 65 years and older found 9% with cognitive impairment on global scores, and 46% with deficits in more than one cognitive domain, with strong scores in any 1 domain mitigating weak scores in other domains.10 A recent report of recredentialing-mandated cognitive testing of clinicians aged 70 years and older in 1 hospital system found that 13% had significant cognitive deficits likely to impair their ability to practice medicine independently.9
Responding to the increasing number of practicing older physicians, the American Medical Association (AMA) Council on Medical Education suggested in 2015 that “perhaps episodic reevaluations (of physicians) after a certain age such as 70, when incidence of declines is known to increase, may be appropriate…(and) should include… neurocognitive testing.11” In 2019, over two-thirds (67%) of the American Society of Surgical Chairs advocated mandatory cognitive and psychomotor testing of surgeons by at least 65 years.12 The American College of Surgeons has recommended age-based evaluations, starting at age 65–70 years, suggesting surgeons “voluntarily assess their neurocognitive function using online tools,” and self-report findings.13
The aim of any such screening of physicians, from an occupational health and safety viewpoint, is to identify cognitive impairment, a condition that increases the risk of poor professional performance and likelihood of patient harm.14 Impaired cognitive status is a factor in the assessment of fitness to work, which reflects a worker's ability to perform, while minimizing the risk to self or others. Since the passage of the Health Care Quality Improvement Act in 1986, health care providers have become among the most regulated workers in America15 and such oversight will likely continue to increase. The impetus for physician groups to develop self-monitoring guidelines is, as the AMA noted, to “head off a call for mandatory retirement ages, as pilots experience, or imposition of guidelines by others.11”
Because of brain function or occupational health expertise, neurologists, psychiatrists, and occupational health physicians will likely participate in both policy-making processes as health care systems grapple with an aging physician population and the assessment of cognitive capacity in physicians at risk.
Although many aging physicians perform as well as their younger counterparts on cognitive tasks,16,17 aging physicians are more likely to be referred for concerns about competence.7,18 Although physicians of any age referred for competency concerns are more often found to be cognitively impaired than their peers,19,20 aging physicians tend to be more severely compromised.19 Of 683 American physicians (mean 53 years, range 32–84 years) referred for competency assessment, primarily by their state licensing board, 86 were found unfit to practice, associated with older age and solo practice (where fewer interactions with colleagues may limit recognition of impairment).18 Another estimate found 6%–12% of physicians to be dyscompetent, with age-related cognitive impairment being one cause.21 Nearly three-quarters (73%) of unsolicited patient complaints about physicians featured words associated with cognitive loss, correlating with objective evidence of possible cognitive impairment in those physicians.22 The number of malpractice claims against a physician, one theoretical metric for evaluating fitness to practice, is poorly correlated with age and is instead associated with physician subspecialty, male sex, and previous malpractice history.23
Many countries, including China, Finland, India, Ireland, and Japan, require surgeons to retire by ages 60–68 years because of age-based performance concerns. However, Britain's National Health Service, acknowledging individual variations in age-related performance, eliminated this rule, with similar lifting of requirements across Europe, further driven by physician shortages. Australia, Canada, and the United States do not enforce age-based retirements for physicians.
Because performance of pilots14 and judges affects public safety, policies regarding these professions could be applicable to medicine. Pilots for major American airlines undergo periodic health screening and must retire after age 65 years—previously age 60 years. Most high court judges worldwide have mandated retirement in their 60s to 70s, although American federal judges do not. Judges in most American states must retire by age 70–75 years, with limited tenure extension in some states on passing periodic brief cognitive evaluations.
Approaches to Cognitive Impairment in Physicians
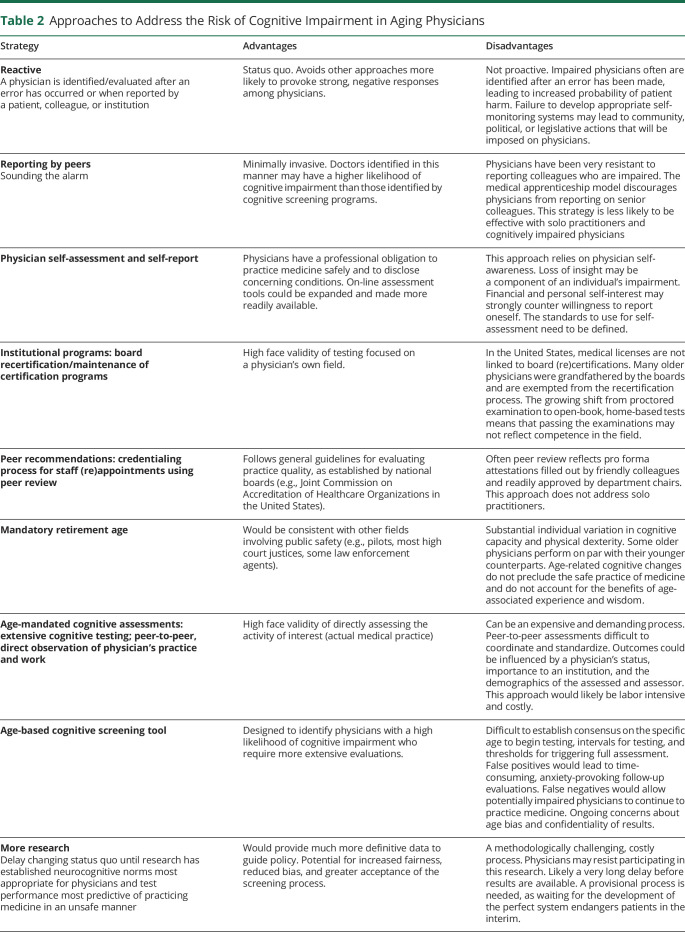
Table 2 delineates the various approaches—extant and proposed, addressing cognitive impairment in aging physicians, along with attendant advantages and limitations.
Table 2.
Approaches to Address the Risk of Cognitive Impairment in Aging Physicians
Reactive Assessment
The current norm in medicine for identifying physicians with cognitive impairment is the reactive assessment.4 Diverse concerns lead to a fitness to work evaluation,15 whereby a physician is evaluated only after an error has occurred or when reported by a patient, colleague, or institution.7,22,24 Unfortunately, this reactive approach leaves many potentially impaired physicians unrecognized,25,26 and is often punitive, driving those with impaired competence underground.4
Sounding the Alarm
Sounding the alarm in a timely manner is one route to earlier identification of impaired colleagues,24 but physicians are loath to report colleagues. Over a third (36%) of physicians in a large American survey did not completely agree that there is a professional responsibility to report impaired colleagues, whereas another third felt very unprepared for dealing with such colleagues.27 The hierarchical medical apprenticeship model and fear of retribution are factors discouraging physicians from reporting senior colleagues.27–29 Physicians are also more reluctant to report cognitive impairment than substance abuse in their peers.30
One way to overcome this challenge is by raising consciousness through educating physicians about the professional duty to report potentially impaired peers, the consequences of keeping silent, and the procedures in place for ensuring fair and supportive assessments.
Physician Self-Assessment and Self-Report
Given every physician's professional obligation to report conditions compromising ability to practice safely, self-reporting seems ideal. Unfortunately, conditions that undermine cognition may erode insight. Moreover, many physicians are poor at self-care, have no primary care provider, and, when they do, often do not fully disclose their problems.14,21,31 When physicians do become aware of a compromising condition, they are resistant to self-report for fear of professional, societal, and legal sanctions.14,32
Institutional Programs
Globally, to measure continued professional competence, prevailing, generally unpopular methods are in place, ranging from the revalidation system in Canada and the United Kingdom, a version of 360-degree assessments of the corporate world, to board recertification and maintenance of certification in the United States.33 American physicians may practice without board certification. Older physicians were grandfathered by their boards, exempting them from recertification, although as these physicians retire, this number will dwindle. Some specialty boards grant recertification with clinical medical education requirements alone. Moreover, with the ongoing shift from proctored examinations to open-book, home-based tests, external assistance is possible.
Peer Recommendations
American medical institutions require periodic peer recommendations for affiliated physicians on reappointment, but these are often pro forma attestations by friendly colleagues and routinely approved by department chairs.
Mandatory Retirement Age
Mandatory retirement age would minimize the likelihood of aging physicians continuing to practice medicine despite having dementia or other age-related conditions that compromise clinical care. However, this is an arbitrary approach, solely age and not performance based. It would ignore the wide variability in capacity among older adults and indiscriminately force capable individuals to stop working.
Age-Mandated Cognitive Assessments
Several American health systems have begun instituting age-based cognitive testing.34,35 Such screening ranges from simple ones, such as the Mini-Mental State Examination and the Montreal Cognitive Assessment, which may misclassify patients and are less sensitive for highly educated patients,36,37 to extensive hours-long neurocognitive testing.1 For example, Nebraska's Children's Hospital requires physicians aged 70 years and older to undergo an assessment by several peers, a complete physical, and unspecified cognitive screening, and every 2 years thereafter, with results reported to institutional authorities, Yale New Haven Hospital requires cognitive screening, whereas Cooper University Health Care requires extensive neurocognitive testing. Although these approaches provide detailed information about physicians' performance,38 complete neurocognitive examinations are labor intensive and costly, and direct peer assessments of clinical care are difficult to standardize—influenced by physician status,14 importance to the institution, and the demographics of the assessed and assessor.27 One option to address these issues would be a standardized cognitive screening tool.
Age-based Cognitive Screening Tool
What constitutes adequate cognitive screening for highly educated groups, such as physicians, who generally perform 1 to 2 SDs above the population mean, has been challenging to determine and difficult to implement.21,39 Even thorough neurocognitive evaluations can miss impairment in such individuals or remain normal early in the course of conditions, such as frontotemporal dementia. A cognitive screen must strike a balance between complexity and ease of use. We propose a simplified, multidomain approach that would likely augment sensitivity by not simply depending on a total score.10
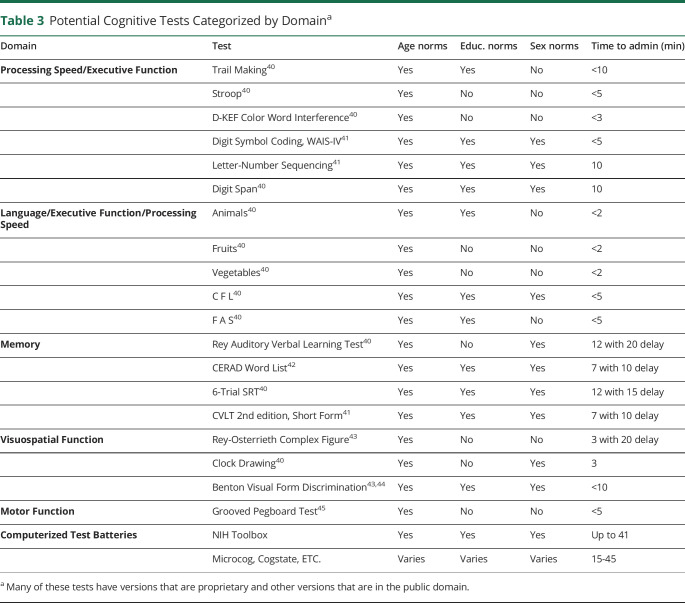
A brief neurocognitive screening protocol should aim to identify physicians with levels of impairment likely to increase the risk of serious harm to their patients. We are not recommending a specific test battery. Rather, we highlight the critical features of cognitive screening including (1) assessment of critical cognitive domains (executive functioning/processing speed,40,41 memory,40–42 semantic access/fluency,40 visuospatial functioning,43,44 and motor dexterity45), (2) utilization of tests with established norms (allowing identification of very poorly performing physicians), and (3) feasibility of completing the evaluation in a relatively short period. Table 3 lists potential tests—including some computerized batteries, specifying available age, education, sex-based norms, and approximate time for administration. Health systems may choose tests predicated on accessibility, cost, duration, and suitability of normative data. We suggest selecting 1 test for each cognitive domain, which would result in screening evaluations between 35 and 50 minutes.
Table 3.
Potential Cognitive Tests Categorized by Domaina
We recommend screening at age 65 years, although earlier screening at age 60 years, or later at age 70 years, may be more palatable to some health systems.3,12,28 Scoring at ≥2 SD below mean for age on any test would trigger a more extensive evaluation. For example, on the 12-word Selective Reminding Test of memory, a 2 SD cutoff signifies that a 70-year-old recalls no more than 3 words after 15 minutes, despite 6 learning trials. In the realm of category/semantic fluency, a 2 SD cutoff would signify that a 70-year-old physician was not able to generate more than 9 animal names in 1 minute. Setting the threshold for further evaluation at 2 SD below the mean for age is a low bar, likely to identify only the most cognitively impaired physicians. We suggest rescreening physicians who score within 1 SD of mean on tests in 5 years. Physicians who score between 1 and 2 SD below mean would require more frequent screening, perhaps every 2 years.
Our goal is to develop a systematic and relatively accessible way to identify seriously impaired practicing physicians. Health systems may opt for more sensitive and less specific cutoffs of 1 SD, apply norms for 60-year-olds to all individuals, creating a less relativistic standard, or maintain a longitudinal record of scores that could help identify a worrisome decline in cognitive performance over time rather than simply detecting scores below an arbitrary cutoff. We emphasize that failing cognitive screening would start, not end, the evaluation process.
The Problem With Age-Based Cognitive Testing
Age-based mandatory cognitive testing has met with understandable physician-led resistance, with the Stanford medical faculty successfully protesting such requirements. Reflecting this mindset, the American Medical Association recanted its suggestion for testing physicians aged 70 years and older,11 stating instead that “the effect of age on any individual physician's competence can be highly variable. While age is one factor in predicting potential competence, other factors such as practice setting, clinical volume, specialty, and stress also can contribute” (AMA, personal communication, March 23, 2018).
This opposition springs from important limitations associated with testing. Screening tests are imperfect tools that can have untoward consequences. False-positive results would require competent physicians to undergo time-consuming, anxiety-provoking evaluations, and false-negative results may lead to erroneous assumptions about clinical competence. In addition, performance on more extensive cognitive testing does not necessarily reflect clinical competence. Good performance does not guarantee acceptable functioning as a physician nor does poor performance make for an unsafe physician. Experience may be as or more relevant than test scores. Other factors, including age-related declines in physical dexterity, are specialty specific, affecting surgeons more than psychiatrists. Finally, age-based screening may be subject to antidiscrimination laws, although increasing numbers of American health systems are opting for mandated testing of aging physicians.
Proactive rather than ad hoc assessments of physicians' fitness to practice, emphasizing the health and well-being of physicians, are crucial14 for physician buy-in. Separate from mandated requirements, some physicians may volitionally seek a confidential cognitive evaluation, for reasons including personal integrity, confidence in one's competence, and minimizing malpractice risk.
Protocol for Follow-Up—If Potential Impairment Identified
Identification of potentially compromised physicians begins the evaluation process. Neutral third parties, possibly appointed by state medical boards, could orchestrate evaluations and maintain confidentiality, avoiding potential conflict of interests from institutions assessing physician employees. Alternatively, occupational health programs within hospitals could require evaluations, with physicians screened by designated providers. In either scenario, designated cognitive specialists would see those needing further evaluation or reported to an overseeing board—such as a committee within the local medical society that makes recommendations to the physician and affiliated institutions. Such committees, physician health programs or their equivalent, exist in 47 American states and numerous countries, including Australia, Canada, New Zealand, and the United Kingdom.25
Crucial to successfully implementing strategies for handling impaired physicians is assuring that health systems approach identified individuals supportively, rather than punitively, and that such colleagues be assisted in their desire to continue practicing for as long as is safely possible.2
The specialist would perform a comprehensive evaluation, including medical, functional, neurocognitive, psychiatric, and socioeconomic assessments, with confidential interviews of colleagues or family—if needed, to determine the physician's ability to continue working, and under what circumstances. Recognizing and addressing medical, psychiatric, and other factors, including substance abuse, affecting cognition is important. Additional tools, if questions remain, include residency training type simulation programs, observing the physician in clinical interactions, chart review, recommendation to take the specialty board examination, or referral for an intensive specialty-specific assessment at a specialized program.4,19,25
Ideally, the consultant should be unaffiliated with both the physician and the employing institution. The physician or the employer bears the cost of evaluations. In the event of a disagreement with recommendations, physicians should have the option of a second opinion. Confidentiality is key, as with any health problem involving a private individual. Should an overseeing authority be unavailable, the consultant should release only recommendations, not the detailed evaluation, to institutions. Storing medical records with physician health programs or another overseeing authority or with the unaffiliated consultant prevents employers from accessing evaluations.
Although initially established for substance abuse, American physician health programs handle behavioral health issues, including dementia. Based on numbers of impaired physicians, these programs may need more resources. Most American state laws provide that physicians' records in such programs are nondiscoverable, provided that participating physicians comply with program recommendations. Waiving confidentiality requirements may backfire—California's physician health program closed after patient advocates successfully lobbied to publicize addiction records.3
Oversight or monitoring of the physician's practice or provisions for a more restricted type of practice may be necessary. The protocol for evaluation and recommendations should resemble that used for other chronic, disabling illnesses such as addiction, stroke, or mental illness, allowing for ongoing monitoring, and focusing on therapy and integration, rather than dismissal.3,14,46 There is tremendous stigma associated with cognitive disorders and a lack of understanding of the heterogeneity and individual variability inherent to these conditions.3,46,47 Although the fear of penalty and stigma with loss of livelihood is real, there is precedent for rehabilitation and return of cognitively impaired physicians to work, with oversight in place, if needed.3,19
For physicians found unable to maintain primary clinical responsibilities, options for continued meaningful engagement based on preserved skills are essential. Activities include teaching trainees the art of history taking and physical examination, career mentoring of younger colleagues, administrative duties, patient or disease advocacy, and leadership of medical groups. Finally, although we focused on physicians, similar principles apply to other patient care providers.
There are many legitimate concerns about proactively assessing for cognitive impairment in aging physicians. As with many taboo subjects, research on this topic has been sparse. Investing in studies, including establishing physician-specific neurocognitive norms, and test performances most predictive of impaired medical practice, is important. However, waiting for the development of the perfect system can have negative consequences for our patients' health and our profession's integrity. Creating systems for the early identification of colleagues with impairment substantial enough to pose unacceptable hazards to health care delivery should have widespread consensus, guided by the Hippocratic principle of primum non nocere. Moreover, failure to develop appropriate self-monitoring systems may result in less nuanced and more disruptive community, political, or legislative actions.3
Among available options, a relatively brief cognitive screening followed by more extensive testing for the most impaired individuals appears most reliable in confidentially identifying truly impaired physicians while minimizing the chance of a falsely flagging unimpaired individuals. This strategy allows aging physicians to continue working while safeguarding both their reputations and their patients' health.7,39 Honoring their seniority, wisdom, and contributions, impaired physicians should be supported in practice settings with proper oversight, or transitioned to other, non–patient care positions, or retirement.
Appendix. Authors

Footnotes
Editorial, page 89
Study Funding
No targeted funding reported.
Disclosure
G. Devi serves on the medical advisory board of 98point6.com. D. Gitelman reports personal fees from Biogen, Global Alzheimer's Platform, Roche, UsAgainstAlzheimer's, Merck, Novartis, AbbVie, Shire, Suven, Eisai, (spouse) Abbott, (spouse) AbbVie, (spouse) Bristol-Myers Squibb, (spouse) Pfizer, and (spouse) UCB Biosciences. D. Press and K.R. Daffner report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Bhatt N, Morris M, O'Neil A, Gillis A, Ridgway P. When should surgeons retire? BJS 2016;103:35–43. [DOI] [PubMed] [Google Scholar]
- 2.California Public Protection and Physician Health, Inc. Assessing Late-Career Practitioners: Policies and Procedures for Age-Based Screening-A Guideline From: California Public Protection and Physician Health. San Francisco, CA: CPPPH; 2015. [Google Scholar]
- 3.Devi G. Alzheimer's disease in physicians: assessing competence and tempering stigma. N Engl J Med 2018;378:1073–1075. [DOI] [PubMed] [Google Scholar]
- 4.Norcross W, Henzel T, Freeman K, Milner-Mares J, Hawkins R. Toward meeting the challenge of physician competence assessment: the University of California, San Diego physician assessment and clinical education (PACE) program. Acad Med 2009;84:1008–1014. [DOI] [PubMed] [Google Scholar]
- 5.Eva K. The aging physician: changes in cognitive processing and thier impact on medical practice. Acad Med 2002;77:S1–S6. [DOI] [PubMed] [Google Scholar]
- 6.Blasier R. The problem of the aging surgeon: when surgeon age becomes a surgical risk factor. Clin Orthop Relat Res 2009;467:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beekman A. Aging affects us all: aging physicians and screening for impaired professional proficiency. Am J Geriatr Psychiatry 2018;26:641–642. [DOI] [PubMed] [Google Scholar]
- 8.Peisah C, Wilhelm K. Physician don't heal thyself: a descriptive study of impaired older doctors. Int Psychogeriatr 2007;19:974–984. [DOI] [PubMed] [Google Scholar]
- 9.Cooney L, Balcezak T. Cognitive testing of older clinicians prior to recredentialing. JAMA 2020;323:179–180. [DOI] [PubMed] [Google Scholar]
- 10.Powell D, Whitla D. Normal cognitive aging: toward empirical perspectives. Curr Dir Psychol Sci 1994;3:27–31. [Google Scholar]
- 11.AMA Council on Medical Education (A-15), Competency and the Aging Physician: Appropriateness of Guidelines for Testing for and Judgment of a Physician's Competence to Care for Patients. Chicago, IL: American Medical Association; 2015. [Google Scholar]
- 12.Rosengart T, Doherty G, Higgins R, Kibbe M, Mosenthal A. Transition planning for the senior surgeon. Guidance and recommendation from the society of surgical chairs. JAMA Surg 2019;1159:E1–E7. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger E, Pellegrini C, Gallagher T. The aging physician and the medical profession-A review. JAMA Surg 2017;152:967–971. [DOI] [PubMed] [Google Scholar]
- 14.Harrison J. Doctors' health and fitness to practise: assessment. Occup Med 2008;58:318–322. [DOI] [PubMed] [Google Scholar]
- 15.Meyer DJ, Price M. Peer review and psychiatric physician fitness for duty evaluations: analyzing the past and forecasting the future. Int J L Psychiatry 2012;445-451:35. [DOI] [PubMed] [Google Scholar]
- 16.Drag L, Bieliauskas L, Langenecker S, Greenfield L. Cognitive functioning, retirement status, and age: results from the cognitive changes and retirement among senior surgeons study. J Am Coll Surg 2010;211:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trunkey D, Botney R. Assessing competency: a tale of two professions. J Am Coll Surg 2001;192:385–395. [DOI] [PubMed] [Google Scholar]
- 18.Grace E, Wenghofer E, Korinek E. Predictors of physician performance on competence assessment: findings from CPEP, the center for personalized education for physicians. Acad Med 2014;89:912–919. [DOI] [PubMed] [Google Scholar]
- 19.Turnbull J, Cunnington J, Unsal A, Norman G, Ferguson B. Competence and cognitive difficulty in physicians: a follow-up study. Acad Med 2006;81:915–918. [DOI] [PubMed] [Google Scholar]
- 20.Korinek L, Thompson L, McRae C, Korinek E. Do physicians referred for competency evaluations have underlying cognitive problems? Acad Med 2009;84:1015–1021. [DOI] [PubMed] [Google Scholar]
- 21.Williams BW, Flanders P, Grace ES, Korinek E, Welindt D, Williams MV. Assessment of fitness for duty of underperforming physicians: the importance of using appropriate norms. PLoS One 2017;12:e0186902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper W, Martinez W, Domenico H, et al. Unsolicited patient complaints identify physicians with evidence of neurocognitive disorders. Am J Geriatr Psychiatry 2018;26:927–936. [DOI] [PubMed] [Google Scholar]
- 23.Taragin MI, Wilczek AP, Karns EM, et al. Physician demographics and the risk of medical malpractice. Amer J Med 1992;93:537–542. [DOI] [PubMed] [Google Scholar]
- 24.Soonsawat A, Tanaka G, Lammando M, Ahmed I, Ellison J. Cognitively impaired physicians: how do we detect them? How do we assist them? Am J Geriatr Psychiatry 2018;26:631–640. [DOI] [PubMed] [Google Scholar]
- 25.St. George I, Kaigas T, McAvoy P. Assessing the competence of practicing physicians in New Zealand, Canada, and the United Kingdom: progress and problems. Fam Med 2004;36:172–177. [PubMed] [Google Scholar]
- 26.Norman G, Davis D, Lamb S, Hanna E, Caulford P, Kaigas T. Competency assessment of primary care physician as part of a peer reivew program. JAMA 1993;270:1046–1051. [PubMed] [Google Scholar]
- 27.DesRoches C, Rao S, Fromson J, et al. Physicians' perceptions, preparedness for reporting, and experiences related to impaired and incompetent colleagues. JAMA 2010;304:187–193. [DOI] [PubMed] [Google Scholar]
- 28.Fortunator J, Menkes D. The aging physician: a practical approach to protect our patients. Ethics 2019;14:46–49. [Google Scholar]
- 29.Reuben D, Noble S. House officer responses to impaired physicians. JAMA 1990;263:958–960. [PubMed] [Google Scholar]
- 30.Farber N, Gilbert S, Abolf B, Collier V, Weiner J, Boyer E. Physicians' willingness to report impaired colleagues. Soc Sci Med 2005;61:1772–1775. [DOI] [PubMed] [Google Scholar]
- 31.Pullen D, Lonie C, Lyle D, Cam D, Doughty M. Medical care of doctors. Med J Aust 1995;162:481–484. [DOI] [PubMed] [Google Scholar]
- 32.Boisaubin E, Levine E. Identifying and assisting the impaired physician. Am J Med Sci 2001;322:31–36. [DOI] [PubMed] [Google Scholar]
- 33.McCartney M. Views and Reviews: we must look at the whole impact of revalidation. BMJ 2018;361:k2323. [DOI] [PubMed] [Google Scholar]
- 34.Moutier C, Bazzo D, Norcross W. Approaching the issue of the aging physician population. J Med Regul 2013;99:10–18. [Google Scholar]
- 35.Heymann W. Assessing the competence of aging physicians who are young at heart. JAMA Dermatol 2018;154:875–876. [DOI] [PubMed] [Google Scholar]
- 36.Ranson J, Kuzma E, Hamilton W, et al. Predictors of dementia misclassification when using brief cognitive screens. Neurology 2019;9:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagnon G, Hansen K, Woolmore-Goodwin S, et al. Correcting the MoCA for education: effect on sensitivity. Can J Neurol Sci 2013;40:678–683. [DOI] [PubMed] [Google Scholar]
- 38.Katlic M, Coleman J. The aging surgeon. Adv Surg 2016;50:93–103. [DOI] [PubMed] [Google Scholar]
- 39.LoboPrabhu S, Molinari V, Hamilton J, Lomax J. The aging physician with cognitive impairment: approaches to oversight, prevention, and remediation. Am J Geriatr Psychiatry 2009;17:445–454. [DOI] [PubMed] [Google Scholar]
- 40.Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 3rd ed. New York: Oxford University Press.; 2006. [Google Scholar]
- 41.Available at: pearsonclinical.com [online]. Accessed March 4, 2020.
- 42.Fillenbaum G, Unverzagt F, Ganguli M, et al. The CERAD Neuropsychological Battery: performance of representative community and tertiary care samples of African American and European American elderly, In: Ferraro FR, editor. Minority and Cross-Cultural Aspects of Neuropsychological Assessment, Lisse: Swets and Zeitlinger; 2002:45–62. [Google Scholar]
- 43.Available at: parinc.com [online]. Accessed March 4, 2020.
- 44.Campo P, Morales M. Reliability and normative data for the Benton visual form discrimination test. Clin Neuropsychol 2003;17:220–225. [DOI] [PubMed] [Google Scholar]
- 45.Grooved Pegboard Test Manual [online]. Available at: www.advys.be/docs/GroovedPegboardTestManual.pdf. Accessed March 4, 2020. [Google Scholar]
- 46.Collier S. Invited perspective on “unsolicited patient complaints identify physicians with evidence of neurocognitive disorders.” Am J Geriatr Psychiatry 2018;26:937–938. [DOI] [PubMed] [Google Scholar]
- 47.Devi G, Scheltens P. Heterogeneity of Alzheimer's disease: consequence for drug trials? Alzheimers Res Ther 2018;10:122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]