PRACTICAL IMPLICATIONS
Patients presenting with symptoms compatible with COVID-19 and encephalitis should be tested for SARS-CoV-2 in addition to the usual pathogens associated with neurologic infection.
Although adults with coronavirus disease 2019 (COVID-19) who require admission to the hospital have often required critical care, infants and children with COVID-19 have generally demonstrated milder disease severity, fewer complications, and overall a much lower case fatality rate. Neurologic manifestations have been reported with COVID-19 in adult patients including acute cerebrovascular disease, ischemic and hemorrhagic strokes, skeletal muscle injury, and rare cases of encephalopathy. We report here an adolescent patient with acute encephalitis associated with COVID-19.
Case
A 16-year-old previously healthy boy presented to the emergency department (ED) at the Joseph M. Sanzari Children's Hospital at Hackensack University Medical Center with fever up to 101°F for 9 days, followed by generalized weakness and somnolence for 2 days. He had pharyngitis and intermittent headaches, which prompted evaluation at a local ED 4 days before admission. Rapid tests for influenza and streptococcal pharyngitis were negative. He was prescribed amoxicillin, and, given the ongoing coronavirus disease 2019 (COVID-19) pandemic, he was instructed to self-isolate at home. Two days later, he developed emesis, severe malaise, progressive somnolence with confusion, and incoherent speech. He was not able to ambulate without assistance, which prompted re-evaluation in our ED.
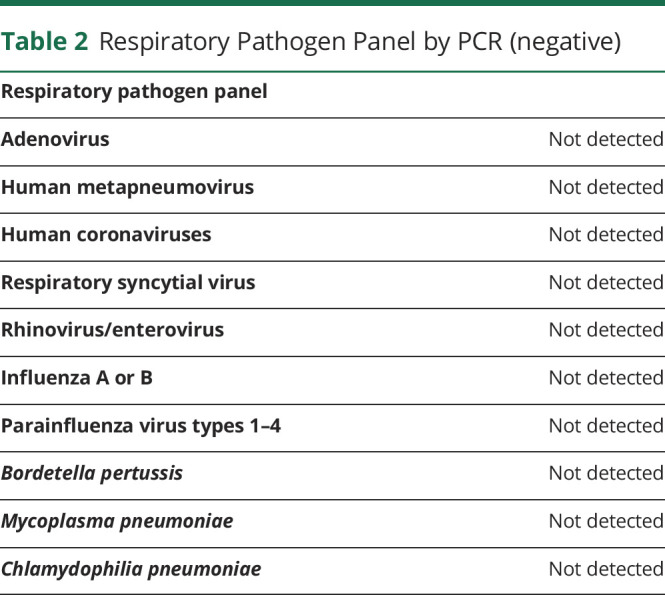
In the ED, he was afebrile, normotensive, and had an arterial oxygen saturation of 98% on room air. On neurologic examination, he was difficult to arouse, confused, speaking incoherently, and following commands inconsistently. No papilledema was noted, and his cranial nerve examination was normal. Motor examination was significant for mild to moderate generalized weakness without any focal findings. His deep tendon reflexes were normal, with negative Babinski bilaterally. He was noted to have frequent episodes of eye rolling, lip smacking, and insuppressible left facial and hand twitching for few seconds, which were treated as clinical seizures with 2 mg of lorazepam. The movements recurred soon after, which resolved after receiving 1 g of levetiracetam. He had a normal cardiopulmonary examination. Abdominal examination was notable for suprapubic tenderness. There was no rash. CT of his head without contrast was normal. A CT scan of his abdomen and pelvis with IV contrast showed bilateral dependent patchy lower lung ground-glass opacities. Chest radiograph had low lung volumes with possible left lower lobe infiltrate. Complete blood count, C-reactive protein, and erythrocyte sedimentation rate obtained secondary to suspicion for infection were normal. A total creatine kinase value was obtained because of his complaint of weakness, which was found to be elevated to 1200 U/L. He had hyponatremia (124 mmol/L) with a serum osmolality of 263 mOsm/kg. A urine osmolality of 900 mOsm/kg was suggestive of the diagnosis of syndrome of inappropriate antidiuretic hormone (table 1). Urine drug screen was negative. Respiratory pathogen panel by PCR was negative for common respiratory viruses and atypical bacterial pathogens (table 2).
Table 1.
Laboratory Results
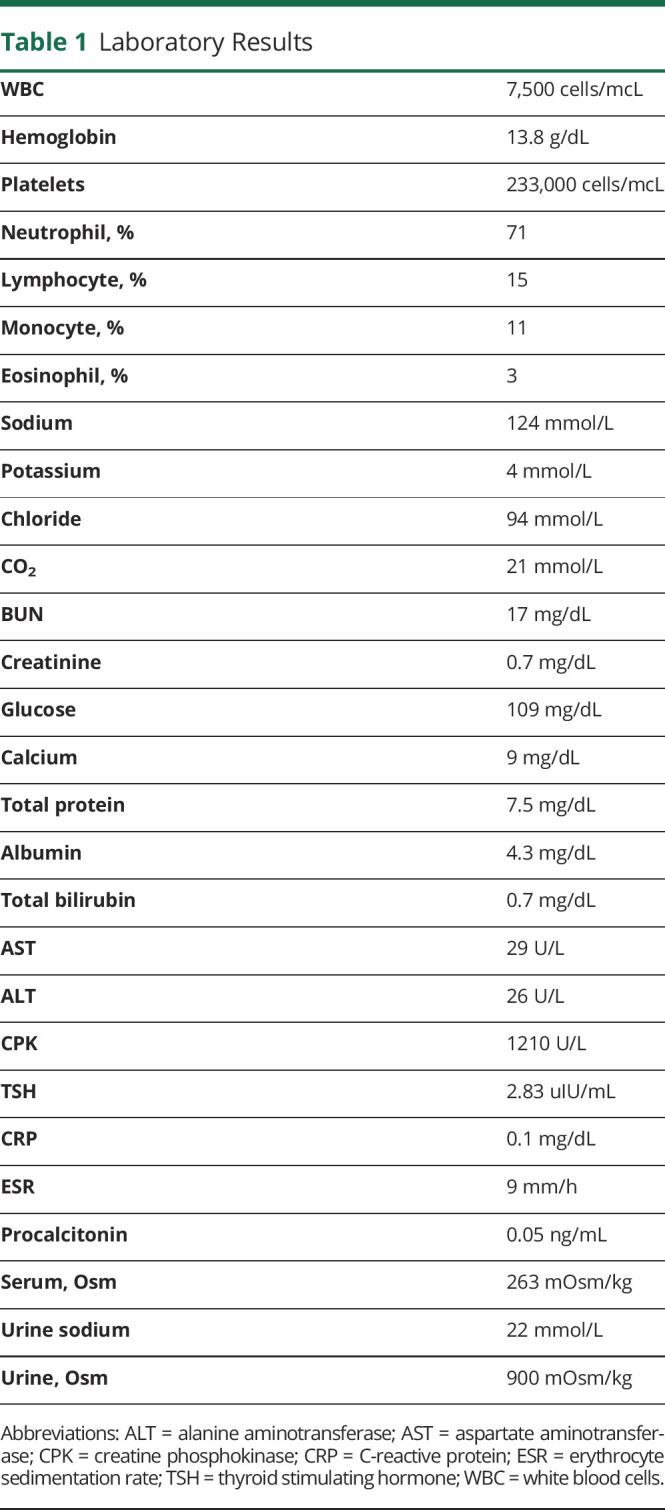
Table 2.
Respiratory Pathogen Panel by PCR (negative)
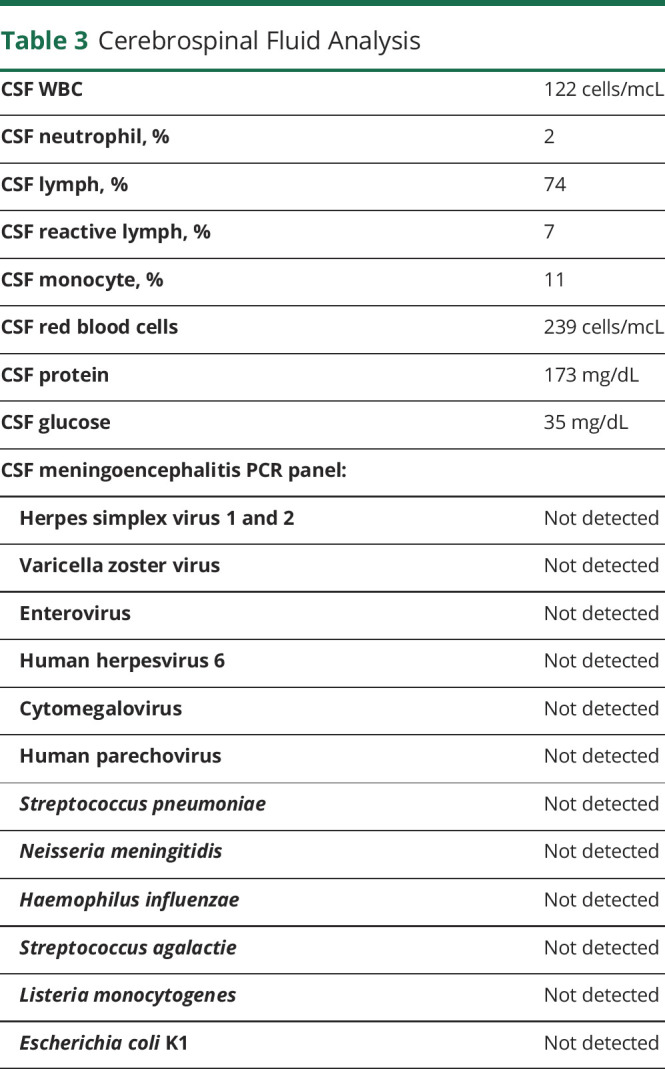
Due to concern for meningoencephalitis, a lumbar puncture was performed. CSF was clear and had 122 white blood cells/μL with mononuclear cells predominance. There were 239 red blood cells/mcL, protein was 173 mg/dL, and glucose was 35 mg/dL (serum glucose 109 mg/dL). The Gram stain of his CSF showed no cells or organisms. A meningoencephalitis PCR panel (Biofire ME Film Array) was negative for common viral and bacterial pathogens (table 3).
Table 3.
Cerebrospinal Fluid Analysis
The patient was started on vancomycin and ceftriaxone and was admitted to the pediatric intensive care unit for neurocritical monitoring for acute encephalitis and correction of hyponatremia. After admission, he became hypothermic with temperatures as low as 91.1°F rectally, which slowly improved to normal with external warming measures. He had bradycardia, with rates ranging between 50-58 beats per minute, but his perfusion remained normal and he was normotensive. He continued to be lethargic, oriented to name only, and inconsistently followed commands and answered questions. Cranial nerve examination was normal, and he displayed generalized weakness with no focal motor or sensory deficits. Supplemental oxygen via nasal cannula was required briefly in concurrence with alteration of mental status, hypoventilation, and difficulty handling oral secretions. Hyponatremia was initially treated with fluid restriction and hypertonic saline. He was placed on video EEG, which was consistent with encephalopathy, showing slow background without epileptiform discharges or seizures. Further investigation for etiology of encephalitis was performed on serum including negative testing for autoimmune encephalopathy antibody panel. Syphilis and human immunodeficiency virus antibodies were nonreactive. Interferon gamma release assay, Lyme enzyme linked immunosorbent assay, and serum Epstein-Barr virus and cytomegalovirus PCRs were negative. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing by in-house RT-PCR from his nasopharynx obtained in the ED resulted as positive. His CSF was experimentally tested for SARS-CoV-2, which was not detected. Antibiotics were discontinued once blood and CSF cultures were negative for 48 hours. Compassionate use of remdesivir for a 10-day course was initiated. The patient's neurologic symptoms started to improve by hospital day 4, and he was transferred to a lower level of care. During his stay, he continued to have moderate hyponatremia with serum sodium level between 129 and 133 mmol/L, which improved with oral sodium chloride supplements. His mental status remained altered, with inconsistent awareness of time and place, despite normalization of his laboratory test results. One week into admission, he developed pain to palpation in his right gastrocnemius. A venous Doppler confirmed an infrapopliteal deep venous thrombosis, and he was started on Lovenox, which prolonged his hospital stay. He was discharged home on day 15 with improved mental status and condition, with outpatient therapies arranged.
Discussion
Previously healthy children have rarely been reported to have severe manifestations with COVID-19.1,2 Our report illustrates an adolescent patient with severe infection with neurologic involvement. Neurologic manifestations have been reported in adult patients with COVID-19 including rare cases of encephalopathy.3–5 However, true encephalitis with evidence of CNS inflammation in CSF analysis has not yet been reported. There are reports of human coronaviruses associated with neurologic manifestations.6 There has been a single report of human coronavirus associated with encephalitis in an 11-month-old boy with severe combined immunodeficiency, which was diagnosed by a postmortem brain biopsy.7 Acute disseminated encephalomyelitis has also been associated with human coronaviruses, including in a 15-year-old boy who presented with irritability, focal neurologic findings (right-hand weakness, left-hand dysmetria, bilateral leg numbness, and difficulty walking), and had brain and spine MRI showing multifocal lesions.8 Brain MRI can be useful in differentiating these conditions. Our report is limited in that a brain MRI was not performed to limit SARS-CoV-2 exposure within the hospital. In light of his normal CT findings, nonfocal neurologic examination and improving neurologic examination, brain MRI was deferred. If he did decompensate, then brain MRI would have been performed. Although our patient did not present typically with respiratory symptoms, his chest imaging was consistent with COVID-19 with the findings of bilateral ground-glass opacities. He also later developed deep vein thrombosis, which coincides with the reports of increased incidence of thrombotic complications in critically ill intensive care unit patients with COVID-19.9
As our patient illustrates, encephalitis may be associated with COVID-19. Patients presenting with symptoms compatible with COVID-19 and encephalitis should be tested for SARS-CoV-2 in addition to the usual pathogens associated with neurologic infections.
Appendix. Authors
Footnotes
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020. doi: 10.1542/peds.2020-0702 [DOI] [Google Scholar]
- 2.Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV2 infection. NEJM 2020;382:2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 2020;12:e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med 2016;375:497–498. [DOI] [PubMed] [Google Scholar]
- 8.Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 2004;113:e73–e76. [DOI] [PubMed] [Google Scholar]
- 9.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]


