Abstract
Purpose of Review
To report the findings in 12 members over 3 generations of a family with dominantly inherited Charcot-Marie-Tooth disease (CMT1B) due to a novel MPZ mutation, who all had moderately severe selective impairment of vestibular function with normal hearing. Methods used were video head impulse testing of the function of all 6 semicircular canals, Romberg test on foam, nerve conduction studies, and whole exome and Sanger sequencing.
Recent Findings
All affected patients had a demyelinating neuropathy and a novel MPZ mutation: c.362A>G (chr1: 161276584, p.D121G). All also had normal hearing for age but a moderately severe impairment of semicircular canal function and a positive Romberg test on foam.
Summary
Some CMT mutations can impair vestibular function, presumably because of a vestibular nerve involvement but spare hearing. In such patients, impairment of vestibular function and impairment of proprioception contribute to imbalance.
Although some patients with Charcot-Marie-Tooth disease (CMT) have obvious hearing impairment from auditory nerve involvement, vestibular impairment will not be obvious because the patient already has imbalance from distal weakness and somatosensory impairment.1,2 Some patients with CMT—CMT1B/MPZ—3 have only auditory impairment, whereas others—CMT4C/H3TC24,5 or CMT4D/NDRG16—have severe, early impairment of both auditory and vestibular function. Here, we report 12 members in 3 generations of a Turkish family with CMT1B demyelinating neuropathy due to a novel MPZ mutation, who have selective bilateral vestibular impairment with normal hearing. In some, vestibular rather than proprioceptive impairment seems the principal cause of postural instability.
Methods
Genetic Testing
Sample Collection and DNA Extraction
Minimum 3 mL of peripheral blood was collected in ethylenediaminetetraacetic acid. The DNA extraction was performed by the commercial QIAcube method (Qiagen, Hilden, Germany) according to the recommended extraction and purification protocols. The DNA was stored −20 °C.
Fragment Analysis for HNPP by STR Markers
The index case (IV.3) was tested for 17p11.2–12 duplication by fragment analysis.
Multiplex Ligation-Dependent Probe Amplification
The multiplex ligation-dependent probe amplification (MLPA) assay was used for 4 family members (III.6, IV.4, IV.3, and V.2). The DNA samples were investigated by SALSA MLPA Probemix P033-B4 CMT1 (MRC-Holland, Holland), which contains 38 probes specific for PMP22, KIF1b genes, and control regions according to the producer's recommendations.
Whole Exome Sequencing
The DNA of index case was studied by whole exome sequencing (WES), and a novel, heterozygous variation of MPZ gene (ENST00000533357.4) was found: c.362A>G (chr1: 161276584, p.N121G), confirmed by Sanger sequencing. The variation is classified as likely pathogenic according to the ACMG criteria.7
Sanger Sequencing
Conventional Sanger sequencing was used for the confirmation of MPZ c.362A>G variation and for screening family members (III.6, IV.3, IV.4, IV.8, IV.9, V.1, V.2, and V.5).
Vestibular Testing
Function of each semicircular canal (SCC) was tested in turn by measuring the vestibulo-ocular reflex (VOR) gain in response to rapid, passive, head accelerations in the plane of each SCC—the Head Impulse Test (GN Otometrics, Tastrup, Denmark).8 The test assesses both VOR gain and any catch-up saccades compensating for low VOR gain.
Balance Testing
In the modified Romberg test, the patient first stands on a firm surface (the floor) and then on a foam surface, with eyes open and then closed. Postural stability is observed for 30 seconds. Sway is graded as follows: 1 = minimal, 2 = mild, 3 = moderate, and 4 = potential fall.9
The study was approved by the Hospital's institutional review board. Each adult patient and the parent of each child gave written permission for testing and for publication of the results.
Results
Clinical Features
The initial problem was imbalance, starting in infancy in 7.10 All except 3 (V.1, V.12, and V.13) had pes cavus; 4 with pes cavus (IV.5, V.5, V.6, and V.7) also had hammer toes. None had appreciable weakness. All those old enough for reliable sensory testing had distal impairment of vibration sense in the lower limbs. None had lower limb tendon reflexes. None had symptomatic hearing loss; 3 (III.6, IV.3, and IV.10) had audiograms—all 3 normal for age.
Nerve Conduction Studies
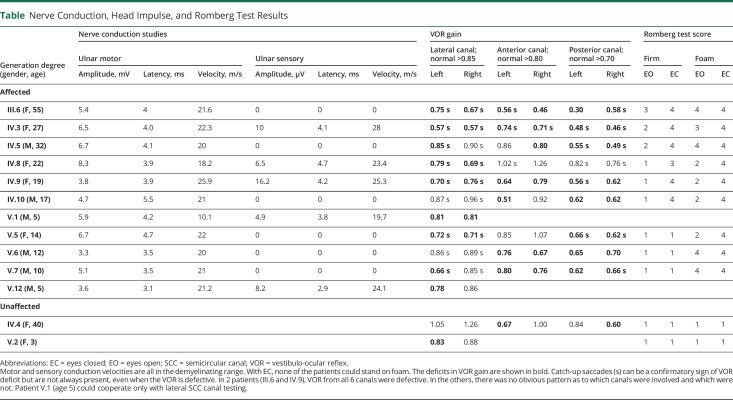
Of the 12 mutation-positive patients tested (table), 11 had marked slowing of motor conduction (10–26 m/s) and also of sensory conduction (20–28 m/s) in the 5 patients (IV.3, IV.8, IV.9, V.1, and V.12) with sensory action potentials.
Table.
Nerve Conduction, Head Impulse, and Romberg Test Results
Vestibular Function
Every patient had some impairment of SCC function in one or more canals. There appeared to be no association of extent or severity with patient age (table). The oldest patient (III.6) had impairment of all 6 SCCs (figure 1), whereas the youngest (V.7) who could have 6 SCC testing had impairment of 4 SCCs. In all patients, the SCC impairment was bilateral and of moderate severity with gains of 0.57–0.85 for the lateral canals (normal >0.85), 0.46–0.80 (normal >0.80) for the anterior canals, and 0.30–0.70 for the posterior canals (normal >0.70).
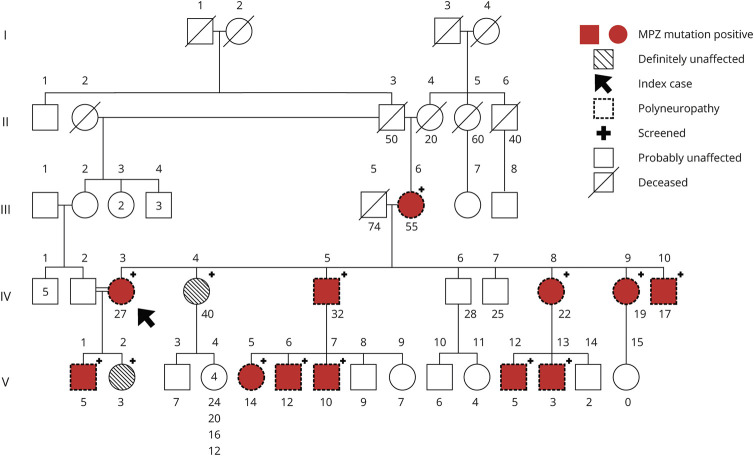
Figure 1. Family Tree.
Each clinically affected patient is also electrophysiologically affected (indicated by dotted symbols) and is MPZ mutation positive (indicated by crimson shading and a plus sign “+”). Conversely, each MPZ mutation-positive patient is clinically and electrophysiologically affected. Patient IV.4 (unaffected) has 5 unaffected children; only the youngest (age 4) is indicated with a symbol; the ages of the other 4 are appended. The only exception is patient V.13, a clinically affected, MPZ-positive 3 years old, too young to cooperate with electrophysiologic or vestibular testing. The arrow indicates index case.
Balance
Of the 12 patients, 9 attempted Romberg testing. On a firm surface, all 9 could stand with eyes open, but 5 (III.6, IV.3, IV.5, IV.9, and IV.10) could not do so with eyes closed—i.e., these 5 had a positive firm Romberg test, indicating proprioceptive impairment. On a foam surface, 5 (IV.3, IV.8, IV.9, IV.10, and V.5) could stand with eyes open but not with eyes closed—i.e., these 5 had a positive foam Romberg test, indicating vestibular impairment. Of the 9 patients, 5 (III.6, IV.3, IV.5, IV.9, and IV.10) had positive firm and foam Romberg tests, indicating that both somatosensory and vestibular impairment contributed to their imbalance.9
Family Tree and Genetics
The index case (IV.3) has a consanguineous marriage with a fourth degree relative, the only consanguineous marriage in the family (figure 2). There is no other genetic disorder in the family. The results of specific fragment analysis targeting for HNPP-related 17p11.2–12 deletion and MLPA analysis for PMP22 are all normal. The WES analysis identified MPZ c.362A>G (chr1: 161276584, p.D121G) variation. Sanger sequencing confirmed the presence of heterozygous MPZ c.362A>G variation in the index case (IV.3) and was used for screening of other family members. The variation was also found in the index case's mother (III.6), son (V.1), brothers (IV.5 and IV.10), sisters (IV.8 and IV.9), and nephews (V.5, V.6, V.7). They all have polyneuropathy. Cosegregation of the variation and the disorder is revealed in the family. Therefore, the variation is considered as likely pathogenic according to the ACMG criteria: PS4, PM1, PM2, and PP3.7 This result was confirmed in the index case (IV.3) at an independent laboratory.
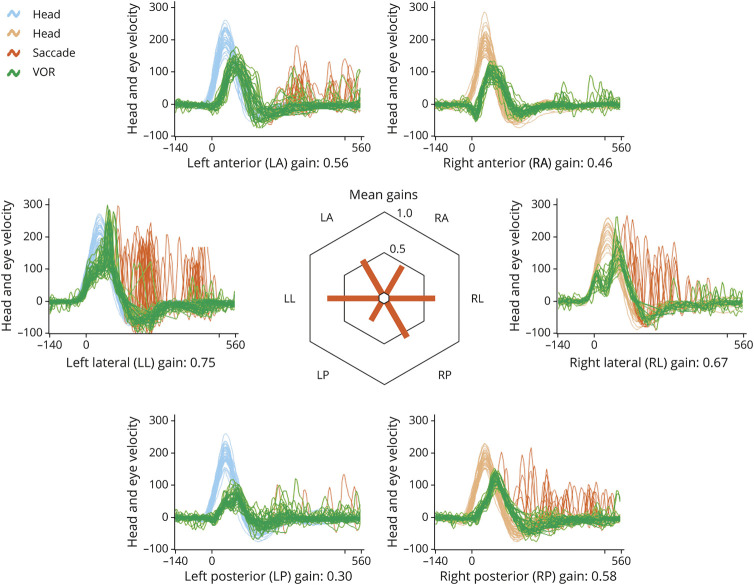
Figure 2. Video Head Impulse Testing.
A typical example, from patient III.6, a woman aged 55. Vestibulo-ocular reflex (VOR) gain is reduced from each of the 6 semicircular canals (SCCs). Head velocity is shown in blue for 30 leftward head impulses and in orange for 30 rightward head impulses in the plane of each SCC. VOR eye velocity is shown in green. Catch-up saccades compensating for any VOR inadequacy are shown in red. This patient happens to make fewer catch-up saccades in response to head impulses in the right anterior-left posterior canal plane. In the centre of the figure, VOR gains are illustrated as a polar plot.
Discussion
CMT1B is a hereditary demyelinating neuropathy due to one of more than 200 possible mutations in the MPZ gene. In patients with early onset disease, the neuropathy is demyelinating.11 In this family with a novel MPZ mutation (exon 3, c.362A>G; p.D121G), there was a contrast between the invariable vestibular impairment and the normal hearing. All our patients had bilateral impairment of SCC function affecting some but not all SCCs. There was no relationship between the patient age and vestibular impairment severity, suggesting that the vestibular impairment is static.
Poretti et al.1 reported bilateral vestibular impairment affecting both otolith function (tested with cervical vestibular-evoked myogenic potentials) and lateral SCC function (tested with head impulses using the scleral search coil method) in 15 patients with CMT. Of these 15, 13 had a demyelinating neuropathy, 4 had CMT1A (PMP22 dupl), 3 had CMTX1 (GJB1x32), one each had DI-CMT (MPZ), CMT2 (undef), CMT4D (undef), and 5 had clinically and genetically undefined CMT but a positive family history. In general, video Head Impulse Test gains were reduced and cervical vestibular evoked myogenic potential latency was increased; findings suggesting that these patients had a demyelinating vestibular and peripheral neuropathy. Of these 15 patients, 6 had auditory and vestibular impairment. In Dejerine-Sottas syndrome (motor conduction velocity 3–4 m/s) because of a T420C mutation predicting an L71P amino acid change of PMP22 protein, there can be total bilateral absence of lateral SCC canal function and a 40–50 dB sensorineural hearing loss.12
Although we cannot distinguish vestibular end-organ from vestibular nerve involvement causing vestibular impairment, physiologic and histologic data indicate that vestibular nerve demyelination is the cause. The marked delay in cVEMPs suggests demyelination.1 Otopathologic examination of 2 separate CMT1A/MPZ cases showed extensive demyelination of the vestibular and cochlear nerves with preservation of receptor hair cells. In one case with a heterozygous missense variant in exon 2 of the MPZ gene (c.193A>G; p.Thr65Ala), both auditory and vestibular function were impaired.13 In the other with a missense mutation in exon 3 of the MPZ gene (c.434A>C; p.Tyr145Ser), neither auditory or vestibular function was evaluated.14
TAKE-HOME POINTS
→ Clinicians should consider a vestibular contribution to patients with CMT imbalance.
→ Video head impulse testing can accurately measure function of each of the 6 semicircular canals.
→ It is well tolerated and takes only approximately 20 minutes.
Appendix. Authors
Study Funding
This work was funded by the Scientific and Technological Research Council of Turkey (TÜBİTAK) under Grant Number (216S658). The content is merely the responsibility of the authors and does not necessarily represent the views of TÜBİTAK.
Disclosure
G. Akdal, K. Koçoğlu, E. Bora, A. Koç, A. Ülgenalp, M. Bedir, R.T. Ala, E. Battaloğlu, G. Kırkım, and İ.Ş. Şengün report no disclosures. G.M. Halmágyi was formerly unpaid consultant to GN Otometrics for the development of video head impulse testing. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Poretti A, Palla A, Tarnutzer AA, et al. Vestibular impairment in patients with Charcot-Marie-tooth disease. Neurology 2013;80:2099–2105. [DOI] [PubMed] [Google Scholar]
- 2.Akdal G, Koçoğlu K, Tanriverdizade T, et al. Vestibular impairment in Charcot-Marie-Tooth disease. J Neurol Epub 2020 Aug 30. [DOI] [PubMed] [Google Scholar]
- 3.Duan X, Gu W, Hao Y, et al. A Novel Asp121Asn mutation of myelin protein zero is associated with late-onset axonal Charcot–Marie–Tooth disease, hearing loss and pupil abnormalities. Front Aging Neurosci 2016;8:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez-Garrigues H, Sivera R, Vílchez JJ, Espinós C, Palau F, Sevilla T. Vestibular impairment in Charcot–Marie–Tooth disease type 4C. J Neurol Neurosurg Psychiatry 2014;85:824–827. [DOI] [PubMed] [Google Scholar]
- 5.Sivera R, Cavalle L, Vílchez JJ, Espinós C, Pérez Garrigues H, Sevilla T. Audiological findings in Charcot-Marie-Tooth disease type 4C. J Int Adv Otol 2017;13:93–99. [DOI] [PubMed] [Google Scholar]
- 6.Dačković J, Keckarević-Marković M, Komazec Z, et al. Hereditary motor and sensory neuropathy Lom type in a Serbian family. Acta Myol 2008;27:59–62. [PMC free article] [PubMed] [Google Scholar]
- 7.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halmagyi GM, Curthoys IS. The video head impulse test in clinical practice. Neurol Sci Neurophysiol 2018;35:1–5. [Google Scholar]
- 9.Horak FB. Clinical measurements of postural control in adults. Phys Ther 1987;67:1881–1885. [DOI] [PubMed] [Google Scholar]
- 10.Estilow T, Glanzman AM, Burns J, et al. Balance impairment in pediatric Charcot-Marie-Tooth disease. Muscle Nerve 2019;60:219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callegari I, Gemelli C, Geroldi A, et al. Mutation update for myelin protein zero-related neuropathies and the increasing role of variants causing a late-onset phenotype. J Neurol 2019;266:2629–2645. [DOI] [PubMed] [Google Scholar]
- 12.Jen J, Baloh RH, Ishiyama A, Baloh RW. Dejerine-Sottas syndrome and vestibular loss due to a point mutation in the PMP22 gene. J Neurol Sci 2005;237:21–24. [DOI] [PubMed] [Google Scholar]
- 13.Nadol JB Jr, Hedley-Whyte ET, Amr SS, O Apos Malley JT, Kamakura T. Histopathology of the inner ear in Charcot-Marie-Tooth syndrome caused by a missense variant (p.Thr65Ala) in the MPZ gene. Audiol Neurootol 2018;23:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starr A, Michalewski HJ, Zeng FG, et al. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser). Brain 2003;126:1604–1619. [DOI] [PubMed] [Google Scholar]