Abstract
Objective
To examine the longitudinal health care resource utilization, in-hospital mortality, and incidence of downstream complications of bacterial meningitis in the United States.
Methods
Using IBM MarketScan, we retrieved data on adult patients with a diagnosis of bacterial meningitis admitted to a US hospital between 2008 and 2015. Patients were stratified into groups (1) with/without prior head trauma/neurosurgical complications, (2) nosocomial/community acquisition, and (3) Gram-negative/positive bacteria. Cost data were collected for up to 2 years and analyzed with descriptive statistics and longitudinal modeling.
Results
Among 4,496 patients with bacterial meningitis, 16.5% and 4.6% had preceding neurosurgical complications and head injuries, respectively. Lumbar punctures were performed in 37.3% of patients without prior trauma/complications who went on to develop nosocomial meningitis, and those with prior head injuries or complications had longer initial hospital stays (17.0 days vs 8.0 days). Within a month of diagnosis, 29.2% of patients with bacterial meningitis had experienced downstream complications, most commonly hydrocephalus (12.7%). The worst 30-day mortality was due to tuberculous (12.3%) and streptococcal meningitis (7.2%). Overall, prior head trauma and complications were associated with higher costs. Community-acquired bacterial meningitis had lower median baseline costs relative to the nosocomial group (no head trauma/complication: $17,152 vs $82,778; head trauma/complication: $92,428 vs $168,309) but higher median costs within 3 months of diagnosis (no head trauma/complication: $47,911 vs $34,202; head trauma/complication: $89,207 vs $58,947). All costs demonstrated a sharp decline thereafter.
Conclusions
Bacterial meningitis remains costly and devastating, especially for those who experience traumatic head injuries or have a complicated progress after neurosurgery.
Bacterial meningitis remains a devastating disease, despite the presence of vaccines against Streptococcus pneumoniae and Haemophilus influenzae type b.1 Meningococcal disease, for instance, affects approximately 2,600 patients in the United States each year, with a mortality rate of 10%–15% despite initiating antibiotics, and a further 10% incidence of neurologic and other sequelae.2 The emergence of antimicrobial resistance to organisms has likely further affected, both clinically and economically, the management of such patients.3
At present, a substantial need for understanding the health economic burden of bacterial meningitis exists, with an emphasis on separating nosocomial from community-acquired meningitis and understanding the role of preceding traumatic head injuries and neurosurgical interventions. This will help characterize the clinical and economic burden of iatrogenic complications and prolonged hospitalizations. Unfortunately, few studies have been conducted to frame these issues.
In the United States, a Nationwide Inpatient Sample (NIS) analysis in 2006 found 15,700 hospitalizations in that year with a mean of 16.6 days of stay for bacterial meningitis, an average of $33,100 per stay, and a total of $520.4 million in aggregated costs.4 The in-hospital death rate was 8.0%. Along these lines of research, but with an emphasis on more updated data, we have examined the longitudinal health economic burden and risk of complications of bacterial meningitis secondary to nosocomial and community-acquired infections. The health care resource utilization (HCRU), demographics and other data associated with bacterial meningitis in adult patients were quantified by analyzing a large commercial database between 2008 and 2015.
Methods
The demographics, economic costs, and other outcomes associated with bacterial meningitis in the United States were retrieved from the IBM MarketScan Research Databases. This database consists of claims data of more than 250 million patients in the United States. It contains deidentified data on inpatient and outpatient claims and outpatient prescription claims. The Commercial Claims and Encounters, Medicare Supplemental and Coordination of Benefits, and Medicaid databases within MarketScan were queried for adult (aged >18 years) patients, between the years 2008 and 2015, using the primary International Classification of Diseases–Ninth Revision (ICD-9-CM) codes. These patients had an inpatient diagnosis of bacterial meningitis, and the first diagnosis date was used as the index date. When patients had a first diagnosis at an outpatient setting, only those with another diagnosis of bacterial meningitis in the hospital within 7 days of the first diagnosis were included. In addition, patients must have had at least a 12-month continuous enrollment before the index date to be included in the study. Continuous enrollment ensures more accurate assessment of baseline comorbidities and estimation of total cost during a certain time period. For example, patients who discontinued enrollment might still have medical costs not recorded in the database. Including these patients in cost analysis will underestimate the total costs. The overall cohort was further stratified into 2 groups based on whether the patients had traumatic head injuries (ICD-9 codes: 800-801.9, 803.0-804.9, 850.0-854.1, and 873.0-873.9) or neurosurgical complications such as cerebral edema and hydrocephalus (ICD-9 codes: 348.5, 331.3, 331.4, 378.54, 324, 324.0, 324.1, 324.9, 348.4, 431, 785.52, 518.82, 388.5, and 286.6) 3 months before the meningitis diagnosis. Additional stratification resulted in 2 subgroups: nosocomial bacterial meningitis, where index date of diagnosis occurred >2 days after hospitalization or within a week of hospital discharge,5 and community-acquired bacterial meningitis (which included all other patients).
Demographics including age, sex, insurance status, geographic region, and employment status were collected at the index bacterial meningitis diagnosis date. The Charlson Comorbidity Index (CCI) was assessed by searching claim records from 12 months before index data to the day before the index date. Gram staining of the bacteria was based on the diagnosis codes of the initial bacterial meningitis diagnosis. When unspecified bacterial meningitis diagnoses (ICD-9: 320.7, 320.81, 320.89, and 320.9) were accompanied by another specific bacterial meningitis diagnosis within 30 days of the initial diagnosis, patients were classified according to the latter, although the date of the first unspecified meningitis diagnosis was used as the index date. When patients had 2 specific diagnoses or multiple diagnoses involving different bacteria, they were defined as “more than one specific” type of meningitis.
The primary outcome was HCRU, consisting of costs accrued and the length of hospital stay during the initial admission for bacterial meningitis. Cost outcomes were collected at baseline (using the 12-month period before diagnosis), 90-day period after the index date, and for up to 2 years following the diagnosis. All the patients included in the HCRU analysis had continuous enrollment for at least 12 months before and 90 days after the initial bacterial meningitis diagnosis. In each of the following time periods after 90 days of the index date, only patients who had continuous enrollment in that time period were included for analysis. Costs assessed included inpatient service costs charged to the hospital, outpatient service costs, outpatient medication costs, and aggregated costs. Furthermore, patients who had fully capitated or partially capitated health plans were excluded from cost analysis, and those with total payments above the 99th percentile were removed as outliers. Costs were adjusted to 2017 US dollars using the consumer price index provided by the U.S. Department of Labor Bureau of Labor Statistics (usinflationcalculator.com/inflation/consumer-price-index-and-annual-percent-changes-from-1913-to-2008/). In a subgroup analysis, patients who did not survive for more than 90 days after the initial bacterial meningitis diagnosis were assessed.
Secondary outcomes were 30-day complications and 30-day in-hospital mortality. Complications included hydrocephalus, intracranial epidural abscess, intracerebral hemorrhage, cerebral edema, respiratory distress syndrome, septic shock, and brain herniation.3,6,7 Patients with continuous enrollment for at least 30 days postindex diagnosis without prior head trauma or neurosurgical complications were included to assess 30-day complications. To assess the 30-day in-hospital mortality, the last hospital discharge status within 30 days of index diagnosis was used. Patients were followed from the time of the initial bacterial meningitis diagnosis to death or discharge and were censored at the time of discontinued enrollment or the end of 30 days, whichever occurred earlier.
Standard Protocol Approvals, Registrations, and Patient Consents
This study uses deidentified data and therefore was approved as an exempt study by the Duke IRB without the need for patient consent (IRB number: Pro00053624).
Statistical Analyses
Variables were summarized with descriptive statistics. The Kaplan-Meier method was used to estimate 30-day in-hospital mortality with 95% confidence intervals (CIs). Log-rank tests were used to evaluate the difference in survival distributions between meningitis groups. In addition, a multivariable longitudinal analysis was conducted to study the relationship between costs and meningitis type after adjusting for CCI, sex, Gram staining, age at diagnosis, and time period using a generalized estimating equation method with log link. Exchangeable correlation structure was used to account for the correlation of patients over time. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Data Availability
Deidentified data not explicitly published within this article were retrieved from a commercial database (MarketScan). Third parties may access the data on behalf of Duke only on entering in a written data agreement with Duke and IBM with access fee.
Results
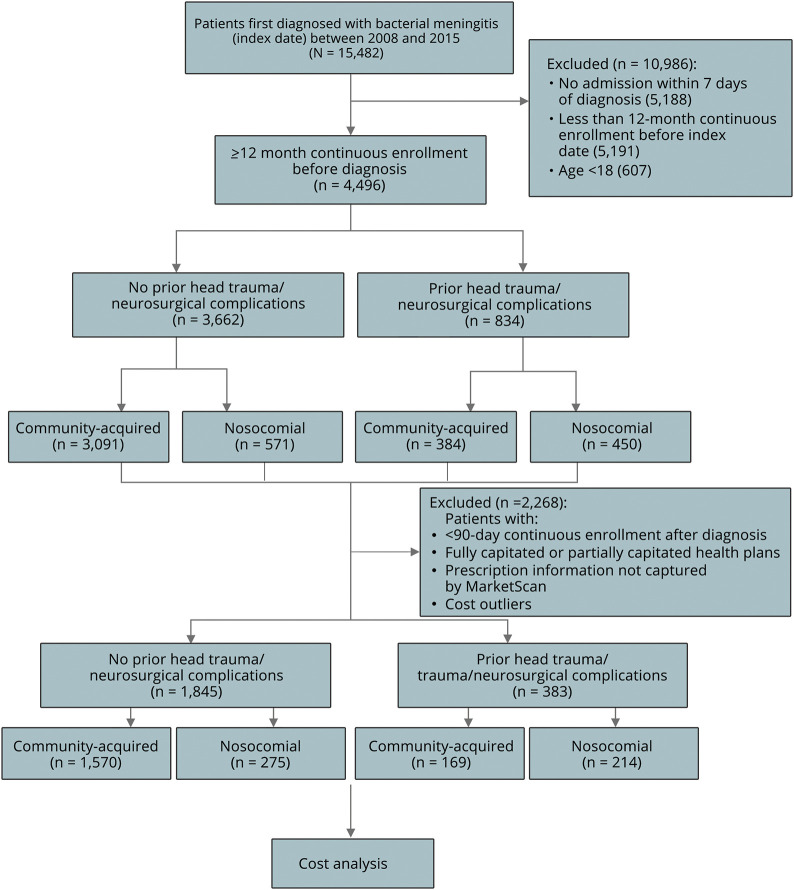
A total of 4,496 adult patients, with 12 months of continuous enrollment before a bacterial meningitis diagnosis between 2008 and 2015 and with a hospital admission within at least 7 days of the initial diagnosis, were identified. Figure 1 represents a flow diagram depicting the selection of cohort in this study. Among them, 834 had either traumatic head injuries (205, 4.6%) or neurosurgical complications (743, 16.5%) in the 3 months leading up to the index date. Patients were further stratified by infection: for 3,662 patients who did not have head trauma or neurosurgical complications, 3,091 (84.4%) had community-acquired bacterial meningitis, whereas 571 (15.6%) had nosocomial meningitis. Compared with those without head trauma/complications, a higher proportion of patients with preceding traumatic head injuries or neurosurgical complications had nosocomial infections (54.0% vs 15.6%). Furthermore, the frequency and percentages of all bacterial types are listed in table e-1 (links.lww.com/CPJ/A177). The most frequent bacterial type besides unspecified bacteria was Streptococcus (20.1%) in atraumatic patients. However, for those with preceding traumatic head injuries or neurosurgical complications, Staphylococcus/Methicillin-resistant Staphylococcus aureus (19.5%) and Gram-negative bacteria (17.4%) were the most common etiologic agents.
Figure 1. CONSORT.
CONSORT diagram of inclusion/exclusion criteria for analysis.
Demographics
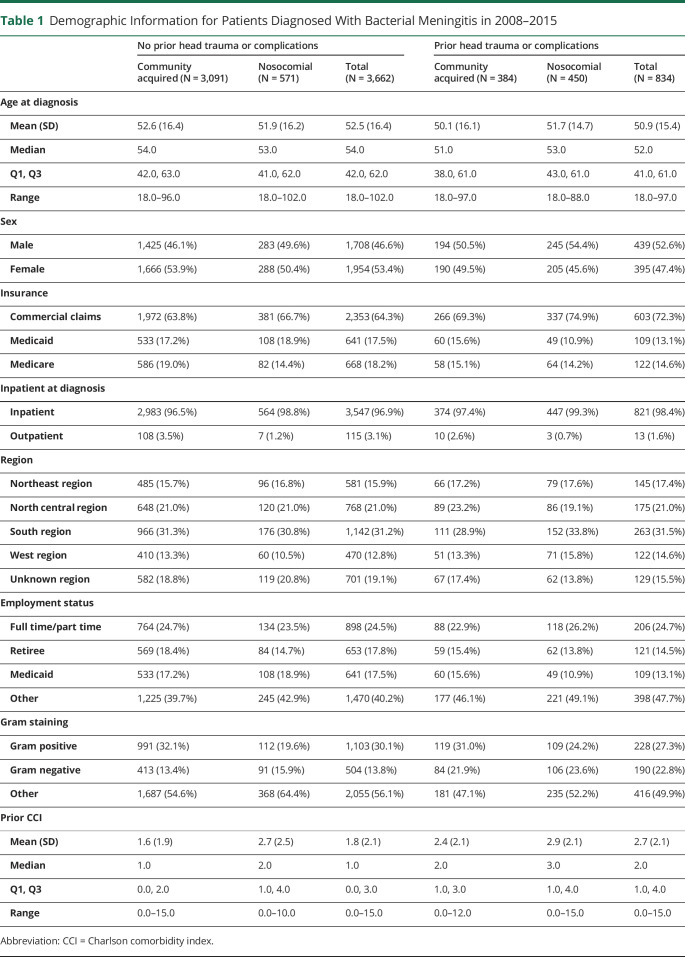
Patient demographics are summarized in table 1. Patients with prior head injuries or complications were more commonly infected with Gram-negative bacteria (22.8% vs 13.8%), were younger (52.0 vs 54.0 year old), were men (52.6% vs 46.6%), and had higher median CCI (2.0 vs 1.0). As expected, patients with nosocomial infection had higher median CCI than those with community-acquired infection (no head trauma/complications: 2.0 vs 1.0; head trauma/complications: 3.0 vs 2.0).
Table 1.
Demographic Information for Patients Diagnosed With Bacterial Meningitis in 2008–2015
Length of Stay, 30-day Discharge Status, and In-hospital Mortality
The overall median length of stay (LOS) for the initial bacterial meningitis admission was longer when prior head injuries or neurosurgical complications occurred (17 [Q1: 9, Q3: 31] vs 8 [Q1: 5, Q3: 16] days) or when nosocomial bacteria were the culprit (head trauma/complications: 21 [Q1: 12, Q3: 35] vs 13 [Q1: 6, Q3: 24] days; no head trauma/complications: 13 [Q1: 7, Q3: 22] vs 8 days [Q1: 4, Q3:15]), as shown in table e-2 (links.lww.com/CPJ/A177). In terms of discharge disposition for the initial admission, most patients with bacterial meningitis (2,623, 58.3%) were discharged home within a month of diagnosis. Compared with those without head trauma/complications, a higher percentage with preceding trauma or complications died (6.8% vs 4.5%) or were subsequently discharged to hospice care (2.5% vs 1.7%).
In-hospital survivals were estimated by the Kaplan-Meier method over a 30-day period (figure 2). The estimates of the survival percentages and 95% CIs stratified by groups are summarized in tables e-3 and e-4 (links.lww.com/CPJ/A177). The overall 30-day in-hospital mortality for bacterial meningitis was 4.7% (95% CI [4.1%–5.3%]). Among patients without prior head trauma/complications, the 30-day mortality rate was comparable between the community-acquired and nosocomial groups (4.5%, 95% CI [3.8%–5.3%] vs 4.4%, 95% CI [3.0%–6.5%]). However, those with streptococcal meningitis had a significantly higher 30-day mortality rate of 7.2% (95% CI [5.6%–9.3%]) compared with 3.8% (95% CI [3.2%–4.5%]) for all other bacterial meningitides. Among patients with prior head trauma/complications, the 30-day mortality rate was considerably higher in the nosocomial group (6.5%, 95% CI [4.5%–9.2%]) compared with the community-acquired group (4.5%, 95% CI [2.8%–7.1%]).
Figure 2. Thirty-day inpatient survival for patients with bacterial meningitis.
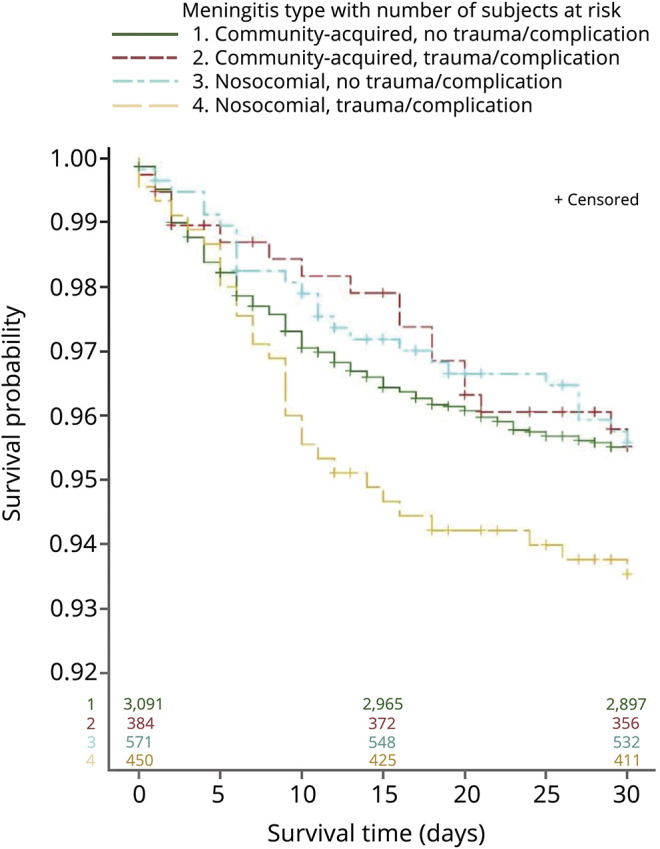
Kaplan-Meier survivals for patients with bacterial meningitis. Patients were censored at the time of disenrollment and represented with a vertical line.
In addition, for patients without prior traumatic injury or neurosurgical complications, tuberculous meningitis and streptococcal meningitis had the worst 30-day mortality rate of 12.3% and 7.2%, respectively, among the different causative bacterial species (table e-4, links.lww.com/CPJ/A177).
Neurosurgical Interventions
Interventions performed within 90 days before and after the initial bacterial meningitis diagnosis are quantified in table e-5 (links.lww.com/CPJ/A177). Before diagnosis, patients with nosocomial bacterial meningitis experienced more procedures (no trauma/complication group: 42.9% vs 14.3%; trauma/complication group: 59.1% vs 41.7%). Specifically, more patients diagnosed with nosocomial meningitis had either diagnostic or therapeutic lumbar punctures (no trauma/complication group: 37.3% vs 12.4%; trauma/complication group: 32.6% vs 19.8%). Within 90 days after the diagnosis of meningitis, community acquisition was associated with more interventions compared with nosocomial acquisition (no trauma/complication group: 55.9% vs 30.3%; trauma/complication group: 48.7% vs 33.1%), with lumbar punctures for diagnostic and therapeutic purposes still being the most common procedures.
Thirty-day Complications
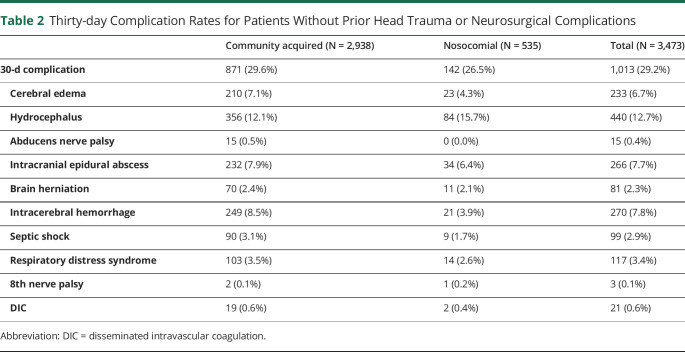
A total of 3,473 out of the 3,662 patients who had no prior head trauma or neurosurgical complication and had continuous enrollment for 30 days after index diagnosis were analyzed. As shown in table 2, patients from the community-acquired group have a higher overall complication rate than those from the nosocomial group (29.6% vs 26.5%), including higher rates of cerebral edema, abducens nerve palsy, intracranial epidural abscess, brain herniation, intracerebral hemorrhage, septic shock, respiratory distress syndrome, and disseminated intravascular coagulation. Hydrocephalus was the most common complication overall, occurring in 440 patients (12.7%) of the cohort, although it was slightly more common in the nosocomial group compared with the community-acquired group (15.7% vs 12.1%).
Table 2.
Thirty-day Complication Rates for Patients Without Prior Head Trauma or Neurosurgical Complications
Cost Analysis
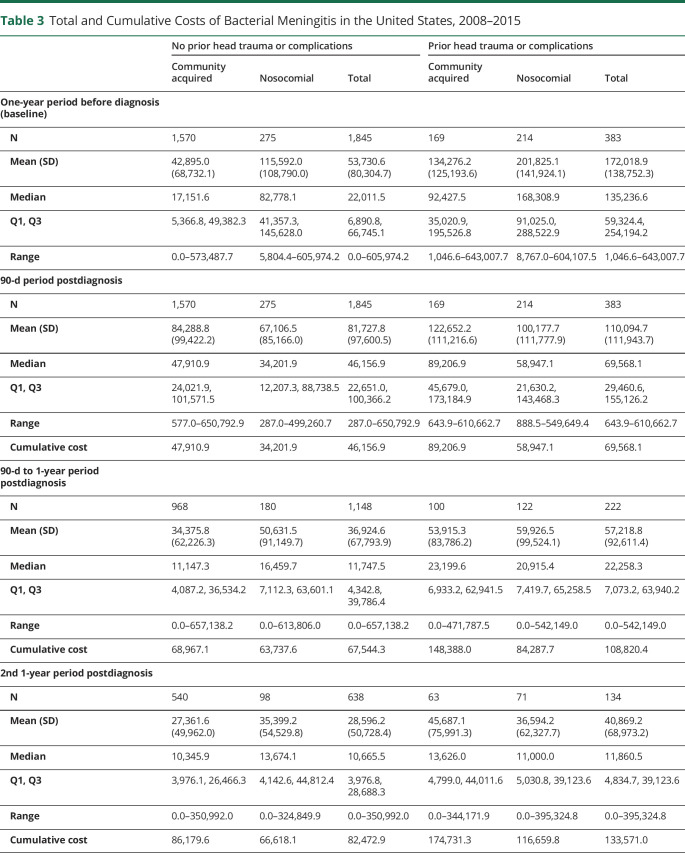
A total of 2,228 patients were included in the cost analysis, with the total costs (including inpatient service, outpatient service, and medication costs) summarized in table 3. In general, prior head trauma and neurosurgical complications were associated with higher baseline costs (median $135,237 vs $22,012), and those infected by nosocomial bacterial meningitis also incurred higher costs than their counterparts with community-acquired meningitis (no head trauma/complications: median $82,778 vs $17,152; head trauma/complications: median $168,309 vs $92,428). Within 3 months of the diagnosis, community acquisition was associated with higher costs compared with the nosocomial group (no head trauma/complications: $47,911 vs $34,202; head trauma/complications: $89,207 vs $58,947). However, these costs declined sharply thereafter.
Table 3.
Total and Cumulative Costs of Bacterial Meningitis in the United States, 2008–2015
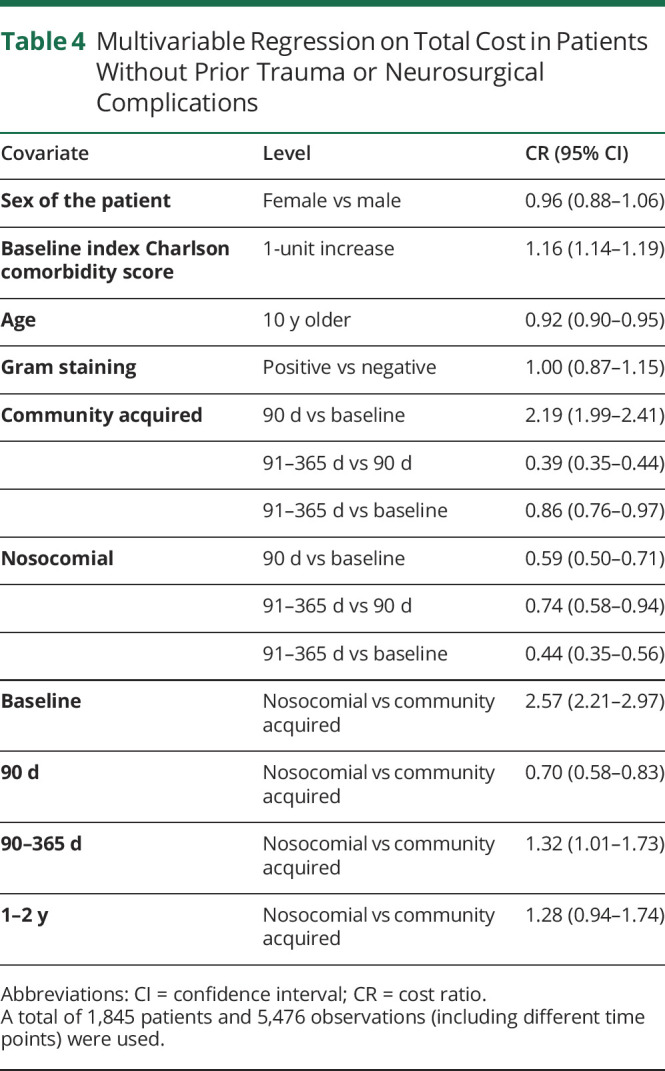
Two separate regression models were used for patients with and without prior traumatic head injury or neurosurgical complications to evaluate the effect of comorbidities, infection sources, and Gram staining on total cost. For patients without preceding head injury or interventions (table 4), the CCI was found to be associated with total cost such that each 1-unit increase in the CCI is expected to raise the total cost by 16% (cost ratio [CR]: 1.16, 95% CI [1.14–1.19]). Age also affected total cost such that each 10-year increase of age is expected to lower cost by 8% (CR: 0.92, 95% CI [0.90–0.95]). Gram staining was not associated with total cost (CR: 1.00, 95% CI [0.87–1.15]). With other covariates held fixed, the total cost for community-acquired bacterial meningitis is expected to be 2.19 times higher within 90 days of diagnosis compared with that at baseline (CR: 2.19, 95% CI [1.99–2.41]). After the first 90 days, total average costs in the 90–365-day period are 39.0% of that in the previous 90-day period (CR: 0.39, 95% CI [0.35–0.44]). On the other hand, in the nosocomial bacterial meningitis group, the total cost within 90 days of diagnosis is expected to be 59% of the baseline cost (CR: 0.59, 95% CI [0.50–0.71]), decreasing further in the 90–365-day period to 74% of the first 90-day cost (CR: 0.74, 95% CI [0.58–0.94]). Overall, the baseline total cost for the nosocomial group is expected to be 2.57 times of that for community-acquired bacterial meningitis (CR: 2.57, 95% CI [2.21–2.97]). In the subsequent 90 days after diagnosis, however, the total cost for the nosocomial group is significantly lower (CR: 0.70, 95% CI [0.58–0.83]). There was no significant difference in cost of nosocomial vs community-acquired meningitis during the 1–2-year period after diagnosis (CR: 1.28, 95% CI [0.94–1.74]).
Table 4.
Multivariable Regression on Total Cost in Patients Without Prior Trauma or Neurosurgical Complications
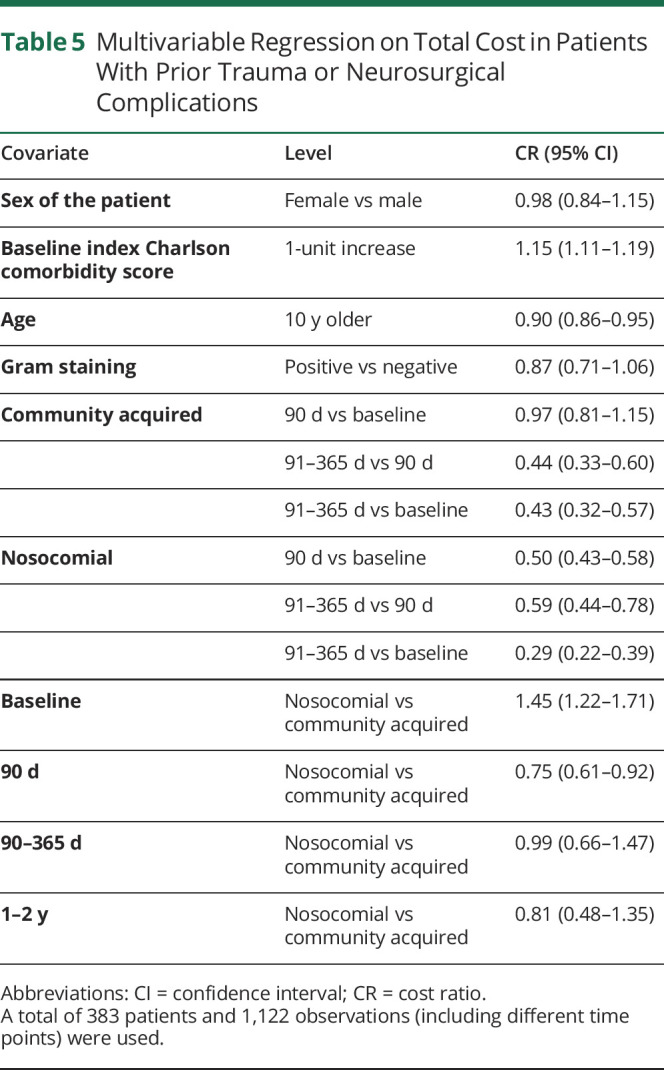
Results differed when traumatic head injuries or interventions were considered, although similar associations of CCI and age with costs were found (table 5). When other covariates are held fixed, the total cost for community-acquired bacterial meningitis within 90 days of diagnosis is as high as the baseline cost (CR: 0.97, 95% CI [0.81–1.15]), whereas in the nosocomial bacterial meningitis group, the total cost within 90 days of diagnosis is not as high as the baseline cost (CR: 0.50, 95% CI [0.43–0.58]). Overall, the baseline total cost for the nosocomial group is expected to be 1.45 times of that for community-acquired bacterial meningitis (CR: 1.45, 95% CI [1.22–1.71]). In the subsequent 90 days after diagnosis, however, the total cost for the nosocomial group is significantly lower (CR: 0.75, 95% CI [0.61–0.92]).
Table 5.
Multivariable Regression on Total Cost in Patients With Prior Trauma or Neurosurgical Complications
Cost Analysis of Patients Who Died Within 90 Days
As table e-6 (links.lww.com/CPJ/A177) indicates, compared with patients who survived within 90 days of the index date, those who died earlier tended to be older (no trauma/complication group: median age 60 vs 54 years; trauma/complication group: median age 55 vs 52 years) and had higher median Charlson comorbidity scores in the year before the diagnosis of meningitis (no trauma/complication group: 2 vs 1; trauma/complication group: 3 vs 2).
Cost analysis (table e-7, links.lww.com/CPJ/A177) revealed that for such patients who died within the first 90 days of index date, their median cost was higher 1-year before diagnosis (no prior head trauma or complication: $44,143 vs $22,011; prior head trauma or complication: $203,469 vs $135,237). At the same time, however, within the 90-day period after diagnosis, the median cost for patients who died and had no prior head trauma/complication was comparable to those who survived ($50,521 vs $46,157), whereas the median cost for patients who died who had head trauma/complications was much lower than those who survived ($27,172 vs $69,568).
Discussion
Our study underscores the substantial public health and economic importance of bacterial meningitis in the United States. This longitudinal study represents a comprehensive characterization of bacterial meningitis morbidity, mortality, and economic burden in the United States in recent years. As the introduction of vaccines and the ever-evolving emergence of resistance among causal organisms have contributed to a changing disease profile,3 a current characterization of the disease is particularly important.
Within the study cohort, we found that a substantial number of bacterial meningitis admissions (up to 84.4%) were a result of community acquisition, although traumatic head injuries or recent neurosurgical procedures resulted in disproportionately more nosocomial infections. Compared with a much older study from 1993,8 in which 60% of the acute bacterial meningitis admissions were secondary to community-acquired bacteria, it appears that the gap between the 2 groups has now widened, likely heralding an era where iatrogenic infections are routinely prevented through the use of empiric antibiotics. At the same time, some studies have demonstrated the inadequacy of prophylactic antibiotic coverage in preventing postoperative bacterial meningitis after craniotomies and other common neurosurgical interventions9—an observation in line with our results in which a high proportion of patients with preceding head injuries or procedures, nevertheless, experienced nosocomial bacterial meningitis.
We also found a higher overall 30-day inpatient mortality for streptococcal meningitis (which is often acquired in the community) relative to other meningitides. Possible explanations might include increasing resistance of Gram-positive community-acquired pathogens to available antimicrobial agents (e.g., beta-lactams and macrolides against Streptococcus) as well as a lower diagnostic threshold (and, therefore, faster initiation of systemic antibiotics and other therapeutic measures) for nosocomial bacterial meningitis in patients with frequent access to health care settings. In addition, that tuberculous meningitis had the worst 30-day mortality in atraumatic patients is not surprising because extrapulmonary tuberculosis is known to adversely affect survival, although updated data quantifying the exact mortality rate are lacking.10,11 Our study, therefore, helps to fill this knowledge gap.
We also emphasized 30-day complication rates because for an acute disease such as bacterial meningitis, which can require emergent intervention, complications are likely to present and be treated early. In addition, the high rates of hydrocephalus found in our study match the pathophysiology of the disease, especially its ability to cause meningeal scarring, inflammation, and resulting ventricular or villi obstruction, thereby impairing CSF absorption.
Our LOS results (17.0 days for traumatic cases and 8.0 days for atraumatic) closely follow prior findings involving the NIS analysis of 2006,4 although we have provided a more thorough understanding of this HCRU aspect by indicating how differences arise in hospital stays when patients with bacterial meningitis experience traumatic head injuries or neurosurgical interventions or when nosocomial bacteria are involved. In addition, our study casts a negative limelight on lumbar punctures due to their association with nosocomial bacterial meningitis following the procedure. These are typically used to treat or prevent CSF leaks, communicating hydrocephalus, and cord ischemia in patients undergoing aortic aneurysm repair, in addition to diagnosing normal pressure hydrocephalus and other conditions.12 We hope that these numbers, rather than impeding neurosurgical interventions, will aid physicians in discerning the risk of such a procedure and ensuring a more informed decision-making process for patients in the future.
We also found that the median baseline costs accrued for nosocomial infections and for those with prior traumatic head injuries or interventions were significantly higher compared with community-acquired infections and atraumatic patients, respectively. Possible reasons for such a difference include higher preceding costs of interventions, hospitalizations, and treatment for those who have traumatic injuries or need neurosurgical procedures, thereby inflating the baseline costs for the nosocomial group. However, within 3 months of the diagnosis, community acquisition became associated with higher costs.
Furthermore, our analysis for patients who died within the first 90 days revealed an older age group that was, unfortunately, more prone to comorbidities and death following a bacterial meningitis diagnosis. The cost analysis also indicated that in the 1-year period before diagnosis, such patients needed treatment with higher associated costs in view of their additional comorbidities compared with those who survived this critical period. The finding that median costs for patients who died were less is likely a reflection of early death after diagnosis and hence lower total costs compared with those who survived.
Retrospective investigations, such as ours, remain limited by the inaccuracies inherent in a database comprised of reported diagnostic codes. In addition, although this study defined mortality as in-hospital deaths due to bacterial meningitis and its complications, the attribution of an in-hospital death to a meningitis diagnosis may not always be correct, as incorrect determination of hospital death is unfortunately not uncommon.13 Moreover, because MarketScan only tracks death discharges, and stopped tracking in-hospital mortality after 2016, mortality (e.g., shortly after being discharged to hospice) may be underestimated in our study. The MarketScan database is also not reflective of the entire US population, and it tends to overrepresent the Southern region and underrepresent the Western region.14 Despite these limitations, we were able to review and quantify morbidity, mortality, and cost for patients diagnosed with bacterial meningitis in recent years.
Our results indicate a substantial health economic burden, morbid complications, and inpatient mortality associated with both nosocomial and community-acquired bacterial meningitis. Although bacterial meningitis is known to be among the top infectious causes of death, the paucity of reporting for associated morbidity, mortality, and economic burden in recent years has resulted in both a knowledge gap and an associated patient outcomes gap. More comprehensive and current disease characterization is essential to improving outcomes while reducing the economic burden of the disease. By addressing the knowledge gap, this study represents the requisite first steps toward ultimately improving patient outcomes for bacterial meningitis.
Appendix. Authors
Study Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002553. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.O'Brien JA, Caro JJ, Getsios D. Managing meningococcal disease in the United States: hospital case characteristics and costs by age. Value Health 2006;9:236–243. [DOI] [PubMed] [Google Scholar]
- 2.Sharip A, Sorvillo F, Redelings MD, Mascola L, Wise M, Nguyen DM. Population-based analysis of meningococcal disease mortality in the United States: 1990–2002. Pediatr Infect Dis J 2006;25:191–194. [DOI] [PubMed] [Google Scholar]
- 3.Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis 2002;2:721–736. [DOI] [PubMed] [Google Scholar]
- 4.Holmquist L, Russo CA, Elixhauser A. Meningitis-related hospitalizations in the United States, 2006: statistical brief# 57. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006–2008. [PubMed] [Google Scholar]
- 5.van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med 2010;362:146–154. [DOI] [PubMed] [Google Scholar]
- 6.Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Impact of bacterial meningitis-associated conditions on pediatric inpatient resource utilization. J Hosp Med 2010;5:E1–E7. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman O, Weber JR. Pathophysiology and treatment of bacterial meningitis. Ther Adv Neurol Disord 2009;2:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults—a review of 493 episodes. N Engl J Med 1993;328:21–28. [DOI] [PubMed] [Google Scholar]
- 9.Korinek AM, Baugnon T, Golmard JL, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery 2006;59:126–133. [DOI] [PubMed] [Google Scholar]
- 10.Garg RK. Tuberculous meningitis. Acta Neurol Scand 2010;122:75–90. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy DH, Fallón RJ. Tuberculous meningitis. JAMA 1979;241:264–268. [PubMed] [Google Scholar]
- 12.Schade RP, Schinkel J, Visser LG, Van Dijk JM, Voormolen JH, Kuijper EJ. Bacterial meningitis caused by the use of ventricular or lumbar cerebrospinal fluid catheters. J Neurosurg 2005;102:229–234. [DOI] [PubMed] [Google Scholar]
- 13.McMillan DA, Lin CY, Aronin SI, Quagliarello VJ. Community-acquired bacterial meningitis in adults: categorization of causes and timing of death. Clin Infect Dis 2001;33:969–975. [DOI] [PubMed] [Google Scholar]
- 14.Ohsfeldt RL, Lage MJ, Rajagopalan K. Medication use, service utilization, and medical costs associated with new episodes of bipolar disorder: evidence from a retrospective claims database. Prim Care Companion J Clin Psychiatry 2007;9:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data not explicitly published within this article were retrieved from a commercial database (MarketScan). Third parties may access the data on behalf of Duke only on entering in a written data agreement with Duke and IBM with access fee.