Abstract
Objective
To evaluate the prevalence of hyposmia and dysgeusia in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and their temporal relationship with the onset of other symptoms.
Methods
We performed a retrospective analysis of patients admitted during the month of March 2020 to the nonintensive COVID unit of Udine University Hospital on the basis of a positive swab test and/or of clinical-radiologic signs of SARS-CoV-2 infection. Patients were interviewed with a standardized questionnaire. Clinical and laboratory data were collected. Data were analyzed with descriptive statistics, and results expressed as point estimates and 95% confidence intervals (CIs).
Results
Of 141 patients admitted, 93 were interviewed. Hyposmia and dysgeusia were present in 58 cases (62.4%). In 22.4% of them, olfactory and gustatory impairment clearly preceded systemic symptoms. The presence of active smoking was very limited in both groups: 8.6% in hyposmic vs 2.9% in normosmic patients (odds ratio 3.2; 95% CI 0.3–28.6). Moreover, total leukocytes and neutrophils count were respectively 23% (effect estimate 1.23; 95% CI 1.06–1.42) and 29% (effect estimate 1.29; 95% CI 1.07–1.54) lower in the hyposmic cohort. No difference was found for other inflammatory biomarkers.
Conclusions
Hyposmia and dysgeusia are common in SARS-CoV-2 infection and can precede systemic symptoms. They should be actively searched and prompt close monitoring and isolation until infection is confirmed or disproven. The lower number of total leukocytes and neutrophils in hyposmic patients might indicate an early-phase virus-induced cytopenia.
Hyposmia and dysgeusia have been described in association with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with recent studies ranging from 33.9% to 85.6%.1–4 This high variability may reflect the different populations considered: in fact, low severity of disease, low comorbidity burden, female sex, and younger age all seem to be associated with a higher prevalence of these symptoms.1,5–7 On the other side, these manifestations may not be recognized when dealing with acute respiratory complications or cannot be evaluated in those who are in intensive care unit (ICU). However, these symptoms could represent an early marker of disease that precedes systemic symptoms.
In our nonintensive care COVID unit, we observed that hyposmia and dysgeusia were frequently reported as initial symptoms, typically before the onset of systemic symptoms, which typically followed these sensory alterations by 24–48 hours. Based on this observation, we conducted a retrospective study of symptom onset and initial laboratory values. Our study extends the clinical observations on hyposmia and dysgeusia in SARS-CoV-2 infection and evaluates the frequency of their presentation as first symptoms of this condition.
Methods
Study Design and Patient Population
We retrospectively analyzed the patients who had been admitted to the nonintensive COVID unit of Santa Maria della Misericordia University Hospital (Udine, Italy) in the period March 1–31, 2020, on the basis of a positive swab test and/or of clinical and radiologic signs of lung involvement (chest x-ray or CT scan). Patients who could not be interviewed due to mechanical ventilation, cognitive impairment, or death were excluded from these analyses. The presence of cognitive impairment was identified by evaluating if the patient was alert and oriented in space, time, and self. Patients who had an initial negative swab and persistence of swab test negativity throughout the hospital stay were excluded from the analysis although infection was suspected based on clinical and radiologic findings.
Data Collection and Interview Structure
At admission, all patients underwent a comprehensive blood panel, including total blood count, C-reactive protein (CRP), lactate dehydrogenase (LDH), creatine phosphokinase, interleukin 6 (IL-6), alanine aminotransferase, aspartate aminotransferase, and gamma glutamyltransferase. The PaO2/FiO2 ratio was calculated from the results of the arterial blood gas analysis at admission.
Patients were personally interviewed at bedside, if still admitted, or by phone call, if transferred to other hospital units or at home. Interviews were all conducted by neurology residents (F.B., A.M., A.S., and G.P.) using a structured interview with open questions including the development of hyposmia and/or dysgeusia, the time of their appearance and temporal relationship with other symptoms, their duration and persistence, smoking habits, history of asthma, allergic rhinitis, and other nasopharyngeal conditions. The interview model form is available as a supplemental file (figure e-1, links.lww.com/CPJ/A240). The time span from the beginning of symptoms and the time in which patients were interviewed has been reported. To have synthetic measure of the disease burden in our population, we calculated the Charlson Comorbidity Index8 using the information obtained from the clinical history and from all the available records. To evaluate the role of pre-existing conditions associated with hyposmia, we recorded whether the patients had a history of Parkinson disease or traumatic brain injury.
Statistical Analysis
Data were analyzed with descriptive statistics. Kolmogorov test and histogram were used to control for normal distribution of variables. Continuous variables were analyzed by the 2-sample t test, whereas categorical variables were analyzed using χ2 test. Since we did not account for a prespecified method for error control, summary statistics is limited to point estimates and 95% confidence intervals (CIs). The widths of the intervals have not been adjusted for multiplicity, and the inferences drawn may not be reproducible. Estimate of effect for normally distributed variables was calculated using Cohen's d test (d). Logarithmic transformation was applied to non-normal distribution, and estimate of effect was calculated as a raw mean difference for continuous variables. Raw mean differences were retransformed by means of antilogarithm and thus expressed as a ratio. Odds ratio (OR) was calculated for categorical variables.
Standard Protocol Approvals, Registrations, and Patient Consents
Owing to the urgent need of data on the new coronavirus disease (COVID-19) infection and the situation of emergency at the peak of the pandemic, it was impossible to ask for a preliminary approval of the regional ethics committee. All patients, however, at the time of the admission gave written general consent to the anonymous use of their clinical data for research purposes. In addition, at the time of the interview, verbal informed consent was obtained on the phone.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
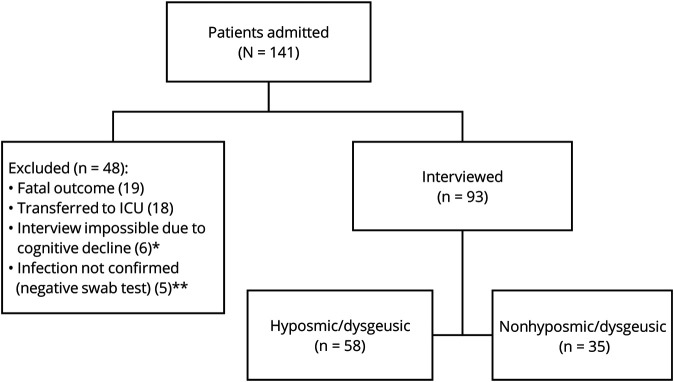
We reviewed 141 consecutive patients, with a mean age of 62.6 ± 15.8 years (range 19–91). 66.7% of the study population was male (n = 94). Thirty-four percent of patients (n = 48) were excluded from the study (figure 1). Interviews were completed for 66% of patients (n = 93) with results from this cohort presented in table 1. Among 141 patients, only one was affected by Parkinson disease but was not interviewed due to concomitant cognitive decline. No patient reported a history of traumatic brain injury. Charlson Comorbidity Index for the entire group of interviewed patients is reported as a supplemental file (figure e-2, links.lww.com/CPJ/A240), and no differences in the score were found in the 2 groups (mean score for hyposmic patients 2.2 vs mean score for normosmic patients 1.72; mean difference 0.48; 95% CI −0.27 to 1.23; d = 0.28; 95% CI −0.15 to 0.71).
Figure 1. Flow Chart of the Study.
* Including 1 patient affected by Parkinson disease. ** Patients with a negative swab test were excluded from the study.
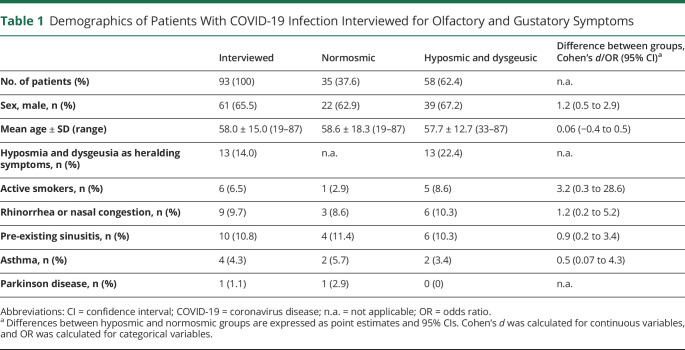
Table 1.
Demographics of Patients With COVID-19 Infection Interviewed for Olfactory and Gustatory Symptoms
Among the interviewed patients, hyposmia and dysgeusia were present in 58 cases (62.4%). Age and sex distribution of the hyposmic group were not different from those of the entire interviewed population (mean difference 0.91 years, 95% CI −6.1 to 7.9; d = 0.06; 95% CI −0.4 to 0.5). The presence of active smoking was very limited in both groups 8.6% in hyposmic vs 2.9% in normosmic patients (OR 3.2; 95% CI 0.3–28.6).
Considering only the group of 58 patients affected by olfactory and gustatory symptoms, hyposmia, and dysgeusia often appeared along with systemic symptoms but in 13 cases (22.4%) clearly preceded them. The interview took place at a variable time interval from onset of symptoms (mean 26.6 ± 7.3 days [range 12–50]) and, at the time of the interview, 25.9% of patients reported persisting olfactory and/or gustatory symptoms. Persistence of symptoms was not associated with a shorter time interval from their onset and the moment of interview (mean interval for patients with persisting symptoms 30.9 days vs mean interval for patients without persisting symptoms 25.0 days; mean difference 5.9 days, 95% CI 1.6–10.2; d = 0.81; 95% CI 0.22–1.40).
Notably, only 6 of 58 hyposmic patients (10.3%) reported rhinorrhea and/or nasal congestion. Similarly, asthma was reported only by 2 of our hyposmic patients (3.4%) in their medical history, whereas the history of allergic rhino-conjunctivitis was present in 6 patients (10.3%). Moreover, 1 patient affected by pre-existing hyposmia reported worsening of his problem 2 days before the onset of systemic symptoms, and this worsening persisted at the time of interview. Among nonhyposmic patients, one had a history of nasal polyposis. Comparative prevalence of medical history elements between hyposmic and normosmic patients is shown in table 1.
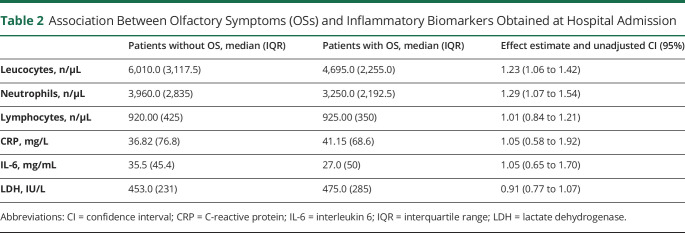
The results regarding biomarkers of inflammation are reported in table 2. Comparing the group of 58 patients with hyposmia with that of 34 nonhyposmic patients (for one more patient, data about leukocytes are missing), we observed that total leukocytes and neutrophils count were respectively 23% (effect estimate 1.23; 95% CI 1.06–1.42) and 29% (effect estimate 1.29; 95% CI 1.07–1.54) lower in the hyposmic cohort. No differences were found in any of the other indexes examined (CRP, IL-6, LDH, and lymphocytes). Similarly, no differences were found between the 2 groups for the presence of dyspnea (46.6% in hyposmic vs 48.6% in normosmic patients; OR 1.08; 95% CI 0.4–2.5) or the PaO2/FiO2 ratio (mean PaO2/FiO2 ratio in hyposmic 351 vs mean PaO2/FiO2 ratio in normosmic 327; mean difference 24, 95% CI −10.5 to 59.3; d = 0.31; 95% CI −0.14 to 0.77). Arterial blood gas analysis was performed in 87% of the interviewed patients at a mean time interval of 6.5 ± 4.3 days from systemic symptoms onset.
Table 2.
Association Between Olfactory Symptoms (OSs) and Inflammatory Biomarkers Obtained at Hospital Admission
Discussion
Our results suggest that olfactory symptoms are prevalent among patients with mild-moderate COVID-19 infection. The observed rate of 63% is consistent with previously published reports. This frequency appears independent of smoking given our cohort had only 8.6% of smokers.
Although olfactory symptoms are encountered in other upper respiratory viral infections (e.g., rhinovirus, influenza virus, adenovirus, and other coronaviruses), their prevalence is higher in SARS-CoV-2 infection.9 Moreover, to our knowledge, a single case of olfactory dysfunction after severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) infection has been reported.10 It would be difficult to attribute hyposmia and dysgeusia only to upper airways involvement, at least for patients with olfactory symptoms preceding systemic symptomatology. On the other side, rhinorrhea and/or nasal congestion were infrequent, whereas only a small proportion of hyposmic patients reported in their medical history the presence of nasal problems preceding the development of olfactory symptoms and of the COVID-19 infection.4 For these reasons, we think that an “obstructive” mechanism in the pathogenesis of hyposmia is unlikely.
In our study, we found that 22.4% of patients with hyposmia and dysgeusia recalled that these symptoms were their initial complaints. Given this, we suggest that these symptoms should be considered as a sentinel of an incumbent infection. At the very least, hyposmia and dysgeusia should be symptoms that prompt close monitoring of patients for subsequent symptoms of COVID-19 infection and consideration for isolation or quarantine until infection is confirmed or disproven.
Recently, it has been reported that 44% of secondary cases of laboratory confirmed COVID-19 are infected during the index case's presymptomatic stage.11 This important observation underlines the usefulness in detecting early signs of infection and should be re-evaluated in the light of the possible presence of olfactory dysfunction.
The temporal presentation of olfactory symptoms raises some questions: Could they represent a clinical correlate of a CNS entry of SARS-CoV-2, alternative to the pulmonary or intestinal ones? Are olfactory nerves uniquely susceptible to SARS-CoV-2 infection? Could asymptomatic viral spread through CSF or glymphatics explain some of the symptoms of this new infection?
In addition, the lower number of total leukocytes and neutrophils in hyposmic patients, compared with nonhyposmic ones, could be suggestive of an earlier viral induced cytopenia. It is not possible for the moment to infer that this represents a damage of the cellular immune system, but its association with hyposmia could at least help in identifying patients at risk.
As of today, a preclinical evidence of CNS invasion in SARS-CoV-2 infection does not exist, and viral RNA isolation in CSF has been published just for 1 case,12 in addition to the case reported by the press.13 Nevertheless, clinical and laboratory evidence exists that beta-coronaviruses14 related to SARS-CoV-2 (i.e., Middle East respiratory syndrome [MERS] and SARS-CoV-1) have access to CNS and may damage nerve cells. Murine models of MERS infection demonstrated viral entry through olfactory neurons and then spread through thalamus to brainstem structures, whereas in SARS-CoV-1 murine models neuronal damage may exist without encephalitis evidence and may drive disease outcome.15
This study has several limitations. First of all, only the patients still admitted in the ward have been personally interviewed. This may have created a difference with the information obtained on the phone from the already discharged patients although we used a standardized questionnaire. Second, we cannot exclude the possibility of recall biases although we performed the interviews in the shortest possible time. For the same reason, all the patients with the evidence of cognitive decline have been excluded from the study. Moreover, detection of olfactory and gustatory symptoms was performed by means of a questionnaire, which has a lower sensitivity compared to olfactory tests.2 This may have led to an underestimation of symptom prevalence. Finally, we cannot rule out that our results might have been different if we had been able to interview the patients who had severe course, leading to ICU admission or fatal outcome.
Although preliminary, our findings suggest that hyposmia and dysgeusia should be considered evidence of COVID-19 infection and should prompt a cross-specialty approach to diagnosis, bearing in mind that there could be more at play than what the lung can tell us.
Appendix. Authors
Footnotes
Contributor Information
Carlo Tascini, Email: c.tascini@gmail.com.
Mariarosaria Valente, Email: mariarosaria.valente@uniud.it.
Alessandro Marini, Email: alemarini00@gmail.com.
Andrea Surcinelli, Email: andsurcinelli@gmail.com.
Gaia Pellitteri, Email: pellitteri.gaia@gmail.com.
Chiara De Carlo, Email: chia.decarlo@gmail.com.
Valentina Gerussi, Email: valentina.gerussi@gmail.com.
Gian Luigi Gigli, Email: gigli.gl@gmail.com.
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis 2020;71:889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol 2020;10:944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID‐19 patients: single‐center experience on 72 cases. Head Neck 2020;42:1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klopfenstein T, Kadiane-Oussou NJ, Toko L, et al. Features of anosmia in COVID-19. Méd Mal Infect 2020;50:436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltrán‐Corbellini Á, Chico‐García JL, Martínez‐Poles J, et al. Acute‐onset smell and taste disorders in the context of Covid‐19: a pilot multicenter PCR‐based case‐control study. Eur J Neurol Epub 2020 Apr 22. [DOI] [PMC free article] [PubMed]
- 7.Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self‐reported olfactory loss associates with outpatient clinical course in Covid‐19. Int Forum Allergy Rhinol 2020;10:821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 9.Wee LE, Chan YFZ, Teo NWY, et al. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur Arch Otorhinolaryngol 2008;277:2389–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwan 2006;15:26–28. [PubMed] [Google Scholar]
- 11.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672–675. Available at: nature.com/articles/s41591-020-0869-5.pdf. Accessed April 20, 2020. [DOI] [PubMed] [Google Scholar]
- 12.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 2020;94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beijing hospital confirms nervous system infections by novel coronavirus—Xinhua | English.news.cn [online]. Available at: xinhuanet.com/english/2020-03/05/c_138846529.htm. Accessed March 28, 2020.
- 14.Li K, Wohlford-Lenane C, Perlman S, et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 2015;213:712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008;82:7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.