Summary
Background:
Biologic therapies in patients with Crohn’s disease often yield low clinical and endoscopic remission rates. After multiple failed therapies, combining two biologic therapies is possibly the sole medical alternative to recurrent surgery. However, data on this approach are limited.
Aims:
Assess the efficacy and safety of concomitant use of two biologic therapies in the largest cohort to date of refractory Crohn’s disease patients.
Methods:
Data were extracted from Crohn’s disease patients started on dual biologic therapy at two referral centers. Biologics utilized include infliximab, adalimumab, vedolizumab, ustekinumab, certolizumab, and golimumab. The primary outcome was endoscopic improvement (>50% reduction in Simplified Endoscopic Score-Crohn’s disease (SES-CD) or explicitly stated). Endoscopic remission (SES-CD<3 or stated), clinical response (Crohn’s disease-patient reported outcome-2 score (PRO2) reduced by 8), clinical remission (PRO2<8), and C-reactive protein were also assessed.
Results:
22 patients with 24 therapeutic trials of dual biologic therapy were identified. The majority of patients had prior surgical resections (91%), stricturing (59%) or penetrating (36%) phenotype, and perianal fistulas (50%). Median number of prior failed biologics was four. Endoscopic improvement occurred in 43% of trials, and 26% achieved endoscopic remission. 50% had clinical response and 41% achieved clinical remission. There were significant post-treatment reductions in median SES-CD [14.0(12.0-17.5) to 6.0(2.5-8.0), p=0.0005], PRO-2 [24.1(20.3-27.0) to 13.4(4.6-21.8), p=0.002], and C-reactive protein [17.0(11.0-24.0) to 9.0(4.0-14.0), p=0.02]. Presence of perianal fistulas decreased from 50% to 33%. Adverse events occurred in 13% of trials.
Conclusion:
Dual biologic therapy was associated with clinical, biomarker, and endoscopic improvements in selected patients with refractory Crohn’s disease who failed multiple biologics. Further studies are needed to validate this approach.
Keywords: Refractory Crohn’s disease, dual biologic therapy, combining biologics, adalimumab, infliximab, vedolizumab, ustekinumab, certolizumab, golimumab
Introduction
Crohn’s disease is a progressive immune-mediated disorder that can involve the entire gastrointestinal tract and presents with an inflammatory, stricturing, or penetrating phenotype.1 Complications of Crohn’s disease – including strictures, fistulas, and abscesses – occur in 50% of patients.2 Additionally, 50% of patients require surgery within ten years of diagnosis.3, 4 Biologic medications have significantly advanced the management of inflammatory bowel disease and have become the cornerstone in the management of moderate-to-severe Crohn’s disease.1 Effective biologic agents for treating Crohn’s disease include tumor necrosis factor-α (TNF)-antagonists to (infliximab, adalimumab, certolizumab pegol), and more recently, antibodies inhibiting the p40 subunit of interleukins (IL)-12 and -23 (ustekinumab) and α4β7 integrins on leukocytes (vedolizumab).5 Randomized controlled trials demonstrate that combination therapy with infliximab and azathioprine is more effective than either treatment alone.6 However, few patients achieve prolonged clinical remission and significant risk for subsequent surgical resections exists despite biologic therapies.1 In refractory Crohn’s disease patients who have failed multiple biologics, therapeutic options are limited. Inability to achieve clinical remission or response deceases quality of life, and recurrent bowel resections can lead to complications such as short bowel syndrome with dependence on parenteral support, bile acid diarrhea, and nutritional deficiencies.1, 7, 8 Thus, just as combination therapy with a biologic and immunomodulator is more effective than either alone6, the concomitant use of dual biologics that target different inflammatory pathways is a potential therapeutic approach in patients with severe and treatment-refractory disease.9 In all Crohn’s disease patients, the simultaneous use of two biologic therapies can potentially lead to improved clinical and endoscopic outcomes.
Biologic agents are effective in treating immune-mediated diseases, but concerns remain regarding adverse events, including infection and malignancy. Data from the TREAT registry demonstrated that while infliximab was associated with an increase in serious infections, stronger associations were shown with corticosteroids, Crohn’s disease severity, and narcotic use.10 Large nationwide cohort studies have demonstrated that both thiopurine and TNF-antagonist monotherapy was associated with an increased risk of lymphoma, and combination therapy further increased this risk.11 While thiopurines are associated with infection and malignancy, their combination with TNF-antagonists is commonplace. Conversely, newer approved biologic agents for Crohn’s disease such as vedolizumab and ustekinumab have not yet demonstrated these risks. Vedolizumab targets the α4β7 integrin receptor to block lymphocyte homing to the intestinal mucosa, and has demonstrated a favorable safety profile in clinical trial12 and real-world13 settings. Ustekinumab inhibits the common p40 subunit of IL-12 and -23 and is safe and effective in Crohn’s disease. Additionally, randomized controlled trials as well as real world data demonstrate a favorable safety profile across multiple diseases.14, 15
Previous randomized controlled trial data analyzing the combination of natalizumab and infliximab in patients with refractory Crohn’s disease did not demonstrate increased adverse events over infliximab monotherapy.16 Although the trial was not designed to analyze efficacy and endoscopic endpoints were not assessed, a trend existed towards symptom-based clinical improvement. Currently, natalizumab is rarely used due to the risk of progressive multifocal leukoencephalopathy and availability of safer and more selectively targeted anti-integrin therapies such as vedolizumab.1 Additional data on dual therapy with conventional biologics remains limited to case reports and series.17–20 Thus, a major knowledge gap exists on the safety and efficacy of combining contemporary biologic therapies in Crohn’s disease.
Despite the safety of newer biologics, simultaneous treatment with two (dual) biologic medications (DBT) is rarely utilized. It is unknown if concomitant use of these newer biologics with one another or with a TNF-antagonist would increase the overall risk for infectious complications compared to current standard of care. We hypothesize that vedolizumab may be an optimal therapeutic anchor to dual biologic therapy due to its favorable safety profile, which is attributed to its gut-specific mechanism. For similar safety considerations, ustekinumab, may be a reasonable alternative anchor.12, 13, 21, 22 We report the largest cohort to date on the preliminary evidence of efficacy and tolerability of DBT in a high-risk group of patients with refractory Crohn’s disease.
Materials and Methods
Patient population
Data were reviewed from patient records at the University of California, San Diego (La Jolla, California) and University of Calgary (Calgary, Alberta). Data were extracted from patients with Crohn’s disease that had started a combination of two different biologics between January 1, 2007 and December 31, 2018. At the University of California, San Diego, we utilized a program, “SlicerDicer”, in the electronic medical record (Epic Systems Corporation (Verona, Wisconsin)), that permits identification of all patients with overlapping dates of biologic prescriptions or medication documentation, regardless of duration. Subsequently, individual chart review was performed to confirm patients received dual biologic therapy. At the University of Calgary, all patients starting dual biologic therapy for any duration were identified prospectively and the data were extracted retrospectively. No patient was started on dual biologic therapy for an indication other than Crohn’s disease, such as rheumatologic or dermatologic diseases. Biologics searched include permutations for combinations of adalimumab, infliximab, certolizumab, golimumab, vedolizumab, natalizumab, and ustekinumab. Chart review and data extraction were performed on each potential combination (E.Y., N.P.).
Data regarding demographics, disease phenotype, prior biologic therapies (prior response, dosing, failures), prior medications (corticosteroids, immunomodulators, antibiotics), prior surgeries, presence of fistulas, laboratory data, and adverse events were obtained thorough chart review. Endpoints were assessed at the time of endoscopic evaluation closest to one year of treatment with DBT or at the time of treatment failure.
Approach to combining biologics
All therapeutic trials consisted of two biologic medications which included either vedolizumab, ustekinumab, or a TNF-antagonist paired with each other. In all therapeutic trials, patients were receiving therapy with a single biologic agent, and the second biologic was added on. Documented infusion of the second concomitant biologic medication was considered the start dates for DBT. Therapeutic drug monitoring was utilized in the majority of cases as standard of care at our institutions – this includes reasons for biologic failure (such as primary non-response, secondary non-response, immunogenicity, and pharmacokinetic or mechanistic failure) and dose escalated regimens.
Outcomes
The primary outcome was endoscopic improvement during maintenance therapy, defined as either >50% reduction in Simplified Endoscopic Score-Crohn’s disease (SES-CD) or explicitly stated on the endoscopy report by an inflammatory bowel disease specialist. Other outcomes included endoscopic remission (SES-CD<3 or clear stated), clinical response (Crohn’s disease–patient reported outcome-2 score (PRO-2) reduced by 8), clinical remission (PRO2<8), and adverse events.23, 24 Need for surgery was considered treatment failure for all endoscopic and clinical outcomes. Per-patient analysis was repeated using the most recent therapeutic trials. PRO-2 scores were calculated using standardized forms routinely given to patients, or from records if clearly stated.23, 24
Statistical analysis
Data analysis included descriptive statistics computed for continuous variables (means, standard deviations [SD]). Percentages were used for categorical variables. Between-groups comparisons were performed using chi-square, Fishers exact test, t-test or Wilcoxon rank testing, as appropriate. P-values ≤0.05 were considered significant. All analyses were done using STATA SE 15.1 (StataCorp, College Station, TX, USA).
Ethics
All authors had access to study data, reviewed, and approved the final manuscript. Study protocol and materials were approved by the institutional review boards at University of California, San Diego and the University of Calgary.
Results
Baseline characteristics
A total of 24 therapeutic trials of DBT among 22 patients with Crohn’s disease were identified. The median age was 35 (IQR 31-43), 95% of patients were diagnosed before the age of 40, and 32% were diagnosed before the age of 16 (Table 1). The majority of patients had either a stricturing (59%, n=13) or penetrating (36%, n=8) phenotype. 55% (n=12) of the total patients had any prior history of perianal fistulas (Table 1). The median number of prior failed biologics was four. Of the 94-total failed single biologic trials among 22 patients, the most common reasons for failure were secondary non-response without immunogenicity (47%, n=44), followed by primary non-response (43%, n=40), immunogenicity (7%, n=7), and adverse drug event (3%, n=3) (Table 2).
Table 1.
Baseline Characteristics
| Median age | 35 |
| Age at diagnosis <16 years old | 32% (7/22) |
| Female gender | 55% (12/22) |
| Crohn’s disease location | |
| Ileal | 18% (4/22) |
| Colonic | 27% (6/22) |
| Ileocolonic | 55% (12/22) |
| Proximal involvement | 5% (1/22) |
| Crohn’s disease phenotype | |
| Inflammatory | 5% (1/22) |
| Stricturing | 59% (13/22) |
| Penetrating | 36% (8/22) |
| Any history of perianal fistulas | 55% (12/22) |
| Mean number of failed biologies | 4 |
| Immunomodulator | 79% (19/24) |
| Steroid | 33% (8/24) |
| Antibiotic | 33% (8/24) |
| Prior surgery | 91% (20/22) |
Table 2.
Description of prior biologic agents and dual biologic therapy (DBT) regimens: the median number of prior failed biologies was four.
| Reasons for prior biologic failure | Biologic at baseline prior to DBT initiation | Combinations of DBT | |||
|---|---|---|---|---|---|
| Primary non-response | 43% (40/96) | vedolizumab | 63% (15/24) | vedolizumab / ustekinumab | 33% (8/24) |
| vedolizumab / infliximab | 25% (6/24) | ||||
| Secondary non-response † | 47% (44/96) | ustekinumab | 33% (8/24) | vedolizumab / adalimumab | 17% (4/24) |
| ustekinumab / adalimumab | 8% (2/24) | ||||
| Immunogenicity | 7% (7/96) | infliximab | 4% (1/24) | vedolizumab / certolizumab | 8% (2/24) |
| ustekinumab / infliximab | 4% (1/24) | ||||
| Adverse event | 3% (3/96) | vedolizumab / golimumab | 4% (1/24) | ||
without immunogenicity.
Prior to initiating DBT, 79% (n=19) of trials were receiving immunomodulators, 33% (n=8) receiving corticosteroids, and 33% (n=8) receiving antibiotics. Additionally, 91% (n=20) of patients had undergone prior inflammatory bowel disease-related surgery, and 50% of trials had ongoing perianal fistulas prior to initiating DBT (Table 1). Prior to initiating DBT, all patients were receiving biologic therapy with either vedolizumab (n=15), ustekinumab (n=8), or infliximab (n=1) (Table 2). No patients were in clinical remission; the median PRO-2 score prior to DBT was 24.1 (IQR 20.0-27.0), with 91% (n=20) having at least moderate disease activity. PRO-2 scores could not be calculated in two patients due the presence of an ostomy. No patients were in endoscopic remission at baseline. At baseline, median SES-CD was 14.0 (IQR 12.0-17.5) and all patients had at least moderate disease activity on endoscopy. Baseline C-reactive protein (CRP) was elevated in 79% (n=19), with a median CRP of 17.0 mg/L (IQR 11.0-24.0 mg/L) (Table 3).
Table 3.
Endpoints for Dual Biologic Therapy (DBT)
| Baseline | Post-Treatment | |
|---|---|---|
| PRO-2 score (median) † | 24.1 | 13.4 |
| Clinical Remission | 0/22 (0%) | 9/22 (41%) |
| Mild | 2/22 (9%) | 2/22 (9%) |
| Moderate | 20/22 (91%) | 9/22 (41%) |
| Severe | 0/22 (0%) | 2/22 (9%) |
| Clinical Response | n/a | 11/22 (50%) |
| Endoscopic ‡ | ||
| Remission | 0/23 (0%) | 6/23 (26%) |
| Improvement | n/a | 10/23 (43%) |
| C-reactive protein (median) | 17 mg/L | 9 mg/L |
| Albumin (median) | 36 g/L | 37 g/L |
| Perianal fistula present | 12/24 (50%) | 8/24 (33%) |
| Required surgery | n/a | 8/24 (33%) |
| Adverse event | n/a | 3/24 (13%) |
| Serious adverse event | n/a | 2/24 (8%) |
PRO-2 (Crohn’s disease-patient reported outcome-2 score) unable to be calculated in two trials due to presence of an ostomy.
Endoscopic endpoint data not yet available in four trials; three were consideredfailure of DBT due to surgery or entering a clinical trial - one was awaiting follow-up but achieved clinical response.
The most common combinations of individual biologics were vedolizumab and ustekinumab (33%, n=8). Vedolizumab was combined with a TNF-antagonist in 13 trials (54%) and ustekinumab was combined with a TNF-antagonist in three trials (12.5%, Table 2). A total of 79% of all DBT regimens included at least one biologic that had induced a prior initial response with subsequent loss of response (secondary non-response) and 29% of DBT trials utilized a biologic that had not been previously used. In therapeutic trials achieving an endoscopic response (n=10), seven trials utilized at least one biologic that demonstrated prior secondary non-response and two trials utilized a biologic that had not been previously used. All baseline biologics and 63% (n=15) of the second biologic agents in each therapeutic trial were administered as a dose-escalated regimen.
Efficacy of dual biologics
Endoscopic improvement occurred in 43% of therapeutic trials, and endoscopic remission was achieved in 26% of trials. The median SES-CD significantly reduced from 14.0 (IQR 12.0-17.5) to 6.0 (IQR 2.5-8.0) (p=0.0005) (Figure 1). The median time to endoscopic assessment was 225 days from initiation of DBT.
Figure 1.

Endpoints of Dual Biologic Therapy (DBT).
A) SES-CD: Simplified Endoscopic Score – Crohn’s Disease. B) PRO-2: Crohn’s Disease – Patient Reported Outcome-2 Score. C) CRP: C-reactive protein.
Clinical response occurred in 50% of therapeutic trials, clinical remission was achieved in 41%, and steroid-free clinical remission was achieved in 36%. The median PRO-2 score significantly reduced from 24.1 (IQR 20.3-27.0) at baseline to 13.4 (IQR 4.6-21.8) after treatment (p=0.002) (Figure 1), and the median CRP concentration declined from 17.0 mg/L (IQR 11.0-24.0) to 9.0 mg/L (IQR 4.0-14.0) (p=0.02) (Table 3). Presence of perianal fistulas declined from 50% at baseline to 33% post-treatment (Table 3). Surgery was required after 33% (n=8) of therapeutic trials and these were considered treatment failures. At the patient level, endoscopic improvement, endoscopic remission, clinical response, and clinical remission rates were 43%, 29%, 50%, and 40%, respectively.
Patients were treated with DBT for a median of 274 days (IQR 191-365) and followed for up to one year. At one year, the proportion of patients remaining on DBT was 38%, all of which were successful and ongoing trials of DBT (Supplementary Figure 1). The most common reasons for ending a trial of DBT were non-response or worsening of disease activity requiring surgery. At the end of DBT, 75% (n=18) of trials were additionally receiving immunomodulators, 17% (n=4) receiving corticosteroids, and 25% (n=6) receiving antibiotics.
The most commonly utilized drug class in DBT was TNF-antagonists, which is also the only drug class for Crohn’s disease with multiple approved medications. Reasons for prior TNF-antagonist failure that were assessed in this study included mechanistic failure (primary non-response and secondary non-response despite adequate drug concentrations), secondary non-response due to immunogenicity, and adverse event (Figure 2). In both trials of DBT that achieved or did not achieve endoscopic improvement utilizing a TNF-antagonists (n=15), all patients had prior failure to both infliximab and adalimumab as separate treatment regimens – five achieved the primary endpoint of endoscopic improvement and ten did not. The most common reason for prior failure of either infliximab or adalimumab was secondary non-response despite adequate drug concentrations, and there was no signal for differences between those with and without improvement. Although specific drug concentration measurements were not available, documentation by expert inflammatory bowel disease specialists that drug concentrations were adequate at the time of treatment failure was utilized.
Figure 2.
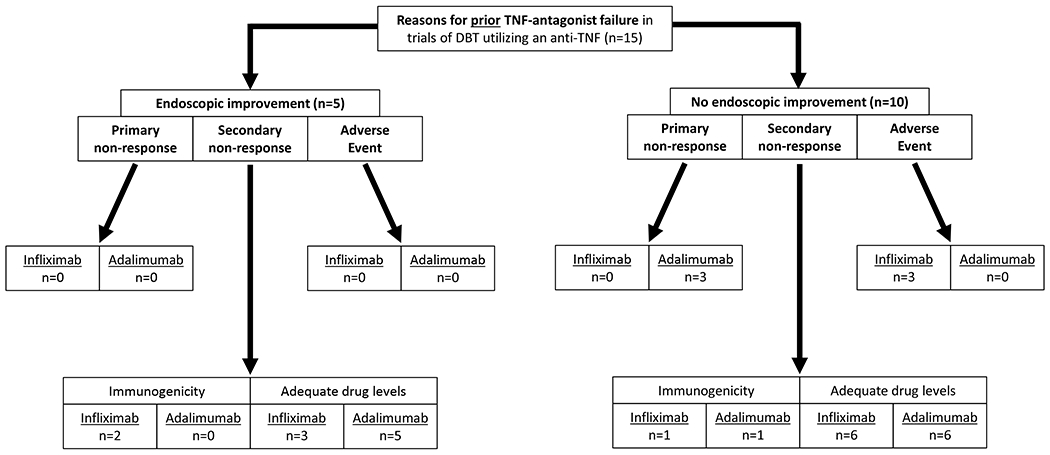
Flowchart of reasons for prior TNF-antagonist failure in dual biologic therapy regimens that utilized a TNF-antagonist
Reasons for prior biologic failure for the entire cohort were assessed (Supplementary Table 1). Among all therapeutic trials that achieved endoscopic response (n=10), nine trials utilized at least one biologic in the regimen that either had demonstrated a prior response (secondary non-response) or had never been exposed to (naive) (Supplementary Table 1). Among therapeutic trials that did not achieve an endoscopic response (n=13), ten trials utilized at least one biologic in the regimen that either had prior secondary non-response or the patient was naïve to (Supplementary Table 1).
Adverse Events
Adverse events occurred in three trials (13%). One trial ended due to drug-induced lupus attributed to adalimumab. One patient developed pneumonia and another had reported basal cell skin cancer, recurrent Clostridium Difficile infection, and Acinetobacter bacteremia in a patient with a recurrent history of all three diseases prior to initiation of DBT.
Amongst the combinations used as DBT, vedolizumab combined with ustekinumab had numerically higher rates of endoscopic improvement, but similar endoscopic remission and adverse event rates (Table 4).
Table 4.
Endpoints for Combinations of Dual Biologic Therapy (DBT)
| Endoscopic Improvement | Endoscopic Remission | Clinical Response | Clinical Remission | Adverse Events | |
|---|---|---|---|---|---|
| vedolizumab / ustekinumab | 63% (5/8) | 25% (2/8) | 71% (5/7)‡ | 57% (4/7)‡ | 13% (1/8) |
| vedolizumab / TNF-antagonist | 33% (4/12)† | 25% (3/12)† | 42% (5/12)‡ | 33% (4/12)‡ | 15% (2/13) |
| ustekinumab / TNF-antagonist | 33% (1/3)† | 33% (1/3)† | 33% (1/3) | 33% (1/3) | 0% (0/3) |
| All DBT trials | 43% (10/23)† | 26% (6/23)† | 50% (11/22)‡ | 41% (9/22)‡ | 13% (3/24) |
Endoscopic endpoint data not yet available in four trials; three were considered failure of DBT due to surgery or entering a clinical trial (two vedolizumab/TNF-antagonist, one ustekinumab/TNF-antagonist) – one was awaiting follow-up but achieved clinical response (vedolizumab/TNF-antagonist).
PRO-2 unable to be calculated in two trials due to presence of an ostomy (one in vedolizumab/ustekinumab and one in vedolizumab/TNF-antagonist).
Discussion
Patients with longstanding Crohn’s disease often fail multiple biologic therapies and require surgical resections. As remission rates of individual biologics are low and limited further options exist, combining biologics may be a reasonable alternative to avoid disease progression and recurrent surgery. However, limited evidence on this approach exists. We report the largest study to date assessing the efficacy and safety of concomitant use of two (dual) biologic therapies in refractory Crohn’s disease. This study reflects a high-risk population, with a large majority having a stricturing or penetrating phenotype and a high prevalence of perianal disease. Objective endoscopic outcomes were achieved in significant proportions of patients and endoscopic scores decreased significantly. Furthermore, objective documentation of fistula resolution existed in many patients. This is consistent with clinical remission rates, PRO-2 reductions, and objective reductions in CRP. Three adverse events (13%) were recorded.
Overall, the preliminary evidence on the efficacy of DBT in this study appears promising. This is particularly evident when considering the limited available therapeutic options in this cohort of refractory Crohn’s disease patients. In these patients, 91% had undergone prior surgical resections and the median number of previously failed biologics was four. In addition, the severe and treatment-refractory disease phenotype described in our cohort is not uncommon in other tertiary academic medical centers. Thus, when considering the range of previously failed medical and surgical therapies in patients with refractory and high-risk Crohn’s disease, an endoscopic improvement rate of 43% may imply a significant beneficial effect of this regimen. Additionally, the proportion of patients remaining on dual biologic therapy at one year was similar to the endoscopic response rate. Although there was no preliminary signal, adverse event rates were comparable to previous TNF-antagonist data.25–27
Previously, pooled data using small case series and case reports in inflammatory bowel disease patients treated with DBT included ten Crohn’s disease patients, with a majority having received vedolizumab and a TNF-antagonist.20 Although prior small case series had similar adverse event rates to the present study, endoscopic data were generally lacking.18, 19 In contrast, the current study significantly increases available data on this topic in patients with severe and high-risk Crohn’s disease phenotypes.
Although a study limitation was its retrospective design, objective endoscopic and biomarker endpoints were evaluated and patient reported outcomes were assessed. Adverse events were documented for a long duration, and the therapeutic impact of a diverse range of biologic combinations and dosing were assessed. While a possibility exists that patients started on dual biologic therapy for very short durations were not captured in this study, all patients receiving DBT were identified using either software from electronic medical records or prospective identification of patients, to permit identification of all patients with overlapping dates of biologic prescriptions or medication documentation, regardless of duration. Additionally, chart review was performed to confirm patients received dual biologic therapy. Lastly, data were not collected for patients in whom DBT was recommended, but not initiated.
The ideal approach to selecting dual biologic regimens remains to be determined. Vedolizumab or ustekinumab may be ideal adjunct therapies in DBT regimens due to their favorable safety profile.12, 13, 21, 22 For this reason, most patients in our study received vedolizumab, and the most frequently used pairing was with ustekinumab. One consideration is an individualized approach that incorporates reasons for prior biologic failures, such as utilizing biologics with prior response (secondary non-response without immunogenicity) in addition to targeting an alternative untreated inflammatory pathway while avoiding biologics that have previously caused immunogenicity. While we did not find a significant difference in endoscopic response rates in this current study when comparing prior reasons for biologic failure or alternate inflammatory targets, this study was not designed to detect this difference. Another consideration may be that the patients in our cohort had a high median number of previously failed biologic agents, which limits therapeutic choices from a mechanistic standpoint.
In summary, DBT was associated with clinical, biomarker, and endoscopic improvements in a highly selective cohort with refractory Crohn’s disease who have failed multiple biologics. While our findings appear promising, additional real world reports and randomized controlled trial data will assist in determining the efficacy and safety of DBT in refractory Crohn’s disease.
Supplementary Material
STROBE Statement—Checklist of items that should be included in reports of cohort studies
| Item No | Recommendation | |
|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract Listed in line 2 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found This is provided – lines 27-45 | ||
| Introduction | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported This is provided – lines 52-99 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses This is provided – lines 92-99 |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper This is provided – lines 100-142 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection This is provided – lines 101-118 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up This is provided – lines 101-118 |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable This is provided – lines 127-134 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group This is provided – lines 127-134 |
| Bias | 9 | Describe any efforts to address potential sources of bias This is provided – lines 100-142 and in discussion lines 236-244 |
| Study size | 10 | Explain how the study size was arrived at This study included all patients that were started on dual biologic therapy in the study inclusion dates. |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why This is provided – lines 135-139 |
| Statistical methods | 12 |
The below are addressed in lines 135-139 (a) Describe all statistical methods, including those used to control for confounding |
| (b) Describe any methods used to examine subgroups and interactions | ||
| (c) Explain how missing data were addressed | ||
| (d) If applicable, explain how loss to follow-up was addressed | ||
| (e) Describe any sensitivity analyses | ||
| Results | ||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed This is provided – lines 145-171 and 184-186 |
| (b) Give reasons for non-participation at each stage N/A | ||
| (c) Consider use of a flow diagram | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders This is provided – lines 146-163 and Table 1 |
| (b) Indicate number of participants with missing data for each variable of interest This is described in Tables 3 and 4: † PRO-2 unable to be calculated in two trials due to presence of an ostomy. ‡ Endoscopic endpoint data not yet available in four trials; three were considered failure of DBT due to surgery or entering a clinical trial - one was awaiting follow-up but achieved clinical response. | ||
| (c) Summarise follow-up time (eg, average and total amount) This is provided – lines 184-186 | ||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time This is provided – lines 172-201 |
| Main results | 16 |
The below are provided in lines 144-208 (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included |
| (b) Report category boundaries when continuous variables were categorized | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses N/A |
| Discussion | ||
| Key results | 18 | Summarise key results with reference to study objectives This is provided – lines 211-220 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias This is provided – lines 236-244 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence This is provided – lines 245-259 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results This is provided – lines 256-259 |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based This is provided – lines 328-373 |
Acknowledgements:
Statement of Interests:
1. Authors’ declaration of personal interests:
E.Y. reports: no potential conflicts of interest.
N.P. reports: no potential conflicts of interest.
N.W. reports: no potential conflicts of interest.
P.S.D. reports: research support from Takeda, Pfizer, AbbVie, Janssen, Polymedco, ALPCO, Buhlmann; consulting fees from Takeda, Pfizer, Abbvie, Janssen.
N.V.C. reports: research and consulting support from Takeda and UCB, research support from R-Biopharm and consulting support from Janssen, Pfizer, Progenity and Prometheus.
S.S. reports: research grant from AbbVie; consulting fees from Takeda and AbbVie; honorarium for adhoc grant review from Pfizer.
B.S.B. reports: consulting fees from Pfizer; research grants from Takeda and Janssen and Prometheus Laboratories.
A.C. reports: honorarium/speaker fees for AbbVie, Janssen, UCB; consulting fees from AbbVie and Takeda.
W.J.S. reports: research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos, Pfizer, Prometheus Laboratories (now Prometheus Biosciences); consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech - consultant, stock options; Progenity - consultant, stock; Oppilan Pharma - employee, stock options; Escalier Biosciences - employee, stock options; Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories) - employee, stock options; Ventyx Biosciences – employee, stock options; Vimalan Biosciences – employee, stock options.
R.P. reports: consultancy fees from AbbVie, Abbott, Alba Therapeutics, Allergan, Amgen, Aptalis, AstraZeneca, Atlantic Healthcare, Baxter, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Cosmo Technologies, Coronado Biosciences, Cubist, Eisau Medical Research, Elan, Eli Lilly, enGene, EnteroMedics, Exagen Diagnostics, Ferring, Genentech, Genzyme, Gilead, Given Imaging, GSK, Hospira, Human Genome Sciences, Janssen, Merck & Co., Merck Research Laboratories, Merck Serono, Millennium, Nisshin Kyorin, Novo Nordisk, Pfizer Inc, Qu Biologics, Receptos, Relypsa, Salient, Salix Pharmaceuticals, Santarus, Shire, Sigmoid Pharma, and Takeda; speakers bureau fees from AbbVie, Aptalis, Celgene, Ferring, Janssen, Merck, Pfizer Inc, Prometheus Laboratories, Shire, and Takeda; financial support for research from AbbVie, Ferring, Janssen, Shire, and Takeda.
R.B. reports: no potential conflicts of interest.
2. Declaration of funding interests:
P.S.D. is supported by an American Gastroenterology Association Research Scholar Award.
N.V.C. holds a Research Scholar Award from the American Gastroenterological Association.
W.J.S. was partially supported by the NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
References:
- 1.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. American Journal of Gastroenterology 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 2.Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg 2000;231:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn’s disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol 2014;109:1739–48. [DOI] [PubMed] [Google Scholar]
- 5.Duijvestein M, Battat R, Vande Casteele N, et al. Novel Therapies and Treatment Strategies for Patients with Inflammatory Bowel Disease. Curr Treat Options Gastroenterol 2018;16:129–146. [DOI] [PubMed] [Google Scholar]
- 6.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. New England Journal of Medicine 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 7.Battat R, Duijvestein M, Vande Casteele N, et al. Serum Concentrations of 7alpha-hydroxy-4-cholesten-3-one Are Associated With Bile Acid Diarrhea in Patients With Crohn’s Disease. Clin Gastroenterol Hepatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battat R, Kopylov U, Szilagyi A, et al. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflammatory bowel diseases 2014;20:1120–1128. [DOI] [PubMed] [Google Scholar]
- 9.Hirten RP, Iacucci M, Shah S, et al. Combining Biologics in Inflammatory Bowel Disease and Other Immune Mediated Inflammatory Disorders. Clin Gastroenterol Hepatol 2018;16:1374–1384. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious Infection and Mortality in Patients With Crohn’s Disease: More Than 5 Years of Follow-Up in the TREAT™ Registry. American Journal of Gastroenterology 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. JAMA 2017;318:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meserve J, Aniwan S, Koliani-Pace JL, et al. Retrospective Analysis of Safety of Vedolizumab in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Alimentary pharmacology & therapeutics 2018;48:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh S, Gensler LS, Yang Z, et al. Ustekinumab Safety in Psoriasis, Psoriatic Arthritis, and Crohn’s Disease: An Integrated Analysis of Phase II/III Clinical Development Programs. Drug Saf 2019;42:751–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sands BE, Kozarek R, Spainhour J, et al. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflammatory Bowel Diseases 2007;13:2–11. [DOI] [PubMed] [Google Scholar]
- 17.Liu EY, Loomes DE. Ustekinumab and Vedolizumab Dual Biologic Therapy in the Treatment of Crohn’s Disease. Case Rep Med 2017;2017:5264216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao EJ, Lewin S, Terdiman JP, et al. Safety of dual biological therapy in Crohn’s disease: a case series of vedolizumab in combination with other biologics. BMJ Open Gastroenterol 2018;5:e000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buer LCT, Høivik ML, Warren DJ, et al. Combining Anti-TNF-α and Vedolizumab in the Treatment of Inflammatory Bowel Disease: A Case Series. Inflammatory Bowel Diseases 2018;24:997–1004. [DOI] [PubMed] [Google Scholar]
- 20.Ribaldone DG, Pellicano R, Vernero M, et al. Dual biological therapy with anti-TNF, vedolizumab or ustekinumab in inflammatory bowel disease: a systematic review with pool analysis. Scand J Gastroenterol 2019:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Ferrer A, Laiz A, Puig L. The safety of ustekinumab for the treatment of psoriatic arthritis. Expert Opin Drug Saf 2017;16:733–742. [DOI] [PubMed] [Google Scholar]
- 22.Wils P, Bouhnik Y, Michetti P, et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: a multicentre experience. Aliment Pharmacol Ther 2018;47:588–595. [DOI] [PubMed] [Google Scholar]
- 23.Khanna R, Zou G, D’Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Alimentary Pharmacology & Therapeutics 2015;41:77–86. [DOI] [PubMed] [Google Scholar]
- 24.Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clinical Gastroenterology and Hepatology 2016;14:348–354.e17. [DOI] [PubMed] [Google Scholar]
- 25.Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. The Lancet 2019;393:1699–1707. [DOI] [PubMed] [Google Scholar]
- 26.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein GR, Feagan BG, Cohen RD, et al. Infliximab for Crohn’s Disease: More Than 13 Years of Real-world Experience. Inflammatory Bowel Diseases 2018;24:490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


