Abstract
Background and aims
Body mass index (BMI) has previously been shown to increase mortality and disease severity in patients with COVID-19, but the pooled effect estimate was heterogeneous. Although BMI is widely used as an indicator, it cannot distinguish visceral from subcutaneous fat. This systematic review and meta-analysis aimed to investigate the association between visceral adiposity, subcutaneous fat, and severe COVID-19.
Methods
We performed a systematic literature search using the databases: PubMed, Embase, and EuropePMC. Data on visceral fat area (VTA), subcutaneous fat area (SFA), and total fat area (TFA) were collected. The outcome of interest was severe COVID-19. We used a REML random-effects model to pool the mean differences and odds ratio (OR).
Results
There were 5 studies comprising of 539 patients. Patients with severe COVID-19 have a higher VTA (mean difference 41.7 cm2 [27.0, 56.4], p < 0.001; I2: 0%) and TFA (mean difference 64.6 cm2 [26.2, 103.1], p = 0.001; I2: 0%). There was no significant difference in terms of SFA between patients with severe and non-severe COVID-19 (mean difference 9.3 cm2 [-4.9, 23.4], p = 0.199; I2: 1.2%). Pooled ORs showed that VTA was associated with severe COVID-19 (OR 1.9 [1.1, 2.2], p = 0.002; I2: 49.3%).
Conclusion
Visceral adiposity was associated with increased COVID-19 severity, while subcutaneous adiposity was not.
Prospero id
CRD42020215876.
Keywords: Adiposity, Coronavirus, Obesity, Visceral fat, Visceral fat area
1. Introduction
The growing number of coronavirus disease 2019 (COVID-19) cases is a concern at population level and a burden on health care workers worldwide [1]. Although most patients only experience minor or no symptoms, a small proportion of patients develop severe and critical illness with complications such as the acute respiratory distress syndrome, cardiopulmonary collapse, sepsis, and multiple-organ failure [2]. Early identification of high-risk patients is crucial because prolonged hospitalization is associated with poor prognosis.
The increasing prevalence of obesity is a major public health issue that has been declared as a global pandemic. Obesity is a widely known risk factor for cardiometabolic events and has been associated with increased severity of COVID-19 [3]. Besides deficiency or under-nutrition, excess or over-nutrition may alter the immune response to infection. Distribution of body fat may have a significant impact on the immune system. Lipids can be stored within skeletal muscle, known as intramuscular fat (IMF), when visceral and subcutaneous fat storage capacity is exceeded [4]. Defective immunity and a proinflammatory state due to excess fat accumulation are thought to place these individuals at a higher risk of contracting severe COVID-19 [3,[5], [6], [7]]. Although body mass index (BMI) is widely used as an indicator of body fat, it cannot distinguish visceral from subcutaneous fat [8]. BMI has previously been shown to increase mortality and severity in patients with COVID-19 [[9], [10], [11]], but the pooled effect estimate was heterogeneous [3]. Computed Tomography (CT) facilitates visceral and subcutaneous fat tissue quantification. This systematic review and meta-analysis aimed to investigate the association between visceral adiposity, subcutaneous fat, and severe COVID-19.
2. Material and methods
This study followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines. This study is registered in the PROSPERO database (CRD42020215876).
2.1. Eligibility criteria
The inclusion criteria for this study was 1) observational studies/research letters that report patients with COVID-19, 2) information on visceral fat area (VTA), subcutaneous fat area (SFA), and total fat area (TFA) 3) grouped based on COVID-19 severity, intensive care unit (ICU) admission, or the need for mechanical intubation/ventilation. These study's exclusion criteria were 1) preprint articles, 2) abstract-only publications, 3) editorial/commentaries, 4) review articles, 5) non-research letters, 6) case reports, 7) non-human studies, and 8) non-English language articles. Preprints were excluded because of varying credibility [12].
2.2. Search strategy and study selection
We performed systematic literature search using the databases: PubMed, Embase, and EuropePMC with search terms “Visceral Fat Area” OR “Visceral Adipose Tissue” AND “COVID-19″ OR “SARS-CoV-2″ OR “2019-NcoV” AND “Mortality” OR “Death” OR “Severity” OR “ICU Admission” OR “Intubation” OR “Mechanical Ventilation” OR “Shock”. Two independent authors performed the literature search. The duplicates were removed by manual screening after initial search and the title/abstracts were then screened for potential relevance. The full-texts of the potential articles were then assessed based on the inclusion and exclusion criteria. Initially, the literature search was up until 22 October 2020. The search was later updated, involving articles up until 7 December 2020.
2.3. Data extraction
Two independent authors conducted data extraction using standardized extraction forms that included author, year, study design, age, gender, body mass index, cardiovascular diseases, diabetes mellitus, hypertension, VTA, SFA, TFA, and the outcome of interest. The parameter for visceral adiposity in this study was VTA.
The outcome of interest was severe COVID-19, defined as patients that fulfil at least one of the following criteria 1) Report of the WHO-China Joint Mission on Coronavirus Disease 2019 Criteria [13], 2) disease progression, 3) ICU admission, and 4) the need for mechanical intubation/ventilation. Non-severe COVID-19 was defined as patients that did not fulfill any of the four criteria. The mean difference in VTA, SFA, TFA between severe and non-severe group was reported in mean difference (cm2). The effect estimate for the association between VTA and severity was reported in odds ratio (OR), the most stringent adjustment was included in the meta-analysis. The included studies' quality and risk of bias were assessed using the Newcastle–Ottawa Scale (NOS).
2.4. Statistical analysis
The meta-analysis of studies was performed using STATA version 16. Continuous variables such as VTA, SFA, and TFA were pooled using the restricted maximum likelihood (REML) method and reported as mean differences and standard deviations (SDs) between the severe COVID-19 and non-severe COVID-19 groups. We used REML method for pooling of OR from the individual studies, it was reported along its 95% confidence intervals (CI). Random-effects model was used for analysis regardless of heterogeneity. P-values ≤0.05 were considered as significant. Regression-based Egger's test was used to assess small-study effects.
3. Results
3.1. Baseline characteristics and study selection
Out of 118 total articles from the initial databases search, 22 were excluded because of duplicates. The remaining articles (n = 96) underwent title and abstract screening, of which 83 articles were excluded based on the exclusion criteria. Finally, from 13 studies remaining, 3 were excluded because of no data on visceral adiposity, 3 were review articles, 1 report thickness (not area), and 1 reported visceral fat by CT density. Five studies comprising of 539 patients were included in the systematic review and meta-analysis [4,[14], [15], [16]]. Fig. 1 depicts the PRISMA diagram. The baseline characteristics of the included studies are displayed in Table 1 . Assessment using NOS indicates a low-moderate risk of bias among the included studies [Table 1].
Fig. 1.
Study flowchart.
Table 1.
Baseline characteristics of the included studies.
| Author | Design | Samples | Age | Male | BMI | Diabetes | Hypertension | Outcome of Interest | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Chandrana 2020 | RC | 51 | 67.9 vs 57.3 | 100 vs 67.5 | 27.6 vs 29.8 | NR | NR | Mechanical Ventilation | 6 |
| Favre 2020 | RC | 165 | 65.8 vs 63.6 | 81 vs 60 | 27.2 vs 25.5 | NR | NR | Severe COVID-19 | 8 |
| Petersen 2020 | CS | 30 | 65.6 | 60 | 26.8 vs 26.1 | 83 | 50 | ICU Care | 8 |
| Watanabe 2020 | RC | 150 | 70.8 vs 62.1 | 65.7 vs 64.3 | NR | 8.6 vs 18.2 | 34.3 vs 46.1 | Intubation | 9 |
| Yang 2020 | RC | 143 | 67 vs 65 | 60 vs 43.9 | 24.8 vs 23 | 22.2 vs 18.4 | 51.1 vs 30.6 | Critical Illness (ARDS/sepsis with organ dysfunction) | 8 |
BMI: Body Mass Index, CS: Cross-Sectional, RC: Retrospective Cohort, NOS: Newcastle–Ottawa Scale, NR: Not Reported.
3.2. Adiposity and severe COVID-19
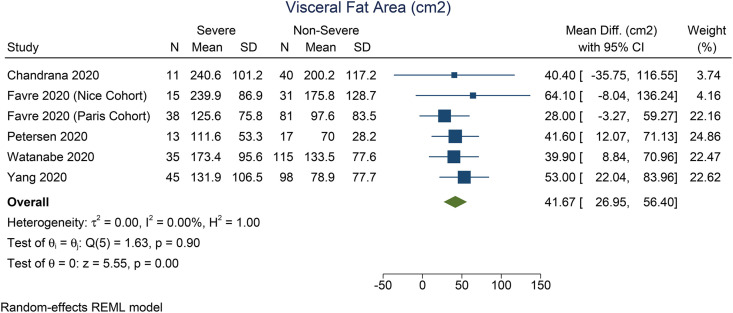
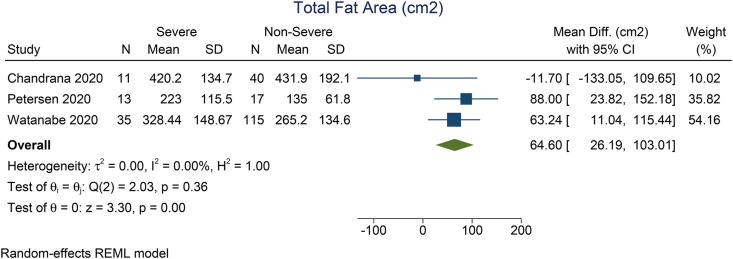
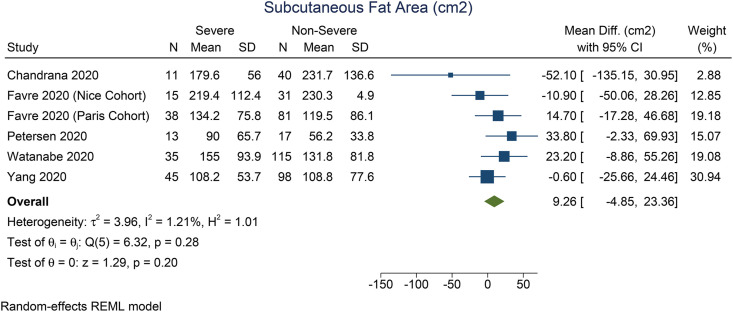
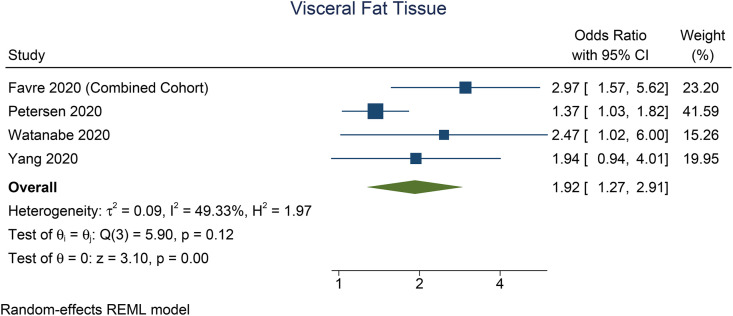
Patients with severe COVID-19 have a higher VTA (mean difference 41.7 cm2 [27.0, 56.4], p < 0.001; I2: 0%, p = 0.897) [Fig. 2 ] and TFA (mean difference 64.6 cm2 [26.2, 103.1], p = 0.001; I2: 0%, p = 0.362) [Fig. 3 ] than the patients with non-severe COVID-19. There was no significant difference in terms of SFA between patients with severe and non-severe COVID-19 (mean difference 9.3 cm2 [-4.9, 23.4], p = 0.199; I2: 1.2%, p = 0.276) [Fig. 4 ]. Pooled ORs showed that VTA was associated with severe COVID-19 (OR 1.9 [1.1, 2.2], p = 0.002; I2: 49.3%, p = 0.117) [Fig. 5 ].
Fig. 2.
Mean difference in visceral fat area between severe and non-severe COVID-19.
Fig. 3.
Mean difference in subcutaneous fat area between severe and non-severe COVID-19.
Fig. 4.
Mean difference in total fat area between severe and non-severe COVID-19.
Fig. 5.
Visceral fat area and risk of severe COVID-19.
Regression-based Egger's test showed no indication of small-study effects for the mean difference of VTA (p = 0.688), SFA (p = 0.259), and TFA (p = 0.277) between the two groups. There was no indication of small-study effects for the association between VTA and severe COVID-19.
4. Discussion
Meta-analysis showed that higher VTA and TFA, but not SFA, was associated with increased COVID-19 severity with low heterogeneity. Which indicates that visceral, rather than subcutaneous lipid deposition, was associated with the severity of COVID-19 illness, similar to the finding in individual studies [4,17].
Excessive BMI is closely related to abdominal circumference and fat (VTA, SFA, and TFA) in the human body. Body composition not only reflects nutritional status and body fatness but also has a significant effect on inflammatory response and metabolic activity [4]. Obesity profoundly increases the risk of many conditions, including insulin resistance, type 2 diabetes mellitus, dyslipidemia, hypertension, cerebrovascular disease and cardiovascular disease, which are significant risk factors for worsening outcomes in COVID-19 [[18], [19], [20], [21], [22], [23], [24]]. Individuals with obesity are prone to respiratory infections due to blunted innate and adaptive immunity and reduced protection from influenza vaccination [25,26]. Obesity independently enhances the risk of developing severe courses of COVID-19 [3,5]. Excess fat buildup makes individuals more likely to develop low-grade, chronic, inflammation, such as in people with comorbidities, which puts them at risk for infections [3].
The renin-angiotensin-aldosterone system (RAAS), which plays a significant role in COVID-19 pathophysiology, is chronically activated in individuals with obesity and excessive lipid accumulation. This condition interferes blood pressure regulation and insulin signaling in peripheral tissues, as well as predisposes the individual to experience a variety of dysfunctions [27]. Recent evidence suggests that the novel coronavirus may exacerbate hyperglycemia by infecting and killing B-cells [28]. In patients with obesity, where white adipose tissue (WAT) is aggravated and brown adipose tissue (BAT) is reduced in size and activation, the likelihood of comorbidities is greatly enhanced. The angiotensin-converting enzyme 2 (ACE2) expressed in adipocytes is crucial for the homeostasis of glucose and lipid metabolism, therefore, high ACE2 expression in fat tissue may increase the susceptibility and accessibility of COVID-19 to the tissue. Also, several medications used to treat coexisting disorders (e.g., hypertension, diabetes mellitus, dyslipidemia) can upregulate ACE2 expression and consequently increase viral uptake [27,29]. However, the measurement of ORs were also based on different comparison, although all of them compared high visceral adiposity versus low visceral adiposity; the magnitude leads to inconsistency of the pooled effect estimate.
However, BMI is an indirect measurement of adiposity, and cannot measure fat distribution or body composition in the human body [4]. Our meta-analysis indicates that visceral fat is a more accurate indicator of prognosis than subcutaneous fat, hence, a distinction is needed. The use of CT-derived quantification of visceral and subcutaneous fat tissue enables a more comprehensive approach in differentiating the fat compartments in the body. Visceral fat is widely recognized as an independent risk factor for cardiometabolic outcomes in the general population. The current study provides a potential explanation for heterogeneous association between BMI and severity/mortality in patients with COVID-19 [3], because BMI does not represent visceral fat. A cross-sectional image of CT scans at the level of the third lumbar vertebra (L3) is currently the most widely used method of measuring distribution of adipose tissue and skeletal muscle, while a single slice and waist circumference obtained at the level of the umbilicus (around L4 or L5) are highly correlated to the total visceral fat volume [4,16]. In addition to VTA and TFA, the use of CT-based upper abdominal circumference may indicate the need for ICU treatment and/or mechanical ventilation in patients with COVID-19 thereby providing a means to predict prognosis in obese patients, although it may result in increased costs [16,17]. Furthermore, abdominal obesity can significantly alter lung function by decreasing exercise capacity and complementing airway resistance, resulting in breathing difficulties which are exacerbated in supine position due to diminished diaphragmatic excursion [3,4].
Additionally, the lack of physical activity is also a concern people with excessive BMI and/or adipose tissue. Exercise is good for boosting the immunity (when done in moderation), reduced physical activity regardless of insulin resistance will impair the immune response against pathogens [30]. Individuals with excessive adiposity typically have a proinflammatory state characterized by high leptin, a proinflammatory adipokine, and low adiponectin, an anti-inflammatory adipokine secreted by subcutaneous adipocytes, which adversely affects the immune system and contributes to the development of obesity-related complications [31]. Adipocyte hypoxia and dysfunction due to low-grade inflammation stimulate the recruitment of immune cells, including B-cells, T-cells, and macrophages, but also the release of proinflammatory cytokines, including interleukin (IL)- 1β, IL-6, IL-8, CRP, and tumor necrosis factor-α (TNF-α), which are mainly secreted by visceral adipocytes [32,33]. This condition causes an auto-regenerating inflammation in a cytokine storm, which is thought to play a role in the pathophysiology of COVID-19-associated complications [3,4]. Damaged immunity leads to insufficient macrophage activation and clearance mechanisms, prolonged viral shedding and increasing the likelihood of spreading the coronavirus to others and enhancing the likelihood of a cytokine storm. Also, reduced interferon generation in these individuals allows for greater viral RNA replication, leading to higher virulence of viral strains [3].
Unfortunately, there is only one study that adequately reported the association between visceral adiposity and mortality; in which patients with high visceral adiposity has higher mortality rate (15.3% vs 5.6%) [4]. Thus, further studies addressing the mortality rate in patients with high visceral adiposity are needed.
5. Conclusion
Visceral adiposity was associated with increased COVID-19 severity, while subcutaneous adiposity was not. Thus, patients with higher VTA and TFA should be monitored more intensively for possible complications.
Consent for publication
Not Applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The corresponding author (R.P.) can be contacted for more information.
Funding
None.
Author's contribution
R.P. conceived and designed the study and drafted the manuscript. R.P. and J.H. acquired the data and drafted the manuscript. E.Y. and R.V. performed data extraction. M.A.L, R.V., R.P., and I.H. interpreted the data, drafted the manuscript, and performed extensive research on the topic. A.A.L, S.A.N, I.A, and B.B.S. reviewed and provide critical revisions for the manuscript. R.P. performed the statistical analysis. All authors contributed to the writing of the manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
None.
References
- 1.World Health Organization . World Health Organization; 2020. Weekly epidemiological update, coronavirus disease 2019 (COVID-19) as of 01 november 2020. Geneva. [Google Scholar]
- 2.Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. 2020;7 doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y., Ding L., Zou X., Shen Y., Hu D., Hu X. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity. 2020;28:2040–2048. doi: 10.1002/oby.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzi L., Radaelli M.G. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57:759–764. doi: 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zbinden-Foncea H., Francaux M., Deldicque L., Hawley J.A. Does high cardiorespiratory fitness confer some protection against pro-inflammatory responses after infection by SARS-CoV-2? Obesity. 2020 doi: 10.1002/oby.22849. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muscogiuri G., Pugliese G., Barrea L., Savastano S., Colao A. Comentary: obesity: the “achilles heel” for COVID-19? Metabolism. 2020;108:154251. doi: 10.1016/j.metabol.2020.154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reilly J.J., El-Hamdouchi A., Diouf A., Monyeki A., Somda S.A. Determining the worldwide prevalence of obesity. Lancet. 2018;391:1773–1774. doi: 10.1016/S0140-6736(18)30794-3. [DOI] [PubMed] [Google Scholar]
- 9.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrina J., Lim M.A., Pranata R. COVID-19 and misinformation: how an infodemic fueled the prominence of vitamin D. Br J Nutr. 2020 doi: 10.1017/S0007114520002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) vol. 2019. 2019. [Google Scholar]
- 14.Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandarana H., Dane B., Mikheev A., Taffel M.T., Feng Y., Rusinek H. Visceral adipose tissue in patients with COVID-19: risk stratification for severity. Abdom Radiol. 2020 doi: 10.1007/s00261-020-02693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen A., Bressem K., Albrecht J., Thieß H.M., Vahldiek J., Hamm B. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battisti S., Pedone C., Napoli N., Russo E., Agnoletti V., Nigra S.G. Computed tomography highlights increased visceral adiposity associated with critical illness in covid-19. Diabetes Care. 2020;43:e129–e130. doi: 10.2337/dc20-1333. [DOI] [PubMed] [Google Scholar]
- 18.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2020;21 doi: 10.1177/1470320320926899. 147032032092689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29:104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yonas E., Alwi I., Pranata R., Huang I., Lim M.A., Gutierrez E.J. Effect of heart failure on the outcome of COVID-19 — a meta analysis and systematic review. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pranata R., Soeroto A.Y., Huang I., Lim M.A., Santoso P., Permana H. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tubercul Lung Dis. 2020;24 doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 23.Pranata R., Supriyadi R., Huang I., Permana H., Lim M.A., Yonas E. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circulatory, Respir Pulm Med. 2020;14 doi: 10.1177/1179548420959165. 1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuty Kuswardhani R.A., Henrina J., Pranata R., Anthonius Lim M., Lawrensia S., Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14:2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green W.D., Beck M.A. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14:S406–S409. doi: 10.1513/AnnalsATS.201706-447AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neidich S.D., Green W.D., Rebeles J., Karlsson E.A., Schultz-Cherry S., Noah T.L. Increased risk of influenza among vaccinated adults who are obese. Int J Obes. 2017;41:1324–1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquarelli-do-Nascimento G., Braz-de-Melo H.A., Faria S.S., Santos I. de O., Kobinger G.P., Magalhães K.G. Hypercoagulopathy and adipose tissue exacerbated inflammation may explain higher mortality in COVID-19 patients with obesity. Front Endocrinol. 2020;11:530. doi: 10.3389/fendo.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L., Han Y., Nilsson-Payant B.E., Gupta V., Wang P., Duan X. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136. doi: 10.1016/j.stem.2020.06.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pranata R., Permana H., Huang I., Lim M.A., Soetedjo N.N.M., Supriyadi R. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14:983–990. doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim M.A., Pranata R. Sports activities during any pandemic lockdown. Ir J Med Sci. 2020 doi: 10.1007/s11845-020-02300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francisco V., Pino J., Campos-Cabaleiro V., Ruiz-Fernández C., Mera A., Gonzalez-Gay M.A. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018;9 doi: 10.3389/fphys.2018.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The corresponding author (R.P.) can be contacted for more information.