Abstract
Background
The prevalence and severity of COVID-19 are greatly reduced in children, yet some pediatric patients develop a syndrome resembling Kawasaki Disease (KD), termed Multisystem Inflammatory Syndrome in Children (MIS-C). With an estimated incidence of 2/100,000 children, MIS-C is relatively rare but can be fatal. Clinical features can include fever, hyperinflammatory state, gastrointestinal symptoms, myocardial dysfunction, and shock. The pathogenesis of MIS-C, although yet to be completely elucidated, appears to be distinct from KD in terms of epidemiology, severity, and biochemical signature.
Aim of Review
Although efficacy of treatments for MIS-C have largely not yet been investigated, we aim to conduct a comprehensive literature search of numerous medical databases (AMED, EBM Reviews, Embase, Healthstar, MEDLINE, ERIC, and Cochrane) to highlight treatments used around the world, their rationale, and outcomes to better inform guidelines in the future. Using the findings, an approach to MIS-C management will be outlined.
Key Scientific Concepts of Review
-
•
MIS-C appears to be a SARS-CoV-2 related post-infection phenomenon that is distinct from Kawasaki disease.
-
•
Although outcomes are largely favorable, there is significant variation in MIS-C treatment. Most management regimens reported to date mirror that of KD; however, targeted therapy based on specific MIS-C phenotypes may have the potential to improve outcomes.
-
•
We recommend close monitoring by a multidisciplinary team, symptomatic treatment (e.g., intravenous immunoglobulin for KD-like symptoms, steroids/immunotherapy for multisystem inflammation), and long-term follow-up.
-
•
Further research is required to evaluate the effectiveness of current MIS-C treatments and to determine more refined therapies.
Keywords: MIS-C, SARS-CoV-2, COVID-19, Kawasaki Disease
1. Introduction
A novel coronavirus was identified in early January 2020 [1] as the causative agent for a cluster of pneumonia cases reported in Wuhan, China on December 31, 2019 [2]. This virus was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in February 2020 by the International Virus Classification Commission [3]. The World Health Organization (WHO) termed the disease resulting from the virus as Coronavirus Disease 2019 (COVID-19) [4] and declared it a pandemic on March 11, 2020 [2]. As of March 2021, there have been over 116 million confirmed cases and just over 2.5 million deaths worldwide [5].
1.1. Virus structure and pathophysiology
SARS-CoV-2 is an enveloped, single stranded, zoonotic RNA virus that is part of the Coronaviridae family, order Nidovirales [6,7]. These groups of viruses have a characteristic crown-like resemblance when viewed using electron microscopy [8], with the spike (S) protein, which is responsible for host cell receptor binding and membrane fusion, forming the spikes of the “crown” [9]. Seven viruses in this family are known to infect humans, four of which cause mild, self-limiting respiratory symptoms (229E, NL63, HKU1, and OC43) [10]. However, three coronaviruses are known to cause severe respiratory illness in individuals: severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and the novel SARS-CoV-2 [3,10]. SARS-CoV-2 enters the host cell similar to SARS-CoV, through binding to the angiotensin-converting enzyme 2 (ACE2) receptor [11] via its S protein [12,13]. The ACE2 receptor is a membrane bound protein expressed by vascular endothelia, renal and cardiovascular tissue, and epithelia of the small intestine [[14], [15], [16]]. However, recent studies show that the novel virus is able to interact more efficiently with the ACE-2 receptor than SARS-CoV [17,18]. Injury to cells occurs due to the host's dysregulated innate and adaptive immune system [19].
1.2. Pediatric transmission of SARS-CoV-2
COVID-19 has been shown to be less common in children thus far, with large countries such as the United States and China reporting an approximate pediatric case rate of 2% [[20], [21], [22], [23], [24]]. Working theories as to why children are less likely to be affected include antibody protection from frequent common mild human coronavirus strains during childhood, the age group having fewer comorbidities, and a potentially different immunological response than in adults resulting in a lower chance of progression to acute hyperinflammatory states [25]. Another theory is that infection rates are lower in pediatric patients due to a relative paucity of ACE-2 receptors, to which the virus attaches [26,27]. Interestingly, the numbers are slightly higher in Canada, with overall 7.7% of COVID-19 cases being in children as of July 23, 2020 [28]. This may be due to increased community testing, which could, in turn, capture more cases of children with mild symptoms or that are asymptomatic.
1.3. Signs and symptoms of SARS-CoV-2
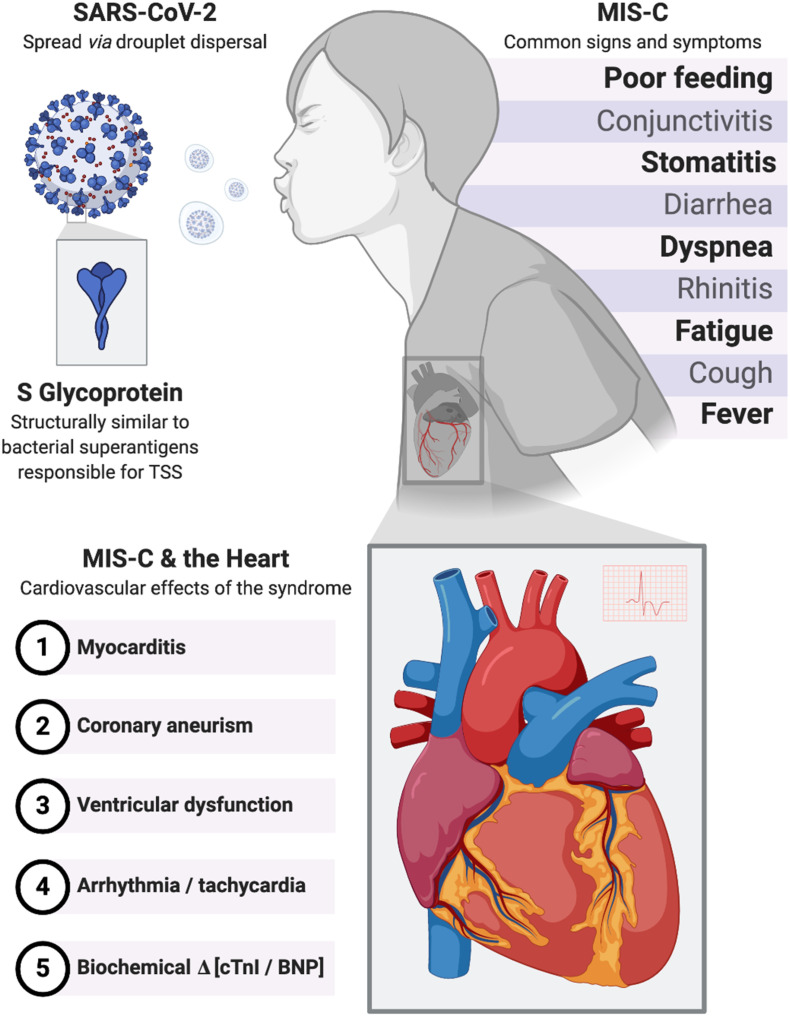
In adults, common symptoms include fever, headache, cough, myalgia, shortness of breath, and fatigue [24,29,30]. Patients with severe symptoms often present to the healthcare system in the second week of the illness, a time when viral loads are decreasing while inflammation markers are rising [[30], [31], [32]], suggesting that the dysregulated immune response is culpable for the observed tissue damage. In children, SARS-CoV-2 presents differently, often with fever, cough, fatigue, headache, rhinitis, diarrhea, dyspnea, cyanosis, and even poor feeding [33,34]. Overall, children tend to have more mild clinical symptoms than adults [21]. According to data from China, only 5.9% of children who have confirmed SARS-CoV-2 develop severe symptoms, and 0.6% present with acute respiratory distress syndrome (ARDS) or multi-organ dysfunction, in comparison to the 18.5% reported in adults [21]. These differences again may be explained by the same theories that try to address the transmissibility discrepancy between adults and children discussed above.
Thankfully, mortality in children remains low. In one multicenter cross-sectional study of PICUs in the USA and Canada, the in-hospital mortality rate was 4.2% [35], which likely overestimates the overall mortality rate as it stems from patients in an acute setting. As of March 10, 2021, the United States reported 217 deaths (0.0426%) in patients 0–17 years old [36]. In Canada, only 4 pediatric patients have passed away from COVID-19 as of March 5, 2021 [28]. Globally, pediatric deaths due to SARS-CoV-2 are also rare [[37], [38], [39], [40]]. However, the disease course in children is not entirely benign as severe complications have been noted.
1.4. Multisystem inflammatory syndrome temporally associated with SARS-CoV-2
On April 27, 2020, the United Kingdom reported a cluster of eight severely ill pediatric cases displaying a phenotype which resembled a combined state of incomplete Kawasaki Disease (KD) and Toxic Shock Syndrome (TSS), including signs of circulatory shock and an overall hyperinflammatory state [41]. Although all children tested negative for SARS-CoV-2 on bronchoalveolar lavage or nasopharyngeal aspirates, four were known to be exposed to COVID-19 within their household previously. The likelihood of the children having contracted the virus recently, in combination with the lack of active infection, caused healthcare professionals to suspect that the hyperinflammatory state is not part of the active COVID-19 process, but rather, a post-infectious phenomenon [41]. The condition was termed multisystem inflammatory syndrome in children (MIS-C) [20,42], also referred to as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) [43], amongst other names [44]. The Canadian Paediatric Surveillance Program (CPSP), Royal College of Paediatrics and Child Health (RCPCH) in the United Kingdom, Centre for Disease Control (CDC) in the United States, and WHO have each determined a case definition for the syndrome (see Table 1 ), with commonalities being fever, clinical evidence of inflammation, and single or multi-organ involvement.
Table 1.
Comparison of the WHO, CDC, RCPCH, and CPSP case definitions.
| WHO [42] | CDC [20] | RCPCH [43] | CPSP [120] | |
|---|---|---|---|---|
| Name | Multisystem inflammatory disorder in children and adolescents | Multisystem inflammatory syndrome in children (MIS-C) | Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) | Pediatric inflammatory multisystem syndrome (PIMS)/Kawasaki Disease temporally associated with COVID-19 |
| Age | 0–19 years old | Less than 21 years old | “Child” | Less than 18 years old |
| Fever | MUST ≥3 days |
MUST ≥24 h Over 38.0 °C or subjective fever reported |
MUST “Persistent fever” Over 38.5 °C |
MUST >/= 3 days Over 38.0 °C |
| Signs and symptoms | MUST have at least two:
|
MUST
|
MUST have at least one:
|
Must have one of both:
|
| Inflammatory markers | MUST have elevated levels of at least one:
|
MUST have at least one, but not limited to:
|
OR one or more inflammatory marker if no additional clinical features:
|
MUST have elevated levels of at least one:
|
| No other cause identified | MUST | MUST | MUST | MUST |
| Documented SARS-CoV-2 infection | MUST RT-PCR, antigen test, serology positive, or likely exposure to patients with COVID-19 |
MUST RT-PCR, serology, antigen test, or known exposure to patients with COVID-19 within 4 weeks in advance of symptom onset Consider in any pediatric death with evidence of SARS-CoV-2 infection |
PCR can be positive or negative, no mention of other forms of infection confirmation | Not necessary |
What is perhaps the most worrisome feature of MIS-C is the syndrome's cardiac involvement. This includes abnormal left ventricular systolic and diastolic function with severity following the patient's clinical status, myocarditis leading to pericardial effusions, and coronary artery aneurysms [41,[45], [46], [47]]. Additionally, cardiac markers such as troponins and BNP/N-terminal pro-BNP were elevated in up to 90% of cases [41,45,[48], [49], [50], [51]]. Even the development of arrhythmias has been observed in a small percentage of cases [41].
1.5. How does MIS-C differ from the inflammatory effect seen in adults?
In adult patients with severe COVID-19, a strong inflammatory response consistent with cytokine storm has been reported [29]. The cytokine storm plays a critical role in the development of ARDS, systemic inflammatory response, and multi-organ failure in adults [52]. Endothelial cell damage is often seen, resulting in coagulation activation that may cause thrombosis in small blood vessels, potentially resulting in pulmonary embolism and a higher cardiac load [53,54]. Amongst deaths from SARS-CoV-2, disseminated intravascular coagulation is very common [55]. However, this inflammatory reaction is different than that seen in children with MIS-C. Although both cause an increase in inflammatory markers [41,47,[53], [54], [55], [56], [57]], levels of ferritin and lactate dehydrogenase are significantly higher in COVID-19 induced ARDS than MIS-C [58]. Additionally, MIS-C patients have less widespread organ damage [57]. Adult patients rarely have gastrointestinal disturbances and usually have respiratory symptoms [29], in contrast to pediatric patients who often experience the inverse [41,47,[54], [55], [56], [57]]. While cardiac involvement has been reported in both extremes of age, the systolic dysfunction and troponin elevation observed in patients with MIS-C is generally rapidly resolved, suggesting that the type of myocardial damage is distinct from that seen in adult patients with acute SARS-CoV-2 infection [50,58,59]. Even the timing of MIS-C onset in the SARS-CoV-2 infectious course differentiates from the inflammatory effect seen in adults. One New York study investigated the difference between patients with mild COVID-19 respiratory disease, those with COVID-19-related ARDS, and a group of children with MIS-C [60]. Analysis of antibody levels to SARS-CoV-2 nucleocapsid protein, the levels of which correlate well with degree of active infection revealed that the MIS-C cohort had a lower levels of the antibody than both other groups [57]. In addition, the MIS-C antibody response shows a significantly higher ratio of IgG to IgM SARS-CoV-2 specific antibody production [57], together providing evidence that MIS-C occurs during a late stage of the disease, while the adult inflammatory reaction occurs during the active initial disease process. This is reflected clinically by the observation that MIS-C tends to occur 2 to 4 weeks after infection with the virus [43]. These significant discrepancies point to entirely distinct pathophysiologies.
1.6. Is MIS-C a form of KD?
KD is generally a self-limiting vasculitis of medium sized vessels, such as the coronary arteries, which is seen almost solely in children, usually under the age of 5 [61]. The main complication that is associated with KD is coronary dilation and aneurysms, which are rarer if patients are treated appropriately and expediently (3–6% of cases), but often no treatment is administered (26–40%) [62]. According to the American Heart Association, there are two classifications of KD, classical or incomplete type. The criteria for KD classic type is as follows: fever for at least 5 days with four or more clinical criteria (bilateral bulbar non-exudative conjunctivitis, changes of the lips or oral cavity, non-suppurative laterocervical lymphadenopathy, polymorphic rash, erythema of the palms and soles, firm induration of the hands and/or feet) [63]. Incomplete KD is similar to classic type, the difference being that only two or three clinical criteria need to be met, along with compatible laboratory or echocardiogram findings [63]. Although there are many similarities between MIS-C and KD, they are believed to be separate entities [41,50,64]. MIS-C occurs more in an older age group and the incidence is higher in different ethnic groups than in KD [41,65,66]. Additionally, gastrointestinal symptoms are much more prominent [41,45,50,65,66], and inflammatory markers tend to be more elevated [66], while lymphocyte and platelet counts are lower on average [67]. The coronary artery effects of MIS-C appear to be different as well, presenting in a distinct pattern of uniformly ectactic vessel dilation and left main coronary artery orifice implication [46]. KD, in contrast, spares the left main coronary artery orifice and has aneurysms that are separated by normal segments of vessel [68,69]. This may suggest that the coronary artery involvement in MIS-C is caused by the fever response and cytokine storm, as opposed to the KD-induced arterial wall changes [46,70,71]. Furthermore, what is most worrisome is that the clinical course for MIS-C appears to be more severe, with myocardial dysfunction, shock, and resistance to single intravenous immunoglobulin (IVIG) treatment occurring more often than in KD [49,67].
1.7. Proposed pathophysiology of MIS-C
The pathophysiology of MIS-C is being actively explored. Theories for the mechanism underlying the myocardial involvement include direct invasion of the myocytes by the virus, systemic inflammatory response causing myocyte injury, and myocardial ischemia from hypotension [72]. Indeed, a recent in vitro report of human induced pluripotent stem cell-derived cardiomyocytes revealed the susceptibility of such cells to SARS-CoV-2 infection, ultimately indicating that viral myocarditis is possible and perhaps partially explanatory of this syndrome [73]. However, given the elevated inflammatory markers observed, the involvement of multiple organ systems [41,47,[54], [55], [56], [57]], and the late onset of the syndrome in the disease course [45,49,66,74], the most likely mechanism is that of a delayed inflammatory response.
To discover the exact pathophysiology, a recent preprint created structural based computational models of SARS-CoV-2 and found that the S protein has a sequence motif that is not present on any other similar coronaviruses [75]. Interestingly, this sequence motif closely resembles, in both sequence and structure, bacterial superantigens that cause toxic shock syndrome [[73], [74], [75]] (Fig. 1 ). The motif is most similar to that of staphylococcal enterotoxin B and as such, would cause large-scale T-cell activation and proliferation, causing an immense release of pro-inflammatory cytokines from T cells (interferon gamma, tumor necrosis factor alpha, interleukin-2) and antigen presenting cells (interleukin-1 and tumor necrosis factor alpha) [[75], [76], [77], [78]]. This cytokine storm results in multi-organ tissue damage as is seen in MIS-C and thus, may be at least in part culpable. The superantigen activity of the SARS-CoV-2 S protein may also be the cause of the cytokine storm seen in adults [75]. Adding further evidence, the signs and symptoms of TSS and MIS-C display notable similarities - including desquamation, altered mental status, gastrointestinal upset, myalgia, low platelets, as well as elevated PT/PTT, d-dimer and CRP - and they both occur in an older age group of children [79]. However, the timeline of MIS-C presentation is not explained by this finding. MIS-C cases are suspected to be post-infectious due to two reasons. One was that MIS-C was observed weeks after the peak of COVID-19 in the community. The other is that MIS-C patients are usually PCR negative and serology positive for SARS-CoV-2. In comparison, TSS is a more acute reaction, occurring within 2 days. Additionally, cardiac manifestations are rare in TSS, unlike in MIS-C [79,80]. That being said, data is still very limited, and further research is needed.
Fig. 1.
Multisystem inflammatory syndrome in children. Signs, symptoms and cardiovascular effects of MIS-C and the proposed underlying pathophysiology related to the S glycoprotein. SARS-CoV-2, severe acute respiratory syndrome coronavirus virus 2; MIS-C, multisystem inflammatory syndrome in children; TSS, toxic shock syndrome; cTnI, cardiac troponin I; BNP, brain natriuretic protein.
2. Purpose/justification
Various institutions, such as The Hospital for Sick Children [81] and Jacobs School of Medicine and Biomedical Sciences [82], have published management algorithms for MIS-C. However, as this is a novel syndrome with limited data, we aim to conduct a comprehensive review to highlight treatments used around the world, their rationale, and outcomes to better inform guidelines in the future. Using our findings, along with expert opinion, we will also outline an approach to MIS-C management and identify areas which require further investigation.
3. Search strategy
The following databases were included in the search strategy: Ovid databases (including AMED, EBM Reviews, Embase, Healthstar, MEDLINE, University of Ottawa Full Text Journals), ERIC, and the Cochrane Library. The search strategy combined the terms for the name of the syndrome (multisystem inflammatory syndrome OR multi-system inflammatory syndrome OR hyper-inflammatory syndrome OR hyperinflammatory syndrome OR hyperinflammatory shock OR hyper-inflammatory shock OR Kawasaki Disease OR multi-system inflammatory disease OR multisystem inflammatory disease OR MISC OR PIMS-TS OR MIS-C) with the name of the virus (COVID-19 OR SARS-CoV-2 OR 2019 novel coronavirus disease OR COVID19 OR 2019-nCoV infection OR 2019-nCoV disease OR coronavirus disease 2019 OR coronavirus disease-19) and the population group (pediatric OR pediatrics OR paediatric OR paediatrics OR children OR adolescent OR infant OR adolescents OR infants OR kids) along with terms for intervention (treatment OR treatment* OR therap* OR manage*). These results were limited to those published in English.
The initial search yielded 57 results on August 6, 2020. The titles and abstracts were manually reviewed with the following inclusion criteria:
-
1)
The paper is a primary study with at least one study participant
-
2)
The paper's target population was children with a multisystem inflammatory disorder associated with SARS-CoV-2
-
3)
The paper detailed the treatment and management given to the pediatric patients
-
4)
The paper mentions the outcome of the treatment, such as symptom resolution, successful hospital discharge, or mortality.
Based on these criteria, 42 were excluded and 15 papers were examined and analyzed in full. Any significant papers published after the date of search are mentioned in the discussion section.
4. Discussion
4.1. Current number of cases and severity
MIS-C is relatively uncommon, with an estimated incidence of 2 per 100,000 individuals less than 21 years old [74]. According to our literature review, there were a total of 386 cases of MIS-C or cases resembling MIS-C (Table 2 ). Roughly 77% were admitted to the PICU, the median length of PICU admission was 5 days, and about 53% needed inotropic support of some sort. Few children were supported with ECMO (n = 19, 5%), and there were 5 fatalities (1.3%) in total. However, these percentages do not accurately reflect the risk in all MIS-C cases as some study criteria included only MIS-C patients that presented to hospital or were admitted to the PICU, thus representing a more acute population. Likely, the risk of PICU admission, fatality, and need for inotropic support or ECMO is more modest.
Table 2.
Summary of MIS-C articles with treatment, outcomes, and key findings.
| Reference | Design | Participants | Case definition | Treatment and outcomes |
|---|---|---|---|---|
| Pouletty et al. Jun 2020 Pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort [67] |
A retrospective case series that included a historical KD cohort as a comparison. | Patients (n = 16) were from 7 Paris region hospitals, and presented between April 7th and 30th, 2020. Their median age was 10 years old, and six had underlying conditions (n = 4 overweight, n = 2 asthma). | Age under 18; complete or incomplete KD; positive testing for SARS-CoV-2 infection by RT-PCR or serology and/or close contact with an individual confirmed with COVID-19. | One patient required no treatment and went into remission spontaneously on day 8. The other 15 patients (94%) received IVIG at a dose of 2 g/kg, three (19%) of which received a second round of IVIG, and one (6%) of which received a second round of IVIG with steroids and was still undergoing steroid treatment upon study completion due to autoimmune hemolytic anemia that developed after the second IVIG infusion. Two (13%) patients received a steroid adjacent post-IVIG infusion. All patients receiving treatment (n = 15, 94%) were given aspirin, with 7 (44%) taking 30-50 mg/kg/day and 8 (50%) taking an anti-aggregant dose. One (6%) received IL-1 receptor antagonist anakinra (4 mg/kg) due to respiratory distress, and another (6%) received IL-6 antagonist tocilizumab (8 mg/kg) for persistent systemic inflammation. Additionally, one patient (6%) was administered hydroxychloroquine, but only due to clinicians initially suspecting systemic lupus erythematosus as the diagnosis. Seven (44%) of all patients were admitted to the pediatric intensive care unit (PICU) and required fluid resuscitation. Six (38%) required inotropic supports as well. All patients that received treatment (n = 15) went into remission within a median of 2 days (IQR 1 to 8) of treatment initiation, with remission defined as absence of fever, disappearance of clinical signs, and complete regression of inflammatory biomarkers. |
| Verdoni et al. May 2020 An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study [45] |
A retrospective observational cohort study that compared a group of patients diagnosed with Kawasaki-like disease during the pandemic (n = 10) to a group presenting with the disease during 5 years before the pandemic (n = 19). | All patients (n = 29) presented to a hospital in Bergamo, Italy. The patients in the pandemic group (n = 10) presented between Feb 19 and April 20, 2020. Their mean age was 7.5 years old. Comorbidities were not mentioned in the paper. | Classic or incomplete KD according to the 2017 American Heart Association criteria. | All patients were treated in accordance with the American Heart Association indications for KD, with IVIG risk stratification done using the Kobayaski score. All 10 patients (100%) were given IVIG at 2 g/kg. In addition, two patients (30%) also received aspirin (50-80 mg/kg/day) for 5 days, and eight (80%) received aspirin (30 mg/kg) with methylprednisolone (2 mg/kg/day) for 5 days, followed by methylprednisolone dose tapering over two weeks. They received steroids as adjuvant due to having features of cytokine storm. Two patients (20%) needed inotropic support. Response to treatment, defined as resolution of signs and symptoms, normal vital signs, normal c-reactive protein levels, and normal blood tests, was observed in all ten patients (100%). |
| Dasgupta and Finch June 2020 A case of pediatric multisystem inflammatory syndrome temporally associated with COVID-19 in South Dakota [121] |
A case report. | An eight-year-old female that presented to the emergency room at a hospital in South Dakota during April 2020. She had no previous comorbidities. | PIMS-TS (RCPCH). No evidence of SARS-CoV-2 in any patients (RT-PCR, serology, or contact history). | The patient was admitted to the PICU, where she received fluid boluses for hypotension, later followed by inotropic supports (low dose norepinephrine). She also received broad spectrum antibiotics. On day three in hospital, the patient developed a new heart murmur, their ejection fraction fell to 47%, and chest radiography showed an enlarged cardiac silhouette, pulmonary edema, and bilateral pleural effusion, so milrinone and diuretics were given along with low dose norepinephrine. The patient showed signs of clinical improvement shortly afterwards. She received IVIG (2 mg/kg) on day four in the hospital along with a single dose of methylprednisolone (1 mg/kg). Within 12 h of receiving the IVIG, the patient's fever went down and she quickly improved. On day five, her cardiac function normalized, and she was prescribed aspirin (30 mg/kg/day). For the next three days, she remained afebrile and was discharged on low dose aspirin. |
| Kaushik et al. June 2020 Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York City [64] |
A multi-centre retrospective observational study. | Patients (n = 33) presented to three New York tertiary care children hospitals between April 23 to May 23, 2020. Their median age was 10 years old, and 16 patients (48%) had comorbid conditions including asthma (n = 5, 15%), obesity (n = 2, 6%), allergic rhinitis/eczema (n = 3, 9%), cardiac (n = 2, 6%), hematologic (n = 2, 6%), and other (n = 3, 9%). | MIS-C (CDC) with confirmed SARS-CoV-2 infection. | All patients (n = 33) required PICU admission. A majority of patients (n = 18, 54%) received IVIG, 17 (51%) were given methylprednisolone due to elevated inflammatory markers, and 2 patients (6%) received both. All patients were given anticoagulation (n = 33), 27 (82%) of which had therapeutic dose enoxaparin, 5 (15%) had prophylactic dose enoxaparin, and 1 (3%) had unfractionated heparin at a therapeutic dose. Vasopressors (norepinephrine n = 10, dopamine n = 9) had to be used in 17 patients (51%), with a mean duration of administration of 72 h. Antibiotics (empiric coverage) were given for less than 48 h in 14 (42%) patients and for more than 48 h in 15 (45%) of patients. In terms of more targeted therapies, 12 (36%) received tocilizumab for high IL-6 levels and 4 (12%) received anakinra. Remdesivir, a nucleoside analog that blocks virus replication during active infection, was compassionately used in 7 patients (21%), all which had a positive RT-PCR. One child (3%) that had severely depressed left ventricle function and was treated with methylprednisolone, also received convalescent plasma along with an intra-aortic balloon pump for 24 h. Another child (3%) required extracorporeal membrane oxygenation (ECMO) due to severely depressed left ventricle function and cardiogenic shock within 24 h of presenting to hospital. All but one patient (n = 32, 97%) improved clinically based on inflammatory markers and myocardial function and were discharged home after a 7.8 day median stay in the hospital and a 4.7 day median stay in the PICU. The patient with the intra-aortic balloon pump was supported by the pump for 24 h and was well enough to be weaned off of invasive mechanical ventilation after 2 days and was discharged home after 12 days. Sadly, the child on ECMO developed an ischemic brain infarction accompanied by a subarachnoid hemorrhage on day 6 of the mechanical circulatory support, resulting in brain death. |
| Toubiana et al. June 2020 Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study [48] |
A single centre prospective cohort study. | All patients (n = 21) were admitted to a hospital in Paris, France, between April 27 to May 11, 2020, and followed up until discharge by May 15, 2020. Their median age was 7.9 years old. Five patients (24%) had a body mass index over the 97th percentile, but presence of other comorbidities was not mentioned. | American Heart Association criteria for complete and incomplete KD. Two patients did not have evidence of recent SARS-CoV-2 infection. | All 21 patients (100%) received IVIG for a median of 5 days, along with aspirin at a low dose (3-5 mg/kg/day). Seven (33%) also received a steroid adjuvant with (2-10 mg/kg). Resistance to IVIG, defined as persistent fever 36 h after the end of initial IVIG infusion and requiring a second round of IVIG, occurred in 5 patients (24%), 4 (19%) of which received a second infusion of corticosteroids (2 mg/kg/day). Broad spectrum antibiotics were given to 18 children (86%). PICU admission due to hemodynamic instability was required in 17 patients (81%) who had higher levels of inflammatory markers, where 11 (52%) received intravenous fluid resuscitation. Eight patients (38%) had hypotension that was unresponsive to fluids, and thus, were given vasoactive agents. Inotropic agents were given to 14 children (67%) because of myocarditis with cardiac dysfunction. All patients were discharged home after a median of 8 days in hospital (IQR 5–17), and 5 days (IQR 3–15) days in the PICU. |
| Grimaud et al. June 2020 Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children [99] |
A multicenter retrospective case series. | Patients (n = 20) were from four tertiary care hospitals in Paris, France, and presented to the healthcare centre between April 15th and April 27th, 2020. Their median age was 10 years old, and no comorbidities were reported. | No published case definition used. Inclusion criteria was less than 18 years old, admission to the PICU with fever and cardiogenic shock secondary to myocarditis, and having a suspected COVID-19 infection. | All patients (n = 20, 100%) received IVIG (2 g/kg) within 2 days at the PICU, 18 (90%) of which had complete fever resolution post-infusion. A small minority of children (n = 2, 10%) were given corticosteroids. One patient (5%) received an interleukin-1 receptor antagonist and another (5%) a monoclonal antibody against the interleukin 6 receptor. A majority (n = 19, 95%) required inotropic support for a median of 3 days. The average PICU stay was 4 days. All children were successfully discharged with cardiac function recovery and a significant decrease in inflammatory markers. |
| Ramcharan et al. June 2020 Pediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK Tertiary Paediatric Hospital [47] |
A single centre retrospective observational study. | All patients (n = 15) were from a tertiary pediatric hospital in the United Kingdom and presented between April 10th and May 9th, 2020. The median age was 8.8 years old, and comorbidities were not mentioned in the study. | PIMS-TS (RCPCH) with referral to cardiology. | Treatment was determined case-by-case by a multidisciplinary team, and supportive treatment was given following the hospital standards. 10 children (67%) received IVIG (2 g/kg) and aspirin (12.5 mg/kg) four times a day. Patients that continued to have persistent fever and increasing inflammatory markers 36 h post initial treatment were given a second dose of IVIG (n = 2, 13%) and/or a 3 day course of methylprednisolone followed by a weaning course of prednisolone (n = 5, 33%). All (n = 15) were given broad spectrum antibiotics for a minimum of 5 days. Ten children (67%) had to be admitted to the PICU and stayed a median of 4 days. In the intensive care unit, all ten (67%) required fluid resuscitation and inotropes/vasopressors for a median of 3 days. In eight patients (53%), hydrocortisone was needed for persistent hypotension. All patients were discharged clinically well with normal or improving laboratory results and cardiac function, on low dose aspirin, with a median hospital stay of 12 days. |
| Blondiaux et al. June 2020 Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series [114] |
A single centre case series focused on examining cardiac MRI findings. | Four patients that were admitted to intensive care at a hospital in Paris were included in the study. The patients presented during the month of April 2020. Their ages were 6, 8, 11, and 12. No cardiac comorbidities were seen, and other comorbidities were not noted in the paper. | No published case definition was used, but the inclusion criteria was ICU admission with cardiogenic and/or septic shock syndrome following myocarditis and undergoing cardiac MRI during their stay. All patients were serology positive for SARS-CoV-2. | All four children received IVIG. Three (75%) were given vasoactive agents along with volume expanders. Also, three children (75%) required prednisolone and aspirin. All patients recovered to normal ventricular function between 48 h to 5 days and were discharged from hospital at 13 to 23 days post symptoms onset. |
| Dolinger et al. May 2020 Pediatric Crohn's disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab [89] |
A case report. | A 14-year-old male recently diagnosed with pediatric Crohn's disease was admitted to a hospital in New York with five days of fever and abdominal pain. | The patient was SARS-CoV-2 PCR positive and suspected to have MIS-C (CDC). | The patient initially received intravenous antibiotics for their perianal abscess, along with hydroxychloroquine (5 day course) and azithromycin (3 days course) for the SARS-CoV-2 infection, prophylactic enoxaparin, and intravenous fluids. Despite initial treatment, the patient remained febrile. On day 8, after consultation with multiple specialties, the anti-tumor necrosis factor alpha treatment infliximab (10 mg/kg) was given as it could address the Crohns' disease and potentially the cytokine storm seen in MIS-C. Just a few hours after infliximab administration, the fever and hypotension resolved, along with inflammatory markers beginning to decrease. He received a second dose of infliximab 5 days later because of active perianal disease and remaining inflammation and was discharged that day. Two weeks after discharge, the patient had complete resolution of all previous clinical findings and normalization of all laboratory values, including inflammatory markers. |
| Cabrero-Hernandez et al. May 2020 Severe SARS-CoV-2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain [88] |
A case series. | Five patients presented to the emergency department and later admitted to PICU at a hospital in Madrid, Spain. The date was not mentioned. Two children were 9 years old, and the other three were 10, 12 and 13. Potential comorbidities were not included in the paper. | No published case definition was used, but the inclusion criteria was severe SARS-CoV-2 infection (suspicion or confirmed), hemodynamic instability, and suspected acute abdomen. | All children (n = 5) were admitted to the PICU, where 4 (80%) received inotropic support. Hydroxychloroquine was given to all patients (n = 5), as was azithromycin and lopinavir/ritonavir. Tocilizumab was administered to 4 children (80%). One child (20%) received therapeutic dose low molecular weight heparin, and 4 (80%) were given the prophylactic dosage. All children were given various antibiotics due to suspected acute abdomen, and all were successfully discharged after a median of 5 days (2–13 range) in the PICU. |
| Belhadjer et al. August 2020 Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic [50] |
A retrospective multi-centre observational study. | All patients (n = 35) were admitted to one of 13 hospitals in France and Switzerland between March 22 and April 30, 2020. The median age was 10 years. Ten patients (28%) had comorbidities, including asthma (n = 3, 9%), lupus (n = 1, 3%), and being overweight (n = 6, 17%). | All patients met MIS-C (CDC) criteria, but the inclusion criteria for the study was even more specific. The criteria were the presence of fever, cardiogenic shock or acute left ventricular dysfunction, with an inflammatory state. SARS-CoV-2 confirmation was not needed, and laboratory evidence of the virus was found in 31 (89%) patients. | A majority of patients (n = 25, 71%) received IVIG. One patient (3%) had persistent fever two days after initial infusion, so was given a second dose of IVIG. Steroids were given to 12 children (34%) because they were deemed to be high risk. Three patients (9%) received anakinra due to persistent severe inflammatory state. Also, 23 patients (66%) were given heparin at a therapeutic dose. Most patients (n = 29, 83%) had to be admitted to the ICU for a median of 7 days, and required inotropic support (n = 28, 80%). Ten (29%) of those in intensive care required mechanical circulatory assistance with venoarterial ECMO but were successfully weaned and removed successfully after 4.5 days on average. Clinical resolution of signs and symptoms occurred in 28 patients (80%) so far. The median hospital stay of patients that were discharged at the time of study publication was 10 days. Seven (20%) children were either still in hospital or had residual impaired cardiac function. |
| Feldstein et al. June 2020 Multisystem inflammatory syndrome in U.S. children and adolescents [49] |
A targeted surveillance (prospective and retrospective) for MIS-C across all pediatric hospitals in the United States. | The study included 186 patients across the United States. Patients presented to healthcare facilities from March 15 to May 20, 2020. Their median age was 8.3 years. Comorbidities were noted in 51 patients (27%), including respiratory (n = 33, 18%), cardiac (n = 5, 3%), immunocompromised or autoimmune (n = 10, 5%), and other (n = 20, 11%). Out of the patients who had BMI reported, 29% were obese (n = 45, out of the 153 patient who had data available). | MIS-C (CDC). | Immunomodulating therapies used included IVIG (n = 144, 77%), glucocorticoids (n = 91, 49%), interleukin-6 inhibitors tocilizumab or siltuximab (n = 14, 8%), and anakinra (n = 24, 13%). A second dose of IVIG was given to 39 children (21%), and anticoagulation therapy was administered to 87 patients (47%). Most children (n = 148, 80%) required admission to intensive care, and many needed vasoactive support (n = 90, 48%). Eight children (4%) needed ECMO. At the end of the study, 130 patients (70%) were successfully discharged, and 52 (28%) were still in hospital. The patients that were discharged spent, on average, 7 days in the hospital. Sadly, 4 patients (2%) passed away. All were above 10 years old, two had underlying comorbidities, and 3 had ECMO support. |
| Ouldali et al. July 2020 Emergence of Kawasaki disease related to SARS-COV-2 infection in an epicentre of the French COVID-19 epidemic: a time series analysis [100] |
A quasi- experimental interrupted time series analysis of KD over the last 15 years in comparison to those presenting during the COVID-19 pandemic. | The study included 230 pediatric patients that presented to hospital with complete or incomplete KD between Dec 1, 2005 and May 20, 2020, with 10 cases presenting during the pandemic (April 15 to May 20, 2020). The children were all admitted to a tertiary centre in Paris, France. Their median age was 11.75 years (range 18 months to 15.8 years). Comorbidities were not mentioned in the paper. | Classic or incomplete KD according to the 2017 American Heart Association criteria. SARS-CoV-19 confirmation was not necessary, but only one patient (10%) had no laboratory evidence of infection or positive exposure history. | Patients were treated based on KD management guidelines. Most children (n = 9, 90%) were given IVIG as first line, from which 6 (60%) was considered unsuccessful and required a second line treatment. The second line treatment included a second dose of IVIG plus methylprednisolone (n = 5, 50%) or tocilizumab (n = 1, 10%). Six patients (60%) required PICU admission, from which 5 (50%) needed inotropic support. All patients were discharged successfully after a median of 13.5 days in hospital (range 4 to 27 days). |
| Lee et al. July 2020 Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children [111] |
A retrospective single centre observational study that compared data with historic cohorts of KD and macrophage activation syndrome. | The study included 28 patients with a median age of 9 years. Children all presented to hospital in Boston, United States between March to June 2020. Half of the patients (n = 14) had comorbidities including asthma (n = 3, 11%), congenital heart disease (n = 1, 4%), KD previously diagnosed (n = 2, 7%), sickle cell anemia (n = 1, 4%), autism (n = 1, 4%), mitochondrial disorder (n = 1, 4%), chromosomal abnormalities (n = 1, 4%), and obesity (n = 4, 14%). | MIS-C (CDC) | Most patients (n = 20, 71%) were given IVIG (n = 13, 46%, 2 g/kg; n = 6, 21%, 1 g/kg; n = 1, 4%, 0.5 g/kg), and 17 (61%) received methylprednisolone (1–4 mg/kg/day). Two patients (7%) required a second dose of IVIG. Anakinra (5–13 mg/kg/day) was administered in 5 children (18%) that were refractory to IVIG and steroids and in one child (4%) that had sickle cell to prevent the use of methylprednisolone. Antibiotics were given to 15 children (54%), remdesivir to 7 (25%), and hydroxychloroquine in one (4%). In terms of anticoagulation therapy, 14 patients (50%) received low dose aspirin (3–6 mg/kg/day), 5 (18%) received high dose aspirin (20–50 mg/kg/day), 13 (46%) were administered enoxaparin at the prophylactic dosing, and 5 (18%) were administered treatment dose enoxaparin. PICU admission was required in 17 patients (61%), where 7 (25%) received inotropes. All cases showed clinical improvement, defined as fever resolution, inflammatory marker improvement, and cessation of intorope need. The median hospital stay was 8 days. In patients that were treated with immunomodulatory therapies, fever resolution was seen after a median of 4 days from admission. Also, 3 of the 6 cases that had coronary artery abnormalities and were treated with IVIG (n = 6, 21%) and methylprednisolone (n = 4, 14%) showed normalization of coronary vessels post-treatment (50%), one showed improvement (17%), and the other two showed stabilization (33%). |
| Jones et al. April 2020 COVID-19 and Kawasaki disease: novel virus and novel case [122] |
A case report. | A 6-month-old female infant that presented to a pediatric hospital in California in early April 2020. She had no comorbidities. | Classic KD with confirmed SARS-CoV-2 infection (RT-PCR positive). | Treated according to KD management guidelines. She was given IVIG (2 g/kg) and high dose acetylsalicylic acid (20 mg/kg four times a day). The patient's fever resolved quite quickly after treatment. There were no cardiac findings, and the patient was discharged 2 days post-IVIG treatment on low dose acetylsalicylic acid (3 mg/kg). |
4.2. Management
Currently, treatment algorithms at renowned tertiary pediatric hospitals recommend management based on severity, congruence with complete KD criteria, presence of macrophage activation syndrome, and inflammatory marker levels [81]. A number of expert societies have created management protocols as well, including the American College of Rheumatology [83], and PICU guidelines have been put forward by an international group of experienced internists [84]. Nevertheless, there is no definitive data on the most appropriate management as of yet [84].
In the studies analyzed, four out of 15 explicitly explained the management guidelines that were followed: three used the American Heart Association 2017 KD guidelines and one used a case-by-case multidisciplinary team approach. All but two studies modeled the KD treatment approach initially, even if not explicitly mentioned. However, as MIS-C is a distinct entity from KD, it is likely to require a different management approach. Additionally, there is the aforementioned possibility that the pathophysiology might be related to that of TSS. However, there is notable overlap between KD and TSS treatment, as the gold-standard for severe KD cases, IVIG, has high affinity for the staphylococcal enterotoxin B [85] and therefore, potentially the SARS-CoV-2 superantigen. If further research does reveal such superantigens to underly this condition, other approved medications for staphylococcal enterotoxin B may be considered for MIS-C treatment, such as the CD28 co-stimulation inhibitor CLTA1-Ig [86] or the mTOR inhibitor rapamycin [87].
Two studies managed cases without using IVIG as the first line therapy, including a case series of five pediatric patients with potentially acute abdomen due to COVID-19 [88] and a case report of a patient with MIS-C and concomitant newly diagnosed Crohn's disease [89]. In the case series, the children received heterogenous treatments for acute COVID-19 infection, such as hydroxychloroquine and lopinavir/ritonavir. These treatments were chosen as either the children were PCR positive or the acuity of symptoms prompted clinicians to begin empiric treatment [88]. As for the case report, the authors opted for infliximab as a treatment that might address both the cytokine storm of MIS-C and the dysregulated immune reaction of Crohn's disease [89].
4.3. Supportive therapy
As mentioned previously, guidelines for PICU management of COVID-19 pediatric patients have been proposed [84]. Eight recommendations from the document were related to MIS-C treatment, with the best practice suggestions being supportive management and close monitoring, use of a multidisciplinary team, laboratory tests (SARS-CoV-2 antigens, inflammatory markers, organ system dysfunction), and empirical antibiotics until bacterial causes are excluded [84]. These were based largely on management of other severe inflammatory diseases [84].
It is important to note that not all MIS-C patients need some form of immunomodulatory therapy [83]; supportive care and close monitoring may be enough. The expert panel of internists was even inconclusive whether IVIG should be given empirically in MIS-C patients [84]. For instance, in one case report of 58 children, 22% of MIS-C patients made a full recovery with supportive care alone [66]. The patient sample comprised of inpatients, with 50% needing ICU admission throughout the length of their stay. However, the study did not stratify data based on who needed further treatment outside of supportive care or not, so it is unclear if there are any commonalities amongst the supportive care group (ex. inflammatory marker levels, presenting symptoms, clinical course, demographics) [66]. In our literature search, there were few patients that received supportive care alone, but this again may be due to the acuity of patients included. Further research will hopefully elucidate what/if specific therapies are superior to supportive care and whether clinical inaction may bring with it serious long-term sequelae, as is the case in KD.
4.4. Immunomodulators
It is well known that IVIG (2 g/kg) prevents coronary artery aneurysm in KD [63,90,91] and there have been reports of benefit from the therapy in COVID-19-associated myocarditis also [92]. In addition, glucocorticoids have been shown to reduce coronary artery aneurysm in KD patients with a high risk of IVIG failure [93,94]. However, since MIS-C is a separate disease entity from KD, using KD management strategies may not be as efficacious, potentially explaining the higher IVIG failure rate in MIS-C patients [45]. As most centers were following KD treatment guidelines, a majority of patients in our literature review did receive IVIG (n = 298, 77%). The treatment appeared reasonably robust, but a substantial percentage required a second dose of IVIG (18%). The reason for second IVIG dose varied based on the study, including failed remission of inflammatory manifestations [67], persistent fever 36 h after the end of initial IVIG infusion [48,49], and persistent fever two days after initial transfusion [51].
Moreover, 171 patients (43%) were given methylprednisolone at some point during admission. According to the COVID-19 PICU recommendations, for patients that meet classical or atypical KD criteria, administering IVIG (2 g/kg) and aspirin (50 m/kg) is strongly encouraged [84]. However, in the context of MIS-C, the use of methylprednisolone and IVIG should be considered with multidisciplinary input as both have insufficient evidence [84]. Similarly, the American Rheumatology College task force recommends IVIG and steroids alone or together, but there is not enough data to compare the effectiveness of one versus the other, or when used in combination [83].
4.5. Specific immunotherapies
It is theorized that IL-6 plays a central role in KD pathophysiology via megakaryocyte maturation, thereby leading to thrombocytosis. The cytokine also potentially causes vasculitis by triggering a cascade that stimulates polyclonal B cell autoantibody production, resulting in acute inflammation and antibody mediated endothelial damage [95,96]. Recent evidence suggested that the cytokine IL-6 may be culpable for myocardial injury in COVID-19 [97] and studies have found IL-6 levels to be significantly elevated in MIS-C patients [48,50,64,67,89]. Therefore, IL-6 inhibitors may prove to be a valuable treatment option in MIS-C. Tocilizumab, a monoclonal antibody that serves an IL-6 inhibitor, is used to treat systemic onset juvenile idiopathic arthritis [98], which shares many features with MIS-C, such as skin rash, major inflammatory syndrome, macrophage activation syndrome, and fever. Several institutions are already using tocilizumab or another IL-6 receptor monoclonal antibody, with 6 of 15 studies administering the immunotherapy to at least one patient, and 33 children (8.55%) receiving it in total, all with promising results [49,64,67,88,99,100]. Also, tocilizumab is already undergoing clinical trial (ClinicalTrials.gov Identifier: NCT04331808) to be used in severely ill COVID-19 adult patients [101,102]. However, formal investigation of tocilizumab use in pediatric MIS-C patients is awaited.
Anakinra is an IL-1 receptor antagonist that is commonly used to treat systemic juvenile arthritis induced cytokine release syndrome [103] and is currently undergoing a Phase 2 clinical trial (ClinicalTrials.gov Identifier: NCT02390596) for its use in KD [104]. The international group of internists recommends anakinra for MIS-C patients who have initially failed IVIG and/or methylprednisolone treatment [83] due to its relative safety in pediatric patients with hyperinflammatory syndromes and success in a small number of patients with IVIG-resistant KD [[105], [106], [107]]. Our literature review reflects this, as 39 patients across all studies received Anakinra or another IL-1 receptor antagonist, with most patients improving thereafter.
TNF-α is elevated in states of cytokine storm, KD, as well as in some reports of MIS-C [75,89,108]. In fact, recent literature has shown that the cytokine can be used to differentiate between MIS-C and severe COVID-19 [67,109]. Therefore, another potential treatment for the inflammatory effects seen in MIS-C patients would to be use infliximab, a TNF-α blocker. The immunotherapy has already been shown to treat IVIG resistant KD, so it may be especially beneficial for MIS-C patients with KD-like symptoms [108]. One case study included in our review used infliximab to successfully treat a patient with concurrent MIS-C and Crohn's, which resulted in symptom resolution [89]. Additionally, a more recent study gave 12 MIS-C patients (52%) infliximab as a second-line therapy due to persistent fever and inflammatory state (defined as increasing inflammatory markers or impaired cardiac function) after initial IVIG therapy [110]. The immunotherapy resulted in a positive outcome as the patients' fevers resolved, cardiac function improved, and any coronary artery dilation was ameliorated [110].
4.6. Antiviral therapy
The antiviral remdesiver was given for compassionate use in several instances, with two studies both treating seven patients with the medication [64,111]. Yet, as the most supported theory for MIS-C pathogenesis is that of post-infection, the potential for benefit of remdesiver in this syndrome may be limited. If the patient is SARS-CoV-2 RT-PCR positive, then remdesiver may theoretically be of use if administered early enough in the infection course [79,112,113]; however, this was the case for only one of the two studies [64].
4.7. Anti-coagulation and anti-platelet
Antiplatelet or anticoagulation therapy (usually aspirin or enoxaparin) was given to over 60% of patients throughout the studies analyzed. Since MIS-C fulfills the high risk of venous thromboembolism criteria, this course of action is entirely appropriate. If there is thrombocytopenia associated with multi-organ failure, which has been seen in MIS-C [67], then the PICU guidelines recommend therapeutic plasma exchange, although the supporting evidence is limited [84]. Additionally, the American College of Rheumatology outlines an algorithm for anticoagulation/antiplatelet medications and dosing based on risk, recommending low-dose aspirin in all patients with KD features, coronary artery aneurysm, and thrombocytosis [63,83]. If a patient has a coronary artery aneurysm with a z-score of 10 or more, then enoxaparin or warfarin is recommended. Additionally, anticoagulation should be considered for patients with moderate to severe left ventricular dysfunction [83].
4.8. Imaging
Ventricular dysfunction is a common occurrence in MIS-C patients [46,114,115]. Therefore, echocardiography and ECG should be performed early upon admission and subsequently at regular intervals. This is supported by the PICU guidelines for MIS-C and pediatric COVID-19 treatment [84]. However, coronary artery enlargement may be missed in echocardiography alone, so CT may be of additional value in indeterminate cases [46]. In terms of MRI use, three case series have investigated its utility and have shown diffuse myocardial edema and hyperemia acutely in the disease course [46,114,116]. In one case series, evidence of myocardial necrosis/fibrosis was also observed [116]. Furthermore, recent evidence shows that subtle cardiac dysfunction may persist past two weeks post PIMS-TS onset [46]. Thus, we recommend structured long-term follow-up with echocardiography supplemented with cardiac MRI or CT as needed.
4.9. New landmark papers and ongoing research
Since the review was compiled, there have been significant articles published that should be mentioned. One such article was a national consensus management pathway for PIM-TS published in February 2021 [117]. It was developed based on 140 consensus statements from UK clinicians who divided PIMS-TS into two phenotypes: KD-like (both complete and incomplete, based on the American Heart Association criteria), and non-specific (shock and/or fever and additional symptoms such as abdominal pain, gastrointestinal, respiratory, or neurologic symptoms not in keeping with KD criteria) [117]. The recommendations made closely resemble the suggestions made in our review. For instance, they emphasize the importance of a multi-disciplinary team, supportive care, IVIG, and methylprednisolone. Additionally, biologic therapies (anakinra, tocilizumab, and infliximab) can be considered [117]. The Lancet went a step further and defined indications for therapy (coronary artery abnormalities, TSS, signs of progressive disease, and fever more than 5 days) and what the first line (IVIG), second line (methylprednisolone), and third line (biologics) treatment should be [117].
Furthermore, the first formal randomized control trial for MIS-C/PIMS-TS treatment has begun as part of the RECOVERY trial in the UK [118]. There is also a clinical trial ongoing for the use of mesenchymal stromal cells as treatment for MIS-C (ClinicalTrials.gov Identifier: NCT04456439). In terms of surveillance, the CDC is putting $2.1 million towards a research study of 800 pediatric patients that were hospitalized after a positive coronavirus test at the Boston Children's Hospital [119]. WHO has created a global clinical platform [42], and MIS-C is actively being investigated by the Canadian Paediatric Surveillance Program [44].
5. Limitations
The results of this comprehensive literature review are limited by the heterogeneity of the studies included. Papers included patients of various levels of acuity, used different case definitions, reported different outcomes, and had assorted study designs of varying quality. Additionally, most of the articles included a small sample size due to the novelty of the condition. Given the few studies published to date and the recent emergence of MIS-C, this could not be avoided in our comprehensive review and must be taken into consideration when interpreting the data. Additionally, the included studies may have been confounded by patients with true KD that presented during this period of pandemic. Finally, it must be emphasized that the data supporting these treatments stems entirely from case series and reports which lack efficacy-establishing controls.
6. Conclusions
MIS-C is an entirely novel disease affecting children globally. The disease appears to be a post-infection phenomenon that is distinct from KD. Most cases have resolved quickly and completely with treatment, and morbidity and mortality have been low. Nevertheless, the data around the effectiveness of various treatment methods (methylprednisolone, IVIG, immunotherapies, etc.) and the best management are limited and of poor quality. Similarly, the exact pathophysiology and the long-term effects of MIS-C are at present unknown. As a result of the present comprehensive review, in combination with expert opinion and newly published protocol suggestions, we propose that pharmacologic treatment be tailored to the individual signs/symptoms observed (i.e., IVIG +/− steroids for Kawasaki disease-like features) and immunotherapies considered in severe cases, with careful evaluation of their appropriateness and potential for harm. In terms of imaging, early echocardiograms and ECGs are deemed essential core management, with cardiac MRI or CT utilized as needed. If patients have moderate to severe symptoms, or their clinical status is rapidly worsening, they should be in a facility that has pediatric intensive care capability. Close monitoring should be performed by an interdisciplinary team and follow-up with echocardiogram and/or CT/MRI is warranted in such cases. Future studies addressing the efficacy of targeted immunotherapies and the long-term sequelae of severe MIS-C are eagerly awaited.
Funding
No funding was sought for the present manuscript.
Declaration of competing interest
The authors have no duality of interest to declare.
Acknowledgements
None.
References
- 1.YZ Zhang. Novel 2019 coronavirus genome. [Internet]. Virological. 2020. Available from: http://virological.org/t/novel-2019-coronavirusgenome/319.
- 2.World Health Organization (WHO). Timeline of WHO's response to COVID-19 [Internet]. World Health Organization. Available from: https://www.who.int/news-room/detail/29-06-2020-covidtimeline.
- 3.Coronaviridae Study Group of the International. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5(4):536. [DOI] [PMC free article] [PubMed]
- 4.World Health Organization. Novel coronavirus (2019-nCoV) situation report – 22 [Internet]. 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf.
- 5.World Health Organization. Weekly operational update on COVID-19 [Internet]. 2021. Available from: https://www.who.int/docs/default-source/coronaviruse/wou-2021-8march-project.pdf?sfvrsn=595b939c_1&.
- 6.Fehr A.R., Perlman S. Coronaviruses. Springer; 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masters P, Perlman S. Coronaviridae. In: Fields virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 825.
- 9.Enjuanes L., Smerdou C., Castilla J., Anton I.M., Torres J.M., Sola I., et al. Corona-and related viruses. Springer; 1995. Development of protection against coronavirus induced diseases; pp. 197–211. [DOI] [PubMed] [Google Scholar]
- 10.America Academy of Pediatrics. Coronaviruses, including SARS and MERS. In: Red Book [Internet]. 31st ed. 2018. Available from: https://redbook.solutions.aap.org/chapter.aspx?sectionid=189640073&bookid=2205.
- 11.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamming I., Timens W., Bulthuis M., Lely A., Navis G. van, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol Soc G B Irel. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 16.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated With Coronavirus Disease 2019 (COVID-19) [Internet]. Center for Disease Control and Prevention. 2020 [cited 2020 Jul 15]. Available from: https://emergency.cdc.gov/han/2020/han00432.asp.
- 21.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID-19 in Shenzhen China: analysis of 391 cases and 1,286 of their close contacts. 2020 Mar 4 [cited 2020 Sep 7]; Available from: 10.1101/2020.03.03.20028423. [DOI] [PMC free article] [PubMed]
- 23.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 25.Colonna C., Monzani N.A., Rocchi A., Gianotti R., Boggio F., Gelmetti C. Chilblains-like lesions in children following suspected Covid-19 infection. Pediatr Dermatol. 2020 doi: 10.1111/pde.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. Jama. 2020 doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L., Lu X., Chen L. Possible causes for decreased susceptibility of children to coronavirus. Pediatr Res. 2020 doi: 10.1038/s41390-020-0892-8. [DOI] [PubMed] [Google Scholar]
- 28.Government of Canada. Coronavirus disease 2019 (COVID-19): epidemiology update [Internet]. Health Infobase. 2021 [cited 2021 Mar 9]. Available from: https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html#a5.
- 29.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan Y., Zhang D., Yang P., Poon L.L., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z.-M., Fu J.-F., Shu Q., Chen Y.-H., Hua C.-Z., Li F.-B., et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020:1–7. doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei M., Yuan J., Liu Y., Fu T., Yu X., Zhang Z.-J. Novel coronavirus infection in hospitalized infants under 1 year of age in China. Jama. 2020;323(13):1313–1314. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Weekly updates by select demographic and geographic characteristics: provisional death counts for coronavirus disease 2019 (COVID-19) [Internet]. CDC. 2021 [cited 2021 Mar 10]. Available from: https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#SexAndAge.
- 37.Yonker L.M., Shen K., Kinane T.B. Lessons unfolding from pediatric cases of COVID-19 disease caused by SARS-CoV-2 infection. Pediatr Pulmonol. 2020;55(5):1085. doi: 10.1002/ppul.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6). [DOI] [PubMed]
- 39.Castagnoli R., Votto M., Licari A., Brambilla I., Bruno R., Perlini S., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 40.Parri N., Lenge M., Buonsenso D. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020 doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 [Internet]. WHO. 2020 [cited 2020 Jul 15]. Available from: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19.
- 43.Royal College of, Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19 [Internet]. [cited 2020 Jul 15]. Available from: https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf.
- 44.Canadian Pediatric Surveillance Program. COVID-19 [Internet]. CPSP. [cited 2020 Jul 15]. Available from: https://www.cpsp.cps.ca/surveillance/study-etude/covid-19.
- 45.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theocharis P., Wong J., Pushparajah K., Mathur S.K., Simpson J.M., Pascall E., et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2020 doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramcharan T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A.G., et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol. 2020:1–11. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020;369. [DOI] [PMC free article] [PubMed]
- 49.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belhadjer Z., Méot M., Bajolle F., Khraiche D., Legendre A., Abakka S., et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 51.Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4(9):669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol Soc G B Irel. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geng Y.-J., Wei Z.-Y., Qian H.-Y., Huang J., Lodato R., Castriotta R.J. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc Pathol. 2020 doi: 10.1016/j.carpath.2020.107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaughan C.J., Cronin H., Ryan P.M., Caplice N.M. Obesity and COVID-19: a Virchow's triad for the 21st century. Thromb Haemost. 2020 doi: 10.1055/s-0040-1714216. [DOI] [PubMed] [Google Scholar]
- 55.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fraser DD, Cepinskas G, Slessarev M, Martin C, Daley M, Miller MR, et al. Inflammation profiling of critically ill coronavirus disease 2019 patients. Crit Care Explor 2020;2(6). [DOI] [PMC free article] [PubMed]
- 57.Weisberg SP, Connors T, Zhu Y, Baldwin M, Lin W-H, Wontakal S, et al. Antibody responses to SARS-CoV2 are distinct in children with MIS-C compared to adults with COVID-19. medRxiv. 2020.
- 58.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 60.Cong Y, Ulasli M, Schepers H, Mauthe M, V'kovski P, Kriegenburg F, et al. Nucleocapsid protein recruitment to replication-transcription complexes plays a crucial role in coronaviral life cycle. J Virol 2020;94(4). [DOI] [PMC free article] [PubMed]
- 61.Kato H., Sugimura T., Akagi T., Sato N., Hashino K., Maeno Y., et al. Long-term consequences of Kawasaki disease: a 10-to 21-year follow-up study of 594 patients. Circulation. 1996;94(6):1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 62.Brogan P., Burns J.C., Cornish J., Diwakar V., Eleftheriou D., Gordon J.B., et al. Lifetime cardiovascular management of patients with previous Kawasaki disease. Heart. 2020;106(6):411–420. doi: 10.1136/heartjnl-2019-315925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 64.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R., et al. Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York City. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020 doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. Jama. 2020 doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pouletty M., Borocco C., Ouldali N., Caseris M., Basmaci R., Lachaume N., et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.JCS Joint Working Group Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008) Circ J. 2010;74(9):1989–2020. doi: 10.1253/circj.cj-10-74-0903. [DOI] [PubMed] [Google Scholar]
- 69.Baltimore R, Gewitz M, Baddour L, Beerman L, Jackson M, Lockhart P, et al. on behalf of the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young and the Council on Cardiovascular and Stroke Nursing. Infective endocarditis in childhood: 2015 update: a scientific statement from the American Heart Association [published online ahead of print September 14, 2015]. Circulation 2015.
- 70.Muniz J.-C.G., Dummer K., Gauvreau K., Colan S.D., Fulton D.R., Newburger J.W. Coronary artery dimensions in febrile children without Kawasaki disease. Circ Cardiovasc Imaging. 2013;6(2):239–244. doi: 10.1161/CIRCIMAGING.112.000159. [DOI] [PubMed] [Google Scholar]
- 71.Bratincsak A., Reddy V.D., Purohit P.J., Tremoulet A.H., Molkara D.P., Frazer J.R., et al. Coronary artery dilation in acute Kawasaki disease and acute illnesses associated with fever. Pediatr Infect Dis J. 2012;31(9):924–926. doi: 10.1097/INF.0b013e31826252b3. [DOI] [PubMed] [Google Scholar]
- 72.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. 2020 doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma A, Garcia G, Arumugaswami V, Svendsen CN. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. bioRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 74.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng MH, Zhang S, Porritt RA, Arditi M, Bahar I. An insertion unique to SARS-CoV-2 exhibits superantigenic character strengthened by recent mutations. bioRxiv. 2020.
- 76.Low D.E. Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin. 2013;29(3):651–675. doi: 10.1016/j.ccc.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 77.Cook A., Janse S., Watson J.R., Erdem G. Manifestations of toxic shock syndrome in children, Columbus, Ohio, USA, 2010–2017. Emerg Infect Dis. 2020;26(6):1077. doi: 10.3201/eid2606.190783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krakauer T. Staphylococcal superantigens: pyrogenic toxins induce toxic shock. Toxins. 2019;11(3):178. doi: 10.3390/toxins11030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Y.-J., Cheng M.-C., Lo M.-H., Chien S.-J. Early differentiation of Kawasaki disease shock syndrome and toxic shock syndrome in a pediatric intensive care unit. Pediatr Infect Dis J. 2015;34(11):1163–1167. doi: 10.1097/INF.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 81.The Hospital for Sick Children. Practice alert: rise in cases of paediatric multisystem inflammatory syndrome in the setting of the COVID-19 outbreak [Internet]. 2020 [cited 2020 Jul 1]. Available from: https://www.pcmch.on.ca/wp-content/uploads/2020/06/SickKids-Practice-alert_COVID-19-Paed-Multisystem-Inflammatory-syndrome.pdf.
- 82.Hennon T.R., Penque M.D., Abdul-Aziz R., Alibrahim O.S., McGreevy M.B., Prout A.J., et al. COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol. 2020 doi: 10.1016/j.ppedcard.2020.101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henderson L.A., Canna S.W., Friedman K.G., Gorelik M., Lapidus S.K., Bassiri H., et al. American College of Rheumatology clinical guidance for pediatric patients with multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 and hyperinflammation in COVID-19. Version 1. Arthritis Rheumatol. 2020 doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kache S., Chisti M.J., Gumbo F., Mupere E., Zhi X., Nallasamy K., et al. COVID-19 PICU guidelines: for high-and limited-resource settings. Pediatr Res. 2020:1–12. doi: 10.1038/s41390-020-1053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nishi J.-I., Kanekura S., Takei S., Kitajima I., Nakajima T., Wahid M.R., et al. B cell epitope mapping of the bacterial superantigen staphylococcal enterotoxin B: the dominant epitope region recognized by intravenous IgG. J Immunol. 1997;158(1):247–254. [PubMed] [Google Scholar]
- 86.Whitfield S.J., Taylor C., Risdall J.E., Griffiths G.D., Jones J.T., Williamson E.D., et al. Interference of the T cell and antigen-presenting cell costimulatory pathway using CTLA4-Ig (abatacept) prevents Staphylococcal enterotoxin B pathology. J Immunol. 2017;198(10):3989–3998. doi: 10.4049/jimmunol.1601525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krakauer T., Buckley M., Issaq H.J., Fox S.D. Rapamycin protects mice from staphylococcal enterotoxin B-induced toxic shock and blocks cytokine release in vitro and in vivo. Antimicrob Agents Chemother. 2010;54(3):1125–1131. doi: 10.1128/AAC.01015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cabrero-Hernández M., García-Salido A., Leoz-Gordillo I., Alonso-Cadenas J.A., Gochi-Valdovinos A., Brabin A.G., et al. Severe SARS-CoV-2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr Infect Dis J. 2020;39(8):e195–e198. doi: 10.1097/INF.0000000000002777. [DOI] [PubMed] [Google Scholar]
- 89.Dolinger M.T., Person H., Smith R., Jarchin L., Pittman N., Dubinsky M.C., et al. Pediatric Crohn's disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. 2020 doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furusho K., Nakano H., Shinomiya K., Tamura T., Manabe Y., Kawarano M., et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;324(8411):1055–1058. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 91.Newburger J.W., Takahashi M., Burns J.C., Beiser A.S., Chung K.J., Duffy C.E., et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 92.Kociol R.D., Cooper L.T., Fang J.C., Moslehi J.J., Pang P.S., Sabe M.A., et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141(6):e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi T., Saji T., Otani T., Takeuchi K., Nakamura T., Arakawa H., et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012;379(9826):1613–1620. doi: 10.1016/S0140-6736(11)61930-2. [DOI] [PubMed] [Google Scholar]
- 94.Wardle A.J., Connolly G.M., Seager M.J., Tulloh R.M. Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2017;1 doi: 10.1002/14651858.CD011188.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ueno Y., Takano N., Kanegane H., Yokoi T., Yachie A., Miyawaki T., et al. The acute phase nature of interleukin 6: studies in Kawasaki disease and other febrile illnesses. Clin Exp Immunol. 1989;76(3):337. [PMC free article] [PubMed] [Google Scholar]
- 96.Lin C.-Y., Lin C.-C., Hwang B., Chiang B. Cytokines predict coronary aneurysm formation in Kawasaki disease patients. Eur J Pediatr. 1993;152(4):309–312. doi: 10.1007/BF01956740. [DOI] [PubMed] [Google Scholar]
- 97.Nandi A., Pal P., Basu S. A comparison of serum IL6 and CRP levels with respect to coronary changes and treatment response in Kawasaki disease patients: a prospective study. Rheumatol Int. 2019;39(10):1797–1801. doi: 10.1007/s00296-019-04375-9. [DOI] [PubMed] [Google Scholar]
- 98.Beukelman T., Patkar N.M., Saag K.G., Tolleson-Rinehart S., Cron R.Q., DeWitt E.M., et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res. 2011;63(4):465–482. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grimaud M., Starck J., Levy M., Marais C., Chareyre J., Khraiche D., et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10(1):1–5. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ouldali N., Pouletty M., Mariani P., Beyler C., Blachier A., Bonacorsi S., et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020;4(9):662–668. doi: 10.1016/S2352-4642(20)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Assistance Publique - Hôpitaux de Paris. Cohort multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients - tocilizumab trial - CORIMUNO-19 - TOCI (CORIMUNO-TOCI) [Internet]. clinicaltrials.gov; 2020 Apr [cited 2020 Jul 15]. Report No.: NCT04331808. Available from: https://clinicaltrials.gov/ct2/show/NCT04331808.
- 103.Food and Drug Administration. Anakinra (kineret) prescribing information [Internet]. 2012 [cited 2020 Jul 15]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103950s5136lbl.pdf.
- 104.Assistance Publique - Hôpitaux de Paris. Anakinra and Kawasaki Disease (KAWAKINRA) [Internet]. clinicaltrials.gov; 2015 Mar [cited 2020 Jul 15]. Report No.: NCT02390596. Available from: https://clinicaltrials.gov/ct2/show/NCT02390596.
- 105.Kone-Paut I., Cimaz R., Herberg J., Bates O., Carbasse A., Saulnier J.P., et al. The use of interleukin 1 receptor antagonist (anakinra) in Kawasaki disease: a retrospective cases series. Autoimmun Rev. 2018;17(8):768–774. doi: 10.1016/j.autrev.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 106.Lind-Holst M., Hartling U.B., Christensen A.E. High-dose anakinra as treatment for macrophage activation syndrome caused by refractory Kawasaki disease in an infant. BMJ Case Rep CP. 2019;12(8) doi: 10.1136/bcr-2019-229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guillaume M.-P., Reumaux H., Dubos F. Usefulness and safety of anakinra in refractory Kawasaki disease complicated by coronary artery aneurysm. Cardiol Young. 2018;28(5):739. doi: 10.1017/S1047951117002864. [DOI] [PubMed] [Google Scholar]
- 108.Mori M., Hara T., Kikuchi M., Shimizu H., Miyamoto T., Iwashima S., et al. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-017-18387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS–CoV-2. J Clin Invest. 2020;130(11). [DOI] [PMC free article] [PubMed]
- 110.Abdel-Haq N., Asmar B.I., Leon M.P.D., McGrath E.J., Arora H.S., Cashen K., et al. SARS-CoV-2-associated multisystem inflammatory syndrome in children: clinical manifestations and the role of infliximab treatment. Eur J Pediatr. 2021:1–11. doi: 10.1007/s00431-021-03935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee P.Y., Day-Lewis M., Henderson L.A., Friedman K., Lo J., Roberts J.E., et al. Distinct clinical and immunological features of SARS-COV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020 doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X., Yuan J., Zheng Y., Chen J., Bao Y., Wang Y., et al. Retracted: clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen. Zhonghua Er Ke Za Zhi Chin J Pediatr. 2020;58:E008. doi: 10.3760/cma.j.issn.0578-1310.2020.0008. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blondiaux E., Parisot P., Redheuil A., Tzaroukian L., Levy Y., Sileo C., et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology. 2020 doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vari D., Miller J.M., Rellosa N., Srivastava S., Frizzola M., Thacker D. Severe cardiac dysfunction in a patient with multisystem inflammatory syndrome in children associated with COVID-19: retrospective diagnosis of a puzzling presentation. A case report. Prog Pediatr Cardiol. 2020;58 doi: 10.1016/j.ppedcard.2020.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jain S, Nolan SM, Biller R, Pinto A, Fuisz AR, Gewitz MH. Cardiovascular magnetic resonance in myocarditis related to multisystem inflammatory syndrome in children associated with COVID-19.
- 117.Harwood R., Allin B., Jones C.E., Whittaker E., Ramnarayan P., Ramanan A.V., et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. 2020 doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.P Hornby. Randomised evaluation of COVID-19 therapy (protocol version 8), recovery trial. [Internet]. 2020. Available from: https://www.recoverytrial.net/files/recovery-protocol-v8-0-2020-07-08.pdf.
- 119.Rollins J.A. Severe inflammatory syndrome in children testing positive for current or recent COVID-19 infection. Pediatr Nurs. 2020;46(3):109. [Google Scholar]
- 120.Canadian Paediatric Surveillance Program. Case definition for COVID-19 study [Internet]. [cited 2020 Jul 15]. Available from: https://www.cpsp.cps.ca/uploads/studies/COVID-19-case-definition-rev-06-2020.pdf.
- 121.Dasgupta K., Finch S.E. A case of pediatric multisystem inflammatory syndrome temporally associated with COVID-19 in South Dakota. SD Med. 2020;73(6):246–251. [PubMed] [Google Scholar]
- 122.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]