Abstract
In addition to being the epicentre of the HIV epidemic, South Africa also has the highest burden of sexually transmitted infections (STIs) in the world. Therefore, understanding the most influential risk factors of STIs is a research priority. Using the data from 9,948 women who resided in KwaZulu Natal, South Africa, we estimated the population attributable risk to quantify the combined impacts of the most influential factors on STI diagnosis. Overal STI Prevalence was 20%, and STI incidence was 15 per 100 person-year. Four factors, age at sexual debut, single/not cohabiting, two or more sex partners and parity<3 were identified as the most influential risk factors for STI prevalence and incidence rates. However, these factors collectively associated with only 51% and 53% of the excess STI prevalence and incidence rates, respectively. These relatively modest impacts provide empirical evidence for the significant impacts of unmeasured factors on STIs. Culturally and socially appropriate prevention programs may be more effective to target those at highest risk of STIs.
Keywords: Sexually Transmitted Infections, women, KwaZulu Natal, South Africa
Introduction
According to the World Health Organisation (WHO), curable sexually transmitted infections (STIs) affect millions of people globally.1,2 Based on the estimates in 2016, more than 376 million men and women aged 15-49 years were infected with one of the four urogenital infections including chlamydia (chlamydia trachomatis), gonorrhoea (Neisseria gonorrhoea), syphilis (Treponema pallidum) or trichomoniasis (trichomonas vaginalis), which reflects an average of more than one million new infections each day. 1,2,3 In addition to creating major social stigma, untreated STIs can also have serious long-term morbidity and mortality including infertility, pre-term birth, ectopic pregnancies and spontaneous abortion.4 Most importantly STIs, have also been reported to facilitate HIV transmission through genital disruption and inflammation.5 These two pathogeneses also share common high-risk sexual behaviours such as low-levels of condom use and multiple/concurrent sex partners. 6–8 Therefore, HIV and STI prevention programs are often considered together due to their overlapping nature.
In addition to be the epicentre of the HIV epidemic in the world, South Africa also has the highest burden of STIs in the region.2 Particularly, KwaZulu Natal, the highly populated province in South Africa has the twin epidemic with the highest HIV and STI rates in the world.9 Despite extensive research to identify those at highest risk of STIs, developing effective programs to reduce the STI acquisitions have proven to be challenging in South Africa. The current evidence suggests that STI related morbidities are still severely affecting young South Africans and contributing to high health care costs nationwide. 10,11Therefore, understanding the most influential risk factors and their contributions to STI prevalence and incidence is an important research priority.
Although there has been extensive research to identify the risk factors associated with increased STI diagnoses in South Africa, to date, there has not been an attempt to quantify the combined (joint) impact of these risk factors on STI positivity. Our objective was two-fold: (1) to identify the correlates and predictors of STI prevalence and incidence the using standard statistical methods; (2) to estimate individual and combined impacts of the most influential risk factors on STI prevalence and incidence rates after accounting for their complex and correlated nature in multifactorial disease setting. 12 This additional information will potentially bring greater insight into the epidemic by informing the extent of the infections in the population and its most influential contributors. Therefore, they collectively will have significant public health implications in reducing STIs by prioritising those at highest risk of STIs.
Materials and Methods
Study design and population
During the period of 2002 to 2016, a total of six phase II/III HIV prevention biomedical intervention trials were conducted in Durban, KwaZulu Natal, South Africa. In this analysis, we included the data from 9,948 women who participated in one of these trials. Details of the study populations were described previously.13–18 All trials used similar STI, HIV and pregnancy testing and diagnostic testing procedures. Interviewer-based demographics, socio-economic and sexual behavioural risk assessments were completed at specified study visits. Women were tested for STIs including chlamydia, gonorrhoea, syphilis and trichomoniasis at baseline and at every study visit. They were classified as STI positive at baseline if they had at least one positive STI test result (i.e. chlamydia, gonorrhoea, syphilis or trichomoniasis). Using the assumption that any STI diagnosis at baseline was adequately treated and resolved per study protocols, any STI positivity during the study follow-up was considered as a new infection.
All women received risk-reduction counselling during the study follow-ups. If women were tested positive for STIs, they were treated at the clinical research sites, as per specific STI results. The current analysis considered risk factors that were measured consistently in all trials.
Measurements
This study has two primary outcomes: STI positivity at baseline and STI incidence during the study follow up period. The date of the incidence of STIs was estimated using the midpoint between the last negative and the first positive test results within the follow-up period. Those who did not have any STIs were censored either at their last visit or end of the study whichever occurred first. Several demographic, socioeconomic characteristics and sexual behaviours were also considered in our analysis: age (<20, 20–24, 25–29, 30-34 and 35+ years); marital/cohabitation status (single/not cohabiting vs. married/cohabiting); level of education (no education, primary education, secondary education); total number of sex partners in past three months (<2 vs 2+ partners); age at sexual debut (<15, 15-19 vs. 20+ years), condom used at last sex (yes/no); method of contraceptive (none, male condoms, oral/pill vs. injectables) parity (nulliparity, primiparity, 2 births vs. 3+ births). We did not analyse the following factors as they were not collected across all the trials: employment status, average number of sexual acts in the past two weeks, language, and reports that their partner had a new partner during the study follow-up period.
Statistical Analysis
We used descriptive statistics (percentages) to characterise the study population according to their characteristics. We identified the correlates of baseline STI diagnoses using the log-binomial regression model. We presented adjusted prevalence odds ratios (aPORs) and 95% CIs. The Cox regression model was used to investigate the associations between the population characteristics and subsequent STI positivity during the follow-up. We presented the adjusted odds ratios (aOR) and adjusted hazard ratios (aHR) and their 95% Confidence Intervals (CI). We also estimated individual and combined impacts of the risk factors in multivariable model setting when all risk factors were included in the model, rather than identifying independent predictors of the outcomes of interest. We used modified version of the population attributable risk (PAR%) which can handle the correlated nature of the risk factors considered in statistical models. 12 Intuitively, PAR% provide proportion of STIs (prevalence or incidence) could be eliminated (at least theoretically) by targeting the combined risk factors in the target population.
Total number of risk factors:
After we identified the significant risk factors for STI positivity (using logistic regression models) and STI incidence (using Cox regression models), we calculated subject-specific total number of risk factors for each outcome separately. Briefly, for each risk factor, we assigned a score of 1 (for the high-risk category) and 0 (otherwise); these scores were added to create the total number of risk factors for each participant. For the analysis, we categorised them as: 0-risk factor, 1-risk factor, 2-risk factors, 3-risk factors, 4-risk factors for STI diagnosis at baseline (or 4+ risk factors for STI incidence during the follow-up). We presented the STI prevalence and incidence rates across the total risk factors by the age groups (<20, 20-24, 25-29, 30-34 and 35+ years) using “heat-maps” (Figure 1a and Figure 1b respectively). We also fit logistic and Cox regression models across the increasing categories of the total subject specific risk factors for both outcomes separately
Figure 1.
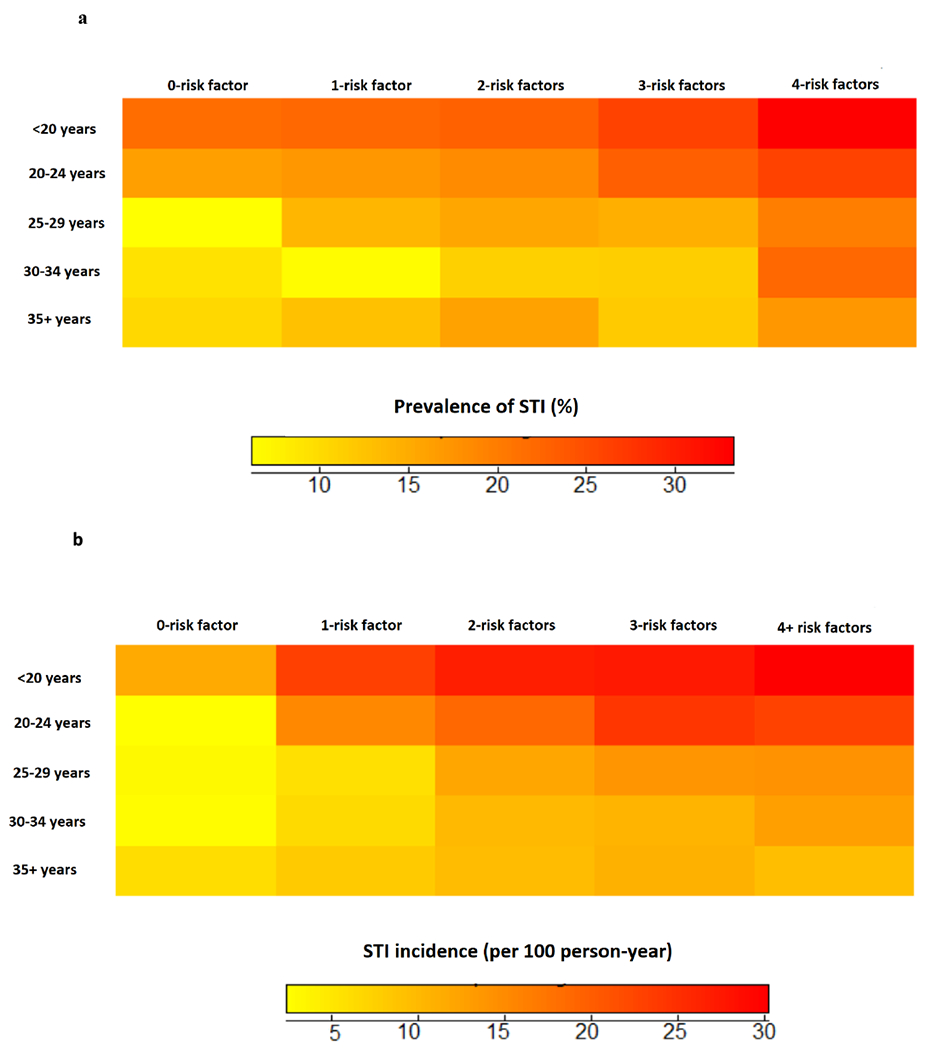
a: STI prevalence by risk factors (overall prevalence: 20%, 95% CI: 17%, 23%)
b: STI incidence by score (overall incidence rate: 15 per 100 person-year 95% CI: 13.7, 16.3)
All analyses were performed using Stata 14.0 and SAS statistical software, version 9.4 (SAS Inc, Cary, NC, USA).
Results
The current analysis included combined data from 9,948 women who enrolled in one of the six HIV biomedical intervention trials conducted during the period of 2002 to 2015. The overall median age was 25 years (Interquartile range (IQR):22-32), more than half of the study population had no education, while more than 90% of women reported being sexually active before the age of 20 years. Most of the study population (77%) was unmarried, 55% of the women had less than two children, 13% of them reported at least two sexual partners in the past three months and 64% of women reported using a condom at the last sex act. Injectable hormonal contraceptives were the most common family planning method (53%).
Correlates of STI diagnosis and predictors of STI incidence
Table 1 presents the results from the adjusted log-binomial and Cox regression models for baseline STI positivity and incidence respectively. Compared to the women 35 years or older, younger women, particularly those <25 years of age were significantly more likely to be diagnosed with STIs at baseline (aPORs: 162 and 1.46 for women <20 and 20-24 years old respectively); they were also at increased risk of STIs during the study follow up (aHRs:3.50, 2.47 and 1.30 for women <20, 20-24 and 25-29 years old respectively). Age at sexual debut (<20 years of age) was identified as a significant predictor of STI positivity at baseline as well as STI incidence during the study follow-up period. Being single/not cohabiting and having a higher number of sex partners in the past three months were also significantly associated with both study outcomes (aPORs: 1.67 and 1.26 and aHRs: 2.55 and 1.52 respectively). Compared to those who had at least three children, women with less than three children were also significantly associated with high STI prevalence and incidence rates (aPORs:1.41, 1.55, 1.17 and aHRs: 2.13, 2.45 and 1.49 for no-children, one-child, two-children respectively); while using hormonal injectables as a family planning method was identified as a significant predictor of STI incidence with aHR: 1.51, 95%CI:1.30, 1.75. Baseline STI positivity was also significantly associated with an increased risk of STIs during the study follow-up period. The p-values for the Hosmer-Lemeshow goodness of fit were 0.542 and 0.183 for the adjusted log-binomial and hazard regression models, respectively.
Table 1:
Factors associated with STI prevalence and incidence
| STI prevalence | STI incidence | ||||
|---|---|---|---|---|---|
| % | Adjusted Prevalence Odds Ratio (95% CI) | p-value | Adjusted hazard ratio (95% CI) | p-value | |
| Age groups | |||||
| <20 years old | 10% | 1.62 (1.39, 1.90) | <0.001 | 3.50 (2.85, 4.29) | <0.001 |
| 20-24 years old | 35% | 1.46 (1.29, 1.66) | <0.001 | 2.47 (2.08, 2.94) | <0.001 |
| 25-29 years old | 22% | 1.07 (0.92, 1.24) | 0.3998 | 1.30 (1.05, 1.60) | 0.014 |
| 30-34 years old | 13% | 0.85 (0.71, 1.24) | 0.0830 | 0.98 (0.77, 1.24) | 0.852 |
| 35+ years | 20% | 1 | |||
| Education | |||||
| None | 54% | 1.02 (0.91, 1.14) | 0.7517 | 0.98 (0.71, 1.35) | 0.861 |
| Primary | 42% | 0.88 (0.78, 1.00) | 0.335 | 0.98 (0.70, 1.36) | 0.908 |
| Secondary or more | 4% | 1 | 1 | ||
| Age at sexual debut | |||||
| <15 years old | 6% | 1.31 (0.96, 1.78) | 0.0900 | 1.65 (1.06, 2.56) | 0.026 |
| 15-19 years old | 78% | 1.24 (1.04, 1.51) | 0.0175 | 1.48 (1.11, 1.96) | 0.007 |
| 20+ years old | 17% | 1 | 1 | ||
| Marital/cohabitation status | |||||
| Married/cohabitating | 23% | 1 | 1 | ||
| Single/not-cohabiting | 77% | 1.67 (1.44, 1.93) | <0.001 | 2.55 (2.16, 3.02) | <0.001 |
| Number of sex partner(s)a | |||||
| None/one | 87% | 1 | 1 | ||
| Two or more | 13% | 1.26 (1.07, 1.49) | 0.0048 | 1.52 (1.26, 1.83) | <0.001 |
| Parity | |||||
| None | 11% | 1.41 (1.16, 183) | <0.001 | 2.13 (1.69, 2.67) | <0.001 |
| One child | 44% | 1.55 (1.34, 1.90) | <0.001 | 2.45 (2.05, 2.92) | <0.001 |
| Two children | 23% | 1.17 (1.03, 1.43) | 0.034 | 1.49 (1.21, 1.83) | <0.001 |
| Three children | 21% | 1 | 1 | ||
| Condom used in last sex | |||||
| No | 36% | 1 | 1 | ||
| Yes | 64% | 0.76 (0.55, 1.05) | 0.0971 | 1.19 (1.05, 1.34) | 0.005 |
| Contraceptive used | |||||
| None/other | 20% | 1 | 1 | ||
| Condom | 15% | 1.01 (0.88, 1.16) | 0.871 | 1.34 (1.11, 1.63) | 0.003 |
| Oral contraceptives | 11% | 0.79 (0.65, 0.94) | 0.0101 | 0.85 (0.66, 1.08) | 0.188 |
| Injectables | 53% | 1.01 (0.91, 1.13) | 0.861 | 1.51 (1.30, 1.75) | <0.001 |
| STI diagnosis at baseline | |||||
| No | 82% | - | - | 1 | |
| Yes | 18% | - | - | 2.81 (2.50, 3.16) | <0.001 |
past 3 months;
After identifying the significant risk factors for both outcomes, we calculated the total risk factors for each participant and categorized them into four groups, as described in the methods section (Table 2). We observed increasing trends in the odds ratios (ranged from 1.38 to 2.58) and hazard ratios (ranged from 2.11 to 4.70) with the increasing number of risk factors (Ptrend<0.001, both outcomes).
Table 2:
Adjusted Odds ratios and Hazard ratios
| Total risk factors | aOR (95% CI)1 | p-value | aHR (95% CI)1 | p-value |
|---|---|---|---|---|
| None | 1 | 1 | ||
| 1-risk factor | 1.38 (1.01, 1.89) | 0.047 | 2.11 (1.46, 3.05) | <0.001 |
| 2-risk factors | 1.83 (1.36, 2.46) | <0.001 | 3.55 (2.51, 5.03) | <0.001 |
| 3-risk factors | 2.07 (1.51, 2.84) | <0.001 | 4.48 (3.13, 6.41) | <0.001 |
| 4+ risk factorsb | 2.58 (1.92, 3.46) | <0.001 | 4.70 (3.30, 6.70) | <0.001 |
Ptrend<0.001;
there were 4-risk factors for the prevalence of STI
Age-specific STI prevalence and incidence
At baseline, a total of 1990 (20%) women were diagnosed with at least one of the STIs including chlamydia, gonorrhoea, syphilis or trichomonas. The overall STI incidence rate was 15 per 100-person years (95%CI: 13.7, 16.3). We presented STI prevalence and incidence rates by the five age groups (<20 years, 20-24 years, 25-29 years, 30-34 years and 35+ years old) across the four categories of the total risk factors (Figure 1a and Figure 1b respectively). In this three-dimensional visual presentation, STI prevalence and incidence rates increased substantially with increasing number of risk factors. For example, STI prevalence was >30% among women who were <20 and between 20-24 years old regardless of how many risk factors they had; while women who had all four risk factors had the highest STI prevalence rates irrespective of their age (Figure 1a). Similar trends were observed in age × risk factors specific STI incidence rates (Figure 1b). Women younger than 20 years of age had the highest STI incidence rates irrespective of their total number of risk factors (STI incidence ranged 10 to 30 per 100-person years); while women 20-24 years old were also at increased risk of STIs if they had at least one of the risk factors (STI incidence rates ranged: 15 to 30 per 100 person years). Women who had at least four risk factors were at the highest risk of STIs irrespective of their age. For example, women 35 years of age with four or more risk factors were almost two to three times more likely to be diagnosed with STIs during the study follow-up compared to those with no risk factors (5 per 100-person years vs 10-15 per 100-person years).
Individual and combined impacts of risk factors on STI prevalence and incidence
At a population level, early age at sexual debut (<20 years of age) was associated with 22% of the STI diagnoses at baseline and 29% of the STI incidence (Table 2); while others, being single/not cohabiting and having a lower number of children were associated with 12% and 32% of the STI diagnoses at baseline and follow-up respectively. Although having a higher number of sexual partners in the past three months has been identified as a significant correlate of both outcomes, it’s population-level impact was only 4% and 7% on STI prevalence and incidence respectively, due to the low prevalence in the population (13%). These four characteristics collectively associated with 51% (95% CI: 49%, 55%) and 53% (95% CI: 49%, 64%) of the STI prevalence and incidence respectively. Considering the other factors such as injectables and baseline STI positivity only had a minimal impact on STI incidence rates with PAR%: 58% (95% CI: 49%, 64%).
Discussion
With millions of infected individuals all over the world, STIs remain a major public health problem globally.1 In addition to the stigma surrounding STIs, they have also been reported to play a significant role in facilitating HIV transmission through genital inflammation.19 In this study, we reported STI prevalence and incidence rates among a large cohort of women who enrolled in various biomedical intervention trials conducted in KwaZulu Natal, South Africa. Overall, the highest STI prevalence and incidence rates were observed among women younger than 20 years of age (25% and 28 per 100-person years, respectively). Besides younger age, increasing STI prevalence and incidence rates were also associated with those who reported being single/not cohabiting and who reported having multiple sex partners in the past three months. These two factors have been previously reported as significant predictors of HIV infections among South African women.20 Although we cannot confirm using the data at hand, these characteristics could have broader implications and interpretations such as transactional and/or intergenerational sex due to poor socio-economic conditions. 21 According to the nationwide estimates, KwaZulu Natal has the highest unemployment rates in the country.22 In our study population more than 50% of the women reported no schooling. These results may collectively indicate low socio-economic conditions and their potential implications regarding women’s vulnerability to risk-taking behaviours.
Early age at sexual debut was identified as the first and the second most influential risk factor for STI positivity and incidence rates, respectively. Given the high levels of unprotected sex and background infection rates in this region, young women may be at risk of STIs as soon as they become sexually active. These results are consistent with the previous research. For example early at sexual debut was frequently identified as significant predictor of subsequent HIV infections. 23,24
Low parity also had a profound impact on both outcomes. Particularly, nulliparous and primiparous women were more likely to test positive for STIs compared to the women who already had three or more children. Although this is a relatively unique finding for STIs, it is not unexpected, since STIs and pregnancy can generally occur with unprotected sex. In fact, in our study population, the highest pregnancy rates were observed among women who were nulliparous and primiparous (22 and 10 per 100 person-years respectively, data not shown). Therefore, these associations can potentially be attributed to the high levels of unprotected sex among women. Consistent with these findings, previous studies also linked the low parity to increased risk of HIV seroconversions in African populations.20 Other risk factors, including hormonal contraceptives such as injectables had a modest impact on STI incidence.
Further analysis revealed substantial age-specific disparities between subject-specific total risk factors and STI positivity. For example, the highest STI prevalence was observed among the youngest age group (i.e. <20 years) irrespective of their total risk factors (25% to 30%); while among women who had no risk factors (i.e. total risk score=0), STI prevalence was five times higher in the youngest age group (i.e. <20 years) compared to the oldest age group (i.e. 35+ years). Similar associations were also observed for STI incidence with a slight shift in the age and risk groups.
This analysis highlighted the most influential key factors associated with the highest burden of STIs among South African women. Four factors, namely, age at sexual debut, being single/not living as married, having two or more sexual partners and low parity were collectively associated with approximately half of the STI prevalence and incidence in our population (PAR%: 51% and 53% respectively). When we also considered injectables and STI positivity at baseline, the PAR% for these six factors was modestly higher than for the four risk factors (increased from 54% to 59%). This relatively modest increase was due to the correlated nature of these risk factors. Our results for condom use was counter-intuitive. Nevertheless, high pregnancy and STI rates in the study population are an indication of high levels of unprotected sex; they also indicate that study participants may be overreporting condom use to avoid further counselling during the trials.25
Although we used established risk factors for STI diagnoses, we were only able to explain just over 50% of the excess STIs in this population. These estimates suggest a substantial impact of unmeasured factors such as community level characteristics, cultural and social norms on STI positivity and incidence rates. For example, if these results were to apply to our study population, a total of 1,020 (i.e. 1990*51%) STI diagnoses at baseline and another 780 (i.e. 1344*58%) new infections would have been (at least theoretically) avoided; while there would still be 970 (i.e. 1990-1,020) STI positive women at baseline; and another 564 newly infected women who had none of the risk factors considered in this study. Although we cannot confirm empirically, these infections could potentially be linked to women’s high-risk sexual partner(s). It is speculative, but other factors such as poverty, employment opportunities, other community-level characteristics including social and sexual mixing, and poor access to the health care system may also have an indirect effect on an individual’s risk for STI acquisition. In addition, religious beliefs, social and cultural norms have also been shown to play crucial roles in shaping individual-level characteristics, particularly those related to sexual behaviours and partnership.
Our study has the following limitations. The data from the women who consented to enrol in HIV prevention trials with certain eligibility criteria, including being at reproductive age and sexually active. Therefore, this population may be at higher risk of STIs compared to the general population. All the characteristics measured in this study were self-reported, except STI diagnosis; therefore, they would be subject recall bias. There were no data available from male partners of the women. The current study assumed that all STI diagnoses at baseline were adequately treated and resolved per study protocols; therefore, any STI positivity during the study follow-up was considered as a new infection. However, we cannot rule out the possibility of counting the same STI(s) as a new infection during the follow-up if they were not successfully treated.
Conclusion
As prevention efforts intensify it is important to target women at the highest risk for STIs; however, it is equally important to consider their combined impacts to quantify the potential influence of unmeasured factors in the population. This strategy may play a crucial role in planning and developing the most beneficial and cost-effective STI prevention programmes to reduce STIs.
Supplementary Material
Table 3:
Population level impacts of STI risk factors: Age adjusted PAR% for STIs by risk factors
| Baseline STI positivity | STI incidence | |
|---|---|---|
| PAR% (95% CI) | PAR% (95% CI) | |
| Age at sexual debut | 22% (16%, 28%) | 29% (22%, 38%) |
| <15 years | 2% (1%, 4%) | 3% (2%, 5%) |
| 15-19 years | 20% (16%, 28%) | 26% (19%, 34%) |
| single/not cohabiting | 12% (10%, 15%) | 32% (29%, 35%) |
| 2 or more sex partners1 | 4% (3%, 5%) | 7% (6%, 8%) |
| Parity categories | 20% (17%, 23%) | 23% (21%, 25%) |
| Nil | 4% (02%, 5%) | 4% (3%, 5%) |
| 1 to 2 children | 16% (14%, 19%) | 19% (17%, 21%) |
| Injectable contraceptives | 3% (2%, 5%) | 18% (16%, 20%) |
| STI baseline | - | 25% (23%, 27%) |
| Combined impacts #1 | 51% (47%, 58%) | 53% (49%, 59%) |
| Age at sexual debut + Single/not cohabiting + two or more sex partners + parity <3 |
Age at sexual debut + Single/not cohabiting + two or more sex partners + parity <3 |
|
| Combined impacts #2 | N/A | 59% (49%, 64%) |
| Age at sexual debut + Single/not cohabiting + two or more sex partners + parity <3 + injectables + STI baseline |
past 3 months;
N/A: not applicable
References
- 1.Global health sector strategy on sexually transmitted infections 2016–2021. Towards ending STIs. Report No.: WHO/RHR/16.09. Geneva: World Health Organization; 2016. (accessed October 2019). [Google Scholar]
- 2.Joint United Nations Program on HIV/AIDS (UNAIDS). Global AIDS Update. Geneva, Switzerland. 2016. (accessed September 2019) [Google Scholar]
- 3.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. WHO Bulletin 2019. August 1;97(8):548–562P. doi: 10.2471/BLT.18.228486. Epub 2019 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moodley D, Moodley P, Sebitloane M, Soowamber D, McNaughton-Reyes H, Groves AK, Maman S. High Prevalence and Incidence of Asymptomatic Sexually Transmitted Infections During Pregnancy and Postdelivery in KwaZulu Natal, South Africa. Sexually Transmitted Diseases: 2015; 42 (1); 43–47. doi: 10.1097/OLQ.0000000000000219 [DOI] [PubMed] [Google Scholar]
- 5.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nature Reviews Microbiology 2003; 1(1):25–34. [DOI] [PubMed] [Google Scholar]
- 6.McClelland R, Sangare L, Hassan W, Lavreys L, Mandaliya K, Kiarie J, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. Journal of Infectious Diseases 2007; 195, 698–702. [DOI] [PubMed] [Google Scholar]
- 7.Shin L, Kaul R. Stay it with flora: Maintaining vaginal health as a possible avenue for prevention of human immunodeficiency virus acquisition. Journal of Infectious Diseases 2008; 197, 1355–1357. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds S, Risbud A, Shepherd M, Rompalo A, Ghate M, Godbole S et al. High rates of syphilis among STI patients are contributing to the spread of HIV-1 in India. Sexually Transmitted Infections 2006; 82, 121–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.South African National Department of Health (DOH). National antenatal sentinel HIV & Syphilis survey report.; 2015. (accessed September 2019).
- 10.Zuma K, Lurie MN, Williams BG, Mkaya-Mwamburi D, Garnett GP, Sturm AW. Risk factors of sexually transmitted infections among migrant and non-migrant sexual partnerships from rural South Africa. Epidemiol Infect 2005; 133(3):421–8. Epub 2005/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korenromp EL, White RG, Orroth KK, et al. Determinants of the impact of sexually transmitted infection treatment on prevention of HIV infection: a synthesis of evidence from the Mwanza, Rakai, and Masaka intervention trials. J Infect Dis. 2005;191(suppl 1):S168–S178. [DOI] [PubMed] [Google Scholar]
- 12.Wand H, Ramjee G. Combined impact of sexual risk behaviors for HIV seroconversion among women in Durban, South Africa: implications for prevention policy and planning. AIDS Behav. 2011;15(2):479–86. [DOI] [PubMed] [Google Scholar]
- 13.Padian NS, van der Straten A, Ramjee G, Chipato T, de Bruyn G, Blanchard K, Shiboski S, Montgomery ET, Fancher H, Cheng H, Rosenblum MA, van der Laan M, Jewell N, McIntyre J. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet 2007;370:251–61. doi: 10.1016/S01406736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Weber J. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet 2010; 376:1329–37. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos M, Friedland B, Govender S, Dekock A, Cassim N, Palanee T. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–87. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 16.Microbicide Trials Network (MTN). MTN statement on decision to discontinue use of Tenofovir gel in VOICE, a major HIV prevention study in women. 2011. http://www.mtnstopshiv.org/node/3909. (Accessed 16 June 2019).
- 17.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Chirenje ZM. Tenofovir-based preexposure prophylaxis for HIV infection among African women. New Eng J Med. 2015;372(6):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, SotoTorres LE, Govender V, Mgodi NM, Kiweewa FM, Nair G, Mhlanga F, Siva S, Bekker LG for the MTN-020-ASPIRE Study Team. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steen R, Wi TE, Kamali A, et al. Control of sexually transmitted infections and prevention of HIV transmission: mending a fractured paradigm. Bull World Health Organ. 2009;87:858–865. [PMC free article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkus JE, Brown E, Palanee T, Nair G, Gafoor Z, Zhang J, et al. An empiric HIV risk scoring tool to predict HIV-1 acquisition in African women. J Acquir Immune Defic Syndr. 2016;72:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunkle K. Transactional sex among women in Soweto, South Africa: prevalence, risk factors and association with HIV infection. Soc Sci Med. 2004;59(8):1581–92. [DOI] [PubMed] [Google Scholar]
- 22.Census 2011: Statistical Release. Statistics South Africa. 2012. (http://www.statssa.gov.za (accessed 24 September 2019. p. 14)
- 23.Pettifor AE, van der Straten A, Dunbar MS, et al. Early age of first sex: a risk factor for HIV infection among women in Zimbabwe. AIDS 2004;18:1435–1442. [DOI] [PubMed] [Google Scholar]
- 24.Wand H, Ramjee G. The relationship between age of coital debut and HIV seroprevalence among women in Durban, South Africa: a cohort study. BMJ Open 2012;2:e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pool R, Montgomery CM, Morar NS, Mweemba O, Ssali A, Gafos M, Lees S, Stadler J, Crook A, Nunn A, Hayes R, McCormack S. A mixed methods and triangulation model for increasing the accuracy of adherence and sexual behaviour data: the microbicides development programme. PLoS One. 2010;5(7):e11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


