Abstract
The thalamic ventral intermediate nucleus (VIM) can be targeted for treatment of tremor by several procedures, including deep brain stimulation (DBS) and, more recently, MR-guided focused ultrasound (MRgFUS). To date, such targeting has relied predominantly on coordinate-based or atlas-based techniques rather than directly targeting the VIM based on imaging features. While general regional differences of features within the thalamus and some related white matter tracts can be distinguished with conventional imaging techniques, internal nuclei such as the VIM are not discretely visualized. Advanced imaging methods such as quantitative susceptibility mapping (QSM) and fast gray matter acquisition T1 inversion recovery (FGATIR) MRI and high-field MRI pulse sequences that improve the ability to image the VIM region are emerging but have not yet been shown to have reliability and accuracy to serve as the primary method of VIM targeting. Currently, the most promising imaging approach to directly identify the VIM region for clinical purposes is MR diffusion tractography.
In this review and update, the capabilities and limitations of conventional and emerging advanced methods for evaluation of internal thalamic anatomy are briefly reviewed. The basic principles of tractography most relevant to VIM targeting are provided for familiarization. Next, the key literature to date addressing applications of DTI and tractography for DBS and MRgFUS is summarized, emphasizing use of direct targeting. This literature includes 1-tract (dentatorubrothalamic tract [DRT]), 2-tract (pyramidal and somatosensory), and 3-tract (DRT, pyramidal, and somatosensory) approaches to VIM region localization through tractography.
The authors introduce a 3-tract technique used at their institution, illustrating the oblique curved course of the DRT within the inferior thalamus as well as the orientation and relationship of the white matter tracts in the axial plane. The utility of this 3-tract tractography approach to facilitate VIM localization is illustrated with case examples of variable VIM location, targeting superior to the anterior commissure–posterior commissure plane, and treatment in the setting of pathologic de-rangement of thalamic anatomy. Finally, concepts demonstrated with these case examples and from the prior literature are synthesized to highlight several potential advantages of tractography for VIM region targeting.
Keywords: ventral intermediate nucleus, MR-guided focused ultrasound, essential tremor, tractography
The thalamic nuclei are not well delineated on traditional imaging modalities such as MRI. Therefore, targeting of thalamic nuclei such as the ventral intermediate nucleus (VIM) for deep brain stimulation (DBS) or MR-guided focused ultrasound (MRgFUS) has relied predominantly on indirect targeting methods based on visible structures such as the anterior commissure (AC) and posterior commissure (PC) and comparing the findings with stereotactic atlas localization rather than direct targeting of MRI features. One major limitation of both of these methods of localization is that they do not account for interindividual anatomical variability.1,2 Clinical experience and correlation with intraoperative microelectrode recording (MER), where tremor cell activity may be recorded, indicate that DBS lead placements using atlas-based coordinates typically need time-consuming adjustments. More recently, the VIM has also been targeted with MRgFUS, a procedure that focuses ultrasound beams from 1024 transducer elements to thermally ablate the VIM. VIM localization for MRgFUS has relied largely on the use of coordinate-based measurements using anatomical landmarks with target adjustment based on patient response to initial subablative sonications.
Development of reliable imaging-based direct methods to target the VIM has been desirable, given the uncertainty of the VIM location, potential for adverse effects, and need for adjustments. Indeed, there are other potential methods to identify or infer the location of thalamic nuclei, including the use of advanced MR pulse sequences and white matter tractography. Of these possibilities, tractography has so far emerged as the most promising for clinical use.
This review and update will briefly summarize the utility and limitations of current MRI techniques for visualization of thalamic nuclei. Given the limitations of these methods for clinical use, the major focus will center on the current reports of diffusion tensor imaging (DTI) and tractography for guiding DBS and MRgFUS treatment. We illustrate the potential utility of tractography with introduction of a 3-tract tractography technique to define the dentatorubrothalamic tract (DRT), somatosensory tract, and pyramidal tract in patients undergoing DBS placement and MRgFUS to the VIM.
MRI Techniques to Directly Identify the Location of Thalamic Nuclei
Thalamic nuclei are not discretely delineated on routine MRI pulse sequences. However, some aspects of the internal thalamic structure can be discerned, as there is variation of histological features such as myelin and iron content (Fig. 1).3,4
FIG. 1.
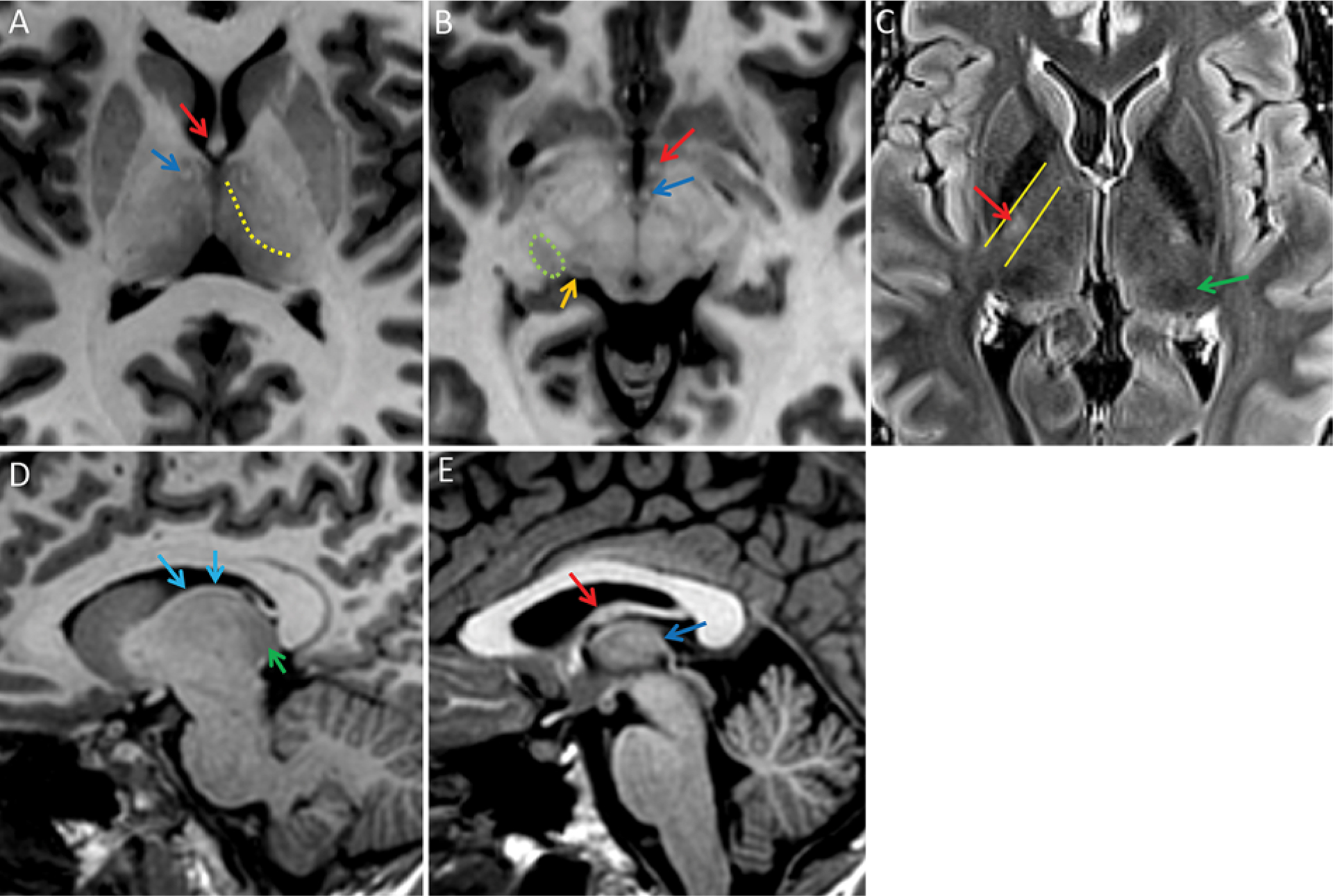
Normal MRI findings of the internal thalamic structure and related white matter tracts on 7T images. A: Axial T1-weighted MR image of the superior and inferior thalamus, demonstrating the dot-like mammillothalamic tracts (blue arrow) and fornix (red arrow). Superiorly, the mammillothalamic tract can be followed to the anterior nucleus, although the anterior nucleus itself is not well delineated (not shown). The internal medullary lamina is faintly perceptible (dotted yellow line). This separates the medial thalamus, which is relatively hypointense, from the lateral thalamus, which is relatively hyperintense with relatively indistinct margins with the internal capsule. This is relevant for VIM targeting because the VIM is located laterally within the thalamus adjacent to the internal capsule and corticospinal tract fibers. B: Axial T1-weighted image. In the inferior thalamus near the mesencephalic-diencephalic junction, the lateral geniculate nucleus (encircled by green dotted line) is vaguely seen as a hypointense area just lateral to the medial geniculate nucleus (orange arrow). The lateral geniculate nucleus is also commonly seen on high-resolution T1-weighted coronal and sagittal images and can be identified by following the optic tract posteriorly from the optic chiasm (not shown). C: Axial T2 FLAIR image in the midthalamus again demonstrating an indistinct margin between the lateral thalamus and the internal capsule. The bright spot of the posterior limb of the internal capsule (red arrow) approximates the width of the internal capsule (yellow lines) and has been reported to be immediately lateral to the VIM near the level of the AC-PC line, but the individual nuclei in the thalamus are not delineated; there is only a general regional area of hypointensity in the region of the pulvinar (green arrow). D: An off-midline sagittal MPRAGE image demonstrating the stria terminalis (blue arrows) as a white matter tract demarcating the superior thalamus border near the caudothalamic groove. Discrete thalamic nuclei are not visualized, although the pulvinar region is relatively hypointense (green arrow). E: A midline sagittal MPRAGE image demonstrating the fornix arching around the thalamus (red arrow) and the stria medullaris thalami (dark blue arrow) arcing anterosuperiorly from the habenula.
Numerous specialized MRI methods to visualize the thalamic nuclei have been attempted over the past couple of decades, both in vivo and ex vivo. These methods were first attempted at 1.5T,4–6 then 3T,7 and more recently at 7T.8–11 While an approximation of VIM location may be inferred with current conventional imaging techniques at all these field strengths, none has demonstrated sufficient quality to serve as the primary method for direct VIM targeting.
For example, Yamada et al. demonstrated that the expected VIM location, as determined with DRT tractography, is located immediately medial to the “bright spot” of the posterior limb of the internal capsule on 1.5T STIR images at the level of the AC-PC line.6 The authors described the VIM itself as corresponding to an oblique, faint stripe of hyperintensity on STIR images and the ventral caudal nucleus (Vc) as an adjacent faint stripe of hypointensity; however, these features are difficult to reliably visualize in routine clinical practice.
More recently, reports of advanced MRI pulse sequences have emerged, such as quantitative susceptibility mapping (QSM) and fast gray matter acquisition T1 inversion recovery (FGATIR).12 For example, Deistung et al. reported that QSM, a technique that highlights differences in susceptibility due to iron content and myelination, can delineate details of thalamic nuclei at 7T.9 FGATIR is a modification of a high-resolution T1-weighted MPRAGE pulse sequence with white matter nulling that can achieve both high spatial resolution and optimized T1 contrast resolution (high conspicuity) of several common DBS targets.12 While limited data suggest that FGATIR can help direct VIM region DBS lead placement, more work is needed to assess the utility.13 Therefore, the remainder of this article will focus on DTI and tractography as reports of direct targeting with several tractography techniques have already been introduced into some clinical practices.
DTI and Tractography: Basic Technique and Outputs
A full description of DTI and tractography is beyond the scope of this review and has been given previously.14 In brief, tractography consists of 3 primary steps: 1) data acquisition, 2) data processing, and 3) white matter tract generation. There are numerous variables within each step; only the most essential terms and concepts will be reviewed here.
Data acquisition (step 1) assesses the diffusion of water with diffusion-weighted imaging (DWI), which characterizes the brownian motion (random motion resulting from collision with adjacent molecules) of water within white matter tracts. The biomolecular basis of white matter DWI and related advanced techniques is that water tends to diffuse longitudinally along the course of white matter tracts. B0 images are also obtained, which do not utilize a directional gradient.
Data processing (step 2) for tractography can be performed using numerous techniques, but most commonly it is performed using DTI, which requires assessment of water diffusion in at least 6, but usually substantially more (typically 12–60), directions. DTI is widely available and easy to implement but is limited, as it computes only a single fiber direction within each voxel, limiting the ability to delineate crossing and complex fibers. The raw DTI data can be used to generate several images, including a fractional anisotropy (FA) color map in which the major white matter tracts are directionally color-coded for visual inspection. The FA color map reveals some detail of thalamic anatomy but is insufficient for localization of targeting coordinates (Fig. 2).
FIG. 2.
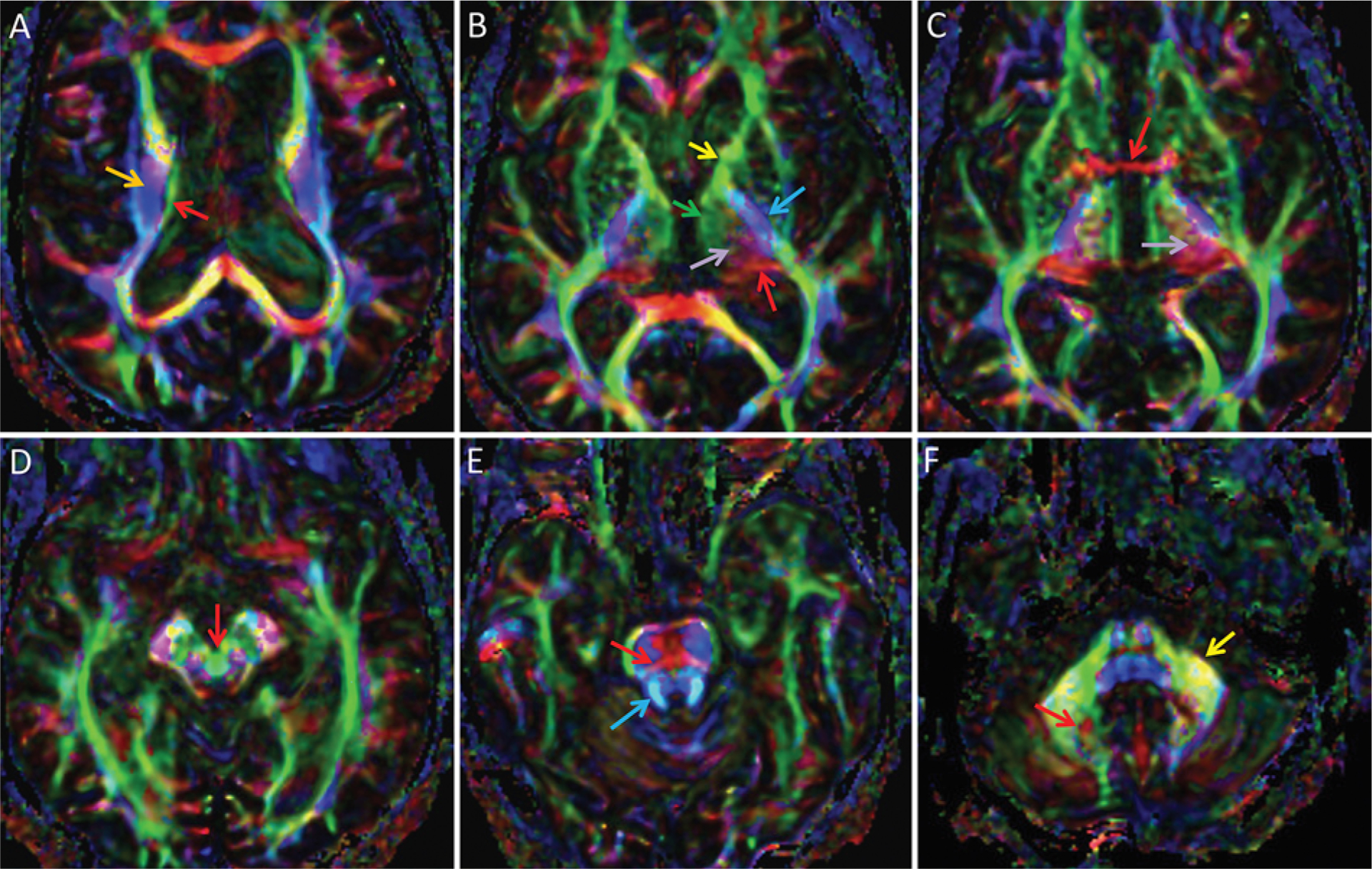
DTI color FA maps of the thalamus and bordering structures, superior to inferior. By standard convention, white matter tracts are color-coded by orientation of their courses: anterior-to-posterior is green, left-to-right is red, and superior-to-inferior is blue; oblique fibers are a blend of these colors. In general, some general regions within the thalamus can be discerned and some important adjacent white matter tracts are identifiable, but individual thalamic nuclei and related white matter tracts are not individually distinguishable. A: Superiorly, the stria terminalis is seen as a green line (red arrow) demarcating the superior thalamus, medial to the corona radiata (orange arrow). B: The medial thalamus is represented in green (green arrow) and is continuous with the anterior limb of the internal capsule (yellow arrow), while the posterior thalamus at the expected location of the pulvinar is red (red arrow). Superolaterally projected tracts between the medial (green) and posterior (red) thalamus are depicted in purple (light purple arrow). This purple region should contain both the DRT and somatosensory fibers associated with VIM and Vc, but these 2 tracts cannot be differentiated. The posterior limb of the internal capsule (blue arrow) contains the pyramidal tract and demarcates the lateral thalamus. C: The inferior thalamus at the level of the AC (red arrow) again demonstrates complex color, but overall there is a green region medially, a red region posteriorly, and a juxtaposed purple region (light purple arrow). D: The decussation of the superior cerebellar peduncles is seen as a circle within the midbrain (red arrow). There, the circle is green, although the color is more typically red, representing predominantly left-to-right crossing fibers including the DRT. E: The superior cerebellar peduncles, which contain the cerebellar efferent fibers such as the DRT (blue arrow) and the transverse pontine fibers (red arrow), are visualized in an image of the superior pons. F: The efferent cerebellar fibers can be followed inferiorly to the region along the medial dentate, which is visualized as an area of complex color (red arrow) near the middle cerebellar peduncle (yellow arrow).
The final step, tract generation with tractography (step 3), can be divided into 2 broad methods: deterministic or probabilistic. Deterministic tractography is more commonly used, less computationally intensive, and provides a single consistent result with fixed input parameters. However, this method lacks the ability to discriminate crossing fibers within a voxel and might not delineate white matter tracts with a complex anatomical course.
Probabilistic tractography can potentially delineate more complex white matter tracts but is computationally complex. Probabilistic tractography does not provide a single result but rather assigns likelihoods to different possible results. Probabilistic tractography results also depend on the user-defined duration of the computational process. There are limited data that indicate deterministic tractography, while less able to show fine details and crossing fibers, results in a similar VIM region target definition compared with probabilistic tractography.15,16
For both tractography methods, several specific tractography parameters are defined to constrain depicted tracts, including a threshold of degree of diffusion, fiber angle, and fiber length. User-defined regions of interest (ROIs) to define key areas of a white matter tract are critical. Overall, tractography is a 3D mathematical construct rather than a direct image of white matter tracts.
Categories of Clinical Utility of DTI and Tractography for DBS and MRgFUS
Although there are other applications of tractography for DBS, this article will primarily focus on the use of tractography for direct targeting.14 Applications of tractography after treatment to assess both lesion location relative to connectivity-based thalamic parcellation and white matter tract integrity (Fig. 3) with FA analysis are also relevant but de-emphasized here. First, the essential technical and clinical aspects of MRgFUS will be reviewed to facilitate understanding of the process and potential advantages of tractography for VIM region treatment for tremor.
FIG. 3.
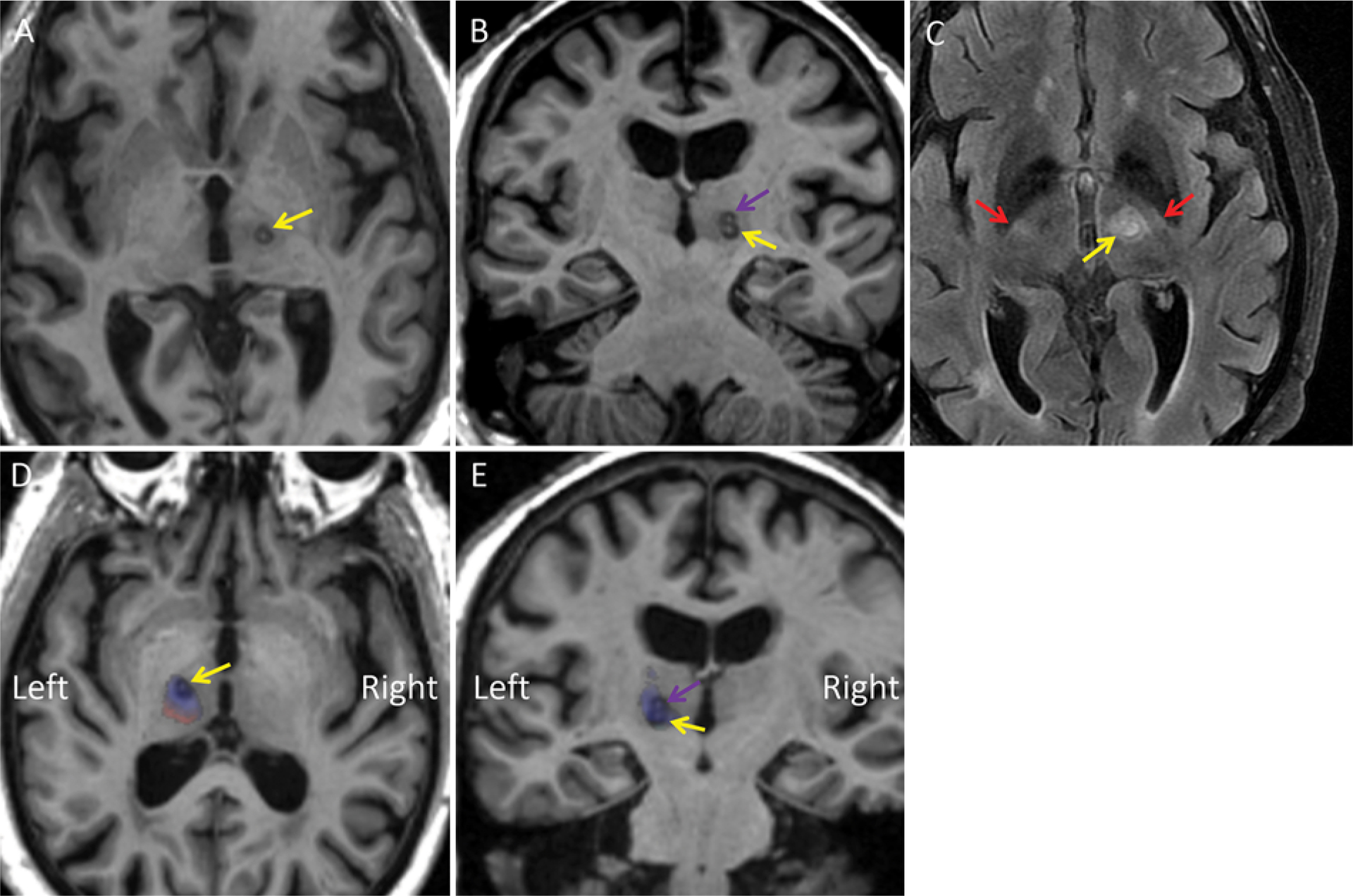
Example of use of a probabilistic tractography atlas overlay after treatment to correlate the lesion area with expected functional connectivity. A 77-year-old man with right ET was treated with a left MRgFUS thalamotomy using coordinate-based targeting both at and 1 mm superior to the level of the AC-PC line. A and B: Axial (A) and coronal (B) MPRAGE images obtained on postoperative day 1, showing a circular ablation in the left VIM region centered just superior to the AC-PC line (yellow arrow) and at the second ablation site immediately superior to it (purple arrow), resulting in a “snowman” lesion. C: Axial T2-weighted FLAIR image demonstrating the ablation site (yellow arrow) with some expected surrounding edema. The edema is located just medial to the bright spot of the posterior limb of the internal capsule in this patient (red arrows). D and E: This MPRAGE sequence was normalized to Montreal Neurological Institute space. The images were obtained after reverse warping the aggregate connectivity atlas described by Akram et al. to MRPAGE images.42 On these axial (D) and coronal (E) MPRAGE images, blue corresponds to expected primary motor cortex connectivity (presumably VIM) and red corresponds to expected primary somatosensory cortex connectivity (presumably Vc). The arrows indicate the same areas as in panels A and B. Note that left-right is inverted due to the warping/normalizing process. This connectivity overlay indicates that the lesion for this patient was in the anterior portion of the VIM, congruent with an excellent clinical response with decreased tremor and lack of side effects.
Tractography for Direct VIM Targeting for DBS or MRgFUS
Reports of the utility of MR diffusion tractography for VIM localization to help guide DBS and MRgFUS are emerging. To identify articles that specifically assessed the utility of tractography for prospective VIM region targeting for this narrative review, we performed searches on PubMed Central with the key terms “Tremor AND Tractography AND Deep Brain Stimulation” on March 9, 2020. The references of each of these articles and all suggested similar articles were screened with the same criteria. Additionally, we performed searches on PubMed Central with the key terms “Tremor AND Tractography AND Focused Ultrasound” on March 9, 2020, which were similarly screened. These search terms identified 56 unique articles in the English language, 9 of which described case reports or studies utilizing tractography to facilitate targeting of either DBS13,17–20 or MRgFUS,21–23 with 3 additional references found on cross-referencing.15,24,25 The full text was screened to characterize the tractography methodology and report of clinical outcomes.
Initially, Coenen et al. published 2 case reports describing the use of 3-tract tractography of the pyramidal tract, DRT, and somatosensory tract to guide DBS for tremor.17,26 The underlying assumptions in this technique, if used for surgical targeting, are that 1) the streamlines produced from diffusion tractography approximate the actual white matter tracts in question; 2) the DRT and the somatosensory tracts coursing through the ventral thalamus essentially fully spatially correspond to the VIM and Vc nuclei, respectively; and 3) targeting these tracts is clinically equivalent to targeting the nuclei through which they run. Since these initial case reports, there have been several reports describing various methods of tractography to prospectively assist targeting for DBS and MRgFUS. These methods include 1-tract tractography of the DRT alone,18,19,23 2-tract tractography of the pyramidal and somatosensory tracts with inference of the relative location of the adjacent DRT,15,22 and 3-tract tractography.17,21,24,26
For DBS targeting, a study by Sammartino et al. exemplifies a 2-tract technique.15 This group delineated the pyramidal and somatosensory tracts in 14 patients with essential tremor (ET) and 15 healthy controls, comparing tractography with conventional targeting and correlating tractography results with intraoperative neurophysiology in a separate cohort of 6 patients (3 ET and 3 tremor-dominant Parkinson disease). The anterior border of the somatosensory tract and medial border of the pyramidal tract were considered to represent the posterior and lateral borders of the DRT, respectively. Tractography-based targets were 1 mm lateral and 1.8 mm anterior to the conventional target on average in the nonsurgical cohort. In the 6-patient surgical cohort, the euclidian distance of adjustment with tractography was 1.6 mm. The Vc was identified by MER in 5 patients and was reported to be at, or, in some cases, slightly anterior to, the tractography-defined anterior border of the somatosensory tract.
Other reports of prospective or retrospective tractography for DBS have also found differences between tractography and atlas-based target locations.13,18,24,25 Interestingly, Schlaier et al. reported that the proximity of the DBS lead contact point to the DRT was not a determinate of clinical outcome in a cohort of 5 patients.25 Additionally, Nowacki et al. also reported that lead location relative to tractography results was not correlated to tremor reduction in 6 patients, each evaluated with 4 different deterministic tractography techniques and each with a different ROI to define the tracts.27 Additionally, the antero-posterior and mediolateral location of the center of the tracts obtained with different techniques differed by several millimeters.27 However, other post hoc studies have reported that lead contact proximity to the DRT is associated with decreased stimulation parameters or improved outcomes.13,24,28–33 Additionally, King et al. reported that MER recordings consistent with VIM found in tremor patients were present in tractography-defined locations.20
For MRgFUS prospective targeting with tractography, there are a couple of reports of tractography using either a 2-tract22 or 3-tract21 approach. There are several additional clinical studies that have included a post hoc DTI and tractography analysis of lesion location relative to DRT location,34 thalamic connectivity maps,31,35–37 and/or treatment impact on FA values31 or tractography appearance21 of related white matter tracts.
First, Krishna et al. performed pyramidal and somatosensory 2-tract tractography to guide MRgFUS at the level of the AC-PC in 10 patients with ET.22 The average inferred DRT location was 2.18 mm anterior and 1.82 mm medial to the coordinate-based approach. The authors reported a reduced tremor score at 3 months without motor or sensory deficit. The authors also performed a post hoc thalamic connectivity analysis showing that the lesion location overlapped with the parcellated predicted location of the VIM. The results of this study raise the possibility that 2-tract tractography could decrease the incidence of motor or sensory adverse events; however, these results need confirmation with larger studies. While 3 patients experienced ataxia, this is potentially accounted for by targeting at the level of the AC-PC plane rather than superior to it.35
Subsequently, Chazen et al. used 3-tract tractography to identify the DRT, the somatosensory tract, and the pyramidal tract in 4 patients undergoing unilateral MRgFUS at the AC-PC level for treatment of ET.21 This group reported excellent immediate postprocedure tremor improvement. Finally, Miller et al. reported substantial tremor reduction in 4 patients undergoing unilateral MRgFUS at the AC-PC level, defining targets with a combination of coordinates and 1-tract approach.23 Overall, several tractography approaches are feasible. These prior reports have included both deterministic19,22,23,25,26,28 and less commonly probabilistic15 approaches for targeting. In our opinion, delineation of all 3 tracts seems intuitively beneficial, although these approaches have not been directly compared. In principle, the 3-tract method facilitates both direct targeting of the DRT and direct avoidance of the somatosensory and pyramidal tracts. Also, visualizing where the pyramidal and somatosensory tracts are expected to be increases confidence in the tractography-suggested boundaries of the DRT, given that these 3 tracts are immediately adjacent to each other.
Illustration of the Utility of a 3-Tract Technique
At our institution, we perform probabilistic tractography of the DRT, pyramidal tract, and somatosensory tract to facilitate targeting of the VIM region for MRgFUS, and we have also applied it to DBS (Figs. 4–7). Diffusion imaging is obtained with isotropic voxels measuring 2–2.5 mm3 with 30-direction DTI, yielding a scan time that patients can generally tolerate well without motion. The raw data are processed with MRTrix version 3.0.38 The diffusion data are then coregistered to anatomical images using the ANTS registration package.39 The coregistered images are displayed in ITKSNAP40 and the following ROIs are manually defined: 1) the precentral gyrus and the ipsilateral middle third of the cerebral peduncle for the pyramidal tract; 2) the precentral gyrus and ipsilateral red nucleus region for the DRT; and 3) the postcentral gyrus and the ipsilateral posterolateral midbrain for the somatosensory tract. The streamlines are generated with MRTrix and then rendered for optimal display with RenderMan (Pixar).
FIG. 4.
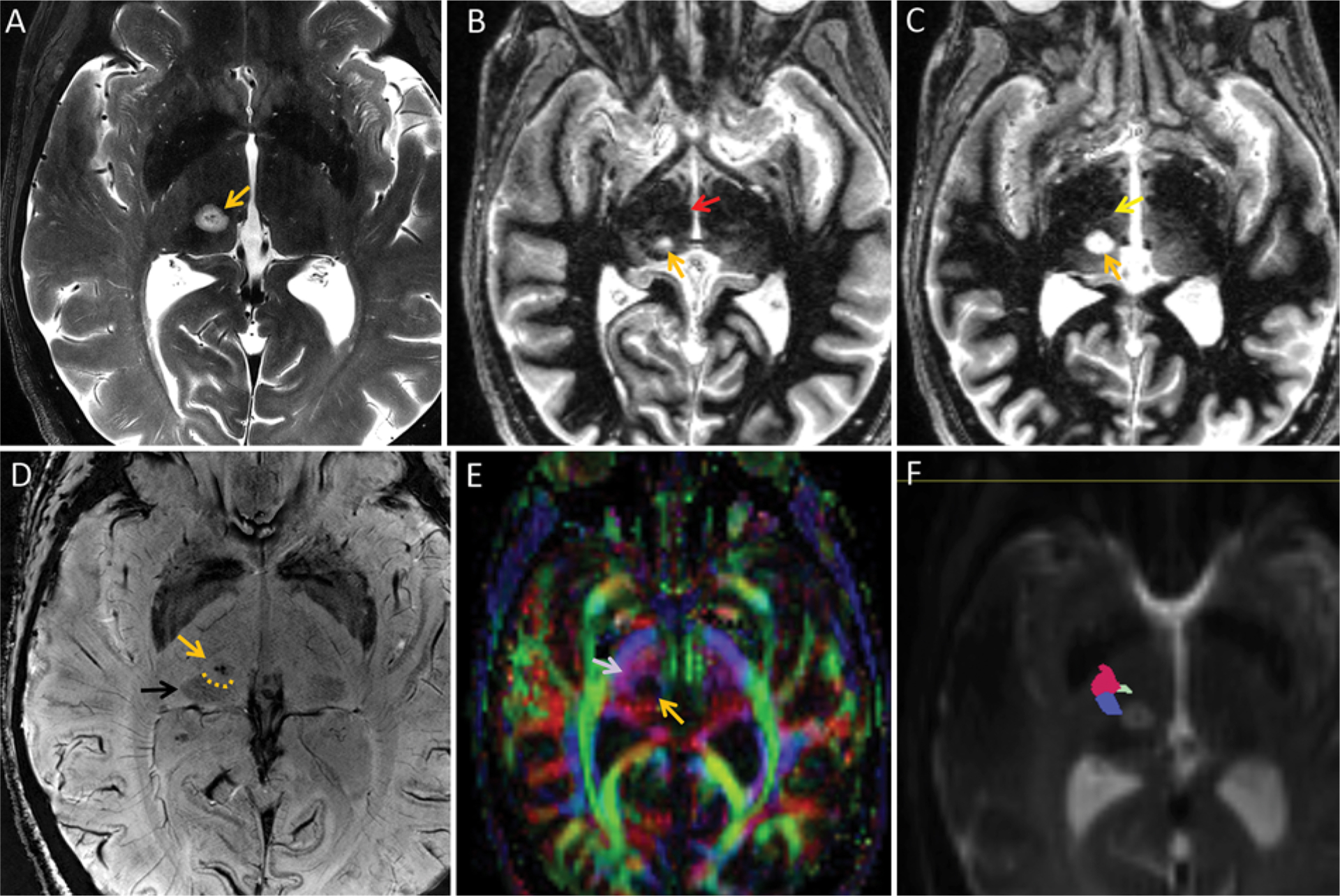
Utility of tractography for DBS in the setting of thalamic pathology in a 61-year-old woman with long-standing bilateral ET and development of voice and head tremor that was refractory to medical treatment. A: Planning 7T MR image demonstrating a 9-mm T2 hyperintense lesion (orange arrow) in the posterior right thalamus near the expected region of the right VIM. The lesion was stable during all available comparison studies over a 3-year time period without mass effect or contrast enhancement (not shown). The lesion was considered nonspecific, but indolent or benign entities such as a hamartoma were considered most likely. B: Axial 7T FGATIR image demonstrating that the inferior aspect of the lesion approaches the mesencephalic-diencephalic junction, where it is located posterior to the superior aspect of the red nucleus (red arrow). C: FGATIR image just superior to the AC-PC line, demonstrating a faint hypointense line (yellow arrow) that presumably corresponds to the DRT, with a thin hyperintense sliver of signal between the presumed DRT and the lesion (orange arrow). D: Axial 7T susceptibility-weighted image demonstrating the contour of the lesion (orange arrow), with slight impression (dotted orange line) of the normally hypointense posterior thalamus (black arrow), indicating some degree of distortion of normal anatomy. E: Axial color FA map demonstrating absent signal (orange arrow) corresponding to the lesion. The purple superolaterally projecting fibers (light purple arrow) that should correspond to the DRT and somatosensory tract are seen along the anteromedial aspect of the lesion, but the individual tracts cannot be resolved. F: A B0 map with superimposed tractography indicated that the DRT (yellow) is slightly anterior to the lesion. The pyramidal tract (red) and somatosensory tracts (blue) are also delineated. The right VIM was targeted directly using tractography, while the left VIM was targeted using an atlas-based approach. Postoperatively, there was immediate reduction of upper-extremity tremor bilaterally.
FIG. 7.
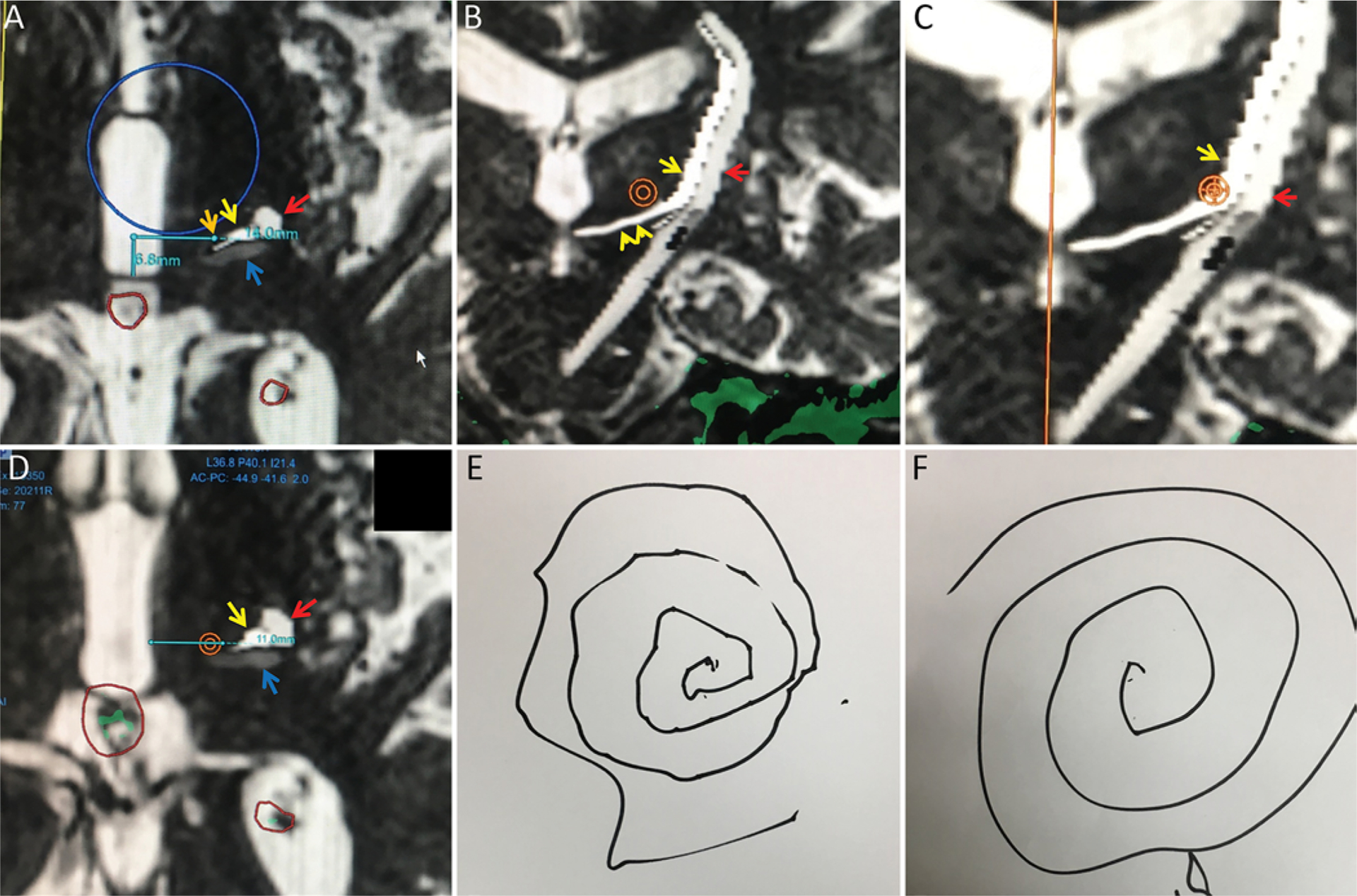
This 85-year-old right-handed man had medically refractory right ET. Intraoperative FIESTA images (A–C) at the level of the AC-PC line with superimposed gray scale tractography of the DRT in white (yellow arrows), pyramidal tract in light gray (red arrow), and somatosensory tract in dark gray (blue arrows). A: Axial image at the AC-PC level, demonstrating that the coordinate-based target 14 mm lateral (horizontal light blue line) to the midline at 25% (6.6 mm, vertical light blue line) of the AC-PC line (orange arrow denotes the target) is medial to the tractography-derived location of the DRT. B: Coronal FIESTA image with superimposed tractography demonstrating a prominent right-left course of the DRT near the level of the AC-PC axial plane (double yellow arrowhead) with an upturn in the lateral thalamus. Superior to the upturn, the DRT is nearly parallel to the pyramidal tract with predominantly vertical orientation. An orange target is placed at a location 14 mm lateral to midline, 2 mm superior to the AC-PC line. At this level, the target is well medial to the DRT. With consideration of tractography results, the target was repositioned laterally 17 mm from midline, still 2 mm superior to the AC-PC line. The green area corresponds to the skull base. C: Magnified coronal image demonstrating this new starting target location, which now overlaps the medial aspect of the DRT. We selected the medial DRT aspect to keep the lesion well removed from pyramidal tract fibers given the laterally adjusted starting position. D: Axial image 2 mm superior to the AC-PC line again demonstrating the orange target centered at 14 mm lateral to midline is medial to the DRT. The third ventricle is prominent (10 mm width), but a location 11 mm lateral to the third ventricle wall (blue line) localizes to the lateral aspect of the orange target, also medial to the DRT. The initial target was also adjusted 1 mm anterior to the coordinate-based location to be located more centrally within the DRT. As an attempt to fully cover the VIM region, a second target location was selected 4 mm superior to the AC-PC level and 18.3–18.8 mm lateral to midline. MR thermography indicated temperature up to 59°C–61°C at all target locations. Green areas represent areas of pineal and choroid plexus calcification. E and F: Preoperative (E) and immediate postoperative (F) Archimedes spiral diagrams demonstrating marked improvement. There were no side effects, including motor deficit, paresthesias, or ataxia, immediately after the procedure.
The remainder of this section will focus on the methods used for MRgFUS on the Exablate 4000 system (InSightec), but tractography can also be applied to DBS planning platforms. Once generated, the 3 tracts are superimposed on preoperative high-resolution anatomical images such as a 3D T2-weighted series for quality assurance and to facilitate initial planning. The confidence of the validity of the white matter tractography is enhanced through comparison of tract locations to findings on both anatomical images (e.g., DRT is located at the red nucleus in the midbrain, is approximately just medial to the bright spot of the internal capsule within the thalamus, and extends to the precentral gyrus) and DTI images (i.e., the DRT and somatosensory tract correspond to the superolateral purple fibers of the thalamus while the pyramidal tract is located within the blue fibers of the internal capsule). The high-resolution anatomical images are transferred to the Exablate console. The white matter tracts are transposed onto these images and displayed in differing shades of gray scale due to software restrictions. Prior to the procedure, the initial target plan is defined by aligning the images to the AC-PC line and locating a point within the DRT either at or approximately 2 mm superior to the AC-PC line.
Finally, during the procedure, the tracts are again transposed onto the intraoperative 3D FIESTA (fast imaging employing steady-state acquisition) images and the planning target is replicated and confirmed and compared with an atlas-derived VIM target (i.e., at the AC-PC plane, 25% of the distance from the PC to the AC, and approximately 14 mm from midline, adjusted for third ventricle size). Since the precise shape, orientation, and interface of the DRT and adjacent tracts as well as the degree of deviation from standard coordinate targets vary among individuals, the precise location within the central DRT is determined by consensus of the neurosurgeon, neuroradiologist, and neurologist using their best clinical judgment. Typically, we opt to favor locations that are safely medial to the pyramidal tract and anterior to the somatosensory fibers but still within the DRT. As with all MRgFUS ablation procedures, initial sonications are performed with subablative energy levels and minor adjustments of target location in any axis are made as needed.
Relative to the coordinate-based target, the tractography-defined target is typically slightly anterior and may be either medial or lateral in our experience. Variability in the medial-to-lateral direction is not surprising given the medial-to-lateral course of the DRT within the inferior thalamus as it courses superiorly, consistent with the prior tractography literature described above. Additionally, small adjustments in the superoinferior (SI) axis can result in relatively large changes in the mediolateral location of the DRT. An initial target may be selected either at or superior to the AC-PC line axial level depending on operator preference. As previously mentioned, incorporation of tractography seems particularly advantageous for targeting superior to the AC-PC level, where the DRT can course laterally.
Potential Advantages and Future Investigations of Tractography for VIM Targeting
The use of tractography for direct targeting in either DBS or MRgFUS has many potential advantages. First, this approach can more directly target a connecting white matter tract, rather than a nucleus, which could improve outcomes as indicated in some post hoc analyses.24,28–31 This approach can also help account for interindividual variability. Intraoperative procedure time may be reduced by defining a more accurate initial target, with decreased need for adjustment of DBS lead or sonication location. Operator confidence of target accuracy and avoidance of the somatosensory and pyramidal tracts are likely increased. Although the treatment location of MRgFUS can be adjusted by response to subablative sonications, adverse effects still occur. Tractography has the potential to decrease the incidence of such adverse effects, as suggested by limited initial studies.21,22 Finally, tractography can help depict the extent and orientation within the axial plane and obliquity within the SI plane, all of which can facilitate precise target location, particularly for sonications superior to the AC-PC line.
Although initial publications and examples herein suggest advantages, further work is needed for validation. There are numerous parameters during each step of MR diffusion tractography that can be varied, but optimal or standardized approaches are not established. Certain technical and anatomical assumptions are required to use tractography for direct VIM targeting, as mentioned previously, but validation of those assumptions will be very important. Finally, the optimal DRT target, both within the axial plane and along the SI axis, needs additional evaluation, for both tremor treatment efficacy and the avoidance of adverse effects. While inadvertently ultrasound ablating a portion of the pyramidal tract or the somatosensory thalamus (Vc) may produce the predictable adverse effects of unilateral weakness or paresthesias with possible anesthesia dolorosa, respectively, the cause of the adverse effect of ataxia is not well understood.41
Considerations for Implementation of Tractography Into Clinical Practice and Future Directions
Since tractography techniques are highly variable and there is evidence that the location of manually placed ROIs can substantially affect tractography results,27 standardization of as many variables as practical would be desirable but likely challenging. Data to direct the locations of ideal ROIs remain scant, and, while some data indicate that deterministic tractography may suffice for targeting purposes, additional work would be useful to confirm this assertion as well.
For now, it is useful for neurosurgeons to keep in mind that there are numerous parameters set at each step that can affect the final tractography appearance. Therefore, a result described in the literature cannot be simply applied to another clinical practice without considering these variables and other user-defined factors. There is also limited validation with true anatomical correlation, and additional work in this realm could be useful. Clinical judgment and consideration of patient-specific factors such as intraoperative feedback and careful assessment of a given technique at each institution during integration of tractography for any functional neurosurgical application into clinical practice seems prudent.
As technique standardization hopefully makes some progress and clinical experience increases, further report of clinical outcomes, complication rates, and impact on procedure time will be desirable. It will also be useful to compare the results with those derived from coordinate or atlas-based methods. Finally, it will be important for neurosurgeons to understand the basic variables and differences of tractography techniques when comparing multiple studies when such technique differs.
Conclusions
Internal thalamic anatomy in the VIM region is not adequately delineated on currently available MRI pulse sequences to allow for confident direct targeting. However, reports of MR diffusion tractography to target the DRT in the VIM region with either DBS or MRgFUS are emerging. Tractography techniques vary on multiple levels and 1-, 2-, and 3-tract tractography approaches have been described. Herein, we discuss and illustrate the potential advantages of the 3-tract approach for targeting at or superior to the AC-PC level and avoiding the pyramidal and somatosensory tracts. The potential advantages are numerous, but further research is needed to validate these assertions and their ultimate impact on clinical outcome.
FIG. 5.
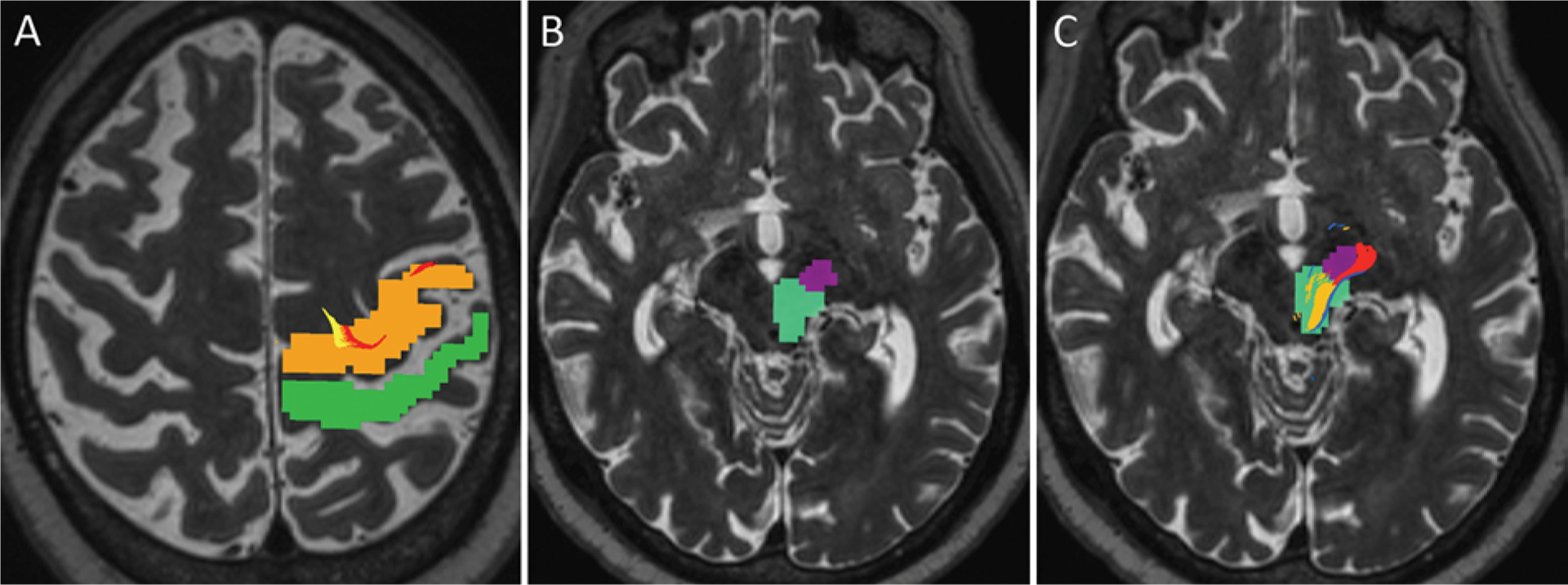
Axial T2-weighted MR images showing an example of manually determined ROIs for tractography in an 82-year-old right-handed man with severe bilateral upper-extremity ET. A: ROIs in the precentral gyrus (orange) and postcentral gyrus (green) are depicted on a preprocedure planning image. The DRT (yellow) and pyramidal tract (red) fibers are seen within the precentral gyrus motor area, while the somatosensory fibers tracked posteriorly near the postcentral gyrus but have withered out at this level in this particular patient. Some variability of precise location, size, and extent of tractography-rendered tracts among patients is expected and must be considered on an individual basis. B: Two ROIs were delineated in the midbrain, including the middle portion of the cerebral peduncle for the pyramidal tract (dark purple) and a larger area encompassing the red nucleus and adjacent posterolateral midbrain (turquois) for the DRT and somatosensory fibers. C: Image of the midbrain at the same level with superimposed pyramidal tract (red), DRT (yellow), and somatosensory fibers just posterior to the DRT (blue). Note that the white matter tracts do not encompass the entire ROIs, but rather smaller connecting regions between ROIs. For this reason, manually determined ROIs can span over a greater distance than the expected regions of the tracts.
FIG. 6.
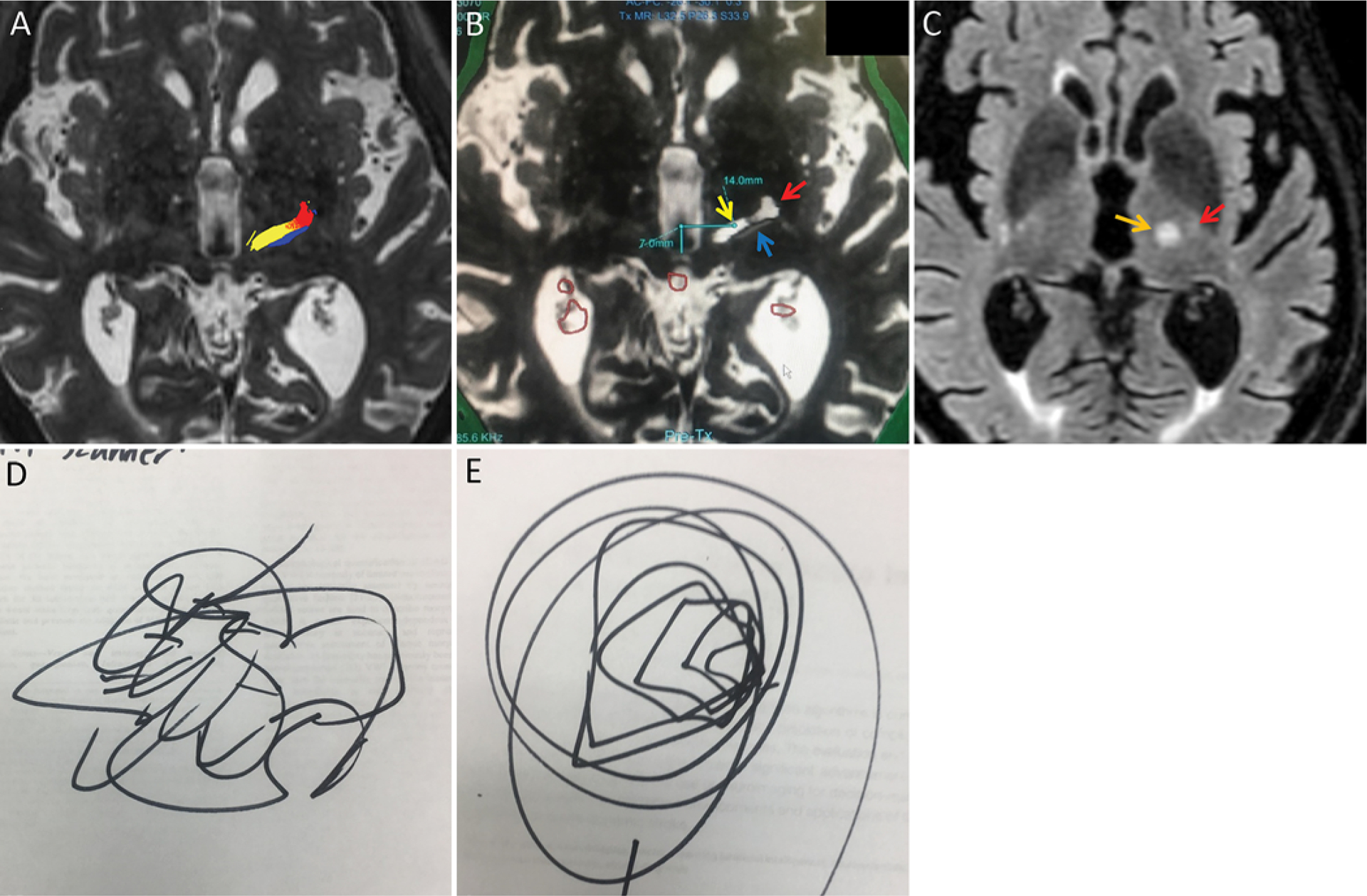
Tractography is well aligned with the coordinate-based target and increases confidence of targeting in an 82-year-old right-handed man with severe bilateral upper-extremity ET (same patient as in Fig. 5). For several clinical reasons, including the use of anticoagulation for atrial fibrillation, the patient and the treatment team elected to perform a left VIM MRgFUS thalamotomy. A: Color-coded tracts are also seen at the AC-PC level on the planning axial T2-weighted image. Yellow indicates the DRT; red, the pyramidal tract; and blue, the somatosensory tract. Note that the DRT is elongated on the left-right axis at this level and that the depicted DRT per tractography results appears thin in the anterior-to-posterior direction in this patient. A small amount of apparent overlap of the DRT and pyramidal tract is present, consistent with the notion that there is some uncertainty of the precise boundaries/edges of the rendered white matter tracts. B: Intraoperative axial FIESTA image with superimposed gray scale tractography at the level of the AC-PC line, demonstrating that the coordinate-based target 14 mm lateral to the midline at 25% of the AC-PC length was located centrally within the DRT depicted in white (yellow arrow) and well removed from the pyramidal tract depicted in light gray (red arrow) or somatosensory tract depicted in dark gray (blue arrow). The initial target location was selected 2 mm superior to the AC-PC level and, with tractography assistance, the target was shifted to 15 mm lateral. After 3 ablative sonications up to 60°C, a second target was selected an additional 1 mm superior to the first target, at 15 mm lateral to the midline, still positioned centrally within the tractography-derived DRT (not shown). The red circles delineate areas of calcification. C: Axial T2-weighted FLAIR reformatted image demonstrating an 8 × 8–mm ablative lesion (orange arrow), located just medial but slightly posterior to the faintly visualized bright spot of the posterior limb of the internal capsule (red arrow). This corresponds to the relationship of the DRT and pyramidal tracts on tractography. At the end of the procedure, the patient had approximately 95% tremor reduction as determined by the attending neurologist. D and E: Preprocedure (D) and postprocedure (E) Archimedes spirals are depicted. There was transient hand weakness during the procedure, which subsequently resolved, but otherwise no weakness, paresthesias, or ataxia. Longer-term follow-up is pending.
Acknowledgments
We thank Sonia Watson, PhD, for assistance with editing the manuscript.
ABBREVIATIONS
- AC
anterior commissure
- DBS
deep brain stimulation
- DRT
dentatorubrothalamic tract
- DTI
diffusion tensor imaging
- DWI
diffusion-weighted imaging
- ET
essential tremor
- FA
fractional anisotropy
- FGATIR
fast gray matter acquisition T1 inversion recovery
- FIESTA
fast imaging employing steady-state acquisition
- MER
microelectrode recording
- MRgFUS
MR-guided focused ultrasound
- PC
posterior commissure
- ROI
region of interest
- SI
superoinferior
- Vc
ventral caudal nucleus
- VIM
ventral intermediate nucleus
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Johansen-Berg H, Behrens TE, Sillery E, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15(1):31–39. [DOI] [PubMed] [Google Scholar]
- 2.Niemann K, Naujokat C, Pohl G, et al. Verification of the Schaltenbrand and Wahren stereotactic atlas. Acta Neurochir (Wien). 1994;129(1–2):72–81. [DOI] [PubMed] [Google Scholar]
- 3.Morel A Stereotactic Atlas of the Human Thalamus and Basal Ganglia. Informa Healthcare; 2007. [Google Scholar]
- 4.Deoni SC, Josseau MJ, Rutt BK, Peters TM. Visualization of thalamic nuclei on high resolution, multi-averaged T1 and T2 maps acquired at 1.5 T. Hum Brain Mapp. 2005;25(3):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vassal F, Coste J, Derost P, et al. Direct stereotactic targeting of the ventrointermediate nucleus of the thalamus based on anatomic 1.5-T MRI mapping with a white matter attenuated inversion recovery (WAIR) sequence. Brain Stimul. 2012;5(4):625–633. [DOI] [PubMed] [Google Scholar]
- 6.Yamada K, Akazawa K, Yuen S, et al. MR imaging of ventral thalamic nuclei. AJNR Am J Neuroradiol. 2010;31(4):732–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iglesias JE, Insausti R, Lerma-Usabiaga G, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage. 2018;183:314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abosch A, Yacoub E, Ugurbil K, Harel N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery. 2010;67(6):1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deistung A, Schäfer A, Schweser F, et al. Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. Neuroimage. 2013;65:299–314. [DOI] [PubMed] [Google Scholar]
- 10.Tourdias T, Saranathan M, Levesque IR, et al. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T. Neuroimage. 2014;84:534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Y, Zitella LM, Duchin Y, et al. Multimodal 7T imaging of thalamic nuclei for preclinical deep brain stimulation applications. Front Neurosci. 2016;10:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudhyadhom A, Haq IU, Foote KD, et al. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR). Neuroimage. 2009;47(suppl 2):T44–T52. [DOI] [PubMed] [Google Scholar]
- 13.Morishita T, Higuchi MA, Kobayashi H, et al. A retrospective evaluation of thalamic targeting for tremor deep brain stimulation using high-resolution anatomical imaging with supplementary fiber tractography. J Neurol Sci. 2019;398:148–156. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese E Diffusion tractography in deep brain stimulation surgery: a review. Front Neuroanat. 2016;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sammartino F, Krishna V, King NK, et al. Tractography based ventral intermediate nucleus targeting: novel methodology and intraoperative validation. Mov Disord. 2016;31(8):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlaier JR, Beer AL, Faltermeier R, et al. Probabilistic vs. deterministic fiber tracking and the influence of different seed regions to delineate cerebellar-thalamic fibers in deep brain stimulation. Eur J Neurosci. 2017;45(12):1623–1633. [DOI] [PubMed] [Google Scholar]
- 17.Coenen VA, Varkuti B, Parpaley Y, et al. Postoperative neuroimaging analysis of DRT deep brain stimulation revision surgery for complicated essential tremor. Acta Neurochir (Wien). 2017;159(5):779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenoy AJ, Schiess MC. Deep brain stimulation of the dentato-rubro-thalamic tract: outcomes of direct targeting for tremor. Neuromodulation. 2017;20(5):429–436. [DOI] [PubMed] [Google Scholar]
- 19.Fenoy AJ, Schiess MC. Comparison of tractography-assisted to atlas-based targeting for deep brain stimulation in essential tremor. Mov Disord. 2018;33(12):1895–1901. [DOI] [PubMed] [Google Scholar]
- 20.King NKK, Krishna V, Basha D, et al. Microelectrode recording findings within the tractography-defined ventral intermediate nucleus. J Neurosurg. 2017;126(5):1669–1675. [DOI] [PubMed] [Google Scholar]
- 21.Chazen JL, Sarva H, Stieg PE, et al. Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. J Neurosurg. 2018;129(2):315–323. [DOI] [PubMed] [Google Scholar]
- 22.Krishna V, Sammartino F, Agrawal P, et al. Prospective tractography-based targeting for improved safety of focused ultrasound thalamotomy. Neurosurgery. 2019;84(1):160–168. [DOI] [PubMed] [Google Scholar]
- 23.Miller TR, Zhuo J, Eisenberg HM, et al. Targeting of the dentato-rubro-thalamic tract for MR-guided focused ultrasound treatment of essential tremor. Neuroradiol J. 2019;32(6):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anthofer J, Steib K, Fellner C, et al. The variability of atlas-based targets in relation to surrounding major fibre tracts in thalamic deep brain stimulation. Acta Neurochir (Wien). 2014;156(8):1497–1504. [DOI] [PubMed] [Google Scholar]
- 25.Schlaier J, Anthofer J, Steib K, et al. Deep brain stimulation for essential tremor: targeting the dentato-rubro-thalamic tract? Neuromodulation. 2015;18(2):105–112. [DOI] [PubMed] [Google Scholar]
- 26.Coenen VA, Mädler B, Schiffbauer H, et al. Individual fiber anatomy of the subthalamic region revealed with diffusion tensor imaging: a concept to identify the deep brain stimulation target for tremor suppression. Neurosurgery. 2011;68(4):1069–1076. [DOI] [PubMed] [Google Scholar]
- 27.Nowacki A, Schlaier J, Debove I, Pollo C. Validation of diffusion tensor imaging tractography to visualize the dentatorubrothalamic tract for surgical planning. J Neurosurg. 2018;130(1):99–108. [DOI] [PubMed] [Google Scholar]
- 28.Coenen VA, Allert N, Paus S, et al. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery. 2014;75(6):657–670. [DOI] [PubMed] [Google Scholar]
- 29.Kincses ZT, Szabó N, Valálik I, et al. Target identification for stereotactic thalamotomy using diffusion tractography. PLoS One. 2012;7(1):e29969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein JC, Barbe MT, Seifried C, et al. The tremor network targeted by successful VIM deep brain stimulation in humans. Neurology. 2012;78(11):787–795. [DOI] [PubMed] [Google Scholar]
- 31.Wintermark M, Huss DS, Shah BB, et al. Thalamic connectivity in patients with essential tremor treated with MR imaging-guided focused ultrasound: in vivo fiber tracking by using diffusion-tensor MR imaging. Radiology. 2014;272(1):202–209. [DOI] [PubMed] [Google Scholar]
- 32.Sweet JA, Walter BL, Gunalan K, et al. Fiber tractography of the axonal pathways linking the basal ganglia and cerebellum in Parkinson disease: implications for targeting in deep brain stimulation. J Neurosurg. 2014;120(4):988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang AI, Buch VP, Heman-Ackah SM, et al. Thalamic deep brain stimulation for essential tremor: relation of the dentatorubrothalamic tract with stimulation parameters. World Neurosurg. 2020;137:e89–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranjan M, Elias GJB, Boutet A, et al. Tractography-based targeting of the ventral intermediate nucleus: accuracy and clinical utility in MRgFUS thalamotomy. J Neurosurg. Published online September 27, 2019. doi: 10.3171/2019.6.JNS19612 [DOI] [PubMed] [Google Scholar]
- 35.Boutet A, Ranjan M, Zhong J, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018;141(12):3405–3414. [DOI] [PubMed] [Google Scholar]
- 36.Tian Q, Wintermark M, Jeffrey Elias W, et al. Diffusion MRI tractography for improved transcranial MRI-guided focused ultrasound thalamotomy targeting for essential tremor. Neuroimage Clin. 2018;19:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsolaki E, Downes A, Speier W, et al. The potential value of probabilistic tractography-based for MR-guided focused ultrasound thalamotomy for essential tremor. Neuroimage Clin. 2017;17:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tournier J-D, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202(November):116137. [DOI] [PubMed] [Google Scholar]
- 39.Avants BB, Tustison NJ, Stauffer M, et al. The Insight ToolKit image registration framework. Front Neuroinform. 2014;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 41.Sprenger T, Seifert CL, Valet M, et al. Assessing the risk of central post-stroke pain of thalamic origin by lesion mapping. Brain. 2012;135(Pt 8):2536–2545. [DOI] [PubMed] [Google Scholar]
- 42.Akram H, Dayal V, Mahlknecht P, et al. Connectivity derived thalamic segmentation in deep brain stimulation for tremor. Neuroimage Clin. 2018;18:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]


