Abstract
Background
While thinner struts are associated with improved clinical outcomes in bare-metal stents (BMS), reducing strut thickness may affect drug delivery from drug-eluting stents (DES) and there are limited data comparing otherwise similar thin and thick strut DES. We assessed 2-year outcomes of patients treated with a thin strut (84–88um) cobalt-chromium, biodegradable polymer, Biolimus A9-eluting stent (CoCr-BP-BES) and compared these to patients treated with a stainless steel, biodegradable polymer, Biolimus A9-eluting stent (SS-BP-BES).
Methods
In total, 1257 patients were studied: 400 patients from 12 centres receiving ≥1 CoCr-BP-BES in the prospective Biomatrix Alpha registry underwent prespecified comparison with 857 patients who received ≥1 Biomatrix Flex SS-BP-BES in the LEADERS study (historical control). The primary outcome was major adverse cardiac events (MACE)—cardiac death, myocardial infarction (MI), or clinically driven target vessel revascularization (cd-TVR). Propensity analysis was used to adjust for differences in baseline variables and a landmark analysis at day-3 to account for differences in periprocedural MI definitions.
Results
MACE at 2 years occurred in 6.65% CoCr-BP-BES versus 13.23% SS-BP-BES groups (unadjusted HR 0.48 [0.31–0.73]; P=0.0005). Following propensity analysis, 2-year adjusted MACE rates were 7.4% versus 13.3% (HR 0.53 [0.35–0.79]; P=0.004). Definite or probable stent thrombosis, adjudicated using identical criteria in both studies, occurred less frequently with CoCr-BP-BES (1.12% vs. 3.22%; adjusted HR 0.32 [0.11–0.9]; P=0.034). In day-3 landmark analysis, the difference in 2-year MACE was no longer significant but there was a lower patient-orientated composite endpoint (11.7% vs. 18.4%; HR 0.6 [0.43–0.83]; P=0.006) and a trend to lower target vessel failure (5.8% vs. 9.1%; HR 0.63 [0.4–1.00]; P=0.078).
Conclusion
At 2-year follow-up, propensity-adjusted analysis showed the thin strut (84–88um) Biomatrix Alpha CoCr-BP-BES was associated with improved clinical outcomes compared with the thicker strut (114–120um) Biomatrix Flex SS-BP-BES.
1. Introduction
Thinner stent struts may improve deliverability and conformability and reduce vessel injury. A comparison of bare-metal stents with identical design apart from strut thickness reported improved clinical outcomes with thinner struts, specifically a lower incidence of angiographic restenosis and repeat revascularization [1]. However, reducing strut thickness may affect drug delivery from drug-eluting stents (DES). Furthermore, most trials evaluating DES with thinner versus thicker struts have compared stents with different designs and/or different polymers and/or different drugs [2]. We thus aimed to assess the clinical impact of reducing strut thickness in DES by comparing two otherwise similar DES, apart from a difference in strut thickness. We also assessed clinical outcomes to 2 years to mitigate the potentially confounding effect of different durations of dual antiplatelet therapy (DAPT) in earlier follow-up.
2. Materials and Methods
2.1. Stent Design
In this paper, we describe 2-year outcomes after a percutaneous coronary intervention (PCI) with the Biomatrix AlphaTM cobalt-chromium, biodegradable polymer, Biolimus A9 eluting stent (CoCr-BP-BES) and the Biomatrix FlexTM stainless steel, biodegradable polymer, Biolimus A9 eluting stent (SS-BP-BES). Both stents are abluminally coated with a mixture of Biolimus A9 and a polylactic acid (PLA) polymer matrix (50 : 50 by weight) in a dose of 15.6 µg/mm stent length. Biolimus A9 is an m-TOR inhibitor with a cytostatic mechanism of action similar to sirolimus but custom-designed with a ligand modification which results in 10-fold increased lipophilicity. PLA is biodegradable and fully absorbed within 6–9 months. While drug and polymer are identical in formulation and dose, the Biomatrix AlphaTM CoCr-BP-BES platform is made from cobalt-chromium (MP35 N) which has enabled a reduction in strut thickness from 114–120 µm (in the 316L stainless steel Biomatrix FlexTM SS-BP-BES) to 84–88 µm while maintaining similar radial strength. All other stent design elements have remained unchanged including the hybrid design of mid-section S-connectors for improved flexibility combined with straight connectors for higher longitudinal strength in the proximal and distal end sections of the stent [3].
2.2. Study Design and Patients
The Biomatrix Alpha™ Registry [3] was a prospective, single-arm, multicentre registry conducted in 12 centres across 4 countries in Europe and Asia, which enrolled 400 patients with stable coronary artery disease or acute coronary syndrome receiving at least one Biomatrix AlphaTM CoCr-BP-BES. Patients were eligible for inclusion if they had undergone PCI in one or more coronary arteries or bypass grafts. There were no limitations as to the number of treated vessels, or the number, type, and length of treated lesions. Patients were excluded if any additional stent(s) different from the study stent were implanted during the index procedure. The registry was managed by the Cardiovascular European Research Center (CERC) in Massy, France. The primary endpoint was the incidence of major adverse cardiac events (MACE) at 9 months—a composite of cardiac death, myocardial infarction (MI), and clinically driven target vessel revascularization (cd-TVR). We previously reported the incidence of MACE at 9 months to be 3.94% (95% CI [2.39–6.47]) which met criteria for noninferiority (P < 0.001) versus the SS-BP-BES arm of the LEADERS study [4]. In this paper, we report clinical outcomes up to 2 years. Secondary endpoints included target vessel failure (TVF), a composite of cardiac death or target vessel (TV), MI, or cd-TVR; the patient-oriented composite endpoint (POCE), a composite of all-cause mortality, any MI, or any revascularization; individual components of the composite endpoints; and ARC definite or probable stent thrombosis.
The LEADERS study [4] was a randomized comparison of 857 patients receiving at least one SS-BP-BES (Biomatrix FlexTM) versus 850 patients receiving a stainless steel, permanent polymer, and sirolimus-eluting stent (SS-PP-SES) (Cypher™, Cordis, Miami Lakes, FL, USA). The SS-BP-BES group showed noninferiority with respect to MACE at 9 months (which was maintained at 5 years) and a significant reduction in very late definite ST from 1 to 5 years compared with the SS-PP-SES group [5]. Key elements of the Biomatrix Alpha registry protocol were intentionally kept the same as in the LEADERS study, to enable a prespecified comparison of patients receiving CoCr-BP-BES stents versus patients in the SS-BP-BES arm of the LEADERS study [4] as a historic control. DAPT with aspirin and a P2Y12 inhibitor was recommended as per clinical practice guidelines in the Biomatrix Alpha registry and for at least one year in the LEADERS study.
2.3. Data and definitions
Both studies were conducted in accordance with good clinical practice (GCP) guidelines and the 1975 Declaration of Helsinki. Both were registered with Clinicaltrials.gov and informed consent from each patient was obtained. Baseline data have been described previously [3, 4]. All reported MACE and stent thrombosis events in both studies were monitored, checked against source documents, and adjudicated by an independent Clinical Event Committee (CEC). Cardiac death was defined as any death due to immediate cardiac cause (e.g., MI, low-output failure, fatal arrhythmia), unwitnessed death, or death of unknown cause. In the Biomatrix Alpha registry, MI was defined by the Third Universal Definition of MI [6]. In the LEADERS study, MI was defined by Minnesota code ECG criteria or creatine kinase (CK) >2x upper limit of normal with elevated CK-MB or troponin. cd-TVR was defined as a repeat PCI or bypass surgery of the target vessel associated with either a ≥ 70% vessel diameter reduction or a ≥ 50% diameter reduction in combination with angina and/or documented ischemia. Stent thrombosis was categorized as definite or probable according to the Academic Research Consortium (ARC) definitions [7, 8] with all relevant angiograms reviewed by the CEC.
2.4. Statistical Analysis
For continuous variables, mean and standard deviation are reported. For categorical variables, counts and percentages are shown. The denominator for the calculation of percentages is based upon the number of the nonmissing values available unless otherwise specified. Clinical events are reported as Kaplan–Meier estimates with corresponding confidence intervals based on the log-log transformation and hazard ratio (HR) derived from the Cox proportional hazard model. All data were analysed using SAS V.9.4 (SAS Institute, Cary, North Carolina, USA).
In order to adjust for potential baseline differences, we conducted a patient-level propensity score analysis [9–11] between the datasets of the CoCr-BP-BES in the Alpha registry and the SS-BP-BES arm of LEADERS. The propensity for each patient was modelled as the probability of being part of the Alpha registry versus being part of the SS-BP-BES arm in LEADERS. The propensity scores were obtained by inverse probability of treatment weight (IPTW). The full list of baseline variables used in the propensity score calculation is provided in Supplementary Table S1.
While the Biomatrix Alpha Registry protocol was designed to match the LEADERS protocol, post-PCI biomarkers were encouraged in the Alpha Registry but mandatory in the LEADERS study and the updated Third Universal Definition of myocardial infarction [6] was used only in the Alpha Registry. Recognizing that different definitions might introduce a potential discrepancy in MI reporting between the Alpha registry and the LEADERS study, particularly for periprocedural MI (within 48 hours), we then conducted a landmark analysis censoring clinical events which were part of the primary endpoint occurring up to day 3.
3. Results
3.1. Patient and Lesion Characteristics
Baseline patient and lesion characteristics of CoCr-BP-BES versus SS-BP-BES are shown in Table 1. The mean age in the two groups was CoCr-BP-BES 64.7 ± 11 years versus SS-BP-BES 64.6 ± 10.8 years and 21% versus 24%, respectively, were current smokers. The proportion of diabetes patients differed between the two groups (CoCr-BP-BES 19.3% vs. SS-BP-BES 26.1%). Over half of the patients in both groups presented with an acute coronary syndrome (acute MI or unstable angina). Renal insufficiency was more common in the CoCr-BP_BES group (11.5% vs. 5.4%) but prior revascularization was more common in the LEADERS study (24.6% vs. 40.9%). Procedural details are listed in Table 1. Use of DAPT in the Alpha registry versus LEADERS study was lower at all time points (96% vs. 98%; P < 0.001 at 30 days, dropping to 69% vs. 93%; P < 0.0001 at 9 months and 0% vs. 21%; P < 0.0001 at 2 years) reflecting evolving treatment guidelines, different proportions of ACS patients, and greater use of single antiplatelet therapy plus oral anticoagulation.
Table 1.
Baseline demographics and procedural details.
| CoCr-BP-BES n = 400 | SS-BP-BES n = 857 | P value | |
|---|---|---|---|
| Baseline demographics | |||
| Mean age (years) | 64.7 ± 11 | 64.6 ± 10.8 | 0.892 |
| Female gender (%) | 21.5 | 25 | 0.178 |
| STEMI or NSTEMI (%) | 41.1 | 32.7 | 0.004 |
| Unstable angina (%) | 14 | 22.2 | <0.001 |
| Prior MI (%) | 18.8 | 32.2 | <0.0001 |
| Previous PCI or CABG (%) | 24.6 | 40.9 | <0.0001 |
| Previous stroke (%) | 6.3 | 4.7 | 0.292 |
| Current smoker (%) | 21 | 24.1 | 0.229 |
| Hypertension (%) | 57.3 | 73.6 | <0.0001 |
| Dyslipidemia (%) | 56.7 | 65.4 | 0.003 |
| Diabetes (%) | 19.3 | 26.1 | 0.009 |
| Renal insufficiency (%) | 11.5 | 5.4 | <0.0001 |
|
| |||
| Procedural details | |||
| Staged procedure (%) | 5.5 | 4.4 | 0.476 |
| Target lesion coronary artery (%) | |||
| LAD | 47.4 | 37.2 | <0.0001 |
| LCX | 20.1 | 28 | <0.001 |
| LM | 2.3 | 2.6 | 0.399 |
| RCA | 26.9 | 30.7 | 0.112 |
| De novo lesions (%) | 95.9 | 94 | 0.123 |
| Bifurcation lesions (%) | 25.8 | 22.4 | 0.132 |
| Number of stents per lesion | 1.34 ± 0.70 | 1.20 ± 0.48 | <0.0001 |
| Severe calcification (%) | 16.2 | 13.1 | 0.09 |
| Lesion length (mm) | 21.7 ± 12.8 | 15.2 ± 11.7 | <0.0001 |
| Reference vessel diameter (mm) | 3.0 ± 0.5 | 2.6 ± 0.61 | <0.0001 |
CABG=coronary artery bypass grafting, CoCr-BP-BES = cobalt-chromium biodegradable polymer Biolimus A9-eluting stent, MI = myocardial infarction, PCI = percutaneous coronary intervention, and SS-BP-BES = stainless steel biodegradable polymer Biolimus A9-eluting stent.
3.2. Clinical Outcomes
Previously published 9-month MACE outcomes met criteria for noninferiority [3]. The unadjusted difference in MACE remained consistent at 2 years (6.6% with CoCr-BP-BES vs. 13.2% with SS-BP-BES; HR 0.48[0.31–0.73]; P=0.0005) (Figure 1). Individual components of the composite MACE endpoint and unadjusted secondary endpoints are listed in Table 2. Each component of MACE and most secondary endpoints were significantly lower in the CoCr-BP-SES group. A Cox regression multivariate analysis, including 15 baseline characteristics (Table S1) and stent type, found the stent type to be an independent predictor of MACE (P=0.0028).
Figure 1.
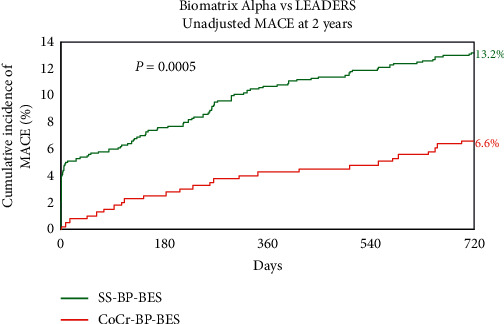
Unadjusted MACE at 2 years. CoCr-BP-BES = cobalt-chromium biodegradable polymer Biolimus A9-eluting stent, MACE = major cardiac adverse events, and SS-BP-BES = stainless steel biodegradable polymer Biolimus A9-eluting stent.
Table 2.
Biomatrix Alpha versus LEADERS : unadjusted MACE at 2 years.
| CoCr-BP-BES (n = 400) | SS-BP-BES (n = 857) | Hazard ratio | P value | |
|---|---|---|---|---|
| MACE | 26 (6.65%) | 112 (13.23%) | 0.48 [0.31–0.73] | 0.0005 |
| - Cardiac death | 4 (1.01%) | 27 (3.21%) | 0.31 [0.11–0.89] | 0.022 |
| - MI | 12 (3.13%) | 55 (6.48%) | 0.46 [0.24–0.85] | 0.012 |
| - cd-TVR | 16 (4.09%) | 65 (7.8%) | 0.51 [0.3–0.89] | 0.0152 |
| All death | 15 (3.82%) | 40 (4.72%) | 0.79 [0.44–1.44] | 0.449 |
| Target vessel MI | 5 (1.29%) | 27 (3.18%) | 0.39 [0.15–1.03] | 0.048 |
| Definite or probable stent thrombosis | 3 (0.81%) | 26 (3.07%) | 0.25 [0.08–0.82] | 0.013 |
| Any revasc | 29 (7.46%) | 143 (17.14%) | 0.40 [0.27–0.60] | <0.0001 |
| TVF (cardiac death or TV-MI or cd-TVR) | 20 (5.09%) | 96 (11.36%) | 0.43 [0.27–0.7] | 0.0004 |
| POCE (all death or any MI or any revasc) | 43 (10.9%) | 192 (22.58%) | 0.44 [0.32–0.61] | <0.0001 |
revasc = revascularization, cd-TVR = clinically driven target vessel revascularization, CoCr-BP-BES = cobalt-chromium biodegradable polymer Biolimus A9-eluting stent, MACE = major adverse cardiac events, MI = myocardial infarction, POCE = patient-oriented composite endpoint, SS-BP-BES = stainless steel biodegradable polymer Biolimus A9-eluting stent, and TVF = target vessel failure.
3.3. Propensity Analysis
Given differences in patient baseline characteristics despite using matching inclusion criteria, a propensity analysis was undertaken and adjusted for 15 variables. Figure 2 shows that the difference in MACE remained after propensity adjustment (7.4% vs. 13.3%; HR 0.52 [0.35 : 0.79]; P=0.0041). Similar to the unadjusted data, the difference in MACE emerged early then remained consistent up to 2 years. Supplementary Figure 1 shows the incidence of MACE during the first month versus months 2–24.
Figure 2.
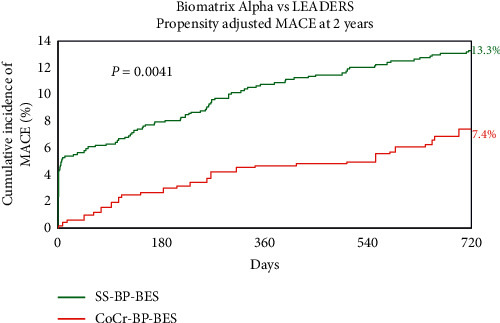
Propensity-adjusted MACE at 2 years. CoCr-BP-BES = cobalt-chromium biodegradable polymer Biolimus A9-eluting stent, MACE = major cardiac adverse events, and SS-BP-BES = stainless steel biodegradable polymer Biolimus A9-eluting stent.
3.4. Stent Thrombosis
The safety endpoint of definite or probable stent thrombosis was adjudicated using identical ARC criteria in both studies. Figure 3 shows that after propensity adjustment, the incidence of definite or probable stent thrombosis was markedly lower at 2 years with CoCr-BP-DES (1.1 vs. 3.2%; HR 0.32 [0.114 : 0.897]; P=0.034) with most of the difference being in early stent thrombosis.
Figure 3.
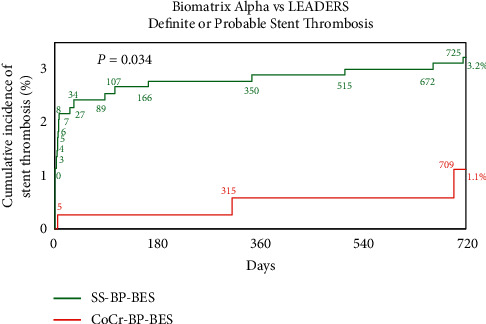
Propensity-adjusted definite or probable stent thrombosis at 2 years. Stent thrombosis was adjudicated using identical criteria in both studies. CoCr-BP-BES = cobalt-chromium biodegradable polymer Biolimus A9-eluting stent, MACE = major cardiac adverse events, and SS-BP-BES = stainless steel biodegradable polymer Biolimus A9-eluting stent.
Of note, the reduction of definite or probable stent thrombosis with CoCr-BP-DES was achieved despite a shorter duration of DAPT.
3.5. Landmark Analysis at Day 3
To account for possible differences in MI reporting, particularly for periprocedural (Type 4a) MI, we conducted a landmark analysis censoring clinical events contributing to the primary endpoint that occurred up to and including day 3. Following landmark analysis, the MACE rate with CoCr-BP-DES remained numerically lower than with SS-BP-BES (Figure 4) but was no longer statistically significant (7.25% vs. 9.34%; HR 0.76 [0.5–1.17]; P=0.25). Individual elements of the composite MACE endpoint and adjusted secondary endpoints following landmark analysis at day 3 are shown in Table 3. The patient-oriented composite endpoint remained significantly lower with CoCr-BP-BES (11.7% vs. 18.7%; HR 0.6[0.43–0.83]; P=0.006) and there was a trend to less frequent target vessel revascularization (5.8% vs. 9.1%; HR 0.63 [0.4–1.00]; P=0.078).
Figure 4.
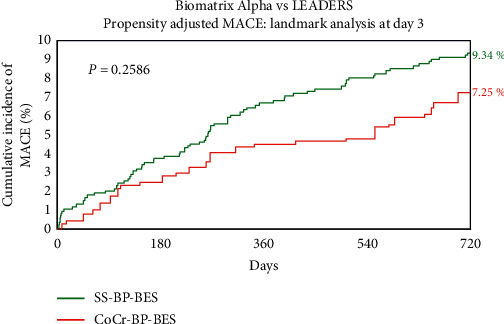
Propensity-adjusted MACE at 2 years with a landmark at day 3. CoCr-BP-BES = cobalt-chromium biodegradable polymer Biolimus A9-eluting stent, MACE = major cardiac adverse events, SS-BP-BES = stainless steel biodegradable polymer Biolimus A9-eluting stent.
Table 3.
Biomatrix Alpha versus LEADERS : propensity-adjusted MACE at 2 years with a landmark at day 3.
| CoCr-BP-BES (%) | SS-BP-BES (%) | Hazard ratio | P value | |
|---|---|---|---|---|
| MACE | 7.25 | 9.34 | 0.76 [0.5–1.17] | 0.259 |
| - Cardiac death | 1.29 | 3.26 | 0.39 [0.15–1.00] | 0.064 |
| - Myocardial infarction | 2.82 | 2.36 | 1.17 [0.56–2.47] | 0.721 |
| - cd-TVR | 4.57 | 6.39 | 0.65 [0.38–1.10] | 0.152 |
| All death | 4.12 | 4.74 | 0.86 [0.49–1.52] | 0.638 |
| Target vessel MI | 0.90 | 0.91 | 1.01 [0.28–3.59] | 0.991 |
| Definite or probable stent thrombosis | 1.12 | 2.11 | 0.5 [0.17–1.45] | 0.238 |
| Any revasc | 8.20 | 15.47 | 0.5 [0.34–0.73] | 0.001 |
| TVF (cardiac death or TV-MI or cd-TVR) | 5.83 | 9.08 | 0.63 [0.4–1.00] | 0.078 |
| POCE (all death or any MI or any revasc) | 11.69 | 18.39 | 0.6 [0.43–0.83] | 0.006 |
4. Discussion
In this first report of longer-term (2-year) outcomes of patients undergoing PCI with CoCr-BP-BES and at a time point when all patients had discontinued dual antiplatelet therapy, the unadjusted event rates with CoCr-BP-BES remained low. These results are in keeping with recent data from studies using other 3rd generation DES. The BIO-RESORT randomized trial of 3514 all-comer patients [12] and reported 2-year TVF rates of 6.8% for the SynergyTM biodegradable polymer everolimus-eluting stent, 6.6% for the OrsiroTM CoCr-BP-SES, and 8.3% for the RESOLUTETM permanent polymer zotarolimus-eluting stent (ZES) (P=ns). In the BIONYX trial [13], 2-year TVF rates were 7.6% with the Resolute OnyxTM permanent polymer ZES and 7.1% with OrsiroTM
While thinner stent struts are associated with factors such as reduced wall shear stress [14] and less malapposition [15], both of which may reduce thrombotic risk, thinner struts are more prone to longitudinal compression [16] and to an increased risk of tissue prolapse increasing the thrombotic risk [17]. Thinner struts may also impact on strut spacing and impact local drug diffusion. It is thus important to study DES strut thickness directly rather than extrapolate from BMS data.
Previous DES studies have compared dissimilar technologies with differences in stent design, polymer, and drug [2] which may confound analysis. While a meta-analysis of thinner strut CoCr versus thicker strut SS-DES showed a reduction in MI at 30 days [18], in SORT OUT VII, the OrsiroTM CoCr-BP-SES failed to show a significant reduction in target lesion failure at 3 years compared with the NoboriTM SS-BP-BES despite a marked difference in strut thickness (60–80 µm vs. 114–120 µm) [19]. While our comparison was not randomized, the prespecified protocol of the Biomatrix Alpha Registry facilitated the comparison of thin (84–88 µm) versus thicker (114–120 µm) struts in the 3rd generation stents while controlling for the stent design, polymer, and drug.
Although MI definitions differed between the 2 studies, definite or probable stent thrombosis was adjudicated using identical criteria. It was thus appropriate to report its incidence without landmark adjustment. The reduction in definite or probable stent thrombosis rates is notable and is consistent with previous literature [2] suggesting that this may be the principal benefit of reduced strut thickness.
The strong trend towards lower cd-TVR with Biomatrix Alpha shows that the antiproliferative effect of Biolimus was not compromised despite the thinner strut platform.
The limitations of this study are the modest sample size and the use of historical rather than prospectively randomized controls. It is possible that some of the outcome benefits described are related to advances in procedural techniques and concomitant drug therapy over the past decade, not fully adjusted for in the propensity analysis. In line with typical registry protocols, only 10% of the patients in the Alpha registry were fully monitored, thus there is a possibility of underreporting of clinical events, although 100% adjudication of MACE events was undertaken.
5. Conclusion
In this analysis, 2-year clinical outcomes with the thin strut (84–88um) Biomatrix AlphaTM CoCr-BP-BES were excellent with rates of MACE at 6.6%, TVF 5.1%, and definite/probable stent thrombosis 0.8%. A prespecified propensity-adjusted analysis showed improved clinical outcomes compared with the thicker strut (114–120 µm) Biomatrix FlexTM SS-BP-BES.
Acknowledgments
The authors acknowledge the work of all medical, nursing, and other hospital staff who cared for the patients. Grant support for the Alpha registry was given by Biosensors SA, Morges, Switzerland.
Data Availability
The data used for the study endpoints may be made available on request from the corresponding author.
Conflicts of Interest
IM has received a speaker honorarium from Biosensors. SC, SSS, and KGO are employed by Biosensors. No other authors have any conflicts to declare.
Authors' Contributions
Each author contributed to the manuscript and takes responsibility for all aspects of the reliability and freedom from bias of the data presented.
Supplementary Materials
The full list of baseline variables used in the propensity score calculation and weighted p values is provided in Supplementary Table S1. Supplementary Figure S1 shows the incidence of MACE (propensity-adjusted) with landmark analysis at 30 days.
References
- 1.Kastrati A., Mehilli J., Dirschinger J., et al. Intracoronary stenting and angiographic results. Circulation. 2001;103(23):2816–2821. doi: 10.1161/01.cir.103.23.2816. [DOI] [PubMed] [Google Scholar]
- 2.Iantorno M., Lipinski M. J., Garcia-Garcia H. M., et al. Meta-analysis of the impact of strut thickness on outcomes in patients with drug-eluting stents in a coronary artery. The American Journal of Cardiology. 2018;122(10):1652–1660. doi: 10.1016/j.amjcard.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Menown I. B. A., Mamas M. A., Cotton J. M., et al. First clinical evidence characterizing safety and efficacy of the new CoCr Biolimus-A9 eluting stent: the Biomatrix Alpha registry. IJC Heart and Vasculature. 2020;26 doi: 10.1016/j.ijcha.2020.100472.100472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windecker S., Serruys P. W., Wandel S., et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. The Lancet. 2008;372(9644):1163–1173. doi: 10.1016/s0140-6736(08)61244-1. [DOI] [PubMed] [Google Scholar]
- 5.Serruys P. W., Farooq V., Kalesan B., et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease. JACC: Cardiovascular Interventions. 2013;6(8):777–789. doi: 10.1016/j.jcin.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K., Alpert J. S., Jaffe A. S., Simoons M. L., Chaitman B. R., White H. D. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/cir.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 7.Cutlip D. E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials. Circulation. 2007;115(17):2344–2351. doi: 10.1161/circulationaha.106.685313. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Garcia H. M., McFadden E. P., Farb A., et al. Standardized end point definitions for coronary intervention trials. European Heart Journal. 2018;39(23):2192–2207. doi: 10.1093/eurheartj/ehy223. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum P. R., Rubin D. B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician. 1985;39(1):33–38. doi: 10.1080/00031305.1985.10479383. [DOI] [Google Scholar]
- 10.D’Agostino R. B. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.D’Agostino R. B. Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 12.Kok M. M., Zocca P., Buiten R. A., et al. Two-year clinical outcome of all-comers treated with three highly dissimilar contemporary coronary drug-eluting stents in the randomised BIO-RESORT trial. EuroIntervention. 2018;14(8):915–923. doi: 10.4244/eij-d-18-00336. [DOI] [PubMed] [Google Scholar]
- 13.Buiten R. A., Ploumen E. H., Zocca P., et al. Thin composite-wire-strut zotarolimus-eluting stents versus ultrathin-strut sirolimus-eluting stents in BIONYX at 2 years. JACC: Cardiovascular Interventions. 2020;13(9):1100–1109. doi: 10.1016/j.jcin.2020.01.230. [DOI] [PubMed] [Google Scholar]
- 14.Kolandaivelu K., Swaminathan R., Gibson W. J., et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123(13):1400–1409. doi: 10.1161/circulationaha.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanigawa J., Barlis P., Dimopoulos K., Dalby M., Moore P., Di Mario C. The influence of strut thickness and cell design on immediate apposition of drug-eluting stents assessed by optical coherence tomography. International Journal of Cardiology. 2009;134(2):180–188. doi: 10.1016/j.ijcard.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 16.Barragan P., Garitey V., Mouneimne K., Rieu R. Longitudinal compression behaviour of coronary stents: a bench-top comparative study. EuroIntervention. 2014;9(12):1454–1462. doi: 10.4244/eijv9i12a243. [DOI] [PubMed] [Google Scholar]
- 17.Vilchez-Tschischke J. P., Salazar C., Gil-Romero J., et al. Stent strut thickness and acute vessel injury during percutaneous coronary interventions. Coronary Artery Disease. 2020 doi: 10.1097/MCA.0000000000000943. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Moreno R., Jimenez-Valero S., Sanchez-Recalde A., et al. Periprocedural (30-day) risk of myocardial infarction after drug-eluting coronary stent implantation: a meta-analysis comparing cobalt-chromium and stainless steel drug-eluting coronary stents. EuroIntervention. 2011;6(8):1003–1010. doi: 10.4244/eijv6i8a173. [DOI] [PubMed] [Google Scholar]
- 19.Ellert J., Maeng M., Raungaard B., et al. Clinical outcomes three-year after revascularization with biodegradable polymer stents: ultrathin-strut sirolimus-eluting stent versus biolimus-eluting stent: from the Scandinavian organization for randomized trials with clinical outcome VII trial. Coronary Artery Disease. 2020;31(6):485–492. doi: 10.1097/mca.0000000000000875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full list of baseline variables used in the propensity score calculation and weighted p values is provided in Supplementary Table S1. Supplementary Figure S1 shows the incidence of MACE (propensity-adjusted) with landmark analysis at 30 days.
Data Availability Statement
The data used for the study endpoints may be made available on request from the corresponding author.


