Abstract
COVID-19 is an airway disease that has affected ~125 million people worldwide, caused by a novel coronavirus termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), spread through respiratory droplets, direct contact, and aerosol transmission. Although most patients presenting with absent or mild symptoms recover completely, the highest morbidity and mortality rates are seen in the elderly, and patients with comorbidities such as cardiovascular diseases, cancer, immunosuppressive diseases, diabetes, and pre-existing respiratory illnesses. Several therapeutic strategies have been examined, but a wide-ranging therapeutic option for particularly severe cases of COVID-19 remains to be elucidated. Considering the indications presented by COVID-19 patients who present similarly with inflammatory conditions, intravenous immunoglobulin (IVIG) administration has been examined as a possible route to reduce proinflammatory markers such as ESR, CRP and ferritin by reducing inflammation, based on its anti-inflammatory effects as indicated by utilisation of IVIG for numerous other inflammatory conditions. Herein, summarising the recent key clinical evaluations of IVIG administration, we present our hypothesis that administration of IVIG within a specific dosage would be extremely beneficial towards reducing mortality and perhaps even the length of hospitalisation of patients exhibiting severe COVID-19 symptoms.
Keywords: COVID-19, SARS-CoV2, Intravenous immunoglobulins (IVIG), Coronavirus, Immunotherapy, Antibodies
Introduction
COVID-19 is an airway disease that has ravaged the medical, social, and economic fabric of the world as we know it, having affected ~125 million people worldwide and resulted in ~2.75 million casualties as of 9:54 am CET, March 27, 2021. The causative underlying factor is a novel coronavirus, termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), spread through respiratory droplets, direct contact, and aerosol transmission [1], [80]. As the seventh member of coronaviruses known to infect humans, SARS-CoV2 is an enveloped positive sense ssRNA virus causing respiratory, gastrointestinal, and neurological symptoms in humans, mammals, and birds. Four coronaviruses cause symptoms in immunocompromised patients, while the other two -SARS-CoV, and the middle east respiratory syndrome (MERS)-CoV- had caused epidemics in 2002–2003 in China and 2012 in the Middle East, respectively [2]. Although most patients presenting with absent or mild symptoms recover completely, some patients further deteriorate into life-threatening conditions including acute respiratory distress syndrome (ARDS), multiple organ dysfunction (MODS), and heart failure, all of which suggest a cytokine storm syndrome (CSS) in such patients [3]. The highest morbidity and mortality rates are seen in the elderly, and patients with comorbidities such as cardiovascular diseases, cancer, immunosuppressive diseases, diabetes, and respiratory illnesses [4].
Pathophysiology of COVID-19
COVID-19 attaches to the respiratory epithelium through spike proteins (S), facilitating viral entry into host cells [2], [5]. The incubation period ranges from 2 to 14 days, during which SARS-CoV2 transmission can occur [4]. Early symptoms of fever and cough may be followed by viremia which can target organs including the heart, renal, and gastrointestinal tract. Common clinical symptoms include flu-like indications such as fever, cough, fatigue, headache, dysgeusia, pleuritic chest pain, conjunctivitis, sore throat, diarrhea, vomiting and myalgia [6], [7]. High resolution CT (HRCT) is the gold standard for radiological diagnosis in COVID-19, while reverse transcription-PCR (RT-PCR) is used as a diagnostic tool using nasal swabs, bronchoalveolar lavage, or tracheal aspirates, as is serology [8], [9].
Patients may recover from any time throughout the course of the disease, or may progress to the next stage, even descending into acute respiratory distress syndrome (ARDS) with or without multiple organ dysfunction syndrome (MODS). In asymptomatic patients, symptoms presented include anosmia, ageusia, asthenia and conjunctivitis, alongside mild lymphopenia. Lung ultrasounds may also show localized B-lines, while HRCT will show localized subpleural ground glass appearance. In patients with mild symptoms, indications such as arthralgia, myalgia, dry cough and fever present with increased lymphopenia, and/or ferritin, LDH and D-Dimer. There is also mild hypoxia (>92, while B-lines are diffuse and lead to pleural line thickening). The CT shows a “ground glass” appearance at this stage [2], [7], [8], [9].
In moderate stages, patients may present with dyspnea, hypoxia, and arrhythmia. Lab tests show progressive increases in D-dimer and ferritin, with worsening hypoxia and hypercapnia, increased transaminases and triglycerides, and increased IL-6 and CRP. There is also a mild increase in NT-pro BNP and troponin, and reduction in platelets. Lung ultrasounds indicate subpleural consolidation, localized pleural effusion and diffuse B-lines, while CTs indicate a “crazy paving” sign. These symptoms necessitate hospital admission. In severe cases of COVID-19, patients show the worst prognosis. ARDS, SIRS, MODS, shock, heart failure, high fever and DIC are some of the clinical symptoms that require urgent care, while blood work show increased pro-inflammatory markers, ferritin, cytopenias, increased NT-proBNT, troponin, and signs of renal failure. Lung US has pleural line thickening due to diffuse B lines and subpleural and alveolar consolidation with air bronchograms [2], [7], [8], [9], [10]. It is no surprise that CT scan shows “white lung” in alignment with the clinical symptoms and lung US [11]. The ARDS seen in the last stage suggests occurrence of a cytokine storm syndrome (CSS) in COVID-19 patients. Some patients may develop secondary hemophagocytic lymphohistiocytosis (sHLH), usually triggered through viral infections, and culminating in CSS or a macrophage activation syndrome (MAS), resulting in increased mortality of up to 60%.
Use of corticosteroids and other treatments in COVID-19
Several strategies and therapeutic options have been tried and tested since the start of COVID-19 outbreak. The use of short courses of corticosteroids at low-to-moderate dose, for critically ill patients with COVID-19 pneumonia, signs of an exaggerated immune response or in patients with symptoms of myocardial involvement has been recommended [12], [13]. A systematic review of 771 publications has concluded that there are no beneficial effects of the use of inhaled corticosteroids in patients with COVID-19. However, withdrawal of ICS in patients with asthma and COPD is not recommended [14]. Dexamethasone is a synthetic corticosteroid approved by the FDA in 1958 for broad-spectrum immunosuppression. The limitations of dexamethasone mainly lies in the fact that although it is helpful in reducing pro-inflammatory cytokines, which is beneficial for the hyper-inflammatory stage in COVID-19 patients, it also suppresses T- and B-cell functions, leading to a loss of immune clearance of the SARS-CoV2 viral load [15].
Antiviral agents such as lopinavir/ritonavir (LPV/RTV) are beneficial for severe or critical cases, especially if used within 12 days of symptom onset as retrieved from retrospective studies from the SARS epidemic, leading to shorter recovery time [16], [17]. Another antiviral added to the treatment regimen recently is remdesivir, which is a broad-spectrum antiviral drug for a wide array of RNA viruses, including SARS/MERS-CoV5 and Ebola [18], [19] The use of antimalarials such as chloroquine and hydroxychloroquine for their antiviral activity and their immunomodulatory effects on 1L-6 and TNF-α have shown some benefit for early stages and mild symptoms [20], [21]. Convalescent plasma containing high-titer neutralizing SARS-CoV2 specific antibody decreases the viral load significantly [22]. Anticoagulant use is indicated through use of heparin considering frequently reported cases of disseminated intravascular coagulation (DIC) and prevalence of abnormal coagulation results, especially markedly elevated D‐dimer and FDP in COVID-19 deaths, suggesting an association of DIC with increased mortality rate [23], [24].
Hypothesis: Intravenous immunoglobulins (IVIG) could prove beneficial for severe COVID-19 patients
COVID-19 is currently divided into four stages: asymptomatic; mild to moderate symptoms including a fever or flu-like symptoms with dry cough; shortness of breath requiring admission; and finally, acute respiratory distress syndrome (ARDS) needing positive pressure oxygen therapy with intensive care therapy. The most severe stage of COVID-19 also presents with disseminated intravascular coagulation (DIC), systemic inflammatory response syndrome (SIRS), multi-organ dysfunction syndrome (MODS), shock, hepatosplenomegaly, among other serious morbidities [11]. The clinical picture of ARDS shows an increase in erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), and serum ferritin, which are markers of inflammation. While limited at present, specific studies show significant promise for the use of IVIG in treating cases of COVID-19, particularly in cases of severe symptoms (Table 1 ) [25], [26], [27], [28], [29], [30]. We can infer from these that the ARDS is driven by proinflammatory markers, for which IVIG may provide symptomatic relief and cure through its immunomodulatory and anti-inflammatory properties [31]. Indeed, considering the indications presented by COVID-19 patients, we posit that perhaps the administration of high-dose IVIG for such patients who present similarly with inflammatory conditions can reduce proinflammatory markers such as ESR, CRP and ferritin by reducing inflammation. The rationale behind use of IVIG for late-stage COVID-19 is based on its biochemical properties, which has anti-inflammatory effects and is utilized for other inflammatory conditions and infectious diseases as well [3], [32].
Table 1.
Summary of conditions applied and relevant findings from key clinical studies examining the efficacy of intravenous immunoglobulin (IVIG) administration in treating various symptoms of COVID-19.
| Study | Number of patients | Severity | Patient treatment | Intravenous Immunoglobulin dosage | Considerations | Outcome |
|---|---|---|---|---|---|---|
| Xie et al, [25] | 58 | Severe/Critical | Oxygen Therapy, Abidor antiviral treatment, Moxifloxacin, Heparin |
20 g/day administered either < 48 h or > 48 h following patient admission |
>48 h group required higher dosage of IVIG | IVIG treatment within 48 h resulted in lower morbidity compared to treatment > 48 h after admission. Length of treatment and ICU stay was shorter if treated < 48 h after admission Proportion of patients requiring mechanical ventilation was lower when treated within 48 h of admission |
| Mohtadi et al., [26] | 5 | Severe |
Combinations of: Hydroxychloroquine, Kaletra, Oseltamivir, Vancomycin, Levofloxacin, Tavanx, Azithromycin, Ceftriaxone, Meropenem, Imipenem |
25–30 g/day for 5 days 9–14 days after admission |
All patients had been intubated | Clinical and respiratory conditions improved Saturated oxygen levels increased resulting in quicker extubating of patients. Obvious improvements in pulmonary Lesions on CT scans. All patients discharged with good general condition and stabilized vital signs. |
| Cao et al., [27] | 3 | Severe | Combinations of: Supportive care and empirical Moxifloxacin, Methylprednisolone, Lopinavir/ritonavir |
25 g/day for 5 days 1–7 days after admission |
All patients exhibited decreased oxygen saturation | Saturated oxygen levels increased resulting in quicker extubating of patients. All patients discharged with good general condition and stabilized vital signs. |
| Shao et al., [28] | 325 | Severe/Critical | N/A | <15 g or > 15 g per day <7 days or > 7 days |
IVIG-treated patients had higher Acute Physiology and Chronic Health Evaluation and Sequential Organ Failure Assessment scores, higher plasma levels of IL-6 and lactate, and lower lymphocyte count and oxygenation index | IVIG significantly reduced the 28-day mortality, the inflammatory response, and improved some organ functions, but only in critical patients >15 g/day IVIG reduced 28-day and 60-day mortality and increased survival time, particularly in critical patients Early administration of IVIG (≤7d) reduced 60-day mortality, total in-hospital stay, and total course of disease, Early administration of IVIG (≤7d) significantly increased survival time and improved inflammatory response and some organ functions. 28-day and 60-day mortality were not improved with IVIG in severe patients in-hospital stay and the total duration of disease were longer in IVIG group in severe patients |
| Aljaberi and Wishah, [29] | 1 | Severe/Critical | Ceftriaxone, Doxycycline, Hydroxychloroquine 2 L/minute oxygen by nasal cannula |
40 g every 2 weeks | Leukopenia (lymphopenia), normal coagulation profile, electrolytes, and liver 17 function. Flu/RSV panels were negative intubation and mechanical ventilation by day 7 |
Extubated on Day 13 and discharged on Day 14 |
| Lanza et al., [30] | 1 | Severe/Critical | Hydroxychloroquine Azithromycin |
450 mL (5 mL/kg) at 36 mL/h × 3 days with premedication with antihistamine and rehydration, followed by a decrease in infusion to 28 mL/h and subsequently extended total administration to 4 days |
Deteriorating respiration and bloodwork | Improvement in clinical and pulmonary function CT scan showed a massive reduction in parenchymal consolidations, Patient discharged with good general condition and stabilized vital signs. |
Evaluation of the hypothesis
The history of IVIG use dates to the 1880s when German Physician E. von Behring first studied the effects of passive immunity through the transfer of serum from sensitized guinea pigs and rabbits to non-immune animals, thus passing on the antitoxins from neutralised tetanus or diphtheria broth to the non-immune animals [33]. The biochemical essence of serotherapy lies in the formation of antigen–antibody complexes, and the specificity and diversity of the binding sites for antigens on these antibodies. The use of immunoglobulins (Ig) was first utilized in intramuscular form, but due to painful injections and other side effects, led to the rise of its intravenous form, IVIG [34], [33]. IVIG went through several modifications in preparation and stabilization techniques to increase its biological half-life, reduce anaphylactoid reactions, increasing efficacy and ensuring safety procedures to reduce viral hepatitis transmission [33].
IVIG mechanism of action
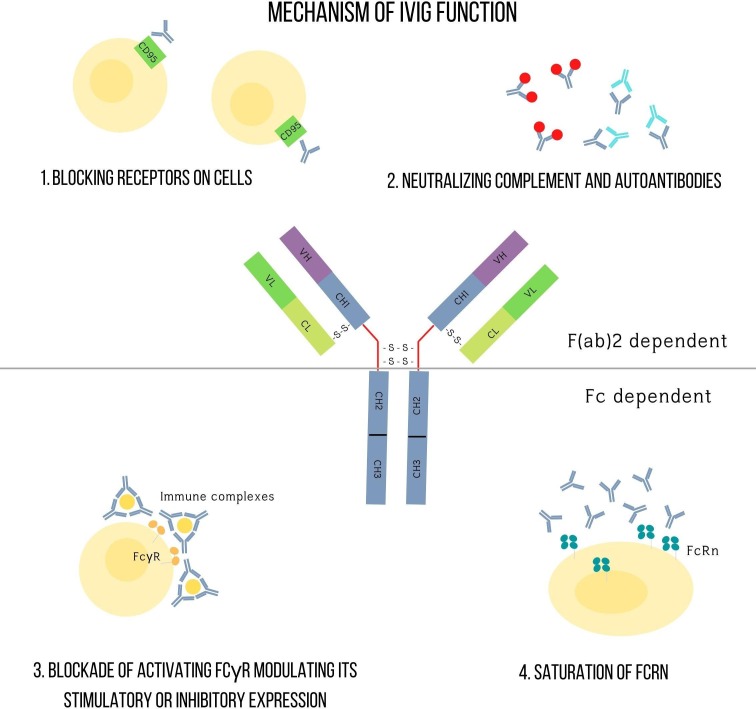
The mechanism of action of IVIG seems to vary amongst differing conditions of autoimmunity but seem to contain a common thread of action (Fig. 1 ; Table 2 ) [35], [36], [37]. Anti-idiotypic antibodies bind to antibodies and neutralize them, while Fc receptors on macrophages are blocked following IVIG. Fcγ receptors on immune cells are saturated at high doses, resulting in their inhibition. FcRn, a protective receptor crucial for regulating IgG half-life, normally binds to IgGs, and is protected from catabolism after being internalised in the endosome. IVIG saturates these receptors, accelerating endogenous IgG breakdown, thus mitigating autoimmune responses [38], [39]. IVIG may also inhibit FcγRI or FcγRIII receptors, and perhaps also upregulate FcγRII receptors [40].
Fig. 1.
Schematic representation summarising the mechanisms of action of intravenous immunoglobulin (IVIG) administration.
Table 2.
Summary of the effects of intravenous immunoglobulin (IVIG) treatment upon components of both innate and acquired immunity.
| Innate Immunity Component | Effect of IVIG on component |
|---|---|
| Natural Killer (NK) cells | - Increased activation - Increased cytokine production and degranulation - Increased anti-tumor effect - Migration from blood to tissue |
| Dendritic cells (DCs) | - Decreased endocytosis - Decreased pro-inflammatory cytokine production - Increased anti-inflammatory cytokine production - Decreased differentiation - Increased NK cell-mediated ADCC - Decreased expression of MHC class II and co-stimulatory molecules - Decreased DC-mediated T cell activation - Decreased expression of activating FcγR - Increased expression of CD1d |
| Macrophages | - Increased production of IL-1Ra - Decreased activation - Decreased production of pro-inflammatory cytokines - Decreased expression of IFN-γR2 - Decreased expression of activating FcγR - Blockade of activating FcγR - Increased expression of inhibitory FcγRIIB |
| Neutrophils | - Decreased activation due to IgG monomers inhibiting FcγR - Increased activation by IgG dimers binding FcγR or by ANCA - Decreased adhesion to endothelium - Increased death by Siglec |
| Acquired Immunity Component | Effect of IVIG on component |
| T cells | - Increased apoptosis - Decreased IL-2 production - Decreased differentiation - Decreased activation and proliferation |
| Treg cells | - Increased production - Increased suppressive action |
| B cells | - Increased apoptosis - Decreased proliferation - Regulation of antibody production - Increase in inhibitory FcγRIIB - Neutralization of survival factors - Inhibition of activating FcγR |
IVIG can also block adhesion molecules on leukocytes, leading to anti-inflammatory effects, while also inhibiting C3a and C5a anaphylatoxins and others, preventing the formation of membrane attack complex (MAC). IVIG administration can also inhibit T cell, dendritic cell, monocytes, and macrophage proliferation and differentiation, while also downregulating Th17 responses and upregulating regulatory T cells (Treg) differentiation [37], [41], [42], [43]. IVIG also exerts cytotoxicity on neutrophils and eosinophils, a process termed antibody-dependent cell-mediated toxicity, or ADCC [35], [36], [37].
In animal models, IVIG reduces IL-2 and interferon-γ production by T-cells [44], [45], while IVIG preparations contain antibodies against CD4 cells, soluble HLA I and II molecules, chemokine receptor, CCR-5, and T-cell receptor β chain [45], [46], [47], [48]. Furthermore, perhaps therapeutic IVIG dosages may restore the balance between Th1 and Th2 cells [49]. IVIG exhibits inhibitory B-cell mediated effects including inhibition of antibody production [50], inhibition of B-cell differentiation [51], and inhibition of production of interleukin-6 and tumour necrosis factor-α [52]. However, IVIG also conversely induces B-cell apoptosis [53], specific B-cells downregulation [54], and regulation of CD5 [55].
IVIG reduces levels of circulating IL-1β, in autoimmune conditions such as Kawasaki syndrome [56], [57], while up to a 1000-fold increase in levels of IL-1 receptor antagonist was observed following IVIG therapy [58]. However, IVIG remains functionally active in mice strains deficient in IL-1R, IL-4, IL-10, IFN-γR, IL-12β and TNF-α [59], indicating that perhaps cytokine modulation is unlikely the major mechanism of action. TNF-α mediated cytotoxicity is also inhibited by IVIG [60], which is also thought to modulate endothelial cell function by interacting with intercellular adhesion molecules (ICAM) [38], [61]. Other possible IVIG mechanisms can also modulate cell migration due to the presence of antibodies against integrins [62] and the argine-glycine-aspargine (RGD) cell adhesion motifs [54]. Infusion of IVIG may also restore levels of sialic acid-rich IgG, inducing an anti-inflammatory action [63], [64], [65].
Structure
Immunoglobulins (Ig) are glycoproteins produced by activated plasma cells in response to antigens and modulate adaptive immune functions in the human body. IgGs are the most abundant immunoglobulin in the human body, with a plasma concentration of 700–1600 mg/dL [35]. The high half-life of IgG (21–25 days) is due to its binding on Fcγ receptors, which in turn protects IgG from lysosomal degradation. One theory on IVIG effect in auto-immune conditions is that IVIG supersaturates these Fcγ receptors leading to marked decrease of auto-IgG antibody half-life. It is the same Fcγ blockade that caused an increase in platelets in immune thrombocytopenic purpura (ITP) through IVIG use [39].
IgG consists of two heavy chains and two light chains, which can be broken down into two regions through the action of proteases: an Fab region, responsible for reversible and noncovalent antigen binding; and an Fc region of IgG which binds to macrophages, monocytes, neutrophils and dendritic cells [66]. The Fab region of IgG works through neutralization of the pathogens, preventing attachment to host cells and opsonization or binding of IgG to a pathogen, leading to macrophagic phagocytosis. The Fc region binds to Fcγ receptors on various immune cells leading to either activation or inhibition of immune response. It can induce phagocytosis in macrophages, for example [67]. Other functions include regulation of apoptosis, release of proinflammatory cytokines, upregulation of Fcγ receptors in B cells, maturation and differentiation of immune cells, modulation of antigen-presenting cells, and regulatory T cell function. The IVIG is a highly purified preparation that can also function as the regular Ig in neutralizing exogenous antigens on pathogens as in the case of SARS-CoV2 or endogenous pathogens [10], usually prepared by pooling serum from three thousand to ten thousand donors, which are processed and stabilized for use in autoimmune, inflammatory and degenerative disorders and refractory bacterial and viral infections [49]. The risk of infectious disease transmission is reduced through screening donors, effectively evaluating donor products for infectious products and usage of virus inactivation methods [68], [69].
Clinical use
IVIG is a standard of care in many primary and secondary immunodeficiency syndromes. Indeed, higher doses of IVIG reduced the occurrence of pneumonia in primary immunodeficiency syndromes. However, this was not the case for chronic airway infections such as chronic sinusitis. IVIG is thought to mount a humoral immune response, which is most commonly deficient in the primary immunodeficiency population. In secondary immunodeficiency syndromes such as AIDS, where patients present with low CD4+ T cell counts, IVIG exhibited protective effects against bacterial, viral and fungal infections [70], [71], [72]. Secondary humoral immunodeficiencies are also seen in malnutrition and cancers for which low-dose IVIG is used to reduce life-threatening infections [73].
IVIG can also exert immunomodulating effects on autoimmune disorders such as immune thrombocytopenic purpura (ITP), whereby a high-dose IVIG increased the platelet count and decreased a patient’s need for frequent platelet transfusions [74]. IVIG is also employed in other autoimmune conditions such as idiopathic juvenile arthritis, dermatomyositis, polymyositis, catastrophic antiphospholipid syndrome (CAS), ANCA-related vasculitis and myasthenia gravis. COVID-19 has shown to resemble inflammatory syndromes in its most serious ICU settings, and this is substantiated not only from the clinical picture, but also through serum inflammatory markers such as ESR and CRP [75].
Dosage
A low-dose of 400 mg/kg IVIG is given once every three to four weeks for most immunodeficiency syndromes, as a typical part of replacement therapy. However, for inflammatory, auto-immune and infectious etiologies, the dosage used can reach of 2000 mg/kg given across a period of two to five days, often referred to as high dosage. This is because IVIG has a paradoxical effect at high-doses; leading to more immunomodulatory and anti-inflammatory effects [10], [35]. A hyperimmune therapy consists of a single intramuscular dose after suspected exposure of a pathogen. Sometimes a hyperimmune therapy is also given as IVIG, such as the respiratory syncytial virus (RSV) IVIG [76].
Adverse reactions
Collectively, while the overall safety profile of IVIG is high, certain risks are also associated with IVIG. For example, IVIG potentially could result in the deposition of antigen–antibody complexes by intradermal injection of antigen into a person who is already sensitized, forming from type-three hypersensitivity reaction, a phenomenon termed the arthus reaction [77]. Most immediate adverse reactions occur within an hour, and range from mild symptoms of headache, fatigue, fever and chills to severe adverse reactions such as myalgia, erythema, flushing, hyper- or hypotension and fluid overload. Transfusion-related acute lung injury (TRALI), acute renal failure, anaphylactic shock, arrhythmias, aseptic meningitis are lesser common immediate adverse reactions. A small percentage of patients have delayed presentation which appear as infections at the transfusion site or renal impairment [78]. Mild to moderate reactions are treated with ceasing the treatment, changing the infusion rate, or applying supportive treatment for the various symptoms. Switching to subcutaneous immunoglobulin (SCIG) is also an option. A non-sugar IVIG preparation may be used to prevent osmotic renal injury in patients with renal failure [79].
Conclusions
Collectively, several strategies and therapeutic options have been examined for efficacy in treatment since the start of COVID-19 outbreak with very mixed results. While promising candidates for treatment have emerged such as Dexamethasone, others have not quite lived up to expectations such as Remdesivir. Even in the case of current success stories, however, modes of treatment that would be able to benefit most COVID-19 patients has yet to be established. To this degree, while a not extensively examined in the context of COVID-19, IVIG presents with a promising avenue of investigation to potentially treat at least those exhibiting severe symptoms. Indeed, the rationale underlying IVIG are its anti-inflammatory effects, being utilized for other inflammatory conditions and infectious diseases, which is a category of disease that COVID-19 seems to increasingly occupy. Perhaps an area which is not yet entirely clear, would be the efficacy of IVIG on addressing/treating novel variants of SARS-CoV-2, as such aspects have thus far generated numerous obstacles to suppressing the spread of the virus. While the proposed mechanisms of IVIG action involve bolstering various aspects of both the innate and acquired immune systems in the body and should thus remain effective against various variants. However, such aspects are purely hypothetical and would need focussed studies to ascertain efficacy rates.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The manuscript was conceived by AY and JK, and all authors contributed towards writing the manuscript, which was submitted following approval of all authors. This work was supported by a COVID-19 project grant (#951) awarded by the Saudi Arabian Ministry of Health awarded to AY and JK.
References
- 1.Bouey, J., From SARS to 2019-Coronavirus (nCoV): U.S.-China Collaborations on Pandemic Response. 2020, RAND Corporation.
- 2.Cdc Weekly C. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Weekly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triggle C.R., Bansal D., Farag E.A.B.A., Ding H., Sultan A.A., Rosenberg H.F. COVID-19: learning from lessons to guide treatment and prevention interventions. mSphere. 2020;5(3) doi: 10.1128/mSphere.00317-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Q., Lin Q., Ni Z., You L. Uncertainties about the transmission routes of 2019 novel coronavirus. Influenza Other Respir Viruses. 2020;14(4):470–471. doi: 10.1111/irv.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F., et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Napoli., M.C.M.R.A.C.S.C.D.R.D., Evaluation, and Treatment of Coronavirus (COVID-19) in Treasure Island (FL): StatPearls Publishing. 2020.
- 10.Prete M., Favoino E., Catacchio G., Racanelli V., Perosa F. SARS-CoV-2 infection complicated by inflammatory syndrome. Could high-dose human immunoglobulin for intravenous use (IVIG) be beneficial? Autoimmun Rev. 2020;19(7):102559. doi: 10.1016/j.autrev.2020.102559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluccio F., Ergonenc T., Garcia Martos A., Allam A.-S., Pérez-Herrero M., Aguilar R., et al. Treatment algorithm for COVID-19: a multidisciplinary point of view. Clin Rheumatol. 2020;39(7):2077–2084. doi: 10.1007/s10067-020-05179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac Involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halpin D.M.G., Singh D., Hadfield R.M. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55(5):2001009. doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theoharides T.C., Conti P. Dexamethasone for COVID-19? Not so fast. J Biol Regul Homeost Agents. 2020;34(3) doi: 10.23812/20-EDITORIAL_1-5. [DOI] [PubMed] [Google Scholar]
- 16.Chan K.S., et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9(6):399–406. [PubMed] [Google Scholar]
- 17.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kupferschmidt K., Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367(6485):1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- 19.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., et al. Cold Spring Harbor Laboratory; 2020. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. [Google Scholar]
- 21.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18(4):786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y., Cao S., Dong H., Li Q., Chen E., Zhang W., et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohtadi N., Ghaysouri A., Shirazi S., Ansari S., Shafiee E., Bastani E., et al. Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: a case series. Virology. 2020;548:1–5. doi: 10.1016/j.virol.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao W., Liu X., Bai T., Fan H., Hong K., Song H., et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao, Z., et al., Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: A multicenter retrospective cohort study. 2020, medRxiv. [DOI] [PMC free article] [PubMed]
- 29.Aljaberi R., Wishah K. Positive outcome in a patient with coronavirus disease 2019 and common variable immunodeficiency after intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2020;125(3):349–350. doi: 10.1016/j.anai.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanza M., Polistina G.E., Imitazione P., Annunziata A., Di Spirito V., Novella C., et al. Successful intravenous immunoglobulin treatment in severe COVID-19 pneumonia. IDCases. 2020;21:e00794. doi: 10.1016/j.idcr.2020.e00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura A., Ikeda K., Hamaoka K. Aetiological significance of infectious stimuli in Kawasaki disease. Frontiers Pediatrics. 2019;7 doi: 10.3389/fped.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan Q., Yang K., Wang W., Jiang L., Song J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(6):1294–1297. doi: 10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.João C., Negi V.S., Kazatchkine M.D., Bayry J., Kaveri S.V. Passive serum therapy to immunomodulation by IVIG: a fascinating journey of antibodies. J Immunol. 2018;200(6):1957–1963. doi: 10.4049/jimmunol.1701271. [DOI] [PubMed] [Google Scholar]
- 34.Marrack J.R. The chemistry of antigens and antibodies. J Phys Chem. 1934;38(7):989. [Google Scholar]
- 35.Arumugham, V.B. and A. Rayi, Intravenous Immunoglobulin (IVIG), in StatPearls. 2020, StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC.: Treasure Island (FL). [PubMed]
- 36.Ballow M. Mechanisms of immune regulation by IVIG. Curr Opin Allergy Clin Immunol. 2014;14(6):509–515. doi: 10.1097/ACI.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 37.Basta M. Modulation of complement-mediated immune damage by intravenous immune globulin. Clin Exp Immunol. 1996;104:21–25. [PubMed] [Google Scholar]
- 38.Xu C., Poirier B., Duong Van Huyen J.-P., Lucchiari N., Michel O., Chevalier J., et al. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins. Am J Pathol. 1998;153(4):1257–1266. doi: 10.1016/S0002-9440(10)65670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Z., Lennon V.A. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med. 1999;340(3):227–228. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- 40.Dalakas M.C. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence-based indications and safety profile. Pharmacol Ther. 2004;102(3):177–193. doi: 10.1016/j.pharmthera.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Lutz H.U., Stammler P., Jelezarova E., Nater M., Spath P.J. High doses of immunoglobulin G attenuate immune aggregate-mediated complement activation by enhancing physiologic cleavage of C3b in C3bn- IgG complexes. Blood. 1996;88(1):184–193. [PubMed] [Google Scholar]
- 42.Mollnes T.E., Hogasen K., Hoaas B.F., Michaelsen T.E., Garred P., Harboe M. Inhibition of complement-mediated red cell lysis by immunoglobulins is dependent on the IG isotype and its Cl binding properties. Scand J Immunol. 1995;41(5):449–456. doi: 10.1111/j.1365-3083.1995.tb03591.x. [DOI] [PubMed] [Google Scholar]
- 43.Frank M.M., Basta M., Fries L.F. The effects of intravenous immune globulin on complement-dependent immune damage of cells and tissues. Clin Immunol Immunopathol. 1992;62(1):S82–S86. doi: 10.1016/0090-1229(92)90045-p. [DOI] [PubMed] [Google Scholar]
- 44.Jacob S., Rajabally Y.A. Current proposed mechanisms of action of intravenous immunoglobulins in inflammatory neuropathies. Curr Neuropharmacol. 2009;7(4):337–342. doi: 10.2174/157015909790031166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saoudi A., Hurez V., de Kozak Y., Kuhn J., Kaveri S.V., Kazatchkine M.D., et al. Human immunoglobulin preparations for intravenous use prevent experimental autoimmune uveoretinitis. Int Immunol. 1993;5(12):1559–1567. doi: 10.1093/intimm/5.12.1559. [DOI] [PubMed] [Google Scholar]
- 46.Grosse-Wilde H., Blasczyk R., Westhoff U. Soluble HLA class I and class II concentrations in commercial immunoglobuh preparations. Tissue Antigens. 1992;39(2):74–77. doi: 10.1111/j.1399-0039.1992.tb01910.x. [DOI] [PubMed] [Google Scholar]
- 47.Bouhlal H., Hocini H., Quillent-Grégoire C., Donkova V., Rose S., Amara A., et al. Antibodies to C-C chemokine receptor 5 in normal human IgG block infection of macrophages and lymphocytes with primary R5-tropic strains of HIV-1. J Immunol. 2001;166(12):7606–7611. doi: 10.4049/jimmunol.166.12.7606. [DOI] [PubMed] [Google Scholar]
- 48.Marchalonis J.J., Kaymaz H., Dedeoglu F., Schluter S.F., Yocum D.E., Edmundson A.B. Human autoantibodies reactive with synthetic autoantigens from T-cell receptor beta chain. Proc Natl Acad Sci. 1992;89(8):3325–3329. doi: 10.1073/pnas.89.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackay I.R., Rosen F.S., Kazatchkine M.D., Kaveri S.V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345(10):747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 50.Kondo N., Kasahara K., Kameyama T., Suzuki Y., Shimozawa N., Tomatsu S., et al. Intravenous immunoglobulins suppress immunoglobulin productions by suppressing Ca2+ -dependent signal transduction through Fc gamma receptors in B lymphocytes. Scand J Immunol. 1994;40(1):37–42. doi: 10.1111/j.1365-3083.1994.tb03430.x. [DOI] [PubMed] [Google Scholar]
- 51.Stohl W., Elliot J.E. In vitro inhibition by intravenous immunoglobulin of human T cell-dependent B cell differentiation induced by staphylococcal superantigens. Clin Immunol Immunopathol. 1996;79(2):122–133. doi: 10.1006/clin.1996.0059. [DOI] [PubMed] [Google Scholar]
- 52.Toungouz M., Denys C.H., Groote D.D., Dupont E. In vitro inhibition of tumour necrosis factor-α and interleukin-6 production by intravenous immunoglobulins. Br J Haematol. 1995;89(4):698–703. doi: 10.1111/j.1365-2141.1995.tb08404.x. [DOI] [PubMed] [Google Scholar]
- 53.Toyoda M., Pao A., Petrosian A., Jordan S.C. Pooled human gammaglobulin modulates surface molecule expression and induces apoptosis in human B cells. Am J Transplant. 2003;3(2):156–166. doi: 10.1034/j.1600-6143.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 54.Vassilev T., Yamamoto M., Aissaoui A., Bonnin E., Berrih-Aknin S., Kazatchkine M.D., et al. Normal human immunoglobulin suppresses experimental myasthenia gravis in SCID mice. Eur J Immunol. 1999;29(8):2436–2442. doi: 10.1002/(SICI)1521-4141(199908)29:08<2436::AID-IMMU2436>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 55.Vassilev T., Gelin C., Kaveri S.V., Zilber M.-T., Boumsell L., Kazatchkine M.D. Antibodies to the CD5 molecule in normal human immunoglobulins for therapeutic use (intravenous immunoglobulins, IVIG) Clin Exp Immunol. 1993;92(3):369–372. doi: 10.1111/j.1365-2249.1993.tb03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung D.Y., Kurt-Jones E., Newburger J., Cotran R., Burns J., Pober J. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989;334(8675):1298–1302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- 57.Sharief M.K., Ingram D.A., Swash M., Thompson E.J. IV immunoglobulin reduces circulating proinflammatory cytokines in Guillain-Barre syndrome. Neurology. 1999;52(9):1833. doi: 10.1212/wnl.52.9.1833. [DOI] [PubMed] [Google Scholar]
- 58.Aukrust P., Froland S.S., Liabakk N.B., Muller F., Nordoy I., Haug C., et al. Release of cytokines, soluble cytokine receptors, and interleukin-1 receptor antagonist after intravenous immunoglobulin administration in vivo. Blood. 1994;84(7):2136–2143. [PubMed] [Google Scholar]
- 59.Crow A.R., et al. A role for IL-1 receptor antagonist or other cytokines in the acute therapeutic effects of IVIG? Blood. 2006;109(1):155–158. doi: 10.1182/blood-2006-05-023796. [DOI] [PubMed] [Google Scholar]
- 60.Stangel M., Schumacher H.C., Ruprecht K., Boegner F., Marx P. Immunoglobulins for intravenous use inhibit TNFα cytotoxicity in vitro. Immunol Invest. 1997;26(5-7):569–578. doi: 10.3109/08820139709088541. [DOI] [PubMed] [Google Scholar]
- 61.Créange A., Gregson N.A., Hughes R.A.C. Intravenous immunoglobulin modulates lymphocyte CD54 and monocyte FcγRII expression in patients with chronic inflammatory neuropathies. J Neuroimmunol. 2003;135(1-2):91–95. doi: 10.1016/s0165-5728(02)00430-7. [DOI] [PubMed] [Google Scholar]
- 62.Lapointe B.M. IVIG therapy in brain inflammation: etiology-dependent differential effects on leucocyte recruitment. Brain. 2004;127(12):2649–2656. doi: 10.1093/brain/awh297. [DOI] [PubMed] [Google Scholar]
- 63.Kaneko Y. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 64.Nimmerjahn F., Anthony R.M., Ravetch J.V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci. 2007;104(20):8433–8437. doi: 10.1073/pnas.0702936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nimmerjahn F., Ravetch J.V. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26(1):513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 66.Ferrara, G., A. Zumla, and M. Maeurer, Intravenous immunoglobulin (IVIG) for refractory and difficult-to-treat infections. Am J Med, 2012. 125(10): p. 1036.e1-1036.e8. [DOI] [PubMed]
- 67.Bournazos S., Ravetch J.V. Diversification of IgG effector functions. Int Immunol. 2017;29(7):303–310. doi: 10.1093/intimm/dxx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vyas G.N. Inactivation and removal of blood-borne viruses. Transfusion. 1995;35(5):367–370. doi: 10.1046/j.1537-2995.1995.35595259143.x. [DOI] [PubMed] [Google Scholar]
- 69.Trejo S.R., Hotta J.A., Lebing W., Stenland C., Storms R.E., Lee D.C., et al. Evaluation of virus and prion reduction in a new intravenous immunoglobulin manufacturing process. Vox Sang. 2003;84(3):176–187. doi: 10.1046/j.1423-0410.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 70.Mofenson L.M. Prophylactic intravenous immunoglobulin in HIV-infected children with CD4+ counts of 0.20 x 10(9)/L or more. Effect on viral, opportunistic, and bacterial infections. The National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. JAMA: J Am Med Assoc. 1992;268(4):483–488. doi: 10.1001/jama.268.4.483. [DOI] [PubMed] [Google Scholar]
- 71.Spector S.A., Gelber R.D., McGrath N., Wara D., Barzilai A., Abrams E., et al. A Controlled trial of intravenous immune globulin for the prevention of serious bacterial infections in children receiving zidovudine for advanced human immunodeficiency virus infection. N Engl J Med. 1994;331(18):1181–1187. doi: 10.1056/NEJM199411033311802. [DOI] [PubMed] [Google Scholar]
- 72.Bruton O.C. Agammaglobulinemia. Pediatrics. 1952;9(6):722–728. [PubMed] [Google Scholar]
- 73.Chinen J., Shearer W.T. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2010;125(2):S195–S203. doi: 10.1016/j.jaci.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solomon C.G., Cooper N., Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945–955. doi: 10.1056/NEJMcp1810479. [DOI] [PubMed] [Google Scholar]
- 75.Lee K.-Y. Pneumonia, acute respiratory distress syndrome, and early immune-modulator therapy. Int J Mol Sci. 2017;18(2):388. doi: 10.3390/ijms18020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hemming V.G. Use of intravenous immunoglobulins for prophylaxis or treatment of infectious diseases. Clin Diagnostic Lab Immunol. 2001;8(5):859–863. doi: 10.1128/CDLI.8.5.859-863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davidoff L.M., Seegal B.C., Seegal D. The arthus phenomenon. J Exp Med. 1932;55(1):163–168. doi: 10.1084/jem.55.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stein M.R. The new generation of liquid intravenous immunoglobulin formulations in patient care: a comparison of intravenous immunoglobulins. Postgrad Med. 2010;122(5):176–184. doi: 10.3810/pgm.2010.09.2214. [DOI] [PubMed] [Google Scholar]
- 79.Gilardin L., Bayry J., Kaveri S.V. Intravenous immunoglobulin as clinical immune-modulating therapy. Can Med Assoc J. 2015;187(4):257–264. doi: 10.1503/cmaj.130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.WHO Coronavirus (COVID-19) Dashboard. (2021). Retrieved 27 March 2021, from https://covid19.who.int/.