Abstract
Worldwide, scientists are looking for specific treatment for COVID-19. Apart from the antiviral approach, the interventions to support healthy immune responses to the virus are feasible through diet, nutrition, and lifestyle approaches. This narrative review explores the recent studies on dietary, nutritional, and lifestyle interventions that influence the microbiota-mediated immunomodulatory effects against viral infections. Cumulative studies reported that the airway microbiota and SARS-CoV-2 leverage each other and determine the pathogen-microbiota-host responses. Cigarette smoking can disrupt microbiota abundance. The composition and diversification of intestinal microbiota influence the airway microbiota and the innate and adaptive immunity, which require supports from the balance of macro- and micronutrients from the diet. Colorful vegetables supplied fermentable prebiotics and anti-inflammatory, antioxidant phytonutrients. Fermented foods and beverages support intestinal microbiota. In sensitive individuals, the avoidance of the high immunoreactive food antigens contributes to antiviral immunity. This review suggests associations between airway and intestinal microbiota, antiviral host immunity, and the influences of dietary, nutritional, and lifestyle interventions to prevent the clinical course toward severe COVID-19.
Keywords: COVID-19, SARS-CoV-2, Microbiota, Gut-lung axis, Probiotics, Prebiotics
1. Introduction
Since its start in December 2019, the coronavirus disease 2019 (COVID-19) outbreak became a global pandemic in just three months due to the high infectivity and transmission of the causative virus—the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [[1], [2], [3]]. According to the WHO-China Joint Mission on Coronavirus Disease 2019, 78% of the infected individuals were asymptomatic [4,5]. 81%, 14%, and 5% of COVID-19 cases had mild, moderate, and severe clinical symptoms, respectively [6]. Individual host metabolic status, including diet, nutrition, lifestyle, and environmental factors, critically determine these different clinical manifestations of SARC-CoV-2 infection [[7], [8], [9], [10]].
The clinical events of COVID-19 include dry cough, fever, breathing difficulties, pneumonia, and respiratory failure [1]. The human airway epithelium is an entering site of SARS-CoV-2 [11]. The microbiome that inhabits the respiratory mucosa, from the upper to the lower respiratory tract, plays a crucial role in health and disease [[12], [13], [14], [15], [16], [17]]. Several studies suggested the mutual relationship between the microbiota to host immunity, respiratory health, viral infection, and the immune responses to viral vaccines [[18], [19], [20]]. The balance of microbiota in the gut, oral cavity, and lung influences the host's immune tolerance to viruses and the clinical severity and duration of the viral infections [20,21]. Alterations in the intestinal microbiome species and metabolites have been highlighted during respiratory viral infections, which may impact the lungs via microbiome-mediated cross-talk along the gut-lung axis [19]. Gut dysbiosis and related lung complications have also been observed during COVID-19 disease progression [[22], [23], [24], [25]]. Among many potential molecular targeted drugs and the newly arrived vaccines, diet and nutrition determine the microbiota's composition and diversity and thus affect host immunity. However, the discussion of these simple options is still limited, as though it is the elephant in the room [[26], [27], [28], [29], [30], [31]]. Herein, we review the dietary and nutritional interventions to maintain the healthy microbiome balances that potentially modulate the host immunity to COVID-19.
2. Microbiota and host immunity
As humans do not live in sterile bubbles, the hosts dynamically interact with their external environments, including foods, environmental toxins, and germs such as bacteria, parasites, fungi, and viruses [[32], [33], [34]]. Microbiota mediates these interactions' healthy balances throughout all surface linings, including skin, gut, lung, cornea, and vagina [19,[35], [36], [37]]. The gastrointestinal tract, particularly the colon, harbors the most populated microbiota in a human body: with the number of microbial cells and their genomes' count at ten and a hundred times more than those of the host, respectively [15].
The barrier linings' integrated functions, protective mucous, and antimicrobial peptides initially protect the internal host environment from microbial translocation [38]. However, the tissue-resident antigen-presenting cells - such as dendritic cells and macrophages- continuously sampling and processing the external microbial antigens and relaying the messages to the host immune cells [38]. These processed molecular patterns comprise the various signals of self, non-self, damaging, or dangerous signals: the pathogen- and the danger-associated molecular patterns [39]. The combined processing of these external signals determines the dynamic internal balance of pro-inflammatory and anti-inflammatory responses, the host tolerances to pathogens, and the composition and diversification of microbiota [38]. These interactions modulate the proliferation, differentiation, and maturation of the various immune cells [[40], [41], [42]].
Apart from those, several microbe metabolites, particularly the short-chain fatty acids (SCFAs), serve as the metabolic fuel for colon cells, the messengers, and the mediators of microbe-host immunity synchronization [13,[40], [41], [42]]. The fermentable dietary fibers and the resulting colonic microbiota compositions determine the SCFAs synthesis, which maintains the intestinal health, barrier functions, and lumen homeostasis: thus, protects the host from several disorders, such as inflammatory bowel disease [[43], [44], [45]]. Interestingly, influenza A infection disrupts these SCFAs production, creating the microbiota imbalances that contribute to the clinical symptoms [46].
3. The lungs and airways microbiota
Far from the historical concept of a sterile environment of lungs, the lung microbiota significantly contributes to the airway tolerance and immune responses to respiratory infection [15,47]. However, the lungs' microbial count is less compared to those in the colon [15]. The changes in microbial abundance and diversification in the upper and lower respiratory tracts correlate to the airway pathologies such as asthma and lung infections [48]. For example, the immunomodulatory Prevotella species promote lung homeostasis. The pro-inflammatory environment in the lung increases the outgrowth of several gram-negative bacteria, such as Gammaproteobacteria, through the nutrient enrichment processes: the mucous production and the enhanced vasculature, and the increased endothelial permeability [49].
The airway-associated lifestyle factors, such as cigarette smoking, significantly change the lung microbial composition toward the increased number of pro-inflammatory Proteobacteria and Firmicutes while decreasing the anti-inflammatory Oceanospirillales, Desulfuromonadales, Nesterenkonia, and Lactobacillaceae [50]. Interestingly, a recent systematic review suggested the associations between cigarette smoking and the negative clinical outcomes of COVID-19 [51]. However, recent studies agree that cigarette smoking increases significantly the risk of progressing and severe viral infection [8]. This finding suggests the close relationship between lifestyle, microbiota, and antiviral immunity.
4. The lung-intestinal microbiota connection
Animal studies reported the increased numbers and the gradual changes of lung microbiota composition during the first two-week of life [52]. These changes influenced the lung immune tolerance toward specific allergens by the increased number of regulatory T-cells [14]. Several studies revealed the association between the lung and intestinal microbiota [53,54]. The neomycin-induced microbiota imbalance, aka gut dysbiosis, increased the lung infection from influenza virus in the animal models through the inactivation of the inflammasome, dendritic cells, and T-cells responses [55]. During the S. pneumoniae-induced lung infection, the microbiota-depleted mice had higher inflammation, more organ damages, and fewer phagocytotic activities of the alveolar macrophages than their controls [56]. The healthy fecal microbiota transfer into the microbiota-depleted mice ameliorated their respiratory symptom severities and reduced the levels of pro-inflammatory cytokines [56]. Intestinal dysbiosis can predispose an individual to respiratory tract infection, including pneumonia and other respiratory conditions such as chronic obstructive pulmonary disease, cystic fibrosis, and lung cancer [57,58]. Despite their different locations, these two microbial communities are closely influencing and interacting with each other.
5. Microbiota and the protection against SARS-CoV-2 infections
Cumulative studies suggested that the microbiota is significantly involved in the host responses to many viral infections, either through the facilitation of viral attachment to host cells or the supports of antiviral immunity such as interferon and innate immune activation [[59], [60], [61]]. Specific evidence of microbiota and infection is starting to be available also for SARS-CoV-2 [[62], [63], [64], [65], [66]].
In the mice's lung, the commensal bacteria enhanced the antiviral activity of type I interferon, which was blunted by antibiotics [67]. While germ-free mice were prone to death from influenza infection, their intestinal microbiota's re-colonization promoted the anti-inflammatory polarization of macrophages [68]. Kanmani et al. reported that the colonization of a gram-positive bacteria, Corynebacterium pseudodiphtheriticum, in mice's nasal cavities reduced their symptoms of respiratory syncytial virus infections and prevented secondary pneumococcal pneumonia [69]. The mechanisms of these antiviral responses were the increased innate immune activation of toll-like receptor 3 signalings in the alveolar macrophages, the interferon signaling, and the T-cells activation [69]. Wu et al. also reported the intestinal microbiota-mediated activation of toll-like receptor 7 signalings in response to the respiratory influenza viral infection [70].
Apart from these mechanisms, the microbiota can exert antiviral effects through their metabolites, particularly the butyrate-an important SCFA. Trompetee et al. demonstrated that SCFAs enhanced the energy metabolism and the activities of effector T-cells and ameliorated influenza infection symptoms [71]. The seven-day pretreatment of SCFAs, including butyrate, restored the CD8+ T-cell functions, increased antibody responses, and prevented the influenza infection in mice [36,72]. Butyrate and propionate treatment in the mice model of asthma increased their production of the master transcription factor for anti-inflammatory regulatory T-cells, FOXP3, while suppressing the pro-inflammatory cytokines, interleukin 9, mast cell activation, and the lung infiltration with eosinophils and Th9 T-helper cells [73]. A study of 360 patients with immunosuppressive treatment during the allogeneic hematopoietic stem cell transplantation reported the five-fold associations between the abundance of butyrate-producing intestinal bacteria and the decreased chances of lower respiratory infection [74].
The intestinal and lung microbiota influence the host antiviral immunity by several immunomodulating mechanisms. The interventions that balance the microbiota composition and diversification, thus, potentially play roles in COVID-19.
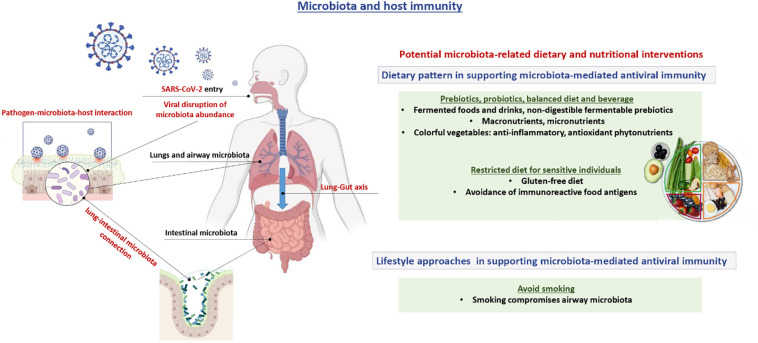
6. Potential microbiota-related dietary and nutritional interventions
6.1. General aspects
Healthy immunity requires integrating supports from various macro- and micronutrients derived from the individual dietary pattern. Specific nutrient deficiencies, such as vitamin D, zinc, and selenium, compromise host antiviral responses when the specific nutrient supplementation may be beneficial [7,10,[75], [76], [77], [78], [79], [80], [81], [82]]. In general, a balanced diet, adequate nutritional status, together with regular consumption of anti-inflammatory and antioxidant phytonutrients, from the colorful vegetables, can support the host responses to SARS-CoV-2 and reduce the severity, duration, and mortality associated with COVID-19 [83,84].
6.2. Prebiotics
Dietary prebiotics is the fermentable and non-digestible fiber compounds that feed the intestinal microbes and contribute to SCFAs production [85]. These prebiotics, including galactooligosaccharides, trans-galactooligosaccharides, and fructooligosaccharides, maintain the number of beneficial microbiota strains and sustain the production of beneficial SCFAs, therefore, potentially modulate the healthy host antiviral immunity. The fiber-rich diet protects mice from the severe pathological symptoms and the respiratory tract inflammation of the influenza virus infection [86]. Mice with a high-fiber diet and butyrate supplementation showed the reduction of chemokine expression, increasing circulating monocytes number, lowering neutrophil infiltration, and promoting T-cells bioenergetics, the reduction of lung inflammation, and enhancing the clearance of influenza virus [86].
Luoto et al. reported the incidence reduction in infants from the rhinovirus-induced respiratory tract infection with the combined usage of prebiotics and a probiotic, Lactobacillus rhamnosus GG [87]. The elders with the supplementation of an experimental formula containing the fermentable oligosaccharides, vitamins, and other antioxidants, showed shorter duration of symptoms to the influenza vaccine, higher antibody titers, and higher lymphocyte proliferation than their controls [88].
A recent study reported that COVID-19 patients showed intestinal dysbiosis and reduced Lactobacillus and Bifidobacterium [89]. Despite a few pieces of supporting evidence, prebiotics is a potential immunomodulatory strategy for COVID-19. However, the regular consumption of colorful vegetables also provides the non-digestible fibers for the prebiotics function and the phytonutrients that provide the anti-inflammatory and antioxidant effects. This dietary pattern can support microbiota-mediated antiviral immunity [89].
6.3. Probiotics
Probiotics are the pre-determined specific strains of beneficial microbiota that may mediate the intestinal microbiome balance and diversification, thus, augment the host immunity against pathogens, including the viral infection [90]. The combined usage of multivitamin and mineral, together with the probiotic strains of Lactobacillus and Bifidobacterium, reduces the duration and the severity of influenza-induced common cold while increasing the number of T-helper cells [85,91]. The administration of the probiotics, Lactobacillus Plantarum DK119 or the Lactobacillus casei DN-114001, protects the host against influenza infection and reduces the duration of common infectious diseases. These relevant mechanisms are the increased production of interleukin 12 and interferon γ, the modulation of macrophages and dendritic cells' activities, and the lowering of host inflammatory responses [[92], [93], [94]].
Therefore, probiotics are the sensible immunomodulatory option for COVID-19. However, the cumulating evidence is premature to conclude the role of specific probiotic strain in therapeutic management [89,95,96].
6.4. Fermented foods and drinks
Despite the limited number of available published studies, an omics-based study reported an association between fermented food consumption and differences in beneficial microbiota [97]. Fermentation processes break down the sugars in foods by bacteria and yeasts. Fermented foods and drinks contain live bacteria and prebiotic fibers. The fermentation of dairy products yields kefir, yogurt, and cottage cheese. Other fermented foods and beverages include fermented vegetables, tempeh, miso, pickles, sauerkraut, kimchi, kombucha, and other drinks such as beet kvass and apple cider. Consumption of these fermented foods is potentially beneficial for the microbiota and host metabolic health [[97], [98], [99]].
Kefir is an inexpensive, homemade, fermented milk drink that modulates the host immunity, reduces the chances of viral and bacterial infections, and benefits many host metabolic conditions [100,101]. The fermentation process of kefir involves the symbiotic activities of lactic acid bacteria and yeast [100,102]. The cell line studies reported the effects of kefir on reducing T-cell proliferation and cytopathic effects of Zika virus exposure [103]. Novel kefir that contains Lactobacillus acted as a natural adjuvant of dendritic cells to enhance the secretion of several cytokines, augmenting the activities of cytotoxic T-cells and acting against the viral infection [84,104].
6.5. Restricted diet
Several studies reported that intestinal microbiota is among other host predisposing factors that influence the food sensitivities through the mechanisms of food antigen degradation, gut barrier integrity, and anti-inflammatory regulatory T cell promotion [105]. The virus-host interactions disrupt these homeostases and induce the pathogenic responses to food antigens [105,106]. This concern is particularly crucial in an individual with a known history of food sensitivities or allergy during the SARS-CoV-2 pandemics.
Apart from those interventions for the healthy microbiota balance, the dietary avoidance of foods that contain high immunoreactive antigens contributes to host antiviral potentials. Gluten sensitivity underlies the development of autoimmune celiac disease [107]. The virus-induced interferon γ responses aggravate celiac disease progression during several virus infections, including adenovirus, enterovirus, rotavirus, and hepatitis C virus [[108], [109], [110], [111]]. Interestingly, a non-virulent species – reovirus– interacts with gluten and initiates the immune responses that lead to celiac disease progression [106].
With these pieces of evidence, an individual with known food sensitivities, allergy, or risk for autoimmune conditions, may consider the restricted diet to promote host tolerance during the outbreak of COVID-19.
7. Conclusion
Scientists are looking for specific treatment for COVID-19 to date. Apart from the antiviral approach, the interventions to support the healthy immune responses to the virus are feasible through diet, nutrition, and lifestyle approaches. As illustrated in Fig. 1 , airway microbiota and SARS-CoV-2 influence each other and determine the host immune responses. Viral infection can disrupt microbiota abundance; however, the intestinal microbiota composition and diversification influence airway microbiota and its immunity. Smoking compromises airway microbiota. Healthy immunity requires the balance of macro- and micronutrients from the diet. Colorful vegetables supplied fermentable prebiotics and anti-inflammatory, antioxidant phytonutrients. Fermented foods and beverages support intestinal microbiota. In the sensitive individual, avoiding the high immunoreactive food antigens contribute to antiviral immunity. These dietary and nutritional interventions can prevent the clinical course toward severe COVID-19.
Fig. 1.
The interrelationship between SARS-CoV-2 and airway microbiota determines the host immune responses. Intestinal microbiota composition and diversification influence airway microbiota, innate, and adaptive immunity. Viral infection disrupts microbiota abundance and the relevant host responses. Smoking compromises airway microbiota and its antiviral responses. Healthy immunity requires the balance of macro- and micronutrients from the diet. Colorful vegetables supplied fermentable prebiotics and anti-inflammatory, antioxidant phytonutrients. Fermented foods and beverages support intestinal microbiota. In the sensitive individual, avoiding the high immunoreactive food antigens contribute to antiviral immunity and prevents the clinical course toward severe COVID-19.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare they have no actual or potential competing financial interests.
References
- 1.Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents. 2020;55:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singhal T. A review of coronavirus Disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y., Shang W., Rao X. Facing the COVID-19 outbreak: what should we know and what could we do? J. Med. Virol. 2020;92:536–537. doi: 10.1002/jmv.25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369:m1375. doi: 10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) p. 40. [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Gasmi A., Noor S., Tippairote T., Dadar M., Menzel A., Bjørklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020;108409 doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasmi A., Peana M., Pivina L., Srinath S., Gasmi Benahmed A., Semenova Y., Menzel A., Dadar M., Bjorklund G. Interrelations between COVID-19 and other disorders. Clin. Immunol. 2021;224:108651. doi: 10.1016/j.clim.2020.108651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tippairote T., Peana M., Chirumbolo S., Bjørklund G. Individual risk management strategy for SARS-CoV2 infection: a step toward personalized healthcare. Int. Immunopharmacol. 2021;107629 doi: 10.1016/j.intimp.2021.107629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasmi A., Chirumbolo S., Peana M., Noor S., Menzel A., Dadar M., Bjorklund G. The role of diet and supplementation of natural products in COVID-19 prevention. Biol. Trace Elem. Res. 2021 doi: 10.1007/s12011-021-02623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin Immunother. 2020:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Hooper L.V. Immune control of the microbiota prevents obesity. Science. 2019;365:316–317. doi: 10.1126/science.aay2057. [DOI] [PubMed] [Google Scholar]
- 13.Levy M., Thaiss C.A., Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Dwyer D.N., Dickson R.P., Moore B.B. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J. Immunol. 2016;196:4839–4847. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu E., Escribano-Vazquez U., Descamps D., Cherbuy C., Langella P., Riffault S., Remot A., Thomas M. Paradigms of lung microbiota functions in health and disease, particularly, in asthma. Front. Physiol. 2018;9:1168. doi: 10.3389/fphys.2018.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamont R.J., Koo H., Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao L., Xu T., Huang G., Jiang S., Gu Y., Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9:488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlasova A.N., Takanashi S., Miyazaki A., Rajashekara G., Saif L.J. How the gut microbiome regulates host immune responses to viral vaccines. Curr. Opin. Virol. 2019;37:16–25. doi: 10.1016/j.coviro.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019 doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 20.Domínguez-Díaz C., García-Orozco A., Riera-Leal A., Padilla-Arellano J.R., Fafutis-Morris M. Microbiota and its role on viral evasion: is it with us or against us? Front. Cell. Infect. Microbiol. 2019;9:256. doi: 10.3389/fcimb.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang A.T., Marsland B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019;12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 22.Haiminen N., Utro F., Seabolt E., Parida L. Functional profiling of COVID-19 respiratory tract microbiomes. Sci. Rep. 2021;11:6433. doi: 10.1038/s41598-021-85750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stavropoulou E., Kantartzi K., Tsigalou C., Konstantinidis T., Voidarou C., Bezirtzoglou E. Unraveling the interconnection patterns across lung microbiome, respiratory diseases, and COVID-19. Front. Cell. Infect. Microbiol. 2020;10:619075. doi: 10.3389/fcimb.2020.619075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu R., Lu R., Zhang T., Wu Q., Cai W., Han X., Wan Z., Jin X., Zhang Z., Zhang C. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun. Biol. 2021;4:240. doi: 10.1038/s42003-021-01796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl. Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinninella E., Cintoni M., Raoul P., Lopetuso L.R., Scaldaferri F., Pulcini G., Miggiano G.A.D., Gasbarrini A., Mele M.C. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11:2393. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimidi E., Cox S.R., Rossi M., Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11:1806. doi: 10.3390/nu11081806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klingbeil E.A., Serre C.B.D.L. Microbiota modulation by eating patterns, dietary and macronutrient composition; impact on food intake. Am. J. Phys. Regul. Integr. Comp. Phys. 2018;315:R1254–R1260. doi: 10.1152/ajpregu.00037.2018. [DOI] [PubMed] [Google Scholar]
- 29.Pereira J.D.C., Rea K., Nolan Y.M., O'Leary O.F., Dinan T.G., Cryan J.F. Depression's unholy trinity: dysregulated stress, immunity, and the microbiome. Annu. Rev. Psychol. 2020;71:49–78. doi: 10.1146/annurev-psych-122216-011613. [DOI] [PubMed] [Google Scholar]
- 30.Rea K., Dinan T.G., Cryan John F. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol. Stress. 2016:1–11. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mailing L.J., Allen J.M., Buford T.W., Fields C.J., Woods J.A. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport Sci. Rev. 2019;47:75–85. doi: 10.1249/jes.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 32.Perdijk O., Marsland B.J. The microbiome: toward preventing allergies and asthma by nutritional intervention. Curr. Opin. Immunol. 2019;60:10–18. doi: 10.1016/j.coi.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 33.McCall L.-I., Callewaert C., Zhu Q., Song S.J., Bouslimani A., Minich J.J., Ernst M., Ruiz-Calderon J.F., Cavallin H., Pereira H.S., Novoselac A., Hernandez J., Rios R., Branch O.H., Blaser M.J., Paulino L.C., Dorrestein P.C., Knight R., Dominguez-Bello M.G. Home chemical and microbial transitions across urbanization. Nat. Microbiol. 2020;5:108–115. doi: 10.1038/s41564-019-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 35.Buchta V. Vaginal microbiome. Ceska Gynekol. 2018;83:371–379. [PubMed] [Google Scholar]
- 36.Lyon J. Even the eye has a microbiome. JAMA. 2017;318:689. doi: 10.1001/jama.2017.10599. [DOI] [PubMed] [Google Scholar]
- 37.Byrd A.L., Belkaid Y., Segre J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018;16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 38.Belkaid Y., Timothy W. Hand, role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herwald H., Egesten A. On PAMPs and DAMPs. J. Innate Immun. 2016;8:427–428. doi: 10.1159/000448437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Reddy D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kałużna-Czaplińska J., Gątarek P., Chartrand M.S., Dadar M., Bjørklund G. Is there a relationship between intestinal microbiota, dietary compounds, and obesity? Trends Food Sci. Technol. 2017;70:105–113. doi: 10.1016/j.tifs.2017.10.010. [DOI] [Google Scholar]
- 43.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venegas D.P., Marjorie K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sencio V., Barthelemy A., Tavares L.P., Machado M.G., Soulard D., Cuinat C., Queiroz-Junior C.M., Noordine M.-L., Salomé-Desnoulez S., Deryuter L. Gut Dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 2020;30:2934–2947. doi: 10.1016/j.celrep.2020.02.013. e2936. [DOI] [PubMed] [Google Scholar]
- 47.Dickson R.P., Erb-Downward J.R., Martinez F.J., Huffnagle G.B. The microbiome and the respiratory tract. Annu. Rev. Physiol. 2016;78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch S.V. The lung microbiome and airway disease. Ann. Am. Thorac. Soc. 2016;13:S462–S465. doi: 10.1513/AnnalsATS.201605-356AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huffnagle G., Dickson R., Lukacs N. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10:299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li K.-j., Chen Z.-l., Huang Y., Zhang R., Luan X.-q., Lei T.-t., Chen L. Dysbiosis of lower respiratory tract microbiome are associated with inflammation and microbial function variety. Respir. Res. 2019;20:1–16. doi: 10.1186/s12931-019-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob. Induc. Dis. 2020;18(20):1–4. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ubags N.D., Marsland B.J. Mechanistic insight into the function of the microbiome in lung diseases. Eur. Respir. J. 2017;50:1602467. doi: 10.1183/13993003.02467-2016. [DOI] [PubMed] [Google Scholar]
- 53.Marsland B.J., Trompette A., Gollwitzer E.S. The gut-lung Axis in respiratory disease. Ann. Am. Thorac. Soc. 2015;12:S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 54.Budden K.F., Gellatly S.L., Wood D.L., Cooper M.A., Morrison M., Hugenholtz P., Hansbro P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 55.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S., Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuijt T.J., Lankelma J.M., Scicluna B.P., Melo F.D.S.E., Roelofs J.J., de Boer J.D., Hoogendijk A.J., de Beer R., de Vos A., Belzer C. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gollwitzer E.S., Saglani S., Trompette A., Yadava K., Sherburn R., McCoy K.D., Nicod L.P., Lloyd C.M., Marsland B.J. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014;20:642. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 58.Zhang D., Li S., Wang N., Tan H.-Y., Zhang Z., Feng Y. The cross-talk between gut microbiota and lungs in common lung diseases. Front. Microbiol. 2020;11:301. doi: 10.3389/fmicb.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilks J., Golovkina T. Influence of microbiota on viral infections. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monedero V., Collado M.C., Rodríguez-Díaz J. Therapeutic opportunities in intestinal microbiota–virus interactions. Trends Biotechnol. 2018;36:645–648. doi: 10.1016/j.tibtech.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Garg R.R., Karst S.M. In: Viral Gastroenteritis. Svensson L., Desselberger U., Greenberg H.B., Estes M.K., editors. Academic Press; Boston: 2016. Chapter 5.2 - interactions between enteric viruses and the gut microbiota; pp. 535–544. [Google Scholar]
- 62.Vignesh R., Swathirajan C.R., Tun Z.H., Rameshkumar M.R., Solomon S.S., Balakrishnan P. Could perturbation of gut microbiota possibly exacerbate the severity of COVID-19 via cytokine storm? Front. Immunol. 2020;11:607734. doi: 10.3389/fimmu.2020.607734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venema K. Foreword - the importance of a healthy microbiota in the era of COVID-19. Benefic. Microbes. 2021;12:1–3. doi: 10.3920/BM2021.x001. [DOI] [PubMed] [Google Scholar]
- 64.Luo J., Liang S., Jin F. Gut microbiota in antiviral strategy from bats to humans: a missing link in COVID-19. Sci. China Life Sci. 2021 doi: 10.1007/s11427-020-1847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Littlejohn P., Finlay B.B. When a pandemic and an epidemic collide: COVID-19, gut microbiota, and the double burden of malnutrition. BMC Med. 2021;19:31. doi: 10.1186/s12916-021-01910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H.S. Do an altered gut microbiota and an associated leaky gut affect COVID-19 severity? mBio. 2021;12 doi: 10.1128/mBio.03022-20. e03022–03020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradley K.C., Finsterbusch K., Schnepf D., Crotta S., Llorian M., Davidson S., Fuchs S.Y., Staeheli P., Wack A. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28:245–256. doi: 10.1016/j.celrep.2019.05.105. e244. [DOI] [PubMed] [Google Scholar]
- 68.Wang J., Li F., Sun R., Gao X., Wei H., Li L.-J., Tian Z. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat. Commun. 2013;4:1–10. doi: 10.1038/ncomms3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanmani P., Clua P., Vizoso-Pinto M.G., Rodriguez C., Alvarez S., Melnikov V., Takahashi H., Kitazawa H., Villena J. Respiratory commensal bacteria Corynebacterium pseudodiphtheriticum improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front. Microbiol. 2017;8:1613. doi: 10.3389/fmicb.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu S., Jiang Z.-Y., Sun Y.-F., Yu B., Chen J., Dai C.-Q., Wu X.-L., Tang X.-L., Chen X.-Y. Microbiota regulates the TLR7 signaling pathway against respiratory tract influenza A virus infection. Curr. Microbiol. 2013;67:414–422. doi: 10.1007/s00284-013-0380-z. [DOI] [PubMed] [Google Scholar]
- 71.Trompette A., Gollwitzer E.S., Pattaroni C., Lopez-Mejia I.C., Riva E., Pernot J., Ubags N., Fajas L., Nicod L.P., Marsland B.J. Dietary fiber confers protection against flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity. 2018;48:992–1005. doi: 10.1016/j.immuni.2018.04.022. e1008. [DOI] [PubMed] [Google Scholar]
- 72.Moriyama M., Ichinohe T. High ambient temperature dampens adaptive immune responses to influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 2019;116:3118–3125. doi: 10.1073/pnas.1815029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vieira R.D.S., Castoldi A., Basso P.J., Hiyane M.I., Câmara N.O.S., Almeida R.R. Butyrate attenuates lung inflammation by negatively modulating Th9 cells. Front. Immunol. 2019;10:67. doi: 10.3389/fimmu.2019.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haak B.W., Littmann E.R., Chaubard J.-L., Pickard A.J., Fontana E., Adhi F., Gyaltshen Y., Ling L., Morjaria S.M., Peled J.U. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131:2978–2986. doi: 10.1182/blood-2018-01-828996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim H., Jang M., Kim Y., Choi J., Jeon J., Kim J., Hwang Y.I., Kang J.S., Lee W.J. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J. Pharm. Pharmacol. 2016;68:406–420. doi: 10.1111/jphp.12529. [DOI] [PubMed] [Google Scholar]
- 76.Hemilä H. Vitamin C and SARS coronavirus. J. Antimicrob. Chemother. 2003;52:1049–1050. doi: 10.1093/jac/dkh002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hansdottir S., Monick M.M., Lovan N., Powers L., Gerke A., Hunninghake G.W. Vitamin D decreases respiratory syncytial virus induction of NF-κB–linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hughes D., Norton R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009;158:20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prasad A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008;43:370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 80.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y., Lin Z., Guo M., Xia Y., Zhao M., Wang C., Xu T., Chen T., Zhu B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomedicine. 2017;12:5733. doi: 10.2147/IJN.S140939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gasmi A., Tippairote T., Mujawdiya P.K., Peana M., Menzel A., Dadar M., Gasmi Benahmed A., Bjorklund G. Micronutrients as immunomodulatory tools for COVID-19 management. Clin. Immunol. 2020;220:108545. doi: 10.1016/j.clim.2020.108545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin S.-C., Ho C.-T., Chuo W.-H., Li S., Wang T.T., Lin C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davani-Davari D., Negahdaripour M., Karimzadeh I., Seifan M., Mohkam M., Masoumi S.J., Berenjian A., Ghasemi Y. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Visan I. Fiber against flu. Nat. Immunol. 2018;19:647. doi: 10.1038/s41590-018-0147-6. [DOI] [PubMed] [Google Scholar]
- 87.Luoto R., Ruuskanen O., Waris M., Kalliomäki M., Salminen S., Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2014;133:405–413. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Langkamp-Henken B., Bender B.S., Gardner E.M., Herrlinger-garcia K.A., Kelley M.J., Murasko D.M., Schaller J.P., Stechmiller J.K., Thomas D.J., Wood S.M. Nutritional formula enhanced immune function and reduced days of symptoms of upper respiratory tract infection in seniors. J. Am. Geriatr. Soc. 2004;52:3–12. doi: 10.1093/jac/dkh002. [DOI] [PubMed] [Google Scholar]
- 89.Olaimat A.N., Aolymat I., Al-Holy M., Ayyash M., Abu Ghoush M., Al-Nabulsi A.A., Osaili T., Apostolopoulos V., Liu S.Q., Shah N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci. Food. 2020;4:17. doi: 10.1038/s41538-020-00078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang H., Yeh C., Jin Z., Ding L., Liu B.Y., Zhang L., Dannelly H.K. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth. Syst. Biotechnol. 2018;3:113–120. doi: 10.1016/j.synbio.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Vrese M., Winkler P., Rautenberg P., Harder T., Noah C., Laue C., Ott S., Hampe J., Schreiber S., Heller K. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine. 2006;24:6670–6674. doi: 10.1016/j.vaccine.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 92.Park M.-K., Vu N., Kwon Y.-M., Lee Y.-T., Yoo S., Cho Y.-H., Hong S.-M., Hwang H.S., Ko E.-J., Jung Y.-J. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rocha-Ramírez L.M., Hernández-Ochoa B., Gómez-Manzo S., Marcial-Quino J., Cárdenas-Rodríguez N., Centeno-Leija S., García-Garibay M. Evaluation of immunomodulatory activities of the heat-killed probiotic strain lactobacillus casei IMAU60214 on macrophages in vitro. Microorganisms. 2020;8:79. doi: 10.3390/microorganisms8010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guillemard E., Tondu F., Lacoin F., Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic lactobacillus casei DN-114 001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2010;103:58–68. doi: 10.1017/S0007114509991395. [DOI] [PubMed] [Google Scholar]
- 95.Hu J., Zhang L., Lin W., Tang W., Chan F.K.L., Ng S.C. Review article: probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021;108:187–196. doi: 10.1016/j.tifs.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Antunes A.E.C., Vinderola G., Xavier-Santos D., Sivieri K. Potential contribution of beneficial microbes to face the COVID-19 pandemic. Food Res. Int. 2020;136:109577. doi: 10.1016/j.foodres.2020.109577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taylor B.C., Lejzerowicz F., Poirel M., Shaffer J.P., Jiang L., Aksenov A., Litwin N., Humphrey G., Martino C., Miller-Montgomery S., Dorrestein P.C., Veiga P., Song S.J., McDonald D., Derrien M., Knight R. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems. 2020;5 doi: 10.1128/mSystems.00901-19. e00901–00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan M., Baxter H., Larsen N., Jespersen L., Ekinci E.I., Howell K. Impact of botanical fermented foods on metabolic biomarkers and gut microbiota in adults with metabolic syndrome and type 2 diabetes: a systematic review protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bell V., Ferrão J., Pimentel L., Pintado M., Fernandes T. One health, fermented foods, and gut microbiota. Foods (Basel, Switzerland) 2018;7:195. doi: 10.3390/foods7120195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bourrie B.C., Willing B.P., Cotter P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016;7:647. doi: 10.3389/fmicb.2016.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walsh A.M., Crispie F., Kilcawley K., O’Sullivan O., O’Sullivan M.G., Claesson M.J., Cotter P.D. Microbial succession and flavor production in the fermented dairy beverage kefir. mSystems. 2016;1 doi: 10.1128/mSystems.00052-16. e00052–00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosa D., Dias M., Grześkowiak Ł., Reis S., Conceição L., MDCG P. Milk kefir: nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017;30:82–96. doi: 10.1017/S0954422416000275. [DOI] [PubMed] [Google Scholar]
- 103.de Andrade G.R., Neves I.V., Marques V.D., Borges M.M., Broring T.A.M., dos Anjos M.T., Astray R.M., de Oliveira M. Influence of a Kefir-derived antimicrobial fraction on Zika virus cytopathic effects and lymphocyte proliferation. J. Virol. Curr. Res. 2017;2:555584. doi: 10.19080/004JOJIV.2017.02.555584. [DOI] [Google Scholar]
- 104.Ghoneum M., Felo N., Agrawal S., Agrawal A. A novel kefir product (PFT) activates dendritic cells to induce CD4+ T and CD8+ T cell responses in vitro. Int. J. Immunopathol. Pharmacol. 2015;28:488–496. doi: 10.1177/0394632015599447. [DOI] [PubMed] [Google Scholar]
- 105.Caminero A., Meisel M., Jabri B., Verdu E.F. Mechanisms by which gut microorganisms influence food sensitivities. Nat. Rev. Gastroenterol. Hepatol. 2019;16:7–18. doi: 10.1038/s41575-018-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bouziat R., Hinterleitner R., Brown J.J., Stencel-Baerenwald J.E., Ikizler M., Mayassi T., Meisel M., Kim S.M., Discepolo V., Pruijssers A.J., Ernest J.D., Iskarpatyoti J.A., Costes L.M., Lawrence I., Palanski B.A., Varma M., Zurenski M.A., Khomandiak S., McAllister N., Aravamudhan P., Boehme K.W., Hu F., Samsom J.N., Reinecker H.C., Kupfer S.S., Guandalini S., Semrad C.E., Abadie V., Khosla C., Barreiro L.B., Xavier R.J., Ng A., Dermody T.S., Jabri B. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356:44–50. doi: 10.1126/science.aah5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tye-Din J.A., Galipeau H.J., Agardh D. Celiac disease: a review of current concepts in pathogenesis, prevention, and novel therapies. Front. Pediatr. 2018;6:350. doi: 10.3389/fped.2018.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown J.J., Jabri B., Dermody T.S. A viral trigger for celiac disease. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Plot L., Amital H. Infectious associations of celiac disease. Autoimmun. Rev. 2009;8:316–319. doi: 10.1016/j.autrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 110.Stene L.C., Honeyman M.C., Hoffenberg E.J., Haas J.E., Sokol R.J., Emery L., Taki I., Norris J.M., Erlich H.A., Eisenbarth G.S. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am. J. Gastroenterol. 2006;101:2333–2340. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 111.Beck M.A. In: Selenium: Its Molecular Biology and Role in Human Health. Hatfield D.L., editor. Springer US; Boston, MA: 2001. Selenium as an antiviral agent; pp. 235–245. [Google Scholar]