Abstract
The prevalence of HIV among young Thai men stabilized at 0.5% from 2005 to 2011. A cross-sectional study was conducted among the male army conscripts in 2018 at 36 military training units nationwide. All new conscripts in each selected unit were invited to participate in the study. Questionnaires were used to determine risk factors to HIV infection that had been developed from related risk factors studies among young Thai men. Among 4629 participants, 44 (1.0%) HIV positive individuals were identified. The proportion subject reporting a history of sex with another man was 10.1%. The prevalence of HIV infection among men who have sex with men (MSM) was 4.0%. The proportion of consistent condom use with a male partner was 39.7%. The risk factors of HIV infection included having sex with another man, history of sexually transmitted infection and history of sex in exchange for gifts/money. Only 1.4% of MSM used pre-exposure prophylaxis (PrEP). HIV prevention programs including PrEP in Thailand should be emphasized among MSM in both rural and urban settings.
Subject terms: HIV infections, Risk factors, Epidemiology
Introduction
According to the UNAIDS DATA 2020, at the end of 2019, approximately 470,000 adults were living with HIV and more than one half were men. In the same year, a total of 5400 new adult HIV cases were identified and more than two thirds comprised men1. The surveillance among newly recruited conscripts has long provided significant information on the status of the epidemic and the behavioral data provide evaluation of existing measures as well as a reference for relevant and up to date preventive measures2.
In 2015, a national behavioral risk factor study was conducted among a sample of young Thai men aged from 17 to 29 years that were inducted in the Royal Thai Army (RTA) during the induction rounds from November 2005 until May 20093. This was followed in May 2011 by similar results2. The prevalence of HIV infection among young Thai men was 0.5% between 2005 and 20112,3. Additionally, sexual behaviors including men who have sex with other men (MSM) were significant risk factors for HIV infection among young Thai men3.
Similarly, the recent report in the US demonstrated that the national HIV prevalence among MSM had a 4.5-fold increase in the past century4. The related study also reported that the prevalence of HIV among MSM in several countries was higher than the prevalence of that among men5. Therefore, HIV transmission among MSM is considered to be an important driver of the global HIV epidemic.
In 2017, the National AIDS Prevention and Alleviation Committee reported that more than 50% of new cases occurred among those aged 15–24 years and 40% were MSM. Sexual transmission remained the primary mode of transmission of which 41.8% were between MSM, 31.3% among married heterosexual couples and 13.3% among nonregular partners6.
The current study aimed to determine prevalence of HIV infection and risk behaviors among the sample of newly inducted army conscripts who entered military service in November 2018 to examine the current epidemiology among young Thai men. The information from a sample of young men in Thailand provided useful information in monitoring the epidemic and implementing effective preventive measures.
Methods
Study participants
The study population was enrolled as previously described2,7. The young Thai men aged 21 years, selected by a lottery system, rendered military service to the RTA. The conscription was conducted annually in April, at the district level of their home province. A small subset of men, found incapable of rendering military service, were excluded including those with severe sickness, transgender women and individuals who participated in alternative military service including the Thai Reserve Officer Training Corps Student program7. The exemption process does not exclude young men with asymptomatic HIV or exclude individuals based on their sexual orientation or drug use. Legal sanctions and penalties are given to those who do not participate in the selection process or do not render military service without a valid exception. Since 2001, men both younger and older than 21 years were accepted to volunteer without going through the lottery system. These men enter military service either in May or November3. All new RTA conscripts were voluntarily invited to participate in the national HIV surveillance program. This includes serological testing for HIV and a short demographics questionnaire that does not include any behavioral risk factor questions3,7.
Data collection
In Thailand, 37 RTA hospitals are located in five regions i.e., north, central, south, northeast, and Bangkok nationwide. Each RTA hospital provided medical care for the responsible basic military training units in the area. The basic military training units were selected using stratified cluster sampling method. A cluster of basic military training units under the supervision of one RTA hospital in each region was selected. Of 323 basic military training units nationwide, 36 basic military training units located in five provinces of five regions; nine units in Chiang Mai (North), seven units in Lopburi (Central), nine units in Songkhla (South), six units in Ubon Ratchathani (Northeast) and five units in Bangkok were selected to be the study sites. All new conscripts in each selected unit were invited to participate in the study. After providing written informed consent, participants were given a self-administered standardized questionnaire that included demographic characteristics and behavioral risk factors for HIV infection. This questionnaire was deployed before the release of the HIV test result to minimize response bias. Anonymity was ensured by using unique codes that could only be decoded by the respective data management personnel of the project. The questionnaires were used to determine risk factors to HIV infection that had been developed from related risk factors studies among young Thai men7. Men having sex with men status was defined as the lifetime sexual activity of man having sex with another man and not as the identity expressed by the person.
Statistical analysis
The individual responses to the self-administered risk factor survey were encoded to a computer- based program with their HIV sero-status added at the end of the process. Data were analyzed using IBM SPSS Statistics for Windows, Version 23.0. Mean and standard deviation were computed to describe continuous data while percentage was used to describe categorical data. Chi-square or Fisher’s exact test was calculated to determine the association of the categorical data while independent t-test was calculated to determine associations of the continuous variables. The odds ratios (OR) and 95% confidence intervals (CI) of demographic and behavioral variables associated with HIV seroprevalence were evaluated using univariate analysis. A multivariate logistic regression model was used to determine the independent effects of significant risk factors for HIV infection. Statistical significance was determined using a p-value less than 0.05.
Ethics consideration
The study protocol was approved by the Institutional Review Boards of the Royal Thai Army Medical Department and the Ethics Subcommittee of Thammasat University (approval number: S009q/61). Written informed consent was obtained from the participants after they read the information sheet and signed the consent form. Identified HIV positive conscripts were given post-test counseling and necessary treatment was provided following Thai national guidelines.
Results
Demographic characteristics
A total of 38,779 conscripts were inducted in November 2018 nationwide. Of these, 38,255 (98.6%) participated in the national surveillance program for HIV. Of 4786 conscripts from the selected 36 basic military training units in five provinces, 4629 (96.72%) participants enrolled in the present study. The demographics and behavioral profiles of the enrolled participants before conscription are presented in Table 1. The average age of the participants was 21.6 ± 1.2 years. In all, 1284 (28.8%) men came from the northern region, 1314 (29.4%) came from the northeastern region, 564 (12.6%) came from the central region, 979 (21.9%) came from the southern region, and 324 (7.3%) came from Bangkok. Of all participants, 58.2% of young men resided in an urban area two years before conscription. The participants obtained bachelor’s degree or higher accounted for 10.7%. In all, 10.1% (95%CI; 9.1–11.0%) of individuals reported history of sex with another man. In terms of sexual preference or sexual desire, 97.6% preferred to have sex with females only while 1.3% preferred to have sex with exclusively with males and 1.1% preferred to have sex with both males and females. Among young men, 5.5% of men knew PrEP and only 0.4% used PrEP. The proportion of history of previous HIV testing among participants was 25.6%. The proportion of consistent condom use with a FSW was 83.7, while that with a male partner was 39.7%.
Table 1.
Demographics and behavioral profiles of the participants before induction.
| Characteristics (n = 4629) | n (%) |
|---|---|
| Age (years) | |
| Mean ± SD | 21.6 ± 1.2 |
| Living pattern | |
| With parents | 3366 (75.1) |
| With lover/wife | 673 (15.0) |
| With relatives and friends | 339 (7.5) |
| Alone | 103 (2.3) |
| Region of residence 2 years before induction | |
| North | 1284 (28.8) |
| Northeast | 1314 (29.4) |
| Central | 564 (12.6) |
| South | 979 (21.9) |
| Bangkok | 324 (7.3) |
| Place of residence | |
| Urban | 1896 (58.2) |
| Rural | 1361 (41.8) |
| Occupation | |
| Employee | 2195 (54.6) |
| Student | 628 (15.6) |
| Agriculture | 609 (15.2) |
| Unemployed | 587 (14.6) |
| Marital status | |
| Single | 3403 (75.0) |
| Married | 1137 (25.0) |
| Educational attainment | |
| No formal to grade 6 | 906 (19.8) |
| Grade 7 to grade 12 | 2165 (47.3) |
| Vocational and diploma | 1104 (22.2) |
| Bachelor’s degree or higher | 492 (10.7) |
| History of injecting drug use | 220 (5.1) |
| History of non-injecting drug use | 1996 (44.8) |
| History of previous HIV testing (lifetime) | 986 (25.6) |
| History of blood transfusion | 228 (5.0) |
| History of circumcision | 625 (14.1) |
| History of sexual intercourse | 4169 (91.0) |
| Average age of first sex (years) | 16.6 ± 2.1 |
| History of sex with a female sex worker | 889 (21.4) |
| Number of lifetime sexual partner | |
| 0–1 | 1035 (23.6) |
| 2–5 | 1504 (34.3) |
| ≥ 6 | 1847 (42.1) |
| Median (min–max) | 5 (0–78) |
| History of sex with another man | 429 (10.1) |
| Sexual experience (lifetime) | |
| Exclusively heterosexual | 3660 (89.6) |
| Bisexual | 361 (8.8) |
| Exclusively homosexual | 66 (1.6) |
| Sexual preference/desire | |
| Female only | 4455 (97.6) |
| Male only | 58 (1.3) |
| Both male and female | 50 (1.1) |
| History of sexually transmitted infection | 231 (5.7) |
| History of sex in exchange for gifts/money | 231 (5.4) |
| History of sexual coercion | 162 (3.8) |
| Knows about PrEP | 251 (5.5) |
| Uses PrEP | 16 (0.4) |
| Knows about PEP | 155 (3.4) |
| Uses PEP | 23 (0.5) |
| History of consistent condom use (n/N) | |
| With female sex workers | 340/406 (83.7) |
| With a male partner | 94/237 (39.7) |
| HIV infected (cases) | 44 (0.95) |
PrEP pre-exposure prophylaxis, PEP post-exposure prophylaxis.
We had the opportunity to identify MSM based on their reported homosexual activity. The demographics and behavioral profiles of MSM compared with non-MSM are shown in Table 2.
Table 2.
Demographics and behavioral profiles of identified men who have sex with men (MSM) and non-MSM among the participants before induction.
| Characteristics | MSM (%) | Non-MSM (%) | p value |
|---|---|---|---|
| n = 429 | n = 3826 | ||
| Age (years) | 0.161a | ||
| Mean (± SD) | 21.6 (± 1.3) | 21.6 (± 1.2) | |
| Living pattern | 0.273b | ||
| With parents | 286 (69.9) | 2764 (74.1) | |
| With lover/wife | 73 (17.8) | 602 (16.1) | |
| With relatives or friends | 37 (9.0) | 281 (7.5) | |
| Alone | 13 (3.2) | 85 (2.3) | |
| Region of residence 2 years before induction | 0.296b | ||
| North | 108 (26.7) | 1073 (28.9) | |
| Northeast | 123 (30.4) | 1114 (30.0) | |
| Central | 43 (10.6) | 488 (13.1) | |
| South | 97 (24.0) | 778 (20.9) | |
| Bangkok | 34 (8.4) | 262 (7.1) | |
| Place of residence | 0.492c | ||
| Urban | 162 (55.9) | 1576 (58.0) | |
| Rural | 128 (44.1) | 1139 (42.0) | |
| Marital status | |||
| Single | 302 (72.4) | 2754 (73.0) | 0.816c |
| Married | 115 (27.6) | 1018 (27.0) | |
| Occupation | 0.672b | ||
| Employee | 200 (54.9) | 1866 (56.0) | |
| Student | 50 (13.7) | 507 (15.2) | |
| Agricultural | 61 (16.8) | 489 (14.7) | |
| Unemployed | 53 (14.6) | 470 (14.1) | |
| Education attainment | < 0.001b | ||
| No formal to grade 6 | 113 (26.5) | 732 (19.3) | |
| Grade 7 to grade 12 | 208 (48.8) | 1811 (47.7) | |
| Vocational and diploma | 57 (13.4) | 866 (22.8) | |
| Bachelor’s degree or higher | 48 (11.3) | 385 (10.1) | |
| History of injecting drug use | < 0.001c | ||
| Yes | 47 (11.8) | 162 (4.5) | |
| No | 353 (88.2) | 3448 (95.5) | |
| History of non-injecting drug use | 0.002c | ||
| Yes | 220 (53.8) | 1687 (45.6) | |
| No | 189 (46.2) | 2016 (54.4) | |
| History of previous HIV testing (lifetime) | |||
| Yes | 118 (36.6) | 812 (25.1) | < 0.001b |
| No | 204 (63.4) | 2425 (74.9) | |
| Age (years) at first sexual intercourse | 0.260a | ||
| Mean (± SD) | 16.8 (± 2.4) | 16.6 (± 2.1) | |
| History of sex with a female sex worker | < 0.001c | ||
| Yes | 131 (31.6) | 753 (20.3) | |
| No | 284 (68.4) | 2958 (79.7) | |
| Number of lifetime sex partner | < 0.001a | ||
| Mean ± SD | 27.7 (± 33.8) | 20.9 (± 30.6) | |
| Median (range) | 6.0 (0–78) | 5.0 (0–78) | |
| Sexual experience (lifetime) | < 0.001b | ||
| Exclusive heterosexual | – | 3660 (100.0) | |
| Exclusive homosexual | 66 (15.5) | – | |
| Bisexual | 361 (84.5) | – | |
| Sexual preference/desire | < 0.001c | ||
| Female only | 339 (81.1) | 3782 (99.7) | |
| Male only | 47 (11.2) | 2 (0.1) | |
| Both male and female | 32 (7.7) | 11 (0.3) | |
| History of sexual transmitted infection | < 0.001c | ||
| Yes | 42 (11.0) | 188 (5.1) | |
| No | 341 (89.0) | 3480 (94.9) | |
| History of sex in exchange for gifts/money | < 0.001c | ||
| Yes | 60 (14.5) | 169 (4.5) | |
| No | 354 (85.5) | 3606 (95.5) | |
| History of sexual coercion | < 0.001c | ||
| Yes | 34 (8.1) | 128 (3.4) | |
| No | 385 (91.9) | 3649 (96.6) | |
| Knowledge of PrEP | 64 (15.5) | 154 (4.1) | < 0.001c |
| Use of PrEP | 6 (1.4) | 8 (0.2) | 0.001c |
| Knowledge of PEP | 41 (9.8) | 102 (2.7) | < 0.001c |
| Use of PEP | 4 (1.0) | 18 (0.5) | 0.159c |
| HIV infected cases | 17 (4.0) | 26 (0.7) | < 0.001c |
PrEP pre-exposure prophylaxis, PEP post-exposure prophylaxis.
ap value for comparison of mean of characteristic between group (independent sample t-test).
bp value for comparison of proportion of characteristics between group (Chi-square test).
cp value for comparison of proportion of characteristics between group (Fisher’s exact test).
The proportion of MSM participants residing in rural communities was comparable with non-MSM participants. Those who reported MSM activity had a higher proportion of risk factors for HIV than of those non-MSM participants, i.e., injecting and non-injecting drug use, number of life time sexual partners, history of sex with a FSW, history of STI, history of sex in exchange for gifts/money, and history of sexual coercion. Among those MSM participants, the proportions of reported exclusive homosexual and bisexual experiences were 15.5 and 84.5%, respectively. However, in terms of sexual preference or sexual desire among MSM participants, 81.1% preferred to have sex with females only while 11.2% preferred to have sex exclusively with males and 7.7% preferred to have sex with both males and females. The proportion of participants reported history of previous HIV testing among MSM and non-MSM was 36.6 and 25.1%, respectively. Among MSM participants, 15.5% knew PrEP and only 1.4% used PrEP.
Prevalence of HIV infection among young Thai men in 2018
Of the 38,255 new RTA conscripts participating in the national HIV surveillance program, we found that 336 (0.9%, 95% CI: 0.8–1.0%) men were HIV positive. Of the enrolled young Thai men, 4629 (96.7%) participated in the study and 44 (1.0%, 95% CI: 0.7–1.3%) HIV positive cases were identified. The prevalence of HIV infection among young Thai men by region of residence two years before induction were 1.5, 1.3, 1.2, 1,1, 0.4 and 0.4% in Bangkok, northeast, lower north, upper north, south, and central, respectively. The prevalence of HIV infection among young Thai men residing in urban and rural areas was 1.1 and 0.8%, respectively.
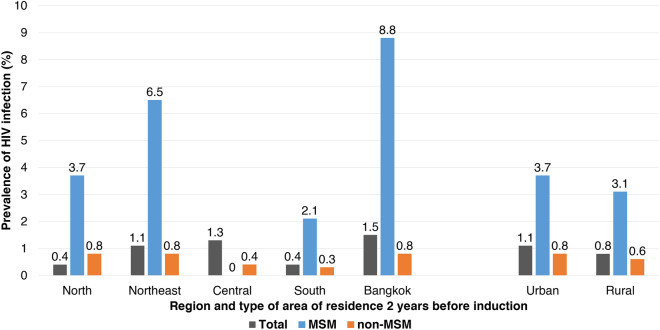
A total of enrolled participants, 429 (10.1%, 95%CI; 9.1–10.9%) young Thai men reported a history of sex between men. Among the total HIV positive cases, 17 (38.6%) were identified to be MSM. HIV prevalence among MSM was 4.0% (95%CI; 2.1–5.8%), while it was 0.7% (95%CI; 0.4.1–0.9%) among non-MSM participants. Of 429 MSM study participants, the prevalence of HIV infection by region of residence two years before induction was 8.8, 6.5, 3.7, 2.1, and 0% in Bangkok, northeast, north, south and central regions, respectively. The prevalence of HIV infection among MSM study participants residing in urban and rural areas was comparable at 3.7 and 3.1%, respectively (Fig. 1).
Figure 1.
Prevalence of HIV infection among participants by region and type of area (rural vs. urban) of residence 2 years before induction.
In terms of sexual preference among MSM participants, the prevalence of HIV infection was 0.9, 6.3, and 23.4% among those who preferred to have sex with females only, both male and female, and male only, respectively. In terms of sexual experience among MSM participants, the prevalence of HIV infection was 1.7 and 16.7% among bisexuals and exclusive homosexuals, respectively (Table 3).
Table 3.
Prevalence of HIV infection among men who have sex with men (MSM) and non-MSM participants in 2018.
| Characteristics | MSM | Non-MSM | ||
|---|---|---|---|---|
| Total | HIV + (%) | Total | HIV + (%) | |
| Total | 429 | 17 (4.0) | 3826 | 26 (0.7) |
| Age (years) | 21.6 ± 1.3 | 21.6 ± 1.0 | 21.6 ± 1.2 | 21.5 ± 1.0 |
| Living pattern | ||||
| Parents | 286 | 11 (3.8) | 2764 | 17 (0.6) |
| Lover/wife | 73 | 1 (1.4) | 602 | 6 (1.0) |
| Relatives and friends | 37 | 1 (2.7) | 281 | 2 (0.7) |
| Alone | 13 | 2 (15.4) | 85 | 1 (1.2) |
| Region of residence 2 years before induction | ||||
| North | 108 | 4 (3.7) | 1073 | 9 (0.8) |
| Northeast | 123 | 8 (6.5) | 1114 | 9 (0.8) |
| Central | 43 | 0 (0.0) | 488 | 2 (0.4) |
| South | 97 | 2 (2.1) | 778 | 2 (0.3) |
| Bangkok | 34 | 3 (8.8) | 262 | 2 (0.8) |
| Place of residence | ||||
| Urban | 162 | 6 (3.7) | 1576 | 13 (0.8) |
| Rural | 128 | 4 (3.1) | 1139 | 7 (0.6) |
| Occupation | ||||
| Employed | 200 | 12 (6.0) | 1866 | 17 (0.9) |
| Student | 50 | 1 (2.0) | 507 | 2 (0.4) |
| Agricultural | 61 | 0 (0.0) | 489 | 3 (0.6) |
| Unemployed | 53 | 2 (3.8) | 470 | 2 (0.4) |
| Education attainment | ||||
| No formal to grade 6 | 113 | 2 (1.8) | 732 | 8 (1.1) |
| Grade 7–grade 12 | 208 | 9 (4.3) | 1811 | 10 (0.6) |
| Vocational and diploma | 57 | 4 (7.8) | 866 | 5 (0.6) |
| Bachelor’s degree | 48 | 2 (4.2) | 385 | 3 (0.8) |
| Marital status | ||||
| Single | 302 | 14 (4.6) | 2754 | 19 (0.7) |
| Married | 115 | 3 (2.6) | 1018 | 7 (0.7) |
| Sexual experience | ||||
| Exclusive heterosexual | – | – | 3660 | 24 (0.7) |
| Exclusive homosexual | 66 | 11 (16.7) | – | – |
| Bisexual | 361 | 6 (1.7) | – | – |
| Sexual experience | ||||
| Female only | 339 | 3 (0.9) | 3782 | 24 (0.6) |
| Male only | 47 | 11 (23.4) | 2 | 0 (0.0) |
| Both male and female | 32 | 2 (6.3) | 11 | 2 (18.2) |
Risk factors for HIV infection among young Thai men
Univariate and multivariate analyses were performed to identify the significant risk factors for HIV infection (Table 4). The independent risk factors for HIV infection among young men included having sex with men (Adjusted odds ratio (AOR): 4.7; 95% CI: 2.3–9.6), having a history of STI (AOR: 2.6; 95% CI: 1.1–6.3) and having a history of sex in exchange for gifts/money (AOR: 3.0; 1.2–7.5). Having a history of sex with a FSW (AOR: 0.5; 95% CI: 0.2–1.2) was found not to constitute a significant risk factor for HIV infection among the participants.
Table 4.
Univariate and multivariate analysis of risk factors for HIV infection among the newly inducted army conscripts that entered into military service in November 2018.
| Characteristics | Total | HIV + (%) | Crude Odds Ratio | Adjusted Odds Ratio |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Age (years) | ||||
| Mean (± SD) | 21.6 ± 1.2 | 21.5 ± 1.0 | 1.1 (0.6–2.1) | 0.8 (0.5–1.3) |
| Living pattern | ||||
| With parents | 3366 | 29 (0.9) | 1 | |
| With lover/wife | 673 | 7 (1.0) | 1.2 (0.7–2.1) | |
| With relatives and friends | 338 | 3 (0.9) | 1.0 (0.5–2.2) | |
| Alone | 103 | 3 (2.9) | 3.4 (1.6–7.6) | |
| Region of residence 2 years before induction | ||||
| Central | 564 | 2 (0.4) | 1 | |
| Northern | 1284 | 14 (1.1) | 3.1 (0.7–13.7) | |
| Northeast | 1314 | 17 (1.3) | 3.7 (0.9–16.0) | |
| Southern | 979 | 4 (0.4) | 1.2 (0.2–6.3) | |
| Bangkok | 324 | 5 (1.5) | 4.4 (0.9–22.8) | |
| Place of residence | ||||
| Rural | 1361 | 11 (0.8) | 1 | |
| Urban | 1896 | 20 (1.1) | 1.3 (0.6–2.7) | |
| Occupation | ||||
| Student | 628 | 3 (0.5) | 1 | |
| Agriculture | 609 | 3 (0.5) | 1.0 (0.2–5.1) | |
| Employee | 2195 | 30 (1.4) | 2.9 (0.9–9.5) | |
| Unemployed | 587 | 4 (0.7) | 1.4 (0.3–6.4) | |
| Single | 3403 | 34 (1.0) | 1.1 (0.6–2.3) | |
| Education attainment | ||||
| No formal to grade 6 | 906 | 10 (1.10) | 1.3 (0.5–3.1) | 1.0 (0.4–2.6) |
| Grade 7–grade 12 | 2165 | 20 (0.9) | 1.0 (0.5–2.3) | 0.8 (0.3–1.7) |
| Vocational and diploma | 1014 | 9 (0.9) | 1 | 1 |
| Bachelor’s degree | 492 | 5 (1.0) | 1.2 (0.4–3.4) | 1.6 (0.3–7.7) |
| History of injecting drug use | ||||
| No | 4126 | 39 (1.0) | 1 | |
| Yes | 220 | 4 (1.8) | 1.9 (0.7–5.5) | |
| History of non-injecting drug use | ||||
| No | 2460 | 21 (0.9) | 1 | |
| Yes | 1996 | 20 (1.0) | 1.2 (0.6–2.2) | |
| History of sex with a female sex worker | ||||
| No | 3269 | 35 (1.1) | 1 | 1 |
| Yes | 889 | 6 (0.7) | 0.6 (0.3–1.5) | 0.5 (0.2–1.2) |
| Number of lifetime sex partner | ||||
| Mean, ± SD | 24.7 ± 39.3 | 29.1 ± 41.0 | 1.0 (1.0–1.0) | |
| History of sex with another man | ||||
| No | 3826 | 26 (0.7) | 1 | 1 |
| Yes | 429 | 17 (4.0) | 6.0 (3.3–11.2) | 4.7 (2.3–9.6) |
| Sexual experience | ||||
| Exclusively heterosexual | 3660 | 24 (0.7) | 1 | |
| Exclusively homosexual | 66 | 11 (16.7) | 30.3 (14.2–69.9) | |
| Bisexual | 361 | 6 (1.7) | 2.5 (1.0–6.3) | |
| Sexual preference/desire | ||||
| Female only | 4455 | 28 (0.6) | 1 | |
| Male only | 58 | 11 (19.0) | 37.0 (17.4–78.7) | |
| Bothe male and female | 50 | 4 (8.0) | 13.7 (4.6–40.8) | |
| History of sexual transmitted infection | ||||
| No | 3846 | 34 (0.9) | 1 | 1 |
| Yes | 231 | 7 (3.0) | 3.5 (1.5–8.0) | 2.6 (1.1–6.3) |
| History of sex in exchange for gifts/money | ||||
| No | 4016 | 34 (0.9) | 1 | 1 |
| Yes | 231 | 8 (3.5) | 4.2 (1.9–9.2) | 3.0 (1.2–7.5) |
| History of sexual coercion | ||||
| No | 4085 | 38 (0.9) | 1 | |
| Yes | 162 | 5 (3.1) | 3.4 (1.3–8.8) | |
The number of HIV + individuals and in the total column in each characteristic was based the response of study participants.
Discussion
Our data exhibited patterns of sexual behavior and risk factors for HIV infection among young Thai men from army training units in selected five provinces in November 2018. Because the conscription process using a lottery system was conducted at the district level of the conscripts’ home province, our study population could to some extent represent young Thai men from both rural and urban areas3,7. We determined that the prevalence of HIV infection among the newly inducted army conscripts from the training units in selected provinces in 2018 was 1.0% (95% CI: 0.7–1.3%), which was comparable to the national HIV prevalence among those participating in the surveillance program for HIV among military conscripts indicating a HIV prevalence of 0.9% (336 of 38,225) (95%CI: 0.8–1.0%) during the same period. The prevalence of HIV infection among RTA new conscripts stabilized at 0.5% since 2004. This was significantly higher than the previous data though still below the 1% mark2,3,8.
We also found that though the prevalence of HIV infection among study participants residing in urban and rural area was comparable. In Thailand, effective HIV prevention intervention programs among MSM have been established especially in venue-based urban settings9–12. Our data suggested that monitoring and prevention measures should also be conducted in the rural areas that usually have more limited resources, as well as more limited ability to access health and social supports in the community13.
The present study illustrated that the proportion of reported cases of history of sex with another man was 10.1% (95%CI; 9.1–11.0%), while it was 7.9% (95%CI; 7.6–8.1%) from 2010 to 20117. Our study reported that MSM played a major role in acquiring HIV infection among the participants. From the total 44 identified HIV infected cases, 17 (38.6%) reported having sex with another man. The prevalence of HIV infection among Thai MSM in our present study was 4.0% which was higher than that among young Thai MSM from 2010 to 2011 almost two fold (2.6%)7. In terms of geographical region resided in two years before conscription, HIV prevalence among MSM participants was significantly high in Bangkok (8.8%) and the northeast region (6.5%) which was higher than that among young Thai MSM from 2010 to 2011 (4.7% in Bangkok and 2.2% in the northeast)7. Nevertheless, the recent study at the Silom Community Clinic in Bangkok reported that HIV declined among Thai MSM between 2010 and 2018 because of PrEP implementation and has expanded substantially in Bangkok9. However, our finding reported that approximately 15% of young Thai MSM knew about PrEP, only 1.4% PrEP. Additionally, a low proportion of consistent condom use with a male partner was found, less than 40%. Our data suggested that effective HIV prevention interventions for MSM in Thailand including PrEP and condom use should be implemented both in rural and urban areas, especially in Bangkok and the northeast.
One of the significant findings related to MSM sexual activity from the present study was that the majority (81.1%) of those having sex with another man reported having exclusively heterosexual desire comparable with the finding in the related study from 2010 to 20117,14. Related studies in China and the UK reported that the proportion of MSM with heterosexual desire were 1.6% and 22.0%, respectively15–17. In addition, we found an increasing proportion of bisexual activity among MSM of 60.6% from 2010 to 20117 to 84.5% in our present study.
Among all participants, 5.4% had sex in exchange for gifts/money, while it was 14.5% among MSM study participants. We found that having sex in exchange for gifts/money was an independent risk factors for HIV infection. This behavior in these young men population may enhance the probability of HIV transmission from the older HIV postitive population.
The proportion of young Thai men reporting a history of sex with a FSW continued to decline since 2010 to 2011 (34.6%) to 21.4% in the present study, and was not associated with HIV infection. Additionally, the reported rate of consistent condom use during sex with a FSW was relatively high at 83.7%.
Because our study participants were homogenous in terms of age distribution; thus, we were unable to explore the effect of age on acquiring HIV infection in this study. The study employed a cross-sectional design, and as such, the results may have limited explanations regarding temporal relationships regarding both the exposure and outcome. Additionally, limitations were encountered related to the use of self-administrated questionnaires that might have provided limited answers especially regarding issues concerning sensitive matters.
Conclusion
In conclusion, we reported the epidemiological data regarding HIV prevalence and related risk factors among randomly selected newly inducted army conscripts entering military service in five regions nationwide in Thailand. An increasing prevalence of HIV was found among young Thai men in 2018. The risk factors of HIV infection included having sex with another man, a history of sexual transmitted infection, and history of sex in exchange for gifts/money. HIV prevention programs including PrEP in Thailand should be emphasized among MSM in both rural and urban areas.
Acknowledgements
The authors express their grateful thanks to Col.Dr. Sutchana Tabprasit for her support to complete the study. The authors are exceedingly grateful to all those involved in the successful field operations in the study, including staff of the Health Promotion and Preventive Medicine Division, Royal Thai Army Medical Department, Ananda Mahidol Hospital, Fort Kawila Hospital, Fort Sunpasitthiprasong Hospital and Fort Senanarong Hospital. The study was funded by Phramongkutklao College of Medicine. Laboratory results received support from the Armed Forces Research Institute of Medical Sciences (AFRIMS) and Royal Thai Army Institute of Pathology (AIP). We acknowledge the Phramongkutklao College of Medicine Research and Development Office, Graduate program of the Faculty of Allied Health Science – Thammasat University, and all the participants. This research was supported by the Scholarships for Foreign Students Studying for a Degree 2559 B.E., Thammasat University.
Abbreviations
- HIV
Human immunodeficiency virus
- AIDS
Acquired immunodeficiency syndrome
- MSM
Men who have sex with men
- FSW
Female sex workers
- STIs
Sexually transmitted infections
- PrEP
Pre-exposure prophylaxis
- PEP
Post-exposure prophylaxis
- CI
Confidence Interval
Author contributions
The concept for study was developed by J.J., B.S., M.M., K.N. and R.R.. J.J., B.S. and R.R. collected the data. J.J. and R.R. analyzed the data. J.J. wrote the first draft. All authors contributed and approved the final version.
Funding
This research was supported by (1) the Phramongkutklao College of Medicine and (2) the Scholarships for Foreign Students Studying for a Degree 2559 B.E., Thammasat University.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available because the data sets containssensitive identifying information including HIV status. Thus, due to ethical restrictions concerning the data sets, they are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The United Nation. UNAIDS DATA 2020. https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf (2020).
- 2.Nelson KE, Rangsin R. The importance of military conscripts for surveillance of human immunodeficiency virus infection and risk behavior in Thailand. Curr. HIV Res. 2017;15:161–169. doi: 10.2174/1570162X15666170517122011. [DOI] [PubMed] [Google Scholar]
- 3.Rangsin R, et al. Risk factors for HIV infection among young Thai men during 2005–2009. PLoS ONE. 2015;10:e0136555. doi: 10.1371/journal.pone.0136555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia Y, Aliyu MH, Jennifer Huang Z. Dynamics of the HIV epidemic in MSM. Biomed. Res. Int. 2014;2014:497543. doi: 10.1155/2014/497543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyrer C, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665–2678. doi: 10.1097/01.aids.0000432449.30239.fe. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Public Health (Thailand). Progress Report for Thailand Ending AIDS 2018. http://azp.ddc.moph.go.th/report.php (2018).
- 7.Jose JEdC, et al. Prevalence of HIV infection and related risk factors among young Thai men between 2010 and 2011. PLoS ONE. 2020;15:e0237649. doi: 10.1371/journal.pone.0237649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torugsa K, et al. HIV epidemic among young Thai Men, 1991–2000. Emerg. Infect. Dis. 2003;9:881–883. doi: 10.3201/eid0907.020653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pattanasin S, et al. Recent declines in HIV infections at Silom community clinic Bangkok, Thailand corresponding to HIV prevention scale up: An open cohort assessment 2005–2018. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;99:131–137. doi: 10.1016/j.ijid.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Sungkanuparph S, et al. Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2010. Asian Biomed. 2010;4:515–528. doi: 10.2478/abm-2010-0066. [DOI] [Google Scholar]
- 11.Committee, T. N. A. Thailand national operational plan accelerating ending AIDS, 2015–2019. Bangkok Thail. Natl. AIDS Comm. (2014).
- 12.Colby D, et al. HIV pre-exposure prophylaxis and health and community systems in the Global South: Thailand case study. J. Int. AIDS Soc. 2015;18:19953. doi: 10.7448/IAS.18.4.19953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrowing, J. N. & Robinson Vollman, A. Seeking serenity: living with HIV/AIDS in rural Western Canada. (2007). [PubMed]
- 14.Rangsin, R. et al. THAC0303: The recent impact of MSM on the prevalence of HIV‐1 infection among young men in Thailand. J. Int. AIDS Soc.15 (2012).
- 15.Yang Z, et al. Current heterosexual marriage is associated with significantly decreased levels of anxiety symptoms among Chinese men who have sex with men. BMC Psychiatry. 2020;20:151. doi: 10.1186/s12888-020-02563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geary RS, et al. Sexual identity, attraction and behaviour in Britain: The implications of using different dimensions of sexual orientation to estimate the size of sexual minority populations and inform public health interventions. PLoS ONE. 2018;13:e0189607. doi: 10.1371/journal.pone.0189607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebecca C Parrish. Heterosexual-identifying men who have sex with men: an understudied population. https://blogs.ucl.ac.uk/igh-blog/2019/05/17/heterosexual-identifying-men-who-have-sex-with-men-an-understudied-population/ (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available because the data sets containssensitive identifying information including HIV status. Thus, due to ethical restrictions concerning the data sets, they are available from the corresponding author on reasonable request.