Abstract
In order to analyze whether the marine macroalga Ulva lactuca can absorb and metabolize anthracene (ANT), the alga was cultivated with 5 µM ANT for 0–72 h, and the level of ANT was detected in the culture medium, and in the alga. The level of ANT rapidly decreased in the culture medium reaching a minimal level at 6 h, and rapidly increased in the alga reaching a maximal level at 12 h and then decreased to reach a minimal level at 48 h of culture. In addition, ANT induced an increase in hydrogen peroxide that remained until 72 h and a higher increase in superoxide anions that reach a maximal level at 24 h and remained unchanged until 72 h, indicating that ANT induced an oxidative stress condition. ANT induced an increase in lipoperoxides that reached a maximal level at 24 h and decreased at 48 h indicating that oxidative stress caused membrane damage. The activity of antioxidant enzymes SOD, CAT, AP, GR and GP increased in the alga treated with ANT whereas DHAR remained unchanged. The level of transcripts encoding these antioxidant enzymes increased and those encoding DHAR did not change. Inhibitors of monooxygenases, dioxygenases, polyphenol oxidases, glutathione-S-transferases and sulfotransferases induced an increase in the level of ANT in the alga cultivated for 24 h. These results strongly suggest that ANT is rapidly absorbed and metabolized in U. lactuca and the latter involves Phase I and II metabolizing enzymes.
Subject terms: Environmental biotechnology, Plant biotechnology, Plant physiology, Plant stress responses
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are molecules commonly formed by two to six benzene rings that can be arranged in linear, angular or clustered forms. Anthracene (ANT) is formed by three benzene rings arranged linearly, phenanthrene (PHE) is constituted by three benzene rings arranged in angle, and both are considered a small PAH1. Benzo[a]pyrene (BaP) is constituted by five benzene rings arranged as a cluster and correspond to a large PAH. PAHs are found in water and soil, they are present in crude oil and industrial effluents and they are produced by fossil fuel combustion, oil refining and automobile emissions1. PAHs are hydrophobic compounds that are very stable and persistent in the environment and, thus, magnified in the food chain1. PAHs are sensitive to sunlight, mainly to UV light, and their derivatives are quinones and hidroxyquinones2,3. PAHs are toxic to animals and plants since they interfere with function of membranes and with proteins and enzymes located in cellular membranes1. In Chile, there are several so-called sacrifice zones located in coastal sites displaying an accumulation of oil refineries and thermo-electric industries, in northern, central and southern Chile and displayed heavy metals and polycyclic aromatic hydrocarbons pollution4,5. In these costal zones successive crude oil spillages have occurred in seawater producing a detrimental effect in human health and in biodiversity4,5. Thus, it is urgently required to find marine species that are capable to bioremediate seawater from crude oil and PAHs.
Regarding PAHs and plants, the aquatic monocot plant Lemna gibba (duckweed) cultivated with 2 µg mL−1 of ANT, PHE and BaP for 24 h showed an inhibition of growth and chlorophyll content, and the absorption of ANT, PHE and BaP of 58%, 66% and 33%, respectively, after 24 h of culture6. In addition, ANT, PHE and BaP exposed to sunlight and UV light showed that its photoxidized derivatives induced a stronger inhibition of growth than the native compounds, and the toxicity of ANT is higher than PHE and higher than BAP in L. gibba2,3. ANT, PHE and BAP exposed to sunlight showed derivatives that are mainly quinones and hydroxyquinones, but also smaller derivatives such as phthalic acid, salicylic acid and benzoic acid7,8. In plants and animals, PAHs can also be degraded at intracellular level by monooxygenases and dioxygenases (Phase I) producing hydroxyl derivatives and hydroxydiols, respectively, and that can be further conjugated with glutathione (GSH), glucose, maltose or glucuronic acid by metabolizing enzymes such as glutathione-S-transferases (GSTs), glucosyl transferases and glucuronyl-transferases, or derivatized with a sulfate, methyl or acetyl group (Phase II) by sulfotransferases, methyltransferases and acetyltransferases. Phase I and Phase II are required to increase solubility of PAHs in water allowing their further degradation and/or excretion in plants and animals9,10.
Regarding PAHs and other hydrocarbons degradation in plants, the tall fescue plant Festuca arundinacea cultivated with 1 µg mL−1 of ANT for 16 days showed the degradation of ANT to anthrone, anthraquinone and hydroanthraquinone that were mainly accumulated in root cells in organelles and cell soluble fraction11. These results are similar to those previously obtained in roots of wheat and tomato exposed to ANT12. The aquatic liverwort Riccia fluitans cultivated with 0.5–10 µM of PHE for 96 h showed an oxidative stress condition, a decrease in chlorophyll and carotenoid contents, a decrease in photosynthesis efficiency and an increase in transcripts encoding antioxidant enzymes and enzymes involved in polyamine synthesis13. In this sense, it is important to point out that polyamines putrescine, spermine and spermidine are involved in protection of cellular and organellar membranes. A. thaliana plants cultivated with 0–250 µM of PHE showed an oxidative stress condition and the increase in the level of transcripts encoding antioxidant enzymes superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (AP) and in the level of transcripts encoding 14 different GSTs, glucosyl- and glucuronyl-transferases, and enzymes involved in polyamines synthesis14. Maize plants cultivated soil contaminated with 1, 2.5 and 5% of petroleum for 30 days showed an increase in the level of transcripts of five GSTs, several-cysteine proteases, and enzymes involved in N assimilation and antioxidant enzymes such as SOD, AP, CAT and glutathione reductase (GR)15. Thus, GSTs and glycosyltransferases may be involved in the solubilization of PAHs and other hydrocarbons in plants.
Regarding PAHs degradation and toxicity in microalgae, the green microalga Selenestartum capricornutum cultivated with ANT showed an inhibition of growth, a IC50 of 16 µg L−1, and the toxicity of ANT increased with the exposure of the alga to UV-A light16. In addition, S. capricornutum exposed to BAP displayed the formation of dihydrodiols, mainly 9,10-dihydrodiol, and the exposure to UV-A light increased the level of dihydrodiols and decreased the level of quinones17. The green microalga S. armatus cultivated with ANT and PHE displayed an oxidative stress condition and the increase in activity of the antioxidant enzyme SOD18. The green microalga C. reinhardtii cultivated with 0.7–4.2 µM of ANT for 24 h displayed an inhibition of growth, a decrease in chlorophyll fluorescence and in the efficiency of photosystem II (PSII) showing a EC50 of 1.6 µM19. The green microalga Tetraselmis chuii exposed to ANT, PHE and naphthalene (NAPH) for 96 h showed an inhibition of growth, the toxicity was PHE > ANT > NAPH and toxicity of these PAHs was enhanced by the increase in 5 °C of temperature20. Recently, it was shown that the microalga Euglena agilis cultivated with increasing concentrations of ANT, ranging from 0.35 to 84 µM, for 96 h showed a decreased in growth, in photosynthetic efficiency and in the content of chlorophylls and carotenoids21.
Regarding PAHs and other hydrocarbons degradation in marine algae, the green macroalgae Ulva intestinalis and Cladophora glomerata metabolize 42–49% of BaP in 6 h22. The green macroalga Ulva lactuca exposed to gasoline containing paraffinic, isoparaffinic, aromatic, naphthenic and olefinic hydrocarbons, constituted by 5–10 C, showed a decrease in chlorophylls, carotenoids and polyphenols levels and an increase in soluble sugars and starch23. Recently, it was shown that U. lactuca exposed to 5 µM of BaP rapidly absorbed and metabolized BaP since 79% of the compound was degraded after 72 h24. The alga displayed an oxidative stress condition reflected in the increase in reactive oxygen species (ROS), mainly superoxide anions, and an increase in activities of antioxidant enzymes such as ascorbate peroxidase (AP), catalase (CAT), glutathione reductase (GR) and glutathione peroxidase (GP) and the metabolizing enzyme GST24. The level of transcripts encoding these antioxidant and metabolizing enzymes were increased in response to BaP suggesting that the increase in activities is due to the enhanced expression of genes encoding these enzymes24. Furthermore, the alga treated with inhibitors of CYP450 and in the synthesis of GSH showed an increase in intracellular BaP level suggesting that CYP450 and GST are involved in Phase I and II of BaP detoxification24. Thus, the marine alga U. lactuca can use other hydrocarbons and PAHs as alternative sources of C.
In this work, we analyze the ability of U. lactuca to absorb and degrade ANT, the induction of an oxidative stress condition, the activation of antioxidant enzymes and the level of transcripts encoding these enzymes. Furthermore, we analyze the effects of inhibitors of Phase I detoxification enzymes such as monooxygenases and dioxygenases and Phase II detoxification enzymes such as polyphenol oxidases (PPOs), GSTs and sulfotransferases (STs), and the level of ANT was determined in U. lactuca.
Results
Viability of U. lactuca exposed to increasing concentrations of ANT
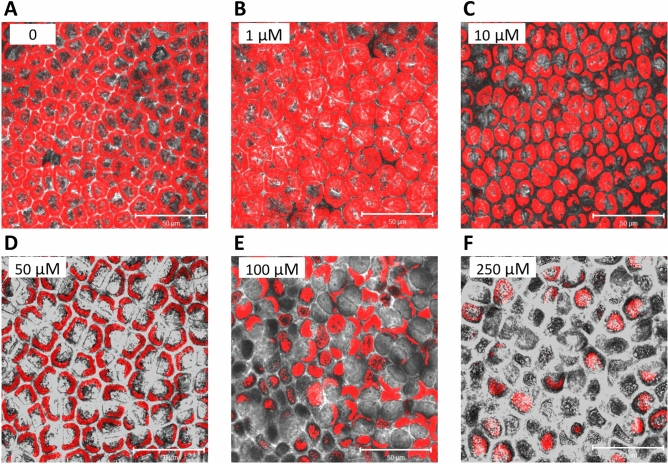
In order to determine a sub-lethal concentration of ANT in U. lactuca, the alga was cultivated in artificial seawater without ANT (control) and with increasing concentrations of ANT corresponding to 1, 10, 50, 100 and 250 µM, for 7 days (Fig. 1). Cell viability was analyzed through visualization of chlorophyll fluorescence in chloroplasts using confocal microscopy. Chlorophyll fluorescence in the algae treated with 10 μM of ANT was similar to the control indicating full viability (Fig. 1A,B). Algae treated with 50–250 µM of ANT showed a progressive decrease in chlorophyll fluorescence compared to the control (Fig. 1C–F). Thus, the chosen sub-lethal concentration of ANT for further experiments was 5 µM.
Figure 1.
Visualization of U. lactuca cells cultivated with 0–250 μM of anthracene for 7 days. The viability of the cells was analyzed based on the red autofluorescence of chlorophylls in chloroplasts by confocal microscopy.
ANT is rapidly incorporated and metabolized in U. lactuca
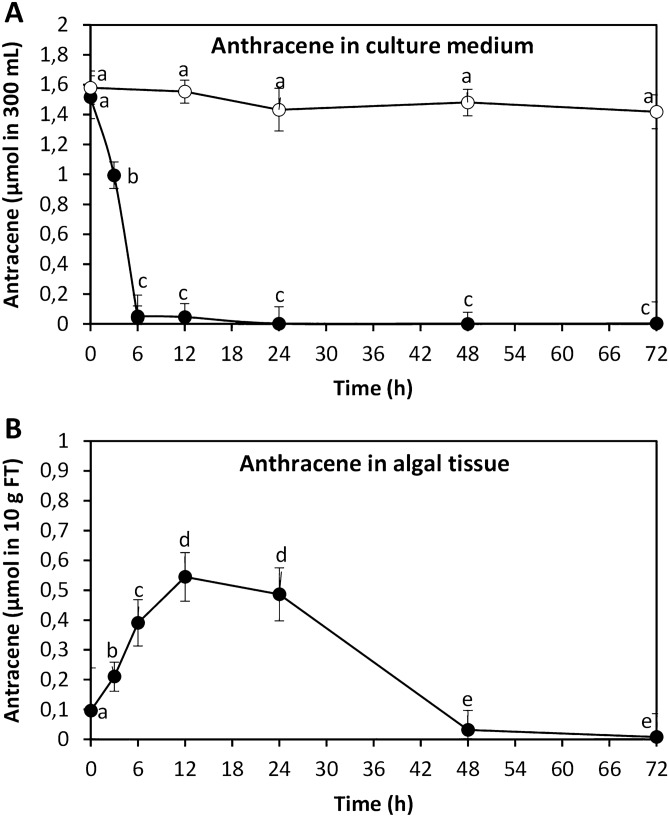
To determine whether U. lactuca can incorporate and metabolize ANT, the alga was cultivated in without ANT (control) and with 5 μM of ANT for 0–72 h, and the level of ANT was determined in the culture medium and in the alga. The level of ANT in seawater was 5 µM, which corresponds to 1.6 µmol in 300 mL of seawater, and it rapidly decreased reaching a minimal level at 6 h of culture corresponding to 0.05 µmol in 300 mL of seawater or 1,6 µM of ANT and almost completely disappeared at 48 h of culture (Fig. 2A) while the amount of ANT in the culture medium without the alga remained constant until the end of the experiment. The level of ANT in the alga rapidly increased reaching a maximal level of 0.54 µmol in 10 g of algal tissue at 12 h of culture and then decreased reaching a minimal level of 0.03 µmol in 10 g of algal tissue at 48 h of culture (Fig. 2B). These results indicate that half of the 5 µM ANT was absorbed by the alga at 6 h culture and almost totally absorbed at 48 h. Thus, ANT is rapidly incorporated in U. lactuca.
Figure 2.
Level of anthracene (ANT) in the culture medium (A) and in U. lactuca (B) cultivated with 1.6 μmol of ANT in 300 mL of culture medium (5 μM) for 0–72. Black circles represent the level of ANT in the culture with ANT and open circles represent the level of ANT in the culture medium with ANT but without the alga. Symbols represent mean values of three independent experiments SD. Letters indicate significant differences among experiments (P < 0.05).
ANT induced an oxidative stress condition in U. lactuca
To analyze whether ANT induce oxidative stress in U. lactuca, the alga was cultivated without ANT (control) and with 5 µM of ANT for 0–72 h, and the level of superoxide anions, hydrogen peroxide and lipoperoxides was determined. The level of superoxide anions increased reaching a maximal level of 7.75 nmol g−1 of fresh tissue (FT) at 24 h of culture and remained increased until 72 h (Fig. 3A). The level of hydrogen peroxide increased reaching a maximal level of 0.8 nmol g−1 of FT at 1.5 h, 1.1 nmol g−1 of FT at 8.5 h and 0.78 at 10 h of culture and remained increased until 72 h (Fig. 3B), The level of lipoperoxides increased reaching a maximal level of 1.25 µmol g−1 of dry tissue (DT) at 24 h of culture, decreased to 0.61 µmol at 48 h, and remained stable until 72 h (Fig. 3C). Thus, ANT induced an oxidative stress condition and membrane damage in U. lactuca.
Figure 3.
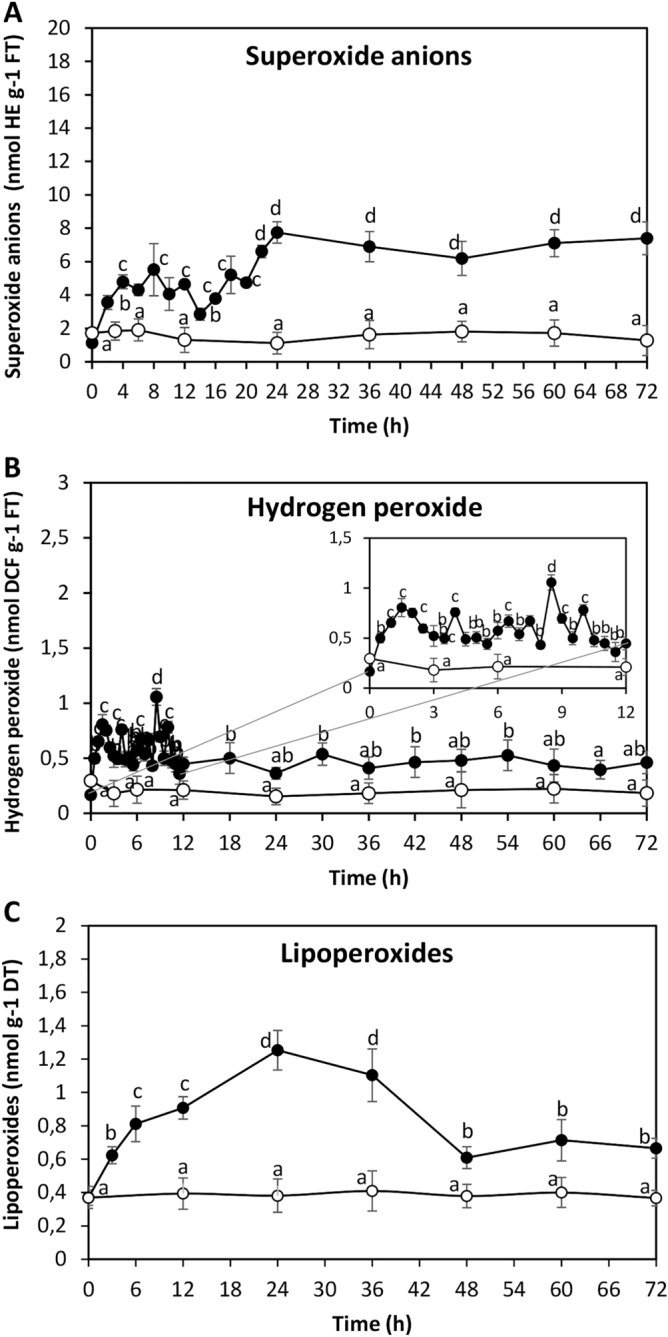
Level of superoxide anions (A), hydrogen peroxide (B) and lipoperoxides (C) in U. lactuca cultivated without ANT (open circles) and with 5 μM of ANT (black circles) for 0–72 h. The level of superoxide anions and hydrogen peroxide are expressed as nanomole per gram of fresh tissue (FT) and lipoperoxides in nanomole per gram of dry tissue (DT) and time in hours. The subframe represents the level of hydrogen peroxide from 0 to 12 h of culture. Symbols represent mean values of three independent experiments SD. Letters indicate significant differences among experiments (P < 0.05).
ANT increased antioxidant enzymes activities in U. lactuca
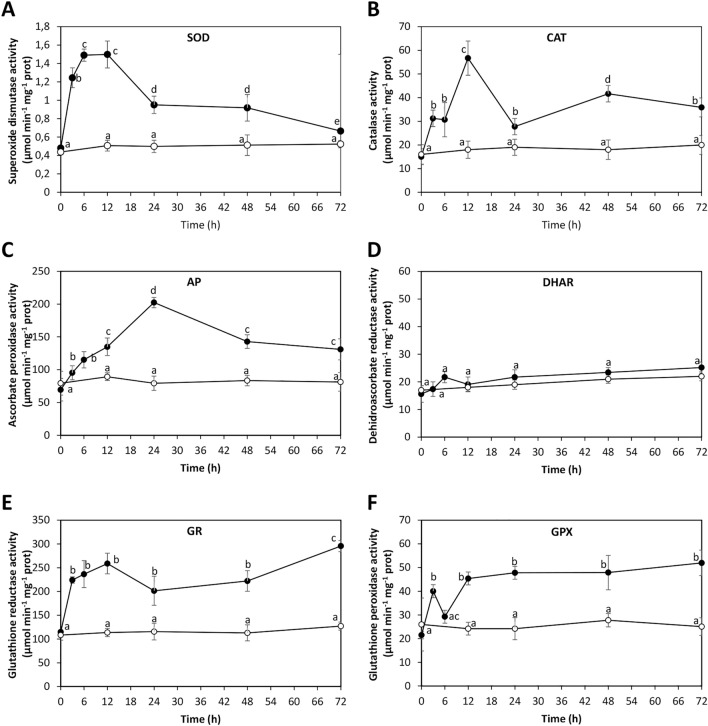
To analyze whether the oxidative stress condition induced the activation of antioxidant enzymes, the alga was cultivated without ANT (control) and with 5 µM of ANT for 0–72 h, and the activities of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (AP), dehydroascorbate reductase (DHAR), glutathione reductase (GR) and glutathione peroxidase (GP) were determined. The activity of SOD was 0.48 µmol of superoxide anions consumed per min−1 mg−1 of protein in control condition and it remained unchanged until 72 h of culture (Fig. 4A). SOD activity increased in treated alga from 0.48 to 1.5 µmol min−1 mg−1 of protein at 6 and12 h of culture and decreased to 0.7 µmol min−1 mg−1 of protein at 72 h (Fig. 4A). The activity of CAT was 15 µmol of hydrogen peroxide consumed per min−1 mg−1 of protein in control condition and it remained unchanged until 72 h (Fig. 4B). CAT activity increased in treated alga from 15 to 58 µmol min−1 mg−1 of protein 12 h of culture and decreased to 28 µmol min−1 mg−1 of protein at 24 h and increased again to 36 µmol min−1 mg−1 of protein at 72 h (Fig. 4B). The activity of AP was 69 µmol min−1 mg−1 of protein in control condition and it remained unchanged until 72 h (Fig. 4C). AP activity increased in treated alga from 69 to 202 µmol of ascorbate consumed per min−1 mg−1 of protein at 24 h of culture and decreased to 131 µmol min−1 mg−1 at 72 h (Fig. 4C). The activity of DHAR was 16 µmol of dehydroascorbate consumed per min−1 mg−1 of protein and it did not change in control or treated conditions (Fig. 4D). The activity of GR was 115 µmol of glutathione consumed per min−1 mg−1 of protein in control condition and it remained unchanged until 72 h of culture (Fig. 4E). GR activity increased from 115 to 259 µmol min−1 mg−1 of protein at 12 h, decreased to 202 µmol min−1 mg−1 of protein at 24 h and increased again to 296 µmol min−1 mg−1 of protein at 72 h (Fig. 4E). The activity of GP was 22 µmol of glutathione consumed per min−1 mg−1 of protein in control condition and it did not change until 72 h of culture (Fig. 4F). GP activity increased from 22 to 40 µmol min−1 mg−1 of protein at 3 h of culture, decreased to 29 µmol min−1 mg−1 of protein at 6 h of culture, increased to 45 µmol min−1 mg−1 of protein at 12 h and remained unchanged until 72 h of culture (Fig. 4F). Thus, the oxidative stress condition induced by ANT is buffered by the activation of several antioxidant enzymes in U. lactuca. The maximal activity of antioxidant enzymes occurred at 12 h of culture, except for AP that reached a maximal level at 24 h of culture.
Figure 4.
Activities of antioxidant enzymes superoxide dismutase (A), catalase (B), ascorbate peroxidase (C), dehydroascorbate reductase (D), glutathione reductase (E) and glutathione reductase (F) in U. lactuca cultivated without anthracene (open circles) and with 5 μM anthracene (black circles) for 0–72 h. Activities of antioxidant enzymes are expressed as micromole per minute per gram of fresh tissue (FT) and time in hours. Symbols represent mean values of three independent experiments SD. Letters indicate significant differences among experiments (P < 0.05).
ANT increased the level of transcripts of antioxidant enzymes in U. lactuca
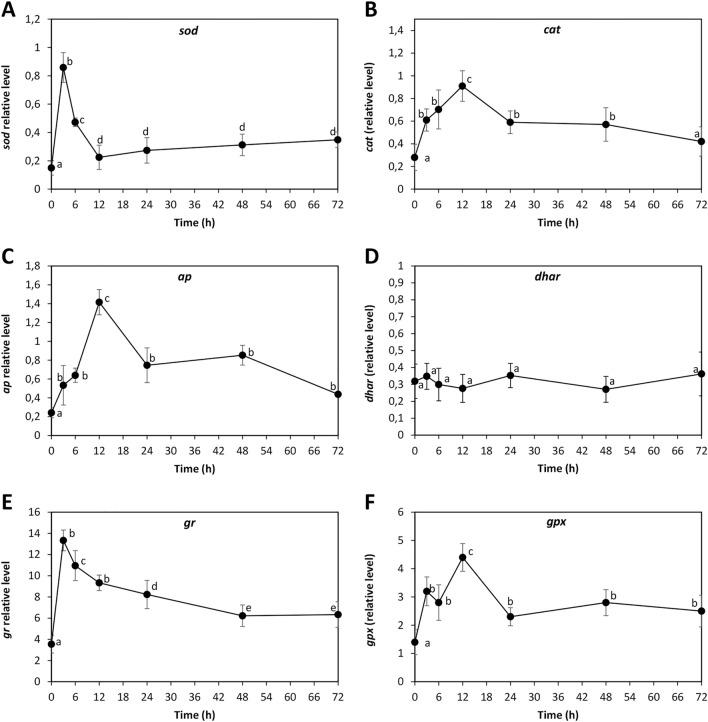
In order to analyze whether the increase in activities of antioxidant enzymes is due to an increase in their expression, the alga was cultivated without ANT (control) and with 5 μM of ANT for 0–72 h, and the relative levels of transcripts encoding antioxidant enzymes SOD, CAT, AP, DHAR, GR and GP were determined. The relative level of transcripts of SOD increased to reach a maximal level of 0.86 times of increase at 3 h of culture, decreased to 0.22 times at 12 h and slightly increased to 0.35 times at 72 h of culture (Fig. 5A). The relative level of transcripts encoding CAT increased to reach a maximal level of 0.91 times of increase at 12 h of culture and decreased to reach 0.42 times of increase at 72 h of culture (Fig. 5B). The relative level of transcripts encoding AP increased to reach a maximal level of 1.42 times of increase at 12 h of culture and decreased to 0.75 times at 24 h and to 0.44 times at 72 h of culture (Fig. 5C). In contrasts, the relative level of transcripts encoding DHAR did not change from 0 to 72 h of culture (Fig. 5D). The relative level of transcripts encoding GR increased to reach a maximal level of 13.3 times of increase at 12 h of culture and decreased to reach 6.3 times of increase at 72 h (Fig. 5E). The relative level of transcripts encoding GP increase to reach a maximal level of 4.4 times of increase at 12 h of culture and decreased to reach 2.5 times at 72 h (Fig. 5F). Thus, the increase in the level of transcripts encoding antioxidant enzymes occurred mainly at 12 h of culture, except those encoding SOD that reached a maximal level at 3 h of culture indicating that the increase in antioxidant enzyme activities is due, at least in part, to the increase in their expression.
Figure 5.
Relative level of transcripts encoding antioxidant enzymes superoxide dismutase (A), catalase (B), ascorbate peroxidase (C), dehydroascorbate reductase (D), glutathione reductase (E) and glutathione reductase (F) in U. lactuca cultivated with 5 μM anthracene for 0–72 h (black circles). The relative level of transcripts is expressed as 2−ΔΔCT. Symbols represent mean values of three independent experiments SD. Letters indicate significant differences among experiments (P < 0.05).
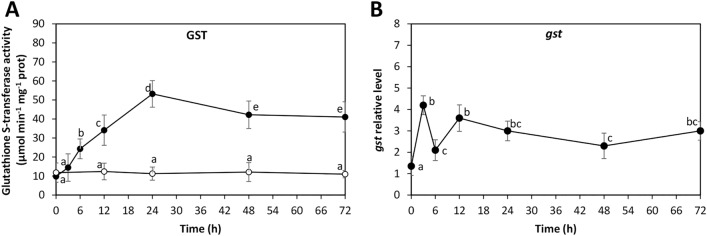
ANT induced an increase in activity and expression of GST metabolizing enzyme
In order to analyze the mechanisms involved in ANT detoxification, the alga was cultivated without ANT (control) and with 5 µM of ANT for 0–72 h, and the activity of the metabolizing enzyme GST and the relative level of transcripts encoding GST were determined. The activity of GST in control condition was 9.8 µmol min−1 mg−1 of protein and it remained unchanged until 72 h of culture (Fig. 6A). GST activity in treated algae increased from 9.8 to 53 µmol min−1 mg−1 of protein at 24 h and decreased to 41 µmol min−1 mg−1 of protein at 72 h of culture (Fig. 6A). The level of transcripts encoding GST increased in 4.2 times at 3 h of culture, decreased to 2.1 times at 6 h, increased to 3.6 times at 12 h and slightly decreased to 3 times at 72 h of culture (Fig. 6B). Thus, ANT induce an increase in the activity of the detoxification enzyme GST and this increase in activity is due, at least in part, to the increase in its expression.
Figure 6.
Activity of glutathione-S-transferase (GST, A) in U. lactuca cultivated without anthracene (open circles) and with 5 μM of ANT (black circles) for 0–72 h. The activity of GST is expressed as micromole per gram of fresh tissue (FT). Relative level of GST transcripts (B) in the alga cultivated with 5 μM of ANT (black circles). The relative level of GST transcripts is expressed as 2−ΔΔCT. Symbols represent mean values of three independent experiments SD. Letters indicate significant differences among experiments (P < 0.05).
ANT induced an increase in Phase I and II metabolizing enzymes activities
In order to analyze the involvement of Phase I and II detoxification enzymes, the alga was cultivated with 5 μM of ANT (control) and 0.5 µM inhibitors of Phase I enzymes CYP450-dependent monooxygenases and dioxygenases, and phase II enzymes PPOs, GSTs and STs and with 5 µM ANT for 24 h, and the amount of ANT in the alga was determined (Fig. 7). The level of ANT in control algae was 0.4 µmol in 10 g of FT and in the alga treated with inhibitors of monooxygenases, dioxygenases, PPO, GST and sulfotransferases it was 0.9, 0.9, 1, 1.1 and 1.1 µmol in 10 g of FT, respectively, which represents increases of 36, 36, 43, 48 and 44% compare to the control (Fig. 7). The level of ANT in the alga increased with all inhibitors, mainly with those that inhibit PPOs, GSTs and STs (Fig. 7). Thus, Phase I enzymes, monooxygenases and dioxygenases, and Phase II enzymes, PPOs, GSTs and STs are involved in ANT detoxification in U. lactuca.
Figure 7.
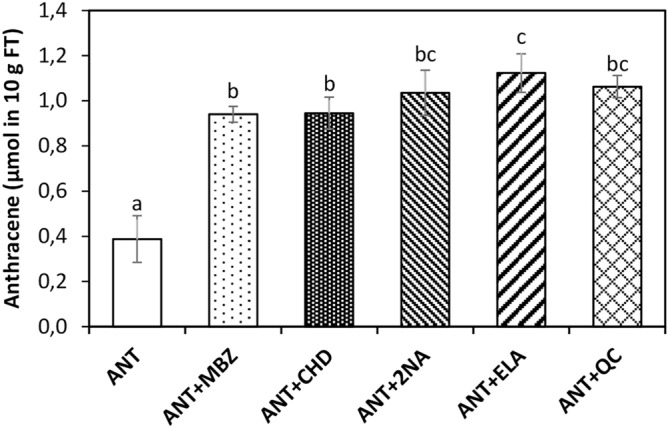
Level of anthracene in U. lactuca cultivated with 5 μM of ANT for 24 h (ANT, open bar) and with 0.5 μM of mebendazole (MBZ), an inhibitor of monooxygenases, cyclohexanedione (CHD), an inhibitor of dioxygenases, 2-naphtoic acid (2-NA), an inhibitor of polyphenol oxidases, ellagic acid (ELA), an inhibitor of GST, and quercetin (QC), an inhibitor of sulfotransferases, and with 5 µM of ANT for 24 h (dashed bars). The level of ANT is expressed as micromole in 10 g of algae fresh tissue (FT). Bars represent mean values of three independent experiments. Letters indicate significant differences among experiments (P < 0.05).
Discussion
In this work, we showed that ANT is rapidly incorporated and metabolized in the marine alga U. lactuca reaching 46% of metabolization at 6 h of culture and 98% at 48 h. These results are accord with those previously obtained in the green macroalgae U. intestinalis and C. glomerata that metabolized 42–49% of BaP in 6 h22. This contrasts with results obtained in brown macroalgae such as Fucus vesiculosus and Chorda filum that metabolized only 5% of BaP after 4 days22. Thus, green macroalgae appeared to be more efficient than brown macroalgae to metabolize PAHs. Recently, it has been shown that U. lactuca metabolized 68% of BaP at 24 h and 75% of BaP at 72 h of culture24. Thus, U. lactuca metabolized more efficiently the three-ring ANT than the five-ring BaP but, interestingly, the alga can metabolize both PAHs.
ANT induced an oxidative stress condition in U. lactuca characterized by the accumulation of ROS, mainly superoxide anions. In addition, ANT increased the level of lipoperoxides with a maximal level of 1.25 µmol g−1 of DT at 24 h of culture which indicate that ROS induced membrane damage in the alga. These results are in accord with those obtained in U. lactuca cultivated with 5 µM of BaP that showed an increase in ROS, mainly superoxide anions, and an increase in lipoperoxides showing a maximal level of 0.68 µmol g−1 of DT at 6 and 12 h of culture24. Thus, it appeared that ANT induced a higher increase of oxidative stress than BaP reflected by membrane damage in U. lactuca. In this sense, it has been shown that ANT induced a higher inhibition of growth and a higher chlorosis than BaP in L. gibba2,3. Thus, it is possible that the higher toxicity of ANT compared to BaP is due to the induction of a higher membrane damage which may affect mainly chloroplasts inducing chlorosis. The aquatic liverwort R. communis cultivated with 0–10 μM of PHE for 72 h showed an increase in amino acid leakage from 2.5 to 10 µM and an increase in liperoxides and carbonylated proteins with 0.5 µM of PHE indicating that PHE also causes an oxidative stress condition and membrane damage13. Thus, PAHs cause oxidative stress and membrane damage in plants and algae and ANT is more toxic than BaP.
ANT induced an increase in activities of antioxidant enzymes SOD, CAT, AP, GR and GP, but not in DHAR. The activation of enzyme antioxidant system may participate in the buffering of ANT-induced oxidative stress. These results are in accord with those obtained in the aquatic plant R. fluitans cultivated with 0.5 μM of PHE for 96 h that showed an oxidative stress condition and the increase in activities of the antioxidant enzymes AP and GR13. A. thaliana plants cultivated with PHE also showed an oxidative stress condition and the increase in the level of transcripts encoding SOD, AP and GR14. Maize plants cultivated soil contaminated with 1–5% of petroleum for 30 days showed an increase in the level of transcripts of antioxidant enzymes such as SOD, AP, CAT and GR15. It has been recently shown that U. lactuca cultivated with BaP for 72 h showed an oxidative stress condition and the activation of antioxidant enzymes SOD, CAT, AP, GR and GP, but not DHAR24. Thus, plants and marine alga exposed to PAHs displayed an oxidative stress condition that is partially buffered by the activation by antioxidant enzymes. As in the case of U. lactuca and BaP, the alga cultivated with ANT did not show and increase in DHAR activity indicating that the Halliwell-Asada-Foyer cycle is uncoupled24,25. DHAR activity uses GSH as substrate and produces oxidized GSH (GSSG) as product24. On the other hand, it was observed that GR and GP showed the higher increase in activities in U. lactuca cultivated with ANT and BaP and, it is important to note, that both enzymes consume GSH. Thus, it is possible that the lack of DHAR activity is due to the high consumption of GSH by enzymes such as GR and GP. Thus, an important molecule for PAHs tolerance in U. lactuca is the potential synthesis and the consumption of GSH.
Interestingly, the level of transcripts of antioxidant enzymes SOD, CAT, AP, GR and GP increased in U. lactuca exposed to ANT, but not those encoding DHAR. The increase in the level of transcript of the latter antioxidant enzymes showed maximal levels that are previous to maximal enzyme activities indicating that the increase in activities is due, at least in part, to their enhanced expression. In addition, the level of transcripts of the metabolizing enzyme GST increased with a maximal level at 3 and 12 h and GST activity showed a maximal level at 24 h. Thus, the increase in GST metabolizing activity is also transcriptionally regulated. It is important to mention that GST also consumes GSH, as GR and GP, and this is another metabolic point of GSH consumption which may also explain the absence of DHAR activity (see above). It was previously shown that BaP also increase activity and expression of GST in U. lactuca24. The involvement of GST in the detoxification of PAHs and other hydrocarbons also occurred in plants since A. thaliana treated with PHE that showed an increase in the level of transcripts encoding 14 different GSTs14 and maize plants cultivated soil contaminated with petroleum showed an increase in the level of transcripts of five GSTs15. Thus, GSTs are key enzymes in degradation of PAHs and other hydrocarbons in plants and algae.
Interestingly, inhibitors of Phase I enzymes, CYP450-dependent monooxygenases and dioxygenases, and Phase II enzymes, PPOs, GSTs and STs are involved in ANT detoxification in U. lactuca mainly those that inhibit PPOs, GSTs and STs. In this sense, it has been shown in the green macroalgae E. intestinalis and C. glomerata metabolize BaP through activation of phenol oxidase activity and peroxidase22. In addition, GSTs are involved in PHE detoxification in willow trees (Salix viminalis) showing and increase in the level of GST transcripts and in those coding for enzymes involved in GSH synthesis26. GST activity was also increased in common beans (Phaseolus vulgaris) exposed to ANT and fluorene27. Regarding STs in plants, A. thaliana genome encode 21 ST genes and rice genome encode 29 functional genes and STs are involved in sulfation of hydroxyl groups present in flavonoids, phenolic acids, terpenes, gibberellic acids and glucosinolates, and in the detoxification of hydroxylated xenobiotics28. In genomes of red and brown micro- and macroalgae, several potential genes of STs have been detected but their substrate preference has not yet been identified29. Thus, plants green macroalgae exhibited Phase I and II enzymes activities that may be involved in PAHs detoxification.
Conclusion
The green macroalga U. lactuca rapidly incorporated and metabolized ANT reaching a complete degradation after 48 h of culture. ANT induced an oxidative stress condition characterized by the accumulation of ROS, mainly superoxide anions, and lipoperoxides. ANT induced the activation of antioxidant enzymes and the metabolizing enzyme GST and their activation was due, at least in part, by the increase in their expression. Using inhibitors, it was shown that monooxygenases, dioxygenases, GSTs, PPOs and STs participate in ANT metabolization in U. lactuca (see model in Fig. 8).
Figure 8.
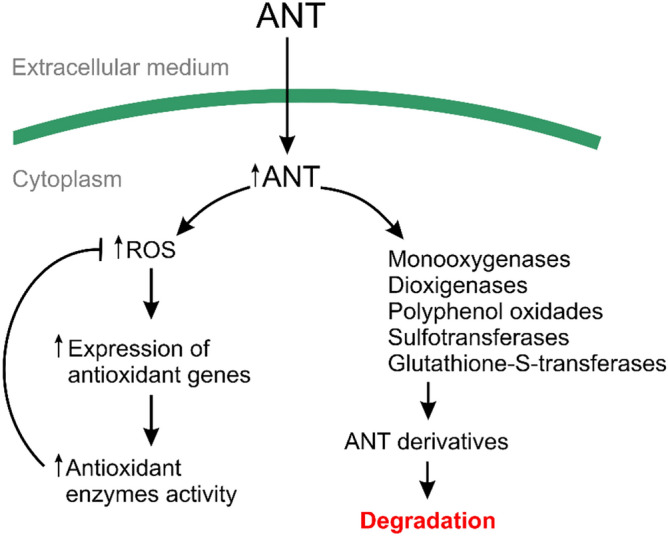
Model of anthracene detoxification in the marine alga U. lactuca. The alga cultivated with ANT rapidly incorporate and degrade this molecule showing an increase in ANT in the alga. ANT induces an oxidative stress condition leading to an increased expression of genes encoding antioxidant enzymes and their activities. Intracellular ANT is the substrate of metabolizing enzymes such as monooxygenases, dioxygenases, polyphenol oxidases, glutathione-S-transferases, and sulfotransferases.
Materials and methods
Alga sampling
Ulva lactuca was collected from Cachagua (32° 34′ S, 71° 27′ W) a non-polluted site from central Chile, transported in a cooler at 4 °C, manually cleaned from other algae species, sonicated twice using 53 Hz for 2 min to remove epiphytic bacteria, and maintained in artificial seawater (Sigma-Aldrich, St Louis, CA, USA).
In vitro cultures
Ulva lactuca (10 g of fresh tissue) was cultivated in 300 mL of artificial sea water without (control) or with 1, 10, 50, 100 or 250 µM of anthracene (ANT) having a purity ≥ 99% (Sigma-Aldrich, St Louis, CA, USA) for 7 days, at 14 °C with air bubbling and a photoperiod of 14 h light and 10 h darkness, as described in González et al.24. Since ANT has low solubility in water, a 25 mM of ANT stock solution was prepared in DMSO with a purity ≥ 99% (Sigma-Aldrich, St Louis, CA, USA) and then an aliquot was dissolved in the culture medium adjusting the DMSO to a final concentration of 0.5% v/v in the medium, including the control cultures. These cultures were used to analyze the accumulation of ANT, for qPCR analyzes, the detection of enzyme activities, and only few fronds were used for cell viability analyzes. All these analyses were performed as independent triplicates.
For assays performed with Phase I and Phase II inhibitors, 10 g of alga were cultivated in 300 mL of artificial seawater without or with the addition of 0.5 µM of mebendazole (MBZ), an inhibitor of CYP450-dependent monooxygenases; cyclohexanedione (CHD), an inhibitor of dioxygenases; 2-naphtoic acid (2-NA), an inhibitor of polyphenol oxidases; ellagic acid (ELA), an inhibitor of GST; and quercetin (QC), an inhibitor of sulfotransferases, and with 5 µM of ANT for 24 h, in triplicates.
Analysis of cell viability
Three algal laminae were cultivated without ANT and with increasing concentrations of ANT for 7 days and cell integrity and morphology were visualized using Axiovert 100 confocal microscope (Zeiss, Oberkochen, Germany) using an emission wavelength of 488 nm from an argon laser and a filter of 505–550 nm for detection of chlorophyll fluorescence. The images were analyzed using the software LSM510 (Zeiss, Oberkochen, Germany).
Extraction of anthracene from algae and seawater
The extraction of ANT from algal tissue was performed as described by Sadowska-Rociek et al.30, with modifications. One gram of algal dried tissue (DT), which corresponds to 7 g of algal fresh tissue (FT), were pulverized in a mortar with liquid nitrogen and homogenized in 15 mL of cyclohexane having HPLC grade purity (Merck, Darmstadt, Germany). Cellular debris were removed using Quechers AOAC method kit (Agilent Technologies, Santa Clara, CA, USA) and centrifugation at 10,000×g for 10 min31. Pigments were removed using Quechers SPE dispersive kit (Agilent Technologies, Santa Clara, CA, USA) (Gratz et al.31). The extracts recovered (7 mL) were filtered using PVDF filters with 0.2 µm pore size (Finetech, Taichung, Taiwan) and stored in amber glass vials at 4 °C.
Extraction of ANT from artificial seawater was performed as described by Colombo et al.32, with modifications. The culture medium (300 mL) was extracted twice with 200 mL of cyclohexane using an extraction funnel, and the extracts were concentrated to 2 mL using a rotatory evaporator HeiVap (Heidolph Industries, Schwabach, Germany). The extracts were filtered using PVDF filters with 0.2 µm pore size (Finetech, Taichung, Taiwan) and stored in amber glass vials at 4 °C.
Extraction of ANT from cultures with inhibitors was performed according to Warshawski et al.17, with modifications. Fresh tissue (10 g) was mixed with 250 mL of ethyl acetate HPLC grade purity (Merck, Darmstadt, Germany) and subjected to sonication for 30 min at room temperature. The solvent was collected and concentrated to 5 mL using a rotatory evaporator HeiVap. For the elimination of chlorophyll and pigments, a reaction mixture of 1 mL was prepared with an aliquot of 100 µL of the extract, 150 mM tert-butyl hydroperoxide and 50 mM sodium hydroxide in acetonitrile. The preparation was agitated in vortex for 60 min and then centrifuged at 10,000×g for 10 min. The supernatant was recovered and filtered using PVDF filters with 0.2 µm pore size and stored at 4 °C.
Quantification of anthracene by HPLC
ANT in extracts was analyzed according to Gratz et al.31 using an Infinity 1260 series HPLC (Agilent Technologies, Santa Clara, CA, USA) having a reverse phase column Zorbax Eclipse XDB-C18 (4.5 mm × 15 mm and particle size of 5 µm, Agilent Technologies, Santa Clara, CA, USA) and a fluorescence detector. The elution program consisted in a mobile phase of H2O/CH3CN with a flux of 0.8 mL min−1 at 25 °C and a step from 0 to 1.5 min of 40% H2O and 60% CH3CN, a step from 1.5 to 7 min of 10% H2O and 90% CH3CN, and a step from 7 to 13 min of 0% H2O and 100% CH3CN. ANT was detected using excitation wavelength of 260 nm and emission wavelength of 520 nm a at retention time of 6.03 min, representative chromatograms for the standard of ANT and for ANT in tissue and in the culture medium are presented in Supplementary Figure S1.
Quantification of hydrogen peroxide, superoxide anions and lipoperoxides
Hydrogen peroxide was determined as described in González et al.33. Algae (1.5 g of FT) were collected from cultures every 30 min and immediately incubated in 100 mM phosphate buffer pH 7.0 containing 10 µM of 2′,7′-dichlorodihydrofluorescein-diacetate (DCF-DA, Invitrogen, Carlsbad, CA, USA) for 30 min. Algae were rinsed with fresh artificial seawater and dried with tissue paper. The samples were pulverized in a mortar with liquid nitrogen, homogenized in 5 mL Tris–HCl pH 7.0 and centrifuged at 10,000×g for 15 min. DCF fluorescence was determined using an excitation wavelength of 480 nm and an emission wavelength of 590 nm using a spectrofluorometer PerkinElmer model LS-5 (PerkinElmer, Waltham, MA, USA) The concentration of hydrogen peroxide was calculated using a calibration curve prepared with 0–1 µM of DCF.
Superoxide ions were determined as described in González et al.33. Algae (1.5 g of FT) were collected from cultures every 2 h and immediately incubated in 100 mM phosphate buffer pH 7.0 containing 100 µM hydroethidine (Invitrogen, Carlsbad, CA, USA) for 30 min. Algae were pulverized in a mortar with liquid nitrogen, homogenized in 5 mL Tris–HCl pH 7,0 and centrifuged at 10,000×g for 15 min. 2-Hydroxy ethidium (2-HE) fluorescence was detected using an excitation wavelength of 488 nm and an emission wavelength of 525 nm using a spectrofluorometer PerkinElmer model LS-5. The concentration of superoxide ions was calculated using the extinction coefficient of 2-HE (ε = 9.4 mM−1 cm−1).
Lipoperoxides were determined as described in Ratkevicius et al.34. Algae (1 g of FT) were pulverized in a mortar with liquid nitrogen and homogenized with 5 mL of 0.1% v/v of trichloroacetic acid (TCA). The mixture was centrifuged at 10,000×g for 20 min, a sample of 200 µL was collected. The sample was mixed with 800 µL of an aqueous solution of 0.5% v/v thiobarbituric acid in 20% m/v TCA and the mixture was incubated at 90 °C for 30 min. The absorbance of malondialdehyde (MDA) adduct was measured at 510 nm using a spectrophotometer model Cary 8520 (Agilent Technologies, Santa Clara, CA, USA). The concentration of lipoperoxides was calculated using the extinction coefficient of MDA adduct (ε = 155 mM−1 cm−1).
Preparation of protein extracts
Protein extracts were prepared González et al.33. Fresh tissue (6 g) was pulverized in a mortar with liquid nitrogen and homogenized with 30 mL of 100 mM sodium phosphate buffer pH 7.4 containing 5 mM 2-mercaptoethanol and the mixture was centrifuged at 10,000×g for 30 min at 4°. The proteins in the supernatant were precipitated with 0.6 g mL−1 ammonium sulphate, shaking at 4° for 1 h and then centrifuged at 10,000×g for 30 min. Protein pellet was solubilized in 1 mL 100 mM sodium phosphate buffer pH 7.4 containing 20% glycerol and 2 mM 2-mercaptoethanol. Protein concentration was determined according to Bradford35.
Detection of antioxidant enzyme activities
Antioxidant enzyme activities were detected as described in González et al.24. Superoxide dismutase (SOD) activity was determined in 1 mL of reaction mixture containing 30 mM Tris–HCl pH 7.0, 0.1 mM EDTA, 20 mM riboflavin, 0.6 mM NBT and without protein extract or with 50 µg of protein extract. The reaction mixture was incubated under white light for 15 min and absorbance of formazan, the reduced form of NBT, was determined at 560 nm. The specific activity of SOD was calculated considering that 1 U of enzyme is the amount of the enzyme that reduces 50% of total NBT which is equal to the amount of dismutated superoxide anions.
Catalase (CAT) activity and other antioxidant enzyme activities were determined according to Ratkevicius et al.34 in 1 mL of reaction mix containing 100 mM sodium phosphate buffer pH 7.0, 1 mM hydrogen peroxide and without protein extract or with 30 µg of protein extract. The decrease in absorbance due to consumption of hydrogen peroxide was detected at 240 nm for 2 min. The specific activity of CAT was calculated using a calibration curve prepared with hydrogen peroxide from 1 nM to 1 mM.
Ascorbate peroxidase (AP) activity was determined in 1 mL of reaction mixture containing 100 mM sodium phosphate buffer pH 7.0, 0.4 mM ascorbate, 1 mM hydrogen peroxide and without protein extract or with 20 µg of protein extract. The decrease in absorbance due to the consumption of ascorbate was detected at 290 nm for 1 min. The specific activity was calculated using the molar extinction coefficient of ascorbate (ε = 2.8 mM−1 cm−1).
Dehydroascorbate reductase (DHAR) activity was determined in 1 mL of reaction mixture containing 100 mM sodium phosphate buffer pH 7.0, 1 mM glutathione, 0.5 mM of dehydroascorbate, and 50 µg of protein extract. The increase in absorbance due to production of ascorbate was detected at 290 was for 2 min. The specific activity was calculated using the molar extinction coefficient of ascorbate (ε = 2.8 mM−1 cm−1).
Glutathione reductase (GR) activity was determined in 1 mL of reaction mix containing 100 mM sodium phosphate buffer pH 7.0, 0.5 mM GSSG, 0.15 mM NADPH and 20 µg of protein extract. The decrease in absorbance due to consumption of NADPH was detected at 340 nm for 1 min. The specific activity was calculated using the NADPH molar extinction coefficient of NADPH (ε = 6.2 mM−1 cm−1).
Glutathione peroxidase (GP) was determined in 1 mL of reaction mix containing 100 mM sodium phosphate buffer pH 7.0, 0.5 mM glutathione, 0.15 mM NADPH, 1 U glutathione reductase, 1 mM hydrogen peroxide and without protein extract or with 20 µg of protein extract. The decrease in absorbance due to consumption of NADPH was detected at 340 nm for 2 min. The specific activity was calculated using the NADPH molar extinction coefficient of NADPH (ε = 6.2 mM−1 cm−1).
Detection of detoxification enzyme
Detection of detoxification enzymes was performed as described in González et al.24. Glutathione-S-transferase (GST) activity was determined in 1 mL of reaction mix containing 100 mM sodium phosphate buffer pH 7.0, 0.5 mM GSH, 1 mM CDNB and 50 µg of protein extract. The increase in absorbance due the formation of the adduct GSH-CDNB was detected at 340 nm 2 min. The specific activity was calculated using molar extinction coefficient of the adduct (ε = 9.6 mM−1 cm−1).
RNA extraction and qPCR analysis
RNA extraction and qPCR analysis was performed as described in González et al.24. Total RNA was extracted from 50 mg of fresh tissue using the FavorPrep Plant Total RNA mini kit (Favorgen, Ping-Tung, Taiwan). Total RNA (2 µg) was used to synthesize cDNA using the Affinityscript qPCR cDNA synthesis kit (Agilent Technologies, Santa Clara, CA, USA). Real time PCR was performed with 50 ng of cDNA, 400 nM of each primer and using Brilliant III ultra-fast SYBR green qPCR kit (Agilent Technologies, Santa Clara, CA, USA) and an Aria MX real-time PCR system (Agilent Technologies, Santa Clara, CA, USA). The amplification program consisted of an initial step of 3 min at 95 °C and 40 cycles of 5 s at 95 °C followed by 10 s at 55 °C. Primers to detect SOD, AP, CAT, GR, GST transcripts were designed using sequences obtained from U. intestinalis, U. fasciata and U. compressa (NCBI GeneBank, U. compressa SRA records SRP145672 and PRJNA557176). Primers were: superoxide dismutase (GeneBank EF437244.1) SOD-F 5′CCT GCT CAG GCA ACC TCC TT3′, SOD-R 5′TGC CAG CCT TCC AGT CTC AC3′, catalase (GeneBank DQ286544.1) CAT-F 5′ACC AAG AGG CGG AGA AAG TG3′, CAT-R 5′CGA AGT CAA ACC GGT CCT CA3′, ascorbate peroxidase (GeneBank DQ286543.1) AP-F 5′CCA CAA GGC TGA GAC CAA GT3′, AP-R 5′CTT TGT CCA CTC AGG GGT CC3′, dehydroascorbate reductase (GeneBank XM_001698323.1) DHAR-F 5′GCG ACT CGT ACT GCT CCT AC3′, DHAR-R 5′GAA AAC GGA ACT CCG CGC TT3′, glutathione reductase (GeneBank DQ286546.1) GR-F 5′AGT TCG GGG ACG TGG ATG TG3′, GR-R 5′CTC TCG TTG CCG GAG ATC GT3′, glutathione peroxidase (GeneBank XM_001698523.1) GPX-F 5′CAC GCG TCC GGT AAC ACT3′, GPX-R 5′GGG ACG AGA AAG GTT GCT GA3′. Glutathione S-transferase (GeneBank FD387475.1) GST-F 5′GGA CCC ATG CAA CGC CAA G3′, GST-R 5′GGT GCG CTG TAC ACC CAA GA3′, and α-tubulin (GeneBank EU701065.1) as housekeeping transcript, TUB-F 5′ACA GGC TCA TTG CTC AGG TC3′, TUB-R 5′TCT CGG CAG AGA TGA CTG GA3’.
Statistical analysis
Experimental results were subject to one-way analysis of variance (ANOVA) and post hoc Tukey Multiple Comparison Test using the statistical software Prism 6 (Graph Pad software Inc., San Diego, CA, USA), previous to the evaluation of the requirements of normality and homogeneity of variance. Significant differences were estimated using three independent replicates at a 95% confidence interval.
Supplementary Information
Acknowledgements
This work was financed by Fondecyt de Iniciación 11180189 and ANID+PAI Concurso Nacional de Inserción de Capital Humano Avanzado en la Academia convocatoria año 2017 PAI79170105 to A.G.
Author contributions
A.G. performed algal cultures, confocal microscopy, ANT extractions, HPLC analyses, ROS determinations, enzyme activities assays, statistical analyses and prepared the figures; C.V. performed ANT extractions and HPLC analyses; D.E. performed qPCR detections; A.M. supervised the work and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87147-5.
References
- 1.Mojiri A, Zhou JI, Ohashi A, Ozaki N, Kindaichi T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatment. Sci. Total Environ. 2019;696:133971. doi: 10.1016/j.scitotenv.2019.133971. [DOI] [PubMed] [Google Scholar]
- 2.Huang XD, Dixon DG, Greenberg BM. Impact of UV radiation and photomodification on the toxicity of PAHs to the higher plant Lemna gibba (duckweed) Environ. Toxicol. Chem. 1993;12:1067–1077. [Google Scholar]
- 3.Huang XD, Dixon DG, Greenberg BM. Increased polycyclic hydrocarbons following their photomodification in natural sunlight: Impact on the duckweed Lemna gibba L. G-3. Ecotox. Environ. Saf. 1995;32:194–200. doi: 10.1006/eesa.1995.1102. [DOI] [PubMed] [Google Scholar]
- 4.Oyarzo-Miranda C, et al. Costal pollution from industrial park Quintero bay of central Chile: Effects on abundance morphology, and development of the kelp Lessonia spicata (Phaeophyceae) PLoS One. 2020;15:e0240581. doi: 10.1371/journal.pone.0240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carranza DM, et al. Socio-environmental conflicts: An underestimated threat to biodiversity conservation in Chile. Environ. Sci. Pol. 2020;110:46–59. doi: 10.1016/j.envsci.2020.04.006. [DOI] [Google Scholar]
- 6.Greenberg BM, Huang XDH, Dixon D. Applications of the aquatic higher plant Lemna gibba for ecotoxicological assessment. J. Aquat. Ecosyst. Health. 1992;1:141–155. doi: 10.1007/BF00044046. [DOI] [Google Scholar]
- 7.Duxbury CL, Dixon DG, Greenberg BM. Effects of stimulated solar radiation on the bioaccumulation of polycyclic aromatic hydrocarbons by the duckweed Lemna gibba. Environ. Ecotoxicol. Chem. 1997;16:1739–1748. doi: 10.1002/etc.5620160824. [DOI] [Google Scholar]
- 8.Mallakin A, et al. Impact of structural photomodification on the toxicity of environmental contaminants: Anthracene photooxidation products. Ecotox. Environ. Saf. 1999;43:204–212. doi: 10.1006/eesa.1999.1764. [DOI] [PubMed] [Google Scholar]
- 9.Jancová P, Azenbacher P, Azembacherova E. Phase II drug metabolizing enzymes. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010;104:103–116. doi: 10.5507/bp.2010.017. [DOI] [PubMed] [Google Scholar]
- 10.Alagic SC, Maluchov BS, Radojicic VB. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation?. A review. Clean Technol. Environ. Pol. 2015;17:597–614. doi: 10.1007/s10098-014-0840-6. [DOI] [Google Scholar]
- 11.Gao Y, Zhang Y, Liu J, Kong H. Metabolism and subcellular distribution of anthracene in tall fescue (Festuca arundinacea Screb.) Plant Soil. 2013;365:171–182. doi: 10.1007/s11104-012-1386-1. [DOI] [Google Scholar]
- 12.Wild E, Dent J, Thomas GO, Jones KC. Direct observation of organic contaminant uptake, storage, and metabolism within plant roots. Env. Sci. Technol. 2005;39:3695–3702. doi: 10.1021/es048136a. [DOI] [PubMed] [Google Scholar]
- 13.Burritt DJ. The polycyclic aromatic hydrocarbon phenanthrene causes oxidative stress and alters polyamine metabolism in the aquatic liverwort Riccia fluitans L. Plant Cell Environ. 2008;31:1416–1431. doi: 10.1111/j.1365-3040.2008.01846.x. [DOI] [PubMed] [Google Scholar]
- 14.Weisman D, Alkio M, Colón-Carmona A. Transcriptional responses to polycyclic aromatic hydrocarbon-induced stress in Arabidopsis thaliana reveal the involvement of hormone and defense signaling pathways. BMC Plant Biol. 2010;10:59. doi: 10.1186/1471-2229-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cevher-Keskin B, et al. Comparative transcriptome analysis of Zea mays in response to petroleum hydrocarbon stress. Environ. Sci. Poll. Res. 2018;25:32660–32674. doi: 10.1007/s11356-018-3078-8. [DOI] [PubMed] [Google Scholar]
- 16.Gala WR, Giesy JP. Flow cytometry determination of the photoinduced toxicity of anthracene to the green microalga Selenestratum capricornutum. Environ. Ecotoxicol. Chem. 1994;5:831–840. doi: 10.1002/etc.5620130519. [DOI] [Google Scholar]
- 17.Warshawski D, et al. Biotransformation of benzo[α]pyrene and other polycyclic aromatic hydrocarbons and heterocyclic analogs by several green algae and other algal species under gold and white light. Chem. Biol. Interact. 1995;97:131–148. doi: 10.1016/0009-2797(95)03610-X. [DOI] [PubMed] [Google Scholar]
- 18.Aksmann A, Tukaj Z. The effect of anthracene and phenanthrene on the growth, photosynthesis, and SOD activity of the green alga Scenedesmus armatus depends on the PAR irradiance and CO2 level. Arch. Environ. Contam. Toxicol. 2004;47:177–184. doi: 10.1007/s00244-004-2297-9. [DOI] [PubMed] [Google Scholar]
- 19.Aksmann A, Tukaj Z. Intact anthracene inhibits photosynthesis in algal cells: A fluorescence induction study on Chlamydomonas reinhardtii cw92 strain. Chemosphere. 2008;74:26–32. doi: 10.1016/j.chemosphere.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 20.Vieira LR, Guilhermino L. Multiple stress effects on marine planktonic organisms: Influence of temperature on the toxicity of polycyclic aromatic hydrocarbons to Tetraselmis chuii. J. Sea Res. 2012;72:94–98. doi: 10.1016/j.seares.2012.02.004. [DOI] [Google Scholar]
- 21.Kottuparambil S, Park J. Anthracene phytoxicity in the freshwater flagellate alga Euglena agilis Carter. Sci. Rep. 2019;9:15323. doi: 10.1038/s41598-019-51451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirso U, Ihra N. Role of algae in fate of carcinogenic polycyclic aromatic hydrocarbons in the aquatic environment. Ecotox. Environ. Saf. 1998;41:83–89. doi: 10.1006/eesa.1998.1671. [DOI] [PubMed] [Google Scholar]
- 23.Kokovicz-Pilatti F, et al. In vitro exposure of Ulva lactuca Linnaeus (Chlorophyta) to gasoline-biochemical and morphological alterations. Chemosphere. 2016;156:428–437. doi: 10.1016/j.chemosphere.2016.04.126. [DOI] [PubMed] [Google Scholar]
- 24.González A, Espinoza D, Vidal C, Moenne A. Benzopyrene induces oxidative stress and increases expression and activities of antioxidant enzymes, and CYP450 and GST metabolizing enzymes in Ulva lactuca (Chlorophyta) Planta. 2020;252:107. doi: 10.1007/s00425-020-03508-w. [DOI] [PubMed] [Google Scholar]
- 25.Foyer C, Noctor G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011;155:2–8. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, et al. Transcriptomic and metabolomic insights into the adaptive response of Salix viminalis to phenanthrene. Chemosphere. 2021;262:127573. doi: 10.1016/j.chemosphere.2020.127573. [DOI] [PubMed] [Google Scholar]
- 27.Pascová V, Hilsherová K, Feldmannová M, Bláha L. Toxic effects and oxidative stress in higher plants exposed to polycyclic aromatic hydrocarbons and their N-heterocyclic derivatives. Environ. Toxicol. Chem. 2006;12:3238–3245. doi: 10.1897/06-162R.1. [DOI] [PubMed] [Google Scholar]
- 28.Hirschmann F, Krause F, Papenbrock J. The multi-protein family of sulfotransferases in plants: Composition, occurrence substrate specificity, and functions. Front. Plant Sci. 2014;5:556. doi: 10.3389/fpls.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho CL. Phylogeny of algal sequences encoding carbohydrate sulfotransferases formylglycine-dependent sulfatase, and putative sulfatase modifying factors. Front. Plant Sci. 2015;6:1057. doi: 10.3389/fpls.2015.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadowska-Rociek A, Surma M, Cieslik E. Application of QuEChERS method for simultaneous determination of pesticide residues and PAHs in fresh herbs. Bull. Environ. Cont. Toxicol. 2013;90:508–513. doi: 10.1007/s00128-012-0951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gratz SR, et al. Screening and determination of polycyclic aromatic hydrocarbons in seafood using QuEChERS-base extraction and high-performance liquid chromatography with fluorescence detection. J. AOAC Int. 2011;94:1601–1616. doi: 10.5740/jaoacint.11-035. [DOI] [PubMed] [Google Scholar]
- 32.Colombo JC, et al. Oil spill in the Río de la Plata estuary, Argentina. 1. Biogeochemical assessment of water, sediments, soils and biota. Environ. Pollut. 2005;134:277–289. doi: 10.1016/j.envpol.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 33.González, et al. Co-occurring increases of calcium and organellar reactive oxygen species determine differential activation of antioxidant and defense enzymes in Ulva compressa (Chlorophyta) exposed to copper excess. Plant Cell Environ. 2010;33:1627–1640. doi: 10.1111/j.1365-3040.2010.02169.x. [DOI] [PubMed] [Google Scholar]
- 34.Ratkevicius N, Correa JA, Moenne A. Copper accumulation, synthesis of ascorbate and activation of ascorbate peroxidase in Enteromorpha compressa (L.) Grev. (Chlorophyta) from heavy-metal-enriched environments in Northern Chile. Plant Cell Environ. 2003;26:1599–1608. doi: 10.1046/j.1365-3040.2003.01073.x. [DOI] [Google Scholar]
- 35.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.