Abstract
Objectives:
The ability of noninvasive brain stimulation to modulate corticospinal excitability and plasticity is influenced by genetic predilections such as the coding for brain-derived neurotrophic factor (BDNF). Otherwise healthy individuals presenting with BDNF Val66Met (Val/Met) polymorphism are less susceptible to changes in excitability in response to repetitive transcranial magnetic (TMS) and paired associative stimulation paradigms, reflecting reduced neuroplasticity, compared to Val homozygotes (Val/Val). In the current study, we investigated whether BDNF polymorphism influences “baseline” excitability under TMS conditions that are not repetitive or plasticity-inducing. Cross-sectional BDNF levels could predict TMS response more generally because of the ongoing plasticity processes.
Materials and Methods:
Forty-five healthy individuals (23 females; age: 25.3±7.0 years) participated in the study, comprising two groups. Motor evoked potentials (MEP) were collected using single-pulse TMS paradigms at fixed stimulation intensities at 110% of the resting motor threshold in one group, and individually-derived intensities based on MEP sizes of 1 mV in the second group. Functional variant Val66Met (rs6265) was genotyped from saliva samples by a technician blinded to the identity of DNA samples.
Results:
Twenty-seven participants (60.0%) were identified with Val/Val, sixteen (35.5%) with Val/Met genotype, and two with Met/Met genotype. MEP amplitudes were significantly diminished in the Val/Met than Val/Val individuals. These results held independent of the single-pulse TMS paradigm of choice (p=0.017 110% group; p=0.035 1mV group), age, and scalp-to-coil distances.
Conclusions:
The findings should be further substantiated in larger-scale studies. If validated, intrinsic differences by BDNF polymorphism status could index response to TMS prior to implementing plasticity-inducing protocols.
Keywords: Brain-derived neurotrophic factor, polymorphisms, single-pulse transcranial magnetic stimulation, neuroplasticity, motor excitability
INTRODUCTION
Since its discovery in the early 1980s, brain-derived neurotrophic factor (BDNF) has been associated with a multitude of survival and growth-promoting functions in the central nervous system (CNS), including but not limited to brain development, neurogenesis, synaptogenesis and synaptic transmission. BDNF is a neurotrophin, belonging to a family of polypeptide growth factors, which along with neurotrophin-3 (NT-3) is highly expressed in the CNS, even in adults1,2. Regulated activity-dependent secretion of BDNF is associated with synaptic plasticity and efficacy3, strengthening excitatory (glutamatergic) synapses and weakening inhibitory (GABAergic) synapses4. BDNF has been shown to play an influential role in several neurological and psychiatric disorders such as mild traumatic brain injury, dementia, bipolar disorder and anxiety-related behaviors2,5–8, and is also linked to impairment in cognitive abilities during normal aging9.
Rs6265 is a common single nucleotide BDNF polymorphism, resulting in a Val66Met gene variation due to a point-substitution of valine to methionine amino acid at position 66 in the pro-region of the BDNF gene10. This polymorphism is found only in humans and is the first mutation in a neurotrophin gene linked to clinical pathology in humans1. Thus, it has received a great deal of attention in the recent years. The presence of BDNF Val66Met polymorphism is associated with decreases in activity-dependent BDNF secretion in response to synaptic activity11. BDNF is involved in early- and late-stage long-term potentiation (LTP) mechanisms, particularly in hippocampal synapses4,11, and its polymorphism then has been shown to affect higher-order cognitive functions such as learning and memory12,13 as well as motor functions14. Individuals carrying the Met allele also have been shown to elicit altered functional brain activation patterns, and volumetric reductions in bilateral hippocampi, dorsolateral prefrontal cortex and caudate nuclei9,15.
Given the important role of BDNF in neuroplasticity mechanisms such as LTP and the profound influence of its polymorphism on brain structure and function, several prior studies have characterized its involvement in induced neuromodulatory processes. These studies employed non-invasive brain stimulation (NIBS)16 to examine the influence of polymorphism on exogenously induced plasticity. We know from this work that the Val66Met polymorphism (Val/Met) affects the ability of NIBS to induce changes in corticospinal excitability17,18. Decreased aftereffects of NIBS are reported in Met carriers compared to Val homozygotes (Val/Val) in multiple studies, using a variety of repetitive transcranial magnetic stimulation (rTMS)18, intermittent theta burst stimulation (iTBS)19, and paired associative stimulation16,20 paradigms. Such differences are hypothesized to reflect lesser susceptibility of the Met carriers to stimulation-induced modulation or cortical excitability. However, there are also reports of null findings indicating no differences in aftereffects based on polymorphism status21–23, and thus the findings regarding the impact of the BDNF polymorphism on stimulation-induced neuroplasticity are mixed.
The rationale for using repetitive or plasticity-inducing NIBS paradigms in these prior studies was to influence the secretion of BDNF in an activity-dependent manner. Insofar as activity-dependent secretion of BDNF is relatively reduced in Met carriers, this may be one mechanism in a complex cascade of synaptic events responsible for differences in neuroplasticity observed in response to exogenous stimulation paradigms. In the current study, we were interested in examining inherent differences in excitability, rather than changes or modulations to it, based on BDNF polymorphism status or presence of Met allele. We reasoned that BDNF polymorphism status might influence ongoing plasticity processes or synaptic activity even without any exogenous manipulation using rTMS paradigms. This effect could potentially be reflected in cross-sectional differences in general responsiveness to single pulses of TMS. We were motivated to explore the role of BDNF polymorphism on baseline cortical excitability based on two anecdotal observations we had made during data collection for a different study. One participant (39-year-old female) had a relatively low resting motor threshold (rMT) at 31%, but was stimulated at up to 152% of rMT in an attempt to increase her motor evoked potential (MEP) to 1 millivolt (mV) averaged amplitude. Even at this high intensity, she only achieved mean MEP amplitudes of 0.52 mV. Another individual (40-year-old male) was stimulated at 100% of the stimulator output yet only achieved a mean MEP amplitude of 0.22 mV. Both of these unusual participants were later determined to have the BDNF Val66Met polymorphism, prompting us to consider how BDNF status might impact baseline MEPs and inspiring the current investigation.
We focused on the examination of baseline intrinsic differences in cortical excitability based on BDNF polymorphism status and whether these differences can be probed under stimulation conditions that are neither plasticity-inducing nor repetitive. Based on the findings from prior TMS plasticity-inducing protocols and our anecdotal observations, we hypothesized reduced excitability in individuals presenting with Val/Met polymorphism. We tested this hypothesis in a retrospective study by measuring differences in transiently evoked motor responses or MEP. MEPs were measured in response to single-pulses of TMS delivered aperiodically from hand muscles at rest, as opposed to a pre-/post-stimulation assessment of MEP amplitudes that is typically done in response to repetitive paradigms.
A sigmoidal input/output relationship between MEP amplitudes and increasing stimulation intensities (SI) is well-documented in the TMS literature24. This relationship indicates that the TMS protocol employed for SI determination could directly impact the MEP amplitudes. Two protocols are widely used in TMS studies: 1) SI is fixed at 110 percent of the resting motor threshold (rMT), and 2) SI is adjusted to obtain a constant MEP amplitude of 1 mV. We adopted both these SI protocols and employed one of each in two different groups of participants (110% group and 1 mV group). The TMS output results, as in the MEP amplitudes, were evaluated separately in the two SI groups for evaluating differences based on BDNF polymorphism status. We deemed this additional level of analyses or grouping necessary to rule out the possibility of differences arising from how SIs were determined and their impact on excitability (i.e. higher the SI, potentially greater the excitability), rather than the BDNF polymorphism status. These study findings can have direct implications for understanding how alterations in BDNF may mediate individual differences in response to TMS, as well as for predicting treatment responsiveness to plasticity-inducing TMS paradigms in clinical populations.
MATERIALS AND METHODS
Participants
Forty-five healthy individuals between the ages of 18 and 45 years (23 females; mean age: 25.3±7.0 years) with no history of neurological or psychiatric disorders participated in this study. None of the participants had any contraindications to receiving TMS. Participants with T1-weighted MRI scans from prior research studies were included. All participants were right-handed. Saliva samples were collected from all participants for genotyping the BDNF polymorphism. As described in detail in the sections below, we employed two single-pulse TMS protocols. In 13 participants, SI for evoking MEPs was set to 110% of the rMT, and in 32 participants, SI was adjusted to evoke MEPs of 1 mV peak-peak (p-p) amplitude. All participants provided informed consent for this study, which was approved by the local Institutional Review Board.
BDNF genotyping procedures
Genomic DNA from human saliva samples was collected in Oragene® DNA collection kits and was then isolated using the prepIT.L2Preagent (cat # PT-L2P-5, DNA Genotek Inc, Canada) and precipitated with ethanol according to manufacturer’s instructions. The DNA samples were genotyped for BDNF (the single nucleotide polymorphism rs6265) using the TaqMan SNP Genotyping Assay (C__11592758_10) designed by Thermo Fisher Scientific. Primers and probes were mixed with TaqMan® Universal PCR Master Mix (Thermo Fisher Scientific). 4.5 μL of genomic DNA (2.5 ng/ μL) was transferred in triplicate to a 384-well plate, with each well containing 5.5 μL of the PCR mixture. The PCR reaction was performed following a protocol provided by ABI. The allele was discriminated by post-PCR plate reading on the ViiA™ 7 System. Data were processed using the ViiA™ 7 Software (Thermo Fisher Scientific)6,25. Genotyping was done by a technician, blinded to the identity of DNA samples.
Single-pulse TMS protocols and MEP acquisition
Stimulation was administered using the Magstim 2002 transcranial magnetic stimulator (monophasic waveform) connected to a 70-mm diameter figure-of-eight, air-cooled coil (Magstim, Whitland, UK). Participants’ T1-weighted MRI scans were uploaded to the Brainsight® Neuronavigation system (Rogue Research, Montreal) and were used to identify the optimal scalp position within the left primary motor cortex (M1) for eliciting MEPs from the right first dorsal interosseous (FDI) muscle; the determination of the optimal scalp position was based on the magnitude of MEPs evaluated in multiple locations around the ‘hand knob’ area. The electromyography (EMG) electrodes for acquiring MEPs were placed using a belly-tendon montage, with the reference electrode on the pointer finger knuckle and the ground electrode on the wrist of the same hand. The MEPs were acquired as participants rested their arms and hands onto the armrest of the TMS chair. RMT was defined as the stimulation intensity at which a minimum of 5 out of 10 MEPs were obtained at or less than 50μV p-p amplitude26; the rMT and SI are expressed as percentage values of the maximum stimulator output (%MSO). In the 1mV group, the starting point for the SI to acquire MEPs was at the rMT, after which the intensity was steadily increased by 1–2% until 10–12 consecutive MEPs were approximately 1 mV p-p amplitude (trial-by-trial variations in MEP amplitudes could be under or slightly above 1 mV). No adjustments in SI were made in the 110% rMT group, beyond determining the rMT. A real-time output of MEP sizes was available for locating target M1 area and for SI determination. In both the 110% and the 1mV groups, 25–35 MEPs were acquired via single-pulse TMS delivered at an inter-pulse interval of 6 seconds (±6% variance). The coil position was maintained at the optimal scalp location and orientation using the neuronavigation system during the entire course of MEP acquisition, which lasted about 6–9 minutes. Cambridge Electronic Design interface was used for digitizing and preprocessing of the EMG signals to obtain MEPs. The computation of the p-p MEP amplitudes was then carried out post-hoc using Signal software (Cambridge Electronic Design Ltd., UK). All experimenters and participants were blind to the BDNF genotype. Scalp-to-TMS coil distance measurements were calculated using the ruler tool in Brainsight. Using the FDI target identified during rMT acquisition, a line along the TMS coil’s modeled magnetic field trajectory was traced, measuring the distance between the FDI target on the scalp and the cortical surface of M1 in millimeters (mm).
Statistical analyses
Wilcoxon rank sum tests were conducted to compare age, scalp-to-coil distances, rMT and SI between Val/Val and Val/Met carriers separately for each SI group (110%, 1mV; Table 1).
Table 1.
Demographics and TMS details by BDNF genotyping
| Val/Val | Val/Met | §p-val | #Met/Met | |
|---|---|---|---|---|
| N = 45 | 27 60.0% |
16 35.5% |
--- | 2 4.4% |
| *N | 7 | 4 | --- | --- |
| Sex (F/M) | 5/2 | 3/1 | --- | --- |
| Caucasian | 4 | 2 | --- | --- |
| Asian | 0 | 1 | --- | --- |
| African-American | 3 | 1 | --- | --- |
| Scalp-to-coil distance (mm) | 17.5 (±2.7) | 18.4 (±2.7) | n.s. | --- |
| rMT (%) | 49.6 (±7.9) | 66.7 (±24.5) | n.s. | --- |
| N | 19 | 11 | --- | 2 |
| Sex (F/M) | 10/9 | 4/7 | --- | 0/2 |
| Caucasian | 6 | 7 | --- | --- |
| Asian | 1 | 3 | --- | --- |
| African-American | 8 | 1 | --- | --- |
| Mix | 2 | 0 | --- | --- |
| Unknown | 2 | --- | --- | 2 |
| Scalp-to-coil distance (mm) | 18.2 (±2.2) | 17.6 (±3.2) | n.s. | 15.1 (±2.4) |
| rMT (%MSO) | 48.7 (±8.3) | 41.6 (±8.1) | 0.050 r=0.36 |
43.5 (±14.8) |
| SI/rMT | 116.0 (±7.6) | 120.2 (±14.2) | n.s. | 117.2 (±7.1) |
two participants in the 110% group were excluded from further analysis because they were deemed outliers; refer to text for more details.
Met/Met carriers excluded from the final analysis because of small sample size
P-values from Wilcoxon rank sum test
Abbreviations: F = Female, M = Male, rMT = Resting motor threshold, SI = stimulation intensity, MSO = maximum stimulator output, r = estimate of effect size for Wilcoxon rank sum test; mm = millimeters
In order to control for potential confounding effects, we ran two Type III ANCOVAs, one per SI group, with BDNF status as an independent variable, age, SI and scalp-to-coil distances as covariates, and MEP amplitudes as the dependent variable (DV). Before running any analyses, trials with MEP amplitudes greater than two standard deviations (SD) of the mean within each participant were excluded to reduce the effects of outliers. The ANCOVA models with and without covariates were compared in order to examine the contribution of each of the covariates to improving the model fit. Results from reduced but best fit models are presented. Each ANCOVA was followed by post-hoc Tukey’s tests to further evaluate significant effects. DVs in ANCOVAs were rank-transformed to ensure that the normality and homogeneity of variance assumptions for parametric tests were satisfied. Rank transformation is especially robust to homogeneity of variance when group sample sizes are unequal, which is expected in our case comparing DVs of interest in Val/Met with sample sizes lower than the Val/Val group. No transformation was applied to DVs when using non-parametric Wilcoxon tests for comparing demographical data and for sub-analyses. All results from model comparisons and ANCOVAs, including partial eta-squared and Cohen’s f as measures of effect sizes and statistical power, are reported in Tables 2 and 3. All analyses were conducted in R and RStudio27,28.
Table 2.
Model fit to analyze effects of BDNF status and covariates
| mod1 = rank(MEP) ~ BDNF | F (df) | p-value |
| mod2 = rank(MEP) ~ Age + BDNF | ||
| mod3 = rank(MEP) ~ SI + BDNF | ||
| mod4 = rank(MEP) ~ Distance + BDNF | ||
| 110% group | ||
|---|---|---|
| anova(mod1, mod2) | 0.322 (1) | 0.586 |
| anova(mod1, mod3) | 0.703 (1) | 0.426 |
| anova(mod1, mod4) | 1.502 (1) | 0.255 |
| mod2, mod3 and mod4 do not contribute significantly to the model fit reduced fit selected: mod2 (to be consistent with 1 mV group selection) | ||
| 1 mV group | ||
| anova(mod1, mod2) | 7.688 (1) | 0.010* |
| anova(mod1, mod3) | 0.126 (1) | 0.725 |
| anova(mod1, mod4) | 0.288 (1) | 0.596 |
| mod3 and mod4 do not contribute significantly to the model fit reduced fit selected: mod2 | ||
BDNF = Brain-derived neurotrophic factor; SI = stimulation intensity (units = %maximum stimulator output); degrees of freedom; Distance = scalp-to-coil distance measurements
Table 3.
ANCOVA (Type III) with BDNF status and covariate
| F (df) | p-value | Partial Eta squared | Cohen’s f | Achieved power | |||
|---|---|---|---|---|---|---|---|
| 110% group rank(MEP) ~ Age + BDNF |
Mean MEP amplitude | ||||||
| Val/Val | Val/Met | ||||||
| BDNF | 8.995 (1, 8) | 0.017* | 0.529 | 1.06 | 0.69 | 1.0 (±0.3) | 0.50 (±0.3) |
| Age | 0.322 (1, 8) | 0.585 | - | - | - | ||
| 1 mV group rank(MEP) ~ Age + BDNF |
Mean MEP amplitude | ||||||
| Val/Val | Val/Met | ||||||
| BDNF | 4.909 (1, 27) | 0.035* | 0.154 | 0.43 | 0.55 | 1.10 (±0.3) | 0.87 (±0.5) |
| Age | 7.689 (1, 27) | 0.010* | 0.222 | 0.53 | 0.57 | ||
ANCOVA = Analysis of covariances; MEP = motor evoked potential; EQ = Excitability quotient; × = interaction; BDNF = Brain-derived neurotrophic factor; df=numerator, denominator (residual) degrees of freedom
The relationship with BDNF status was also examined in the 1 mV SI group in a subset of 20 participants, 10 in each of the Val/Val and Val/Met groups, matched by age and sex using Wilcoxon rank sums tests to further verify the differences in MEP amplitudes by polymorphism status. We assessed differences in rMT and SIs between matched groups.
RESULTS
On average, less than 5% of the trials were excluded from the analysis with the cutoff at two SDs of the mean MEP amplitudes. There were no differences in the percentage of rejected trials (computed as a ratio of number of rejected trials to the total number of trials collected) and between the SI (110%: 4.0 ± 2.3%; 1 mV: 4.3 ± 2.7%; p = 0.73) or the BDNF groups (Val/Val: 3.8 ± 2.6%; Val/Met: 4.9 ± 2.5%; p = 0.27).
Twenty-seven participants (60.0%) were identified with Val/Val, 16 (35.5%) with Val/Met and 2 (4.4%) with Met/Met genotypes. SIs were determined using 110% protocol in 8 participants with Val/Val and 5 with Val/Met, and using 1 mV protocol in 19 Val/Val, 11 Val/Met and 2 Met/Met carriers. The two Met/Met carriers were excluded from our main analysis because of relatively small sample sizes; note that these individuals were not included with the Val/Met group to form a single Met carrier group because of reported differences between these groups in studies examining activity dependent secretion of BDNF11,16.
Two participants from the 110% group—one Val/Val (mean MEP amplitude = 2.5 ± 1.4 mV) and one Val/Met carrier—were also excluded as they were deemed outliers based on the mean MEP amplitudes in that group (Supplementary Figure 1) and because of technical difficulties during data collection. In the final analysis, 11 participants in the 110% group and 30 participants in the 1mV group were included. Table 1 summarizes the demographics and TMS output details by BDNF genotyping for the included sample.
Analyses comparing rMT, SI, ratio SI/rMT and scalp-to-coil distances (where applicable) between Val/Val and Val/Met carriers are summarized in Table 1. The SI/rMT ratios indicate %MSO increases beyond that of the rMT: in the 110% group the ratio would be expected to be close to 110, but this value in the 1 mV group was expected to be variable. RMT and SI appeared to be lower in Val/Met (rMT: 41.6 ± 8.1%) than Val/Val (rMT: 48.7 ± 8.3%) in the 1 mV group (Figure 1B), but not in the 110% SI group (Table 1). Because rMTs were determined using the same procedure (5 out of 10 MEPs) in both SI groups, we directly compared combined data from both groups, which revealed no differences in rMT (p=0.21; Val/Met: 48.3 ± 17.5%; Val/Val: 48.9 ± 8.1%). Scalp-to-coil distances did not significantly differ by BDNF status in the 110% (Val/Met: 18.4 ± 2.7 mm; Val/Val: 17.5 ± 2.7 mm) or the 1 mV group (Val/Met: 17.6 ± 3.2 mm; Val/Val: 18.2 ± 2.2 mm).
Figure 1.
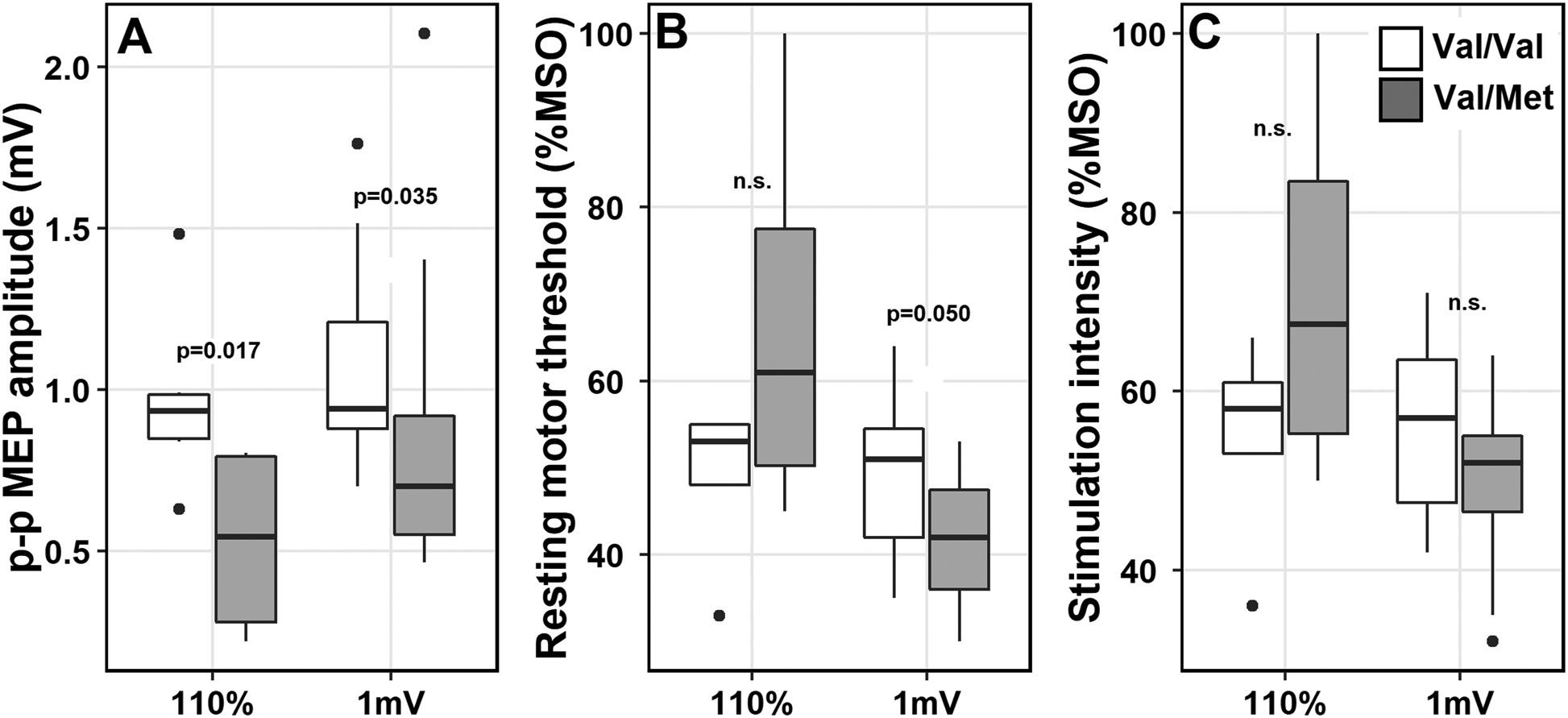
Boxplots displaying differences in TMS parameters in two stimulation intensity protocol groups (110% and 1 mV). A, Peak-to-peak (p-p) MEP amplitudes were lower in the Val/Met individuals compared to Val/Val; B, resting motor thresholds were lower for Val/Met compared to Val/Val in the 1mV group but not in the 110% group; C, No differences were found in stimulation intensities. mV = millivolts; %MSO = maximum stimulator output; n.s. = not significant. P-values reported from the ANCOVA model outputs for MEP amplitudes and from Wilcoxon rank sum test for resting motor thresholds and stimulation intensities.
In the 1 mV group, model comparisons with and without each of the covariates (Table 2) revealed that while age contributed significantly (F(1) = 7.69; p=0.010), inclusion of SI (F(1) = 0.13, p=0.725) or distance measurements (F(1) = 0.29; p=0.596) did not improve the model fit. With MEP amplitudes as DV, the main effect of BDNF polymorphism status was significant in the 1mV group (F(1,27) = 4.9, p = 0.035), after controlling for age (Table 3). The effect of age as a covariate was significant in the 1 mV group (F(1,27) = 7.7, p = 0.010) (Figure 3A). MEP amplitudes and age were negatively correlated (Spearman’s rho = −0.47), indicating diminished excitability with increasing age across both Val/Val and Val/Met carriers (Figure 3B); including individuals <40 years of age in this analysis did not change the outcome (rho = −0.48). In the 110% group, a significant main effect of BDNF status (F(1,8) = 9.0, p=0.017) was found after controlling for age; the effect of age was not significant in this group (F(1,8) = 0.322, p=0.585). Posthoc Tukey’s contrasts revealed that MEP amplitudes were lower in Val/Met carriers (110% group: 0.53 ± 0.31 mV; 1 mV group: 0.87 ± 0.49 mV; Figure 1A) than Val/Val carriers (110% group: 0.96 ± 0.26 mV; 1 mV group: 1.05 ± 0.28 mV), suggesting reduced motor excitability in Met carriers in both SI groups.
Figure 3.
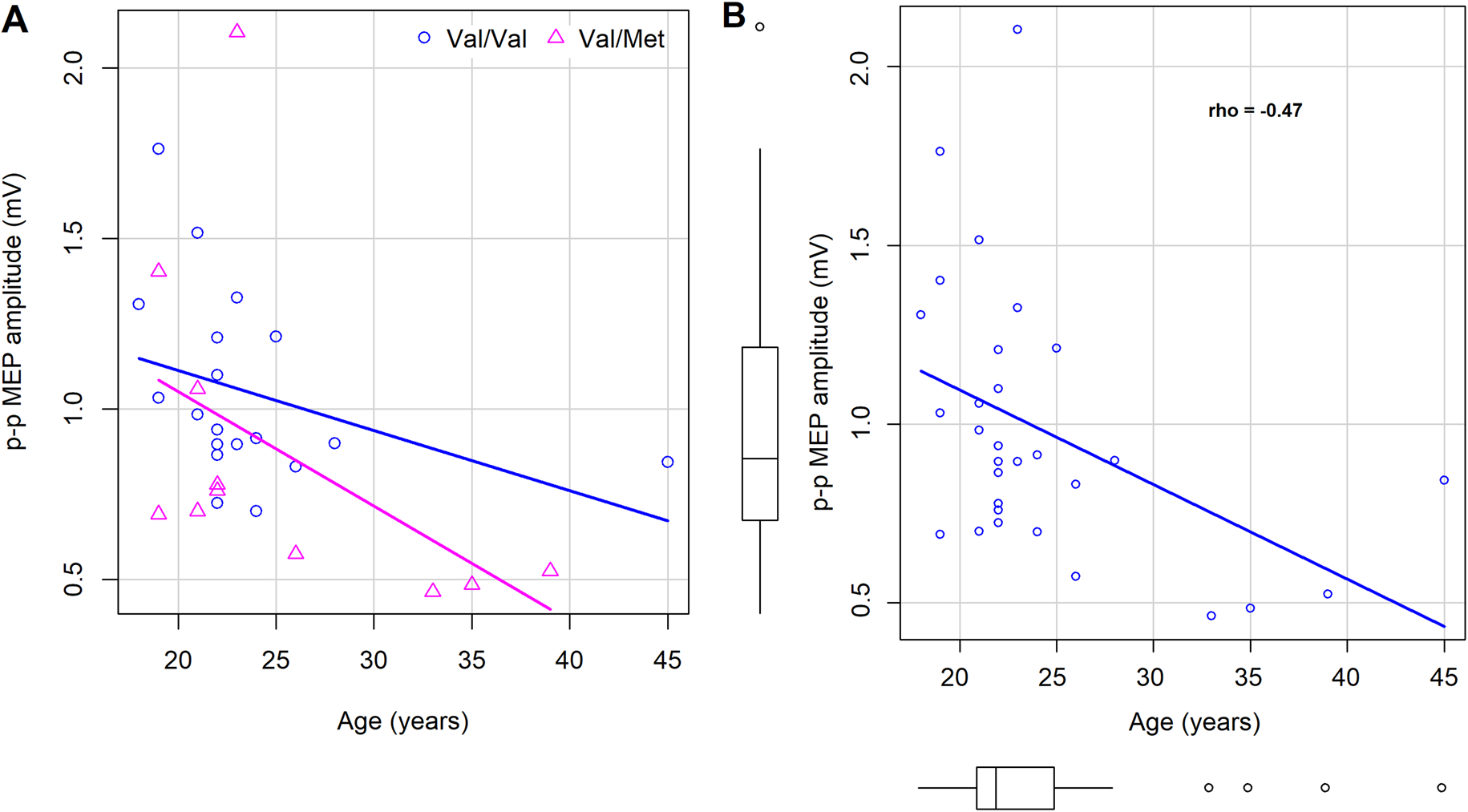
Effect of age on cortical excitability. A, Negative relationship between age and MEP amplitudes was found, suggesting hypoexcitability with aging, expressed equivalently in both Val/Met and Val/Val individuals. B, Negative relationship displayed in data collapsed across BDNF polymorphism groups; boxplots in x- and y-axis show the distribution of age and MEP amplitudes.
Sub-analysis in age- and sex-matched groups from the 1 mV SI group was conducted, including 10 Val/Met and matched 10 Val/Val carriers; mean ages were 25.7 (± 7.3) and 25.6 (± 7.2) years, respectively (p>0.05), and each group consisted of 7 males and 3 females (Table 4). Wilcoxon tests revealed that mean MEP amplitudes (p=0.009) were significantly lower in Val/Met than Val/Val carriers (Figure 2). No differences in rMT, SI or SI/rMT ratios or scalp-to-coil distances (p>0.05) were found. This further confirmed our observations, indicating diminished motor excitability in Val/Met carriers.
Table 4.
Age- and sex-matched groups for the subanalysis
| BDNF genotyping | Age (y) | Sex | Race | Distance (mm) | rMT (%MSO) | SI (%MSO) | SI/rMT | MEP amplitude (mV) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | ||||||||
| Val/Val | 23 | F | AA | 16.5 | 43 | 57 | 132.56 | 1.33 | 0.52 | 1.25 |
| Val/Val | 22 | M | Mix | 18.3 | 35 | 44 | 125.71 | 0.86 | 0.27 | 0.82 |
| Val/Val | 21 | M | AA | 21.5 | 56 | 66 | 117.86 | 1.52 | 0.96 | 1.15 |
| Val/Val | 22 | M | NA | 20.6 | 42 | 49 | 116.67 | 1.10 | 0.46 | 1.04 |
| Val/Val | 45 | M | AA | 20.4 | 51 | 56 | 109.80 | 0.84 | 0.77 | 0.49 |
| Val/Val | 28 | M | Cau | 17.6 | 48 | 61 | 127.08 | 0.90 | 0.34 | 0.93 |
| Val/Val | 22 | F | AA | 18.5 | 49 | 57 | 116.33 | 0.72 | 0.32 | 0.72 |
| Val/Val | 26 | M | Cau | 14.1 | 38 | 42 | 110.53 | 0.83 | 0.49 | 0.74 |
| Val/Val | 25 | F | AA | 20.2 | 52 | 57 | 109.62 | 1.21 | 0.66 | 1.05 |
| Val/Val | 22 | M | Cau | 17.0 | 51 | 56 | 109.80 | 1.21 | 0.79 | 0.91 |
| Val/Met | 26 | M | Asi | 18.5 | 45 | 52 | 115.56 | 0.57 | 0.43 | 0.56 |
| Val/Met | 39 | F | Cau | 14.0 | 31 | 47 | 151.61 | 0.52 | 0.19 | 0.47 |
| Val/Met | 35 | M | Cau | 18.0 | 30 | 32 | 106.67 | 0.48 | 0.33 | 0.43 |
| Val/Met | 19 | M | Asi | 16.9 | 49 | 53 | 108.16 | 0.69 | 0.45 | 0.72 |
| Val/Met | 21 | F | AA | 22.1 | 42 | 56 | 133.33 | 1.06 | 0.71 | 0.98 |
| Val/Met | 33 | M | Cau | 20.7 | 42 | 54 | 128.57 | 0.46 | 0.33 | 0.33 |
| Val/Met | 19 | M | Cau | 14.8 | 49 | 64 | 130.61 | 1.40 | 0.81 | 1.28 |
| Val/Met | 22 | F | Asi | 17.6 | 41 | 46 | 112.20 | 0.78 | 0.31 | 0.74 |
| Val/Met | 21 | M | Cau | 13.5 | 53 | 56 | 105.66 | 0.70 | 0.43 | 0.45 |
| Val/Met | 22 | M | Cau | 22.8 | 46 | 52 | 113.04 | 0.76 | 0.51 | 0.96 |
| Val/Val | ||||||||||
| Mean | 25.6 | 3F | 18.5 | 46.5 | 54.5 | 117.6 | 1.05 | |||
| SD | 7.2 | 2.3 | 6.7 | 7.4 | 8.3 | 0.26 | ||||
| Val/Met | ||||||||||
| Mean | 25.7 | 3F | 17.9 | 42.8 | 51.2 | 120.5 | 0.74 | |||
| SD | 7.3 | 3.2 | 7.5 | 8.4 | 15.0 | 0.29 | ||||
| p-values | n.s. | n.s. | 0.271 | 0.210 | n.s. | 0.009 | ||||
MSO = maximum stimulator output; AA = African-American; Cau = Caucasian; Asi = Asian; Distance = scalp-to-coil distance in millimeters (mm)
Figure 2.
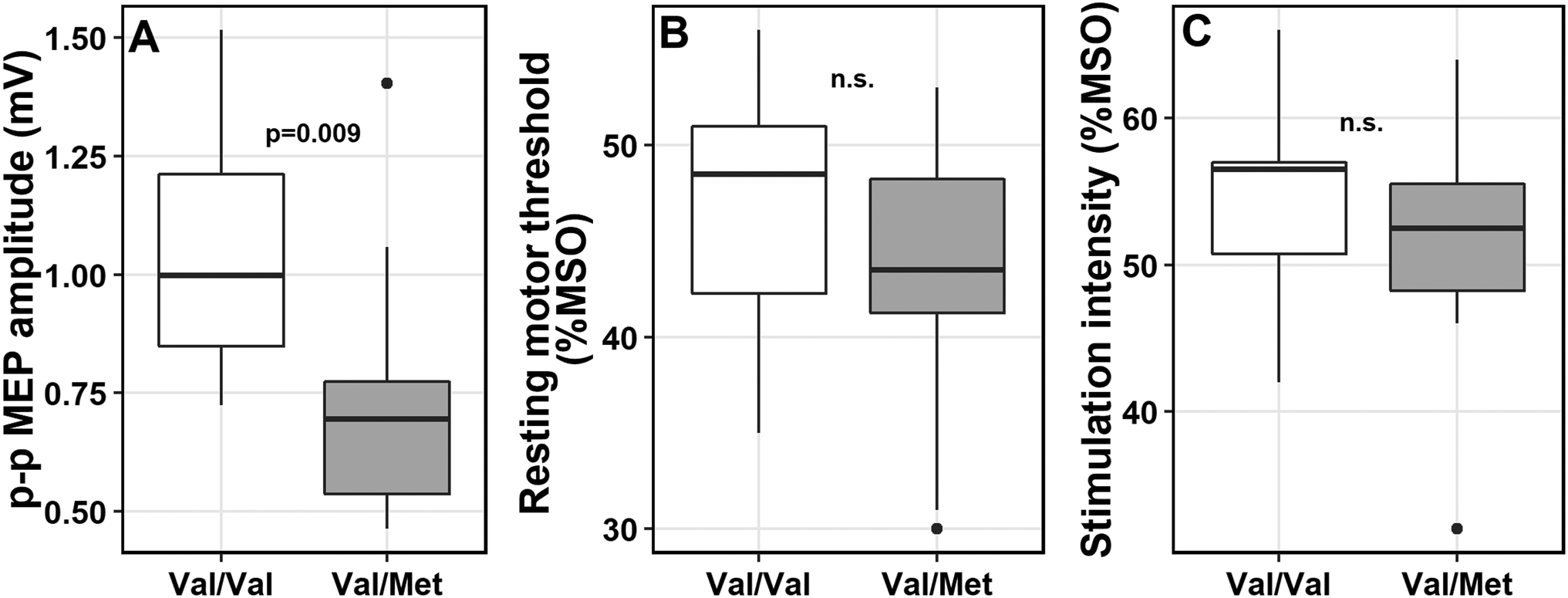
Boxplots displaying differences in TMS parameters in age- and sex-matched groups by BDNF polymorphism (Val/Val and Val/Met). A, Peak-to-peak (p-p) MEP amplitudes were lower in the Val/Met individuals compared to Val/Val; No differences were found in B, resting motor threshold or C, stimulation intensities between matched groups. mV = millivolts; %MSO = maximum stimulator output, n.s. = not significant. P-values reported from the Wilcoxon rank sum test.
DISCUSSION
We examined inherent differences in corticospinal excitability based on BDNF Val66Met polymorphism status in a sample of 41 healthy individuals. Our results indicated that homozygous Val/Val carriers and heterozygous Val/Met carriers differed on a measure of cortical excitability. Specifically, MEP sizes were diminished in Val/Met carriers compared to Val/Val carriers. These results held independent of the single-pulse TMS paradigm used to acquire MEPs, whereby the intensities of stimulation were either fixed at 110% of the resting motor threshold or were individually-derived based on the MEP sizes (at 1 mV). Overall, our findings suggest that differences in excitability associated with BDNF polymorphism can be observed in the absence of stimulation paradigms that explicitly rely on modulation of that excitability.
Differences in motor excitability at baseline i.e., prior to delivering repetitive or rapid TMS, based on BDNF Val66Met polymorphism have been evaluated previously. Mean baseline MEP amplitudes in Cheeran et al. (2008) across different experiments, involving repetitive or paired associative stimulation, were reported in the range of 0.55– 1.1 mV in Val/Val group (mean: 0.860 ± 0.18) and 0.57–1.0 mV in the Non-Val/Val group (which included homozygous Met allele carriers; mean: 0.842 ± 0.15)17. No significant differences in MEP amplitudes or rMT between BDNF groups were noted in this study, whereby stimulation intensities were individually adjusted to produce stable MEPs between 0.5–1mV at rest. Another study by Morin-Moncet et al. (2018) also reported no differences between Val/Val and Val/Met groups in MEPs acquired at rMT, MEPs acquired at 1 mV intensity, or those acquired at 130%, 140% and 150% of rMT29. Kleim et al. (2006) found no differences in rMT across the Val/Val, Val/Met and Met/Met carriers. Recruitment curves generated at 90%, 130%, 110% and 150% rMT also did not show any differences across the different intensities between BDNF groups3. The authors concluded based on these null findings at baseline that the physiological effects of BDNF polymorphism could only be evident in response to “behaviorally driven increases in neural activity.” Cirillo et al. (2012) also did not note any significant baseline differences across Val/Val, Val/Met and Met/Met groups in electrically induced muscle responses or M-waves, or rMT or test intensity as determined at 10% maximal M-wave16. Finally, Strube et al., (2015) did not find any differences in baseline parameters including rMT, TMS intensity (derived at 1 mV), cortical silent period and short-interval intracortical inhibition between Val/Val and Val/Met groups. The only difference that they found at baseline was in intracortical facilitation (ICF), indicating greater levels at baseline in Met compared to Val/Val carriers30. The authors speculated that higher ICF, mediated by glutamatergic neurotransmission, could suggest “cortical disinhibition or motor-cortical hyperexcitability” in Met carriers.
Given these null findings from several prior studies, it is not clear what might be driving the observed differences in motor excitability by BDNF polymorphism in our cohort. Perhaps, there is a more mundane explanation for reduced MEP amplitudes in Val/Met carriers related to lower SIs. Val/Met carriers may not have been stimulated to the same extent as Val/Val participants. Here we emphasize that all team members involved in data collection were blinded to subject genotyping during MEP collection visits making it highly unlikely that SIs were systematically biased by genotyping at the time of data collection. That said, there is a possibility that Val/Met carriers exhibit lower rMTs and thus provide lower starting points for determining SIs, resulting in lower SIs and lower MEP amplitudes. Given the small sample size that we studied, we cannot completely rule out the possibility that observed differences are related to sampling error, where our cohort may not be representative of the population data, thus may be responsible for the present findings. Nonetheless, our data provide an interesting hypothesis related to reduced baseline excitability based on BDNF polymorphism status that should be evaluated in future, larger-scale studies.
Another reason may be related to differences in individual neuroanatomy, independent of the BDNF status that can influence excitability. Prior evidence suggests that the distance from the scalp to the motor cortex can influence motor excitability, such that rMT increases as the scalp-to-cortex distance increases31,32. Our findings held after controlling for the scalp-to-coil distance measurements. Evidence also suggests that white matter fiber orientation of the corticospinal tract, particularly its anterior-posterior trajectory, is highly predictive of rMTs33. While it seems unlikely that individual variation in these structural properties of cortical anatomy would systematically influence MEP amplitudes in a manner highly correlated with yet independent of BDNF status, the possibility cannot be ruled out and should be explored further in future studies.
Differences between population groups could also influence cortical excitability,34 and the frequency of BDNF polymorphism has been observed to vary widely based on ancestry. Both of these factors could have influenced our results. According to dbSNP35 and ALFA Allele Frequency database36, the frequency of the Val/Met BDNF variant is 0.047 in African-Americans, 0.49 in East Asians, and 0.19 in Europeans. In our cohort of 41 participants for whom self-identified racial data were available, the frequency of the variant was 0.048 in African-Americans, 0.097 in Asians, and 0.244 in Caucasians. However, because of low sample sizes in each demographic category, we are unable to directly compare or control for these differences. Larger future studies could overcome this weakness of our study in order to examine this potentially important feature.
In our group analysis, we found averaged response to be lower in Val/Met carriers independent of the relationship with age. Overall, we found age-dependent reduction in cortical excitability (equivalently expressed in both Val/Val and Val/Met carriers [Figure 3]), which has been reported previously37,38. For example, a meta-analysis by Bhandari and colleagues (2006) that evaluated changes in multiple TMS measures of cortical excitability and plasticity as a function of age found hypoexcitability in older adults, consistent with our findings. The authors of the meta-analysis study speculated that this hypoexcitability may stem from age-related changes in anatomical and functional integrity38.
One limitation of the current study is the relatively small sample sizes (as a whole and in each SI group), although they are comparable to previously reported studies exploring the effects of BDNF polymorphism on response to TMS29,30. The achieved power of the findings related to the cortical excitability differences by BDNF polymorphism was low: 55% in the 1 mV and 69% in the 110% SI group. Future studies with larger sample sizes are necessary to draw conclusions regarding the generalizability and reproducibility of our results. Another limitation of the study is that we did not control for cortical anatomy, the time of day, menstrual cycle in female participants, and caffeine intake, all factors that may also influence excitability, which we also recommend in future studies to further substantiate the role of BDNF polymorphism independent of other potential factors.
CONCLUSIONS
We demonstrate diminished physiologic response to single-pulse TMS in polymorphic BDNF-genotype in healthy individuals. Given these results, we urge researchers to report and closely examine the so-called baseline TMS parameters in the context of BDNF polymorphisms, focusing not only on the changes in excitability from baseline but also differences at baseline. If substantiated in a larger-scale study and after controlling for other important factors, our findings have important implications for clinical populations whereby baseline TMS measures could index response to TMS or act as indicators of the capacity for clinically-relevant neuroplasticity.
Supplementary Material
Acknowledgments
We would like to extend our gratitude towards Dr. Gabriella Garcia and Caitlin Maura Breslin and other members of the Laboratory of Cognition and Neural Stimulation (LCNS) at the University of Pennsylvania who contributed to the implementation of this study.
Sources of financial support: The funding for this study came from the Dana Foundation and the NIH/NINDS 1R01DC012780-01A1 (PI: Hamilton).
Footnotes
Conflict of interest: None to disclose
References
- 1.Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci. 2006;6(1):79–85. [DOI] [PubMed] [Google Scholar]
- 2.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature neuroscience. 2006;9(6):735–737. [DOI] [PubMed] [Google Scholar]
- 4.Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda). 2005;20:70–78. [DOI] [PubMed] [Google Scholar]
- 5.McAllister TW, Tyler AL, Flashman LA, et al. Polymorphisms in the brain-derived neurotrophic factor gene influence memory and processing speed one month after brain injury. Journal of neurotrauma. 2012;29(6):1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohoff FW, Sander T, Ferraro TN, Dahl JP, Gallinat J, Berrettini WH. Confirmation of association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) gene and bipolar I disorder. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2005;139B(1):51–53. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZY, Bath K, McEwen B, Hempstead B, Lee F. Impact of genetic variant BDNF (Val66Met) on brain structure and function. Novartis Found Symp. 2008;289:180–188; discussion 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DT, Vickers JC, Stuart KE, Cechova K, Ward DD. The BDNF Val66Met Polymorphism Modulates Resilience of Neurological Functioning to Brain Ageing and Dementia: A Narrative Review. Brain Sci. 2020;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyajima F, Ollier W, Mayes A, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7(4):411–417. [DOI] [PubMed] [Google Scholar]
- 10.Chaieb L, Antal A, Ambrus GG, Paulus W. Brain-derived neurotrophic factor: its impact upon neuroplasticity and neuroplasticity inducing transcranial brain stimulation protocols. Neurogenetics. 2014;15(1):1–11. [DOI] [PubMed] [Google Scholar]
- 11.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. [DOI] [PubMed] [Google Scholar]
- 12.Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(17):6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toh YL, Ng T, Tan M, Tan A, Chan A. Impact of brain-derived neurotrophic factor genetic polymorphism on cognition: A systematic review. Brain and behavior. 2018;8(7):e01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHughen SA, Rodriguez PF, Kleim JA, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cerebral cortex. 2010;20(5):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(45):10099–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo J, Hughes J, Ridding M, Thomas PQ, Semmler JG. Differential modulation of motor cortex excitability in BDNF Met allele carriers following experimentally induced and use-dependent plasticity. The European journal of neuroscience. 2012;36(5):2640–2649. [DOI] [PubMed] [Google Scholar]
- 17.Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of physiology. 2008;586(23):5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antal A, Chaieb L, Moliadze V, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain stimulation. 2010;3(4):230–237. [DOI] [PubMed] [Google Scholar]
- 19.Lee M, Kim SE, Kim WS, et al. Interaction of motor training and intermittent theta burst stimulation in modulating motor cortical plasticity: influence of BDNF Val66Met polymorphism. PloS one. 2013;8(2):e57690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witte AV, Kurten J, Jansen S, et al. Interaction of BDNF and COMT polymorphisms on paired-associative stimulation-induced cortical plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(13):4553–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Voti P, Conte A, Suppa A, et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Experimental brain research. 2011;212(1):91–99. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Enomoto H, Hanajima R, et al. Quadri-pulse stimulation (QPS) induced LTP/LTD was not affected by Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) gene. Neuroscience letters. 2011;487(3):264–267. [DOI] [PubMed] [Google Scholar]
- 23.Mastroeni C, Bergmann TO, Rizzo V, et al. Brain-derived neurotrophic factor--a major player in stimulation-induced homeostatic metaplasticity of human motor cortex? PloS one. 2013;8(2):e57957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Experimental brain research. 1997;114(2):329–338. [DOI] [PubMed] [Google Scholar]
- 25.Lohoff FW, Ferraro TN, Dahl JP, et al. Lack of association between variations in the brain-derived neurotrophic factor (BDNF) gene and temporal lobe epilepsy. Epilepsy research. 2005;66(1–3):59–62. [DOI] [PubMed] [Google Scholar]
- 26.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and clinical neurophysiology. 1994;91(2):79–92. [DOI] [PubMed] [Google Scholar]
- 27.RStudio: Integrated Development for R Studio, Inc. [computer program]. Boston, MA: 2015. [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. . R Foundation for Statistical Computing; 2017; https://www.R-project.org/. [Google Scholar]
- 29.Morin-Moncet O, Latulipe-Loiselle A, Therrien-Blanchet JM, Theoret H. BDNF Val66Met polymorphism is associated with altered activity-dependent modulation of short-interval intracortical inhibition in bilateral M1. PloS one. 2018;13(6):e0197505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strube W, Nitsche MA, Wobrock T, et al. BDNF-Val66Met-Polymorphism Impact on Cortical Plasticity in Schizophrenia Patients: A Proof-of-Concept Study. Int J Neuropsychoph. 2015;18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. The Journal of neuropsychiatry and clinical neurosciences. 2000;12(3):376–384. [DOI] [PubMed] [Google Scholar]
- 32.McConnell KA, Nahas Z, Shastri A, et al. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry. 2001;49(5):454–459. [DOI] [PubMed] [Google Scholar]
- 33.Herbsman T, Forster L, Molnar C, et al. Motor threshold in transcranial magnetic stimulation: the impact of white matter fiber orientation and skull-to-cortex distance. Human brain mapping. 2009;30(7):2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi X, Fisher KM, Lai M, Mansoor K, Bicker R, Baker SN. Differences between Han Chinese and Caucasians in transcranial magnetic stimulation parameters. Experimental brain research. 2014;232(2):545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherry ST, Ward M, Sirotkin K. dbSNP—Database for Single Nucleotide Polymorphisms and Other Classes of Minor Genetic Variation. Genome Res. 1999;9:677–679. [PubMed] [Google Scholar]
- 36.Phan L, Jin Y, Zhang H, et al. ALFA: Allele Frequency Aggregator. 2020; www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/. [Google Scholar]
- 37.Grunhaus L, Polak D, Amiaz R, Dannon PN. Motor-evoked potential amplitudes elicited by transcranial magnetic stimulation do not differentiate between patients and normal controls. Int J Neuropsychoph. 2003;6(4):371–378. [DOI] [PubMed] [Google Scholar]
- 38.Bhandari A, Radhu N, Farzan F, et al. A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clinical Neurophysiology. 2016;127(8):2834–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


