Abstract
Objective:
Factors that lead to metabolic dysregulation are associated with increased risk of early-onset colorectal cancer (CRC diagnosed under age 50). However, the association between metabolic syndrome (MetS) and early-onset CRC remains unexamined.
Design:
We conducted a nested case-control study among participants aged 18–64 in the IBM® MarketScan® Commercial Database (2006–2015). Incident CRC was identified using pathologist-coded ICD-9-CM codes, and controls were frequency matched. MetS was defined as presence of ≥3 conditions among obesity, hypertension, hyperlipidemia, and hyperglycemia/type 2 diabetes, based on ICD-9-CM and use of medications. Multivariable logistic regressions were used to estimate odds ratios and 95% confidence intervals.
Results:
MetS was associated with increased risk of early-onset CRC (N=4,673; multivariable adjusted OR 1.25; 95% CI 1.09 to 1.43), similar to CRC diagnosed at 50–64 (N=14,928; OR 1.21; 1.15 to 1.27). Compared to individuals without a metabolic comorbid condition, those with 1, 2, or ≥3 conditions had a 9% (1.09; 1.00 to 1.17), 12% (1.12; 1.01 to 1.24), and 31% (1.31; 1.13 to 1.51) higher risk of early-onset CRC (Ptrend<0.001). No associations were observed for 1 or 2 metabolic comorbid conditions and CRC diagnosed at 50–64. These positive associations were driven by proximal (OR per condition 1.14; 1.06 to 1.23) and distal colon cancer (OR 1.09; 1.00 to 1.18), but not rectal cancer (OR 1.03; 0.97 to 1.09).
Conclusions:
Metabolic dysregulation was associated with increased risk of early-onset CRC, driven by proximal and distal colon cancer, thus at least in part contribute to the rising incidence of early-onset CRC.
Keywords: metabolic syndrome, early-onset, colorectal cancer
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and cause of cancer death globally.1 Even though the incidence rate of CRC declined rapidly among screening-aged individuals 65 years or older,1 the incidence of early-onset CRC in adults younger than 50 has been increasing in the US,1 Europe2 and worldwide.3 In the US, early-onset CRC has been increasing since the mid-1990s,1 driven largely by rectal tumors. However, data collected between 2012 and 2016 suggests that incidence rates rose by 1.8% annually for tumors in the proximal and distal colon as well as in the rectum.1 Compared with older cases, early-onset CRCs are more likely to present with unique histopathological (e.g. mucosal and signet cell4) and molecular features4,5 (e.g. higher rates of TP53/CTNNB16 and consensus molecular type 1,7 and deregulated redox homeostasis8). These emerging distinct features along with an alarming increase across all anatomic sites highlight the urgent need to re-evaluate putative risk factors associated with average-onset CRC.
Accumulating evidence suggests that obesity9 and sedentary lifestyle10 may contribute to the development of early-onset CRC. Although the underlying mechanisms have not been fully elucidated, obesity11 and sedentary behavior12 both lead to metabolic dysregulation. Metabolic syndrome (MetS),13 a constellation of metabolic disorders including high blood pressure, high triglyceride, central obesity, and low high-density lipoprotein, has been linked with about 13% increased risk of CRC.14–17 However, these studies did not specifically evaluate the associations with early-onset CRC. Notably, in the past decades, the prevalence of MetS has increased dramatically worldwide as a consequence of urbanization, increasing obesity, and sedentary lifestyle.18 In the US, the prevalence of MetS was 6.7% among aged 20–29 and approximately 13% among aged 30–39 between 1988 and 1994,19,20 while the numbers rose to 19% for the combined age group between 2003 and 2012.21 Besides obesity,22 the rising incidence of other metabolic comorbid conditions such as hypertension23 and diabetes24 in younger adults has also been reported in the US and other countries,25 where a similar rise in early-onset CRC has been documented.3 Thus far, the association between MetS and early-onset CRC remains unexamined in the US population.
To address this knowledge gap that is critical for both the etiology, prevention, and early detection of CRC, we utilized the IBM® MarketScan® Commercial Databases (2006–2015), a longitudinal database that contains individual-level, deidentified healthcare claims data of over 113 million young and middle-aged adults from all geographic areas of the US to examine comprehensively the associations between MetS, number of metabolic comorbid conditions, and risk of early-onset CRC.
METHODS
Study population
We conducted a nested case-control study in the MarketScan databases, a longitudinal database that contains de-identified, individual-level healthcare claims data of commercially insured individuals from all geographic areas of the US.26 The database captures information on outpatient and inpatient insurance-reimbursable services, prescription drugs, demographic information, eligibility status and type of health plan. As information for individuals aged 65 and above was not available, we restricted our analyses to 113 million adults aged 18–64 between 2006 and 2015, and required at least 2 years of enrollment and prescription drug coverage prior to the index dates, as well as 90 days of enrollment after index to derive metastatic status. Institutional Review Board approval was not required for this deidentified limited dataset analysis.
Ascertainment of cases and controls
All patients aged 18 to 64 years diagnosed with incident CRC from 2006 to 2015 were identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM code: 153.0–153.9, 154.0, 154.1, and 154.8). To reduce false positives, we required pathologist coding for CRC and assigned the first pathology date as the index date. CRCs were further classified into proximal colon (hepatic flexure: 153.0; transverse colon: 153.1; cecum: 153.4; appendix: 153.5; ascending colon: 153.6), distal colon (descending colon: 153.2; sigmoid colon: 153.3; splenic flexure: 153.7), unspecified colon (153.8–153.9) and rectal cancer (rectosigmoid junction: 154.0; rectum: 154.1) according to anatomical site. CRC metastatic status was also derived based on diagnosis or liver/lung metastatic treatment up to 90 days after the index date.27
We excluded CRC patients with one or more codes for personal history of any cancer or genetic susceptibility to other malignant neoplasm as identified by ICD-9-CM codes prior to 2 years of the index dates. We further excluded CRC patients with cancer in the two years before index as identified by cancer diagnosis codes from the Healthcare Cost and Utilization Project’s Clinical Classification Software (HCUP CCS).28 We excluded all cancers except CRC and non-melanoma skin cancer. One inpatient facility claims and/or two outpatient provider claims 31–365 days apart were required to identify pre-index cancer for exclusion based on Klabunde et al.29
Individuals without CRC were identified as controls and were matched up to 8:1 ratio with the CRC cases using frequency matching on age group, sex, geographical region (Northeast, North Central, South, West, unknown), and full years of health insurance enrollment before index. Controls were required to have two years of prior and at minimum 90 days of post-index medical coverage. The following age groups were used for matching: 18–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, and 60–64. Controls were selected such that the distribution of their index dates matched the distribution of case index dates. To do this, potential controls were assigned random index dates after the first two years of enrollment but prior to the last 90 days of enrollment to allow time to identify outcomes. Frequency matching was then performed by year along with the matching variables mentioned above. Potential controls were excluded if they had a code of any of the following: personal history of any cancer, genetic susceptibility to other malignant neoplasm, cancers other than non-melanoma skin cancer in the two years before index.
Ascertainment of MetS and metabolic comorbid conditions
Since around 95% of cancer patients received treatment within 90 days from diagnosis in the US,30 we restricted our exposures to start from 91 days before the index dates to reduce the influence of clinical visits before CRC diagnosis that may differentially increase the detection of MetS among the case group. MetS was defined using either ICD-9-CM code (277.7) or the presence of at least 3 of the following 4 conditions: obesity/overweight31 (278.00–278.03, 649.1, 793.91, V85.3-V85.4, V85.54), hypertension (401–405), hyperlipidemia (272.0–272.2, 272.4, 272.9), and hyperglycemia/type 2 diabetes (790.2, 790.21–790.22, 790.29, 250.00, 250.02, 250.10, 250.12, 250.20, 250.22, 250.30, 250.32, 250.40, 250.42, 250.50, 250.52, 250.60, 250.62, 250.70, 250.72, 250.80, 250.82, 250.90, 250.92). The identification of diabetes also used an algorithm based on Klabunde et al.29 Based on NCEP ATP III criteria,32 we also defined MetS based upon use of prescription medications for hypertension, hyperlipidemia, and hyperglycemia. Specifically, we defined individuals who used ≥90 days of any anti-hypertensive, lipid lowering, anti-diabetic medications as regular users. Our estimates of the prevalence of hypertension, hyperlipidemia, and hyperglycemia/type 2 diabetes were comparable to other national estimates.33,34
Assessment of covariates
We extracted information on sociodemographic factors: employment status, residence, and health plan. In addition, potential confounders, including inflammatory bowel diseases (IBD), family history of gastrointestinal neoplasm, and regular use of prescription non-steroidal anti-inflammatory drugs (Rx NSAIDs) between 91 days and 2 years before the index dates were extracted.30 Regular Rx NSAIDs user included individuals who received more than ≥90 days of Rx NSAIDs among this period. We also extracted information related to healthcare utilization within 91 days and 2 years before the index dates, including outpatient visits, screening colonoscopies, other colonoscopies, fecal occult blood test, and the Charlson Comorbidity Index35 (derived without taking into account of type 2 diabetes and confirmed using the algorithm based on Klabunde et al.29).
Statistical analyses
We evaluated the association between MetS and risk of early-onset CRC as the main analyses. In addition, we examined the dose-response relationship between number of metabolic comorbid conditions and risk of early-onset CRC. As secondary analyses, we investigated the above associations according to anatomical site of the tumor and metastatic status, and whether the identified associations differ according to sex, age,8 residence, geographic region,36 and outpatient visits.
Multivariable logistic regressions were used to estimate odds ratios 37 and 95% confidence intervals (CIs). We first adjusted for the matching factors including age (year), sex (male, female), duration of insurance enrollment (years), and region (Northeast, North Central, South, West, unknown). We then additionally adjusted for health insurance plan (Preferred Provider Organization [PPO], Health Maintenance Organization [HMO], others), residence (rural, urban, unknown), and the following factors between 91 days and 2 years prior to the index dates: outpatient visits (>5 times, ≤5 times), Charlson Comorbidity Index without diabetes (continuous), IBD (yes, no), family history of gastrointestinal neoplasm (yes, no), screening colonoscopy (yes, no), other colonoscopies (yes, no), fecal occult blood test (yes, no), and regular Rx NSAIDs use (yes, no). Tests for trend were conducted using the number of comorbid conditions as a continuous variable. P for heterogeneity was calculated with polytomous logistic regression to examine whether the association between metabolic syndrome and risk of CRC differed by anatomic sites (colon, rectal cancer; proximal, distal colon, unspecific colon) and metastatic status (metastasis, non-metastasis). P for interaction was calculated by Wald test using the cross-product terms of MetS and each stratification factor. All analyses were performed in SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
RESULTS
A total of 4,673 early-onset CRC cases and 40,832 frequency matched controls were included in our primary analyses for early-onset CRC (table 1). In addition, 14,928 CRC patients diagnosed between age 50 and 64 years old were frequency matched to 132,120 matched controls. The mean age of diagnoses of the early-onset CRC cases was 43, of which 64.4% were colon cancer and 35.6% were rectal cancer. Compared to controls, early-onset CRC cases had more outpatient visits and greater Charlson Comorbidity Index between 91 days to up to 2 years before the index dates.30 They were also more likely to have IBD, colonoscopies other than for screening, and fecal occult blood test from 91 days to up to 2 years before the index dates. Among participants under age 50, 6.0% of the early-onset CRC had MetS compared to only 4.3% of the controls.
Table 1.
Characteristics of participants according to colorectal cancer status, MarketScan database (2006–2015)*
| Age 18–49, Participants No. (%) |
Age 50–64, Participants No. (%) |
|||
|---|---|---|---|---|
| Case (N=4,673) | Control (N=40,832) | Case (N=14,928) | Control (N=132,120) | |
| Age at index date, mean, y | 43.0±5.8 | 42.8±5.8 | 57.2±4.3 | 57.2±4.3 |
| Female | 2248 (48.1) | 19920 (48.8) | 6619 (44.3) | 59576 (45.1) |
| Region | ||||
| Northeast | 684 (14.6) | 5920 (14.5) | 2092 (14.0) | 18624 (14.1) |
| North Central | 1088 (23.3) | 9240 (22.6) | 3957 (26.5) | 33856 (25.6) |
| South | 2085 (44.6) | 18024 (44.1) | 6423 (43.0) | 56096 (42.5) |
| West | 776 (16.6) | 7304 (17.9) | 2342 (15.7) | 22592 (17.1) |
| Unknown | 40 (0.9) | 344 (0.8) | 114 (0.8) | 952 (0.7) |
| Residence | ||||
| Urban | 3935 (84.2) | 34773 (85.2) | 12044 (80.7) | 109413 (82.8) |
| Rural | 738 (15.0) | 6059 (14.1) | 2884 (18.6) | 21792 (16.5) |
| Unknown | 36 (0.8) | 317 (0.8) | 106 (0.7) | 915 (0.7) |
| Full time employment | 3002 (64.2) | 27260 (66.8) | 7157 (47.9) | 65070 (49.3) |
| Insurance enrollment, mean, y | 4.2±1.8 | 4.1±1.8 | 4.4±1.9 | 4.4±1.9 |
| Health insurance plan | ||||
| PPO | 2847 (60.9) | 24002 (58.8) | 8917 (59.7) | 76997 (58.3) |
| HMO | 698 (14.9) | 6787 (16.6) | 1795 (12.0) | 19343 (14.6) |
| Other | 1128 (24.1) | 10043 (24.6) | 4216 (28.2) | 35780 (27.1) |
| Number of outpatient visits, mean† | 6.0±6.4 | 5.3±6.0 | 6.8±7.1 | 7.0±7.3 |
| Charlson Comorbidity Index, mean†‡ | 0.10±0.5 | 0.06±0.4 | 0.15±0.6 | 0.12±0.5 |
| Metabolic syndrome§ | 280 (6.0) | 1763 (4.3) | 2195 (14.7) | 16602 (12.6) |
| Hypertension¶ | 1207 (25.8) | 8917 (21.8) | 7397 (49.6) | 62242 (47.1) |
| Hyperlipidemia¶ | 982 (21.0) | 7824 (19.2) | 6133 (41.1) | 56838 (43.0) |
| Hyperglycemia/type 2 diabetes¶ | 384 (8.2) | 2652 (6.5) | 2731 (18.3) | 20774 (15.7) |
| Obesity† | 321 (6.9) | 2155 (5.3) | 983 (6.6) | 7625 (5.8) |
| Inflammatory bowel disease† | 219 (4.7) | 1059 (2.6) | 470 (3.2) | 3415 (2.6) |
| Family history of gastrointestinal neoplasm† | 51 (1.1) | 402 (1.0) | 150 (1.0) | 2483 (1.9) |
| Screening colonoscopy† | 49 (1.1) | 830 (2.0) | 223 (1.5) | 10074 (7.6) |
| Other colonoscopy† | 173 (3.7) | 740 (1.8) | 678 (4.5) | 6773 (5.1) |
| Fecal occult blood test† | 335 (7.2) | 2432 (6.0) | 1777 (11.9) | 18304 (13.9) |
| Regular Rx NSAID use|| | 159 (3.4) | 1462 (3.6) | 943 (6.3) | 10244 (7.8) |
| Tumor site | ||||
| Colon | 3009 (64.4) | - | 10166 (68.1) | - |
| Rectal | 1664 (35.6) | - | 4762 (31.9) | - |
Abbreviations: CRC, colorectal cancer; HMO, Health Maintenance Organization; NSAID, nonsteroidal anti-inflammatory drug; PPO, Preferred Provider Organization; SD, standard deviation.
Mean±SD and percentages were presented for continuous and categorical variables, respectively.
Between 91 days and 2 years before the index dates.
Charlson Comorbidity Index was calculated without accounting for diabetes.
Metabolic syndrome was defined using ICD-9-CM codes or the presence of at least three of the following conditions: obesity, hypertension, hyperlipidemia, and hyperglycemia/type 2 diabetes.
Hypertension, hyperlipidemia, and hyperglycemia/type 2 diabetes were identified based on ICD-9-CM codes between 91 days and 2 years before the index dates or regular use of medications (≥90 days of use between 91 days and 2 years before the index dates) for the corresponding condition.
Regular Rx NSAID use was defined as use of ≥90 days between 91 days and 2 years before the index dates.
MetS was associated with increased risk of early-onset CRC (OR 1.25; 95% CI 1.09 to 1.43), after adjusting for matching factors, potential confounders, and factors associated with healthcare utilization (table 2). Also, the number of metabolic comorbid conditions was positively associated with risk of early-onset CRC in a dose-dependent fashion. Compared to individuals without any metabolic comorbid conditions, those with 1, 2, or ≥3 metabolic conditions had a 9% (OR 1.09; 95% CI 1.00 to 1.17), 12% (OR 1.12; 95 CI% 1.01 to 1.24), and 31% (OR 1.31; 95% CI 1.13 to 1.51) higher risk of early-onset CRC (Ptrend<0.001), respectively. The positive association between MetS and risk of early-onset CRC was similar when restricted to individuals without IBD (OR 1.23; 95% CI 1.07 to 1.42) (supplementary table S1), and according to sex (female, male), residence (rural, urban), geographic region (South, others), or outpatient visits (>5 times, ≤5 times) (figure 1 and supplementary table S2) (all Pinteraction>0.5). The association between MetS and CRC appeared stronger for individuals aged 40–44 (OR 1.45; 95% CI 1.12 to 1.89), followed by age 45–49 (OR 1.22; 95% CI 1. 03 to 1.44). However, although OR was similar for individuals under 40, the association was not significant for this age group (OR 1.18; 95% CI 0.77 to 1.79), which could be due to limited power. No interaction between age and MetS was identified (Pinteraction=0.883).
Table 2.
Metabolic syndrome, metabolic comorbid conditions and risk of colorectal cancer
| Participants with conditions, No. (%) |
Multivariable-adjusted OR (95% CI)† | Multivariable-adjusted OR (95% CI)‡ | ||
|---|---|---|---|---|
| Cases | Controls | |||
| Age 18–49 | ||||
| Metabolic syndrome | 280 (6.0) | 1763 (4.3) | 1.39 (1.22 to 1.60) | 1.25 (1.09 to 1.43) |
| No. of comorbid conditions* | ||||
| 0 | 2847 (60.9) | 26729 (65.5) | 1 (reference) | 1 (reference) |
| 1 | 1048 (22.4) | 8525 (20.9) | 1.15 (1.06 to 1.24) | 1.09 (1.00 to 1.17) |
| 2 | 519 (11.1) | 3957 (9.7) | 1.22 (1.10 to 1.35) | 1.12 (1.01 to 1.24) |
| ≥3 | 259 (5.5) | 1621 (4.0) | 1.48 (1.29 to 1.70) | 1.31 (1.13 to 1.51) |
| Per condition | 1.12 (1.08 to 1.16) | 1.07 (1.03 to 1.11) | ||
| Ptrend | <0.001 | <0.001 | ||
| Age 50–64 | ||||
| Metabolic syndrome | 2195 (14.7) | 16602 (12.6) | 1.20 (1.14 to 1.26) | 1.21 (1.15 to 1.27) |
| No. of comorbid conditions* | ||||
| 0 | 5520 (37.0) | 49434 (37.4) | 1 (reference) | 1 (reference) |
| 1 | 3987 (26.7) | 36165 (27.4) | 0.99 (0.95 to 1.03) | 1.03 (0.99 to 1.08) |
| 2 | 3282 (21.9) | 30304 (22.9) | 0.97 (0.92 to 1.01) | 1.01 (0.96 to 1.06) |
| ≥3 | 2139 (14.3) | 16217 (12.3) | 1.18 (1.12 to 1.24) | 1.22 (1.15 to 1.29) |
| Per condition | 1.03 (1.02 to 1.05) | 1.05 (1.03 to 1.07) | ||
| Ptrend | <0.001 | <0.001 | ||
Abbreviations: CI, confidence interval; HMO, Health Maintenance Organization; OR, odds ratio; PPO, Preferred Provider Organization; Rx NSAIDs, prescription non-steroidal anti-inflammatory drugs.
Metabolic comorbid conditions included obesity, hypertension, hyperlipidemia, and hyperglycemia/type 2 diabetes.
Adjusted for matching factors including age (year), sex (male, female), duration of insurance enrollment (year), and region (Northeast, North Central, South, West, unknown).
In addition to matching factors, the models were also adjusted for health insurance plan (PPO, HMO, others), residence (rural, urban, unknown), and the following factors within 91 days to 2 years prior to the index dates: outpatient visits (>5 times, ≤5 times), Charlson Comorbidity Index without diabetes (continuous), inflammatory bowel disease (yes, no), family history of gastrointestinal neoplasm (yes, no), screening colonoscopy (yes, no), other colonoscopy (yes, no), fecal occult blood test (yes, no), and regular Rx NSAIDs use (yes, no)
Figure 1.
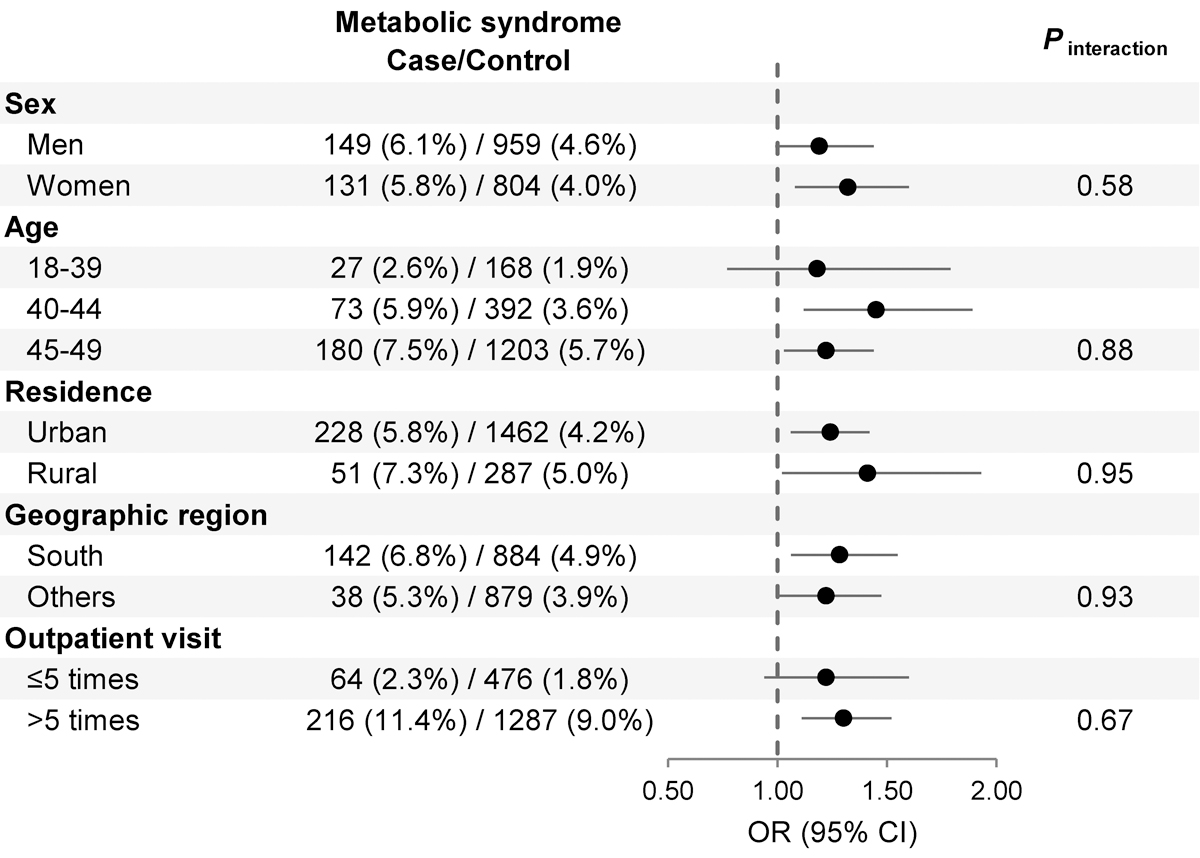
Stratified analyses for MetS and risk of early-onset CRC. The multivariable models were adjusted for the same set of covariates as in model † of Table 2, except for the stratification factor. CRC, colorectal cancer; MetS, metabolic syndrome.
We further evaluated these associations according to anatomical site and metastatic status (table 3 and supplementary table S2). Overall, the association between MetS and early-onset CRC was significant for colon cancer (OR 1.38; 95% CI 1.18 to 1.62) but not for rectal cancer (OR 1.04; 95% CI 0.83 to 1.32), although p for heterogeneity was not significant (Pheterogeneity=0.076). A closer investigation within colon cancer showed similar associations between MetS and risk for proximal, distal, and unspecified colon cancer (Pheterogeneity=0.627). Similar associations were also observed with the number of metabolic comorbid conditions. Each additional metabolic comorbid condition was associated with 10% increased risk of overall colon cancer (OR 1.10; 95% CI 1.05 to 1.15), 14% increased risk for proximal cancer (OR 1.14; 95% CI 1.06 to 1.23), 9% increased risk for distal cancer (OR 1.09; 95% CI 1.00 to 1.18), and 8% increased risk for unspecified colon cancer (OR 1.08; 95% CI 1.01 to 1.15). However, the positive linear association between metabolic comorbid condition was not observed for rectal cancer (OR 1.03; 95% CI 0.97 to 1.09). The association between MetS and risk of CRC was not significantly different by metastatic status (Pheterogeneity=0.951, supplementary table S2).
Table 3.
Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer according to anatomical site
| Participants with conditions, No. (%) |
Multivariable-adjusted OR (95% CI)† | Pheterogeneity | ||
|---|---|---|---|---|
| Cases | Controls | |||
| Colon cancer | ||||
| Metabolic syndrome | 197 (6.6) | 1763 (4.3) | 1.38 (1.18 to 1.62) | |
| Per comorbid condition* | 1.10 (1.05 to 1.15) | |||
| Ptrend | <0.001 | |||
| Proximal | ||||
| Metabolic syndrome | 59 (6.6) | 1763 (4.3) | 1.37 (1.04 to 1.81) | 0.627‡ |
| Per comorbid condition* | 1.14 (1.06 to 1.23) | |||
| Ptrend | <0.001 | |||
| Distal | ||||
| Metabolic syndrome | 52 (6.0) | 1763 (4.3) | 1.25 (0.94 to 1.68) | |
| Per comorbid condition* | 1.09 (1.00 to 1.18) | |||
| Ptrend | 0.040 | |||
| Unspecified | ||||
| Metabolic syndrome | 86 (7.0) | 1763 (4.3) | 1.48 (1.18 to 1.87) | |
| Per comorbid condition* | 1.08 (1.01 to 1.15) | |||
| Ptrend | 0.028 | |||
| Rectal cancer | ||||
| Metabolic syndrome | 81 (5.0) | 1763 (4.3) | 1.04 (0.83 to 1.32) | 0.076§ |
| Per comorbid condition* | 1.03 (0.97 to 1.09) | |||
| Ptrend | 0.375 | |||
Abbreviations: CI, confidence interval; CRC, colorectal cancer; OR, odds ratio.
Metabolic comorbid conditions included obesity, hypertension, hyperlipidemia, hyperglycemia/type 2 diabetes.
Adjusted for the same set of covariates in the multivariate model ‡ of Table 2.
p for heterogeneity was calculated using polytomous logistic regression to examine whether the association between metabolic syndrome and risk of early-onset CRC differed by anatomic site (proximal, distal, unspecific colon), adjusting for the same set of covariates as in model †.
p for heterogeneity was calculated using polytomous logistic regression to examine whether the association between metabolic syndrome and risk of early-onset CRC differed by anatomic site (colon, rectum), adjusting for the same set of covariates as in model †.
Among adults aged 50–64, MetS was identified among 14.7% of the cases and 12.6% of the controls (table 2). Similar to that among younger adults, MetS was associated with increased risk of CRC diagnosed at 50–64 (OR 1.21; 95% CI 1.15 to 1.27). In comparison to individuals without any metabolic comorbid condition, adults with 1 or 2 metabolic comorbid conditions were not at higher risk of CRC, and increased risk of CRC was only observed among individuals with ≥3 metabolic conditions (OR 1.22; 95% CI 1.15 to 1.29; Ptrend<0.001), with stronger associations for colon compared to rectal cancer (supplementary table S3).
DISCUSSION
Leveraging real-world healthcare claims data that covers 113 million US adults aged 18–64 and 4,673 early-onset CRC, we found that MetS and metabolic comorbid conditions were associated with increased risk of early-onset CRC, and the findings remained significant after adjusting for potential confounders and indicators for health care utilization. We also found that the positive associations were largely driven by proximal and distal colon cancer but not rectal cancer. Due to the substantial rise in the prevalence of metabolic syndrome among younger populations19–21 and the increase in early-onset CRC across all the anatomic sites of the colon and rectum,1 our findings suggest that MetS and metabolic dysregulation may contribute in part to the rising incidence of early-onset CRC.
Studies examining the association between MetS and early-onset CRC are thus far limited. In line with our findings, prior colonoscopy-based cross-sectional studies from Europe38 and Korea39 reported that among individuals under age 50, MetS was associated with increased risk of colonic lesions primarily consisting of adenomas. We also examined the association between MetS and risk of CRC among adults aged 50–64, and reported similar strength of association compared to prior studies that reported positive associations between MetS and CRC among an older population.14 It is worth noting that we observed a stronger association for CRC diagnosed at age 40–44 compared to that for CRC diagnosed at age 45–49. We also identified a more apparent linear relationship between the number of metabolic comorbid conditions and early-onset CRC compared to that for CRC diagnosed at age 50–64. Collectively, these findings reiterate the importance of MetS in CRC etiology and prevention. Our findings also lend preliminary support to a stronger role of metabolic dysregulation in early-onset CRC. Interestingly, the positive associations between MetS and CRC appeared stronger for proximal and distal colon cancers as compared to rectal cancer, for both early-onset CRC and CRC diagnosed at 50–64, the latter also being in-line with prior studies among older adults.16,40 Although studies have reported higher prevalence of MetS41 and early-onset CRC36 in the southern states, our stratified analysis suggests that the associations between MetS and early-onset CRC were similar in the South vs in other geographic regions.
Mechanisms linking MetS and CRC risk remain to be fully elucidated. Insulin resistance has been indicated as one of the most important mediators, in which insulin and insulin like growth factors may promote cancer development through their proliferative and anti-apoptotic effects.42,43 Obesity, especially central/visceral, in association with high blood free fatty acids and peripheral insulin resistance, has been also suggested as an underlying factor of MetS as well as CRC carcinogenesis.42 In obesity, a chronic low-grade inflammatory state mediated by elevated cytokines such as tumor necrosis factor-ɑ, interleukin-6, and C-reactive protein would trigger immune cell response and promote cancer development.44 The gut bacterial microbiome, mechanistically involved in CRC pathogenesis,45 was found to capture substantial (~22–36%) variations in metabolic disorders.46 Bile acids as key regulators of systemic metabolism may play direct roles in nutrient absorption, and also link the gut microbiota to hepatic and intestinal metabolism.47 Dysregulation of bile acids and bile acid-microbiota crosstalk disruption in MetS may contribute to the development of CRC.47,48 In addition, MetS could also serve as a surrogate for other established lifestyle factors for CRC, such as sedentary behavior,10,49 western diet,50,51 chronic stress,52,53 and circadian disruption.54,55 A recent large-scale Mendelian randomization analysis showed that genetically predicted waist circumference (independent of body-mass index) and concentrations of low-density lipoprotein and total cholesterol, were independently associated with risk of CRC, serving as strong support for the causal role of metabolic dysregulation in CRC.56 Mechanistic investigations into MetS and colorectal carcinogenesis at younger ages are thus far lacking and warranted. As mucinous adenocarcinoma and signet ring cell carcinoma are more common with younger age at onset57 and the majority of early-onset CRC are microsatellite stable,4 future investigations according to tumor histopathologic and molecular characteristics will be critical in elucidating the mechanisms linking MetS and early-onset CRC.
Our study has several strengths. First, we conducted a nested case-control study within a large, prospectively maintained, real-world claim-based dataset to examine the association between MetS and pathologically confirmed early-onset CRC. Second, MetS and metabolic comorbid conditions were defined by both ICD-9-CM and use of prescription medications, whereas prior claim-based studies that focused on MetS utilized only ICD-9 diagnosis codes.58,59 Third, to reduce influence of detection bias such that CRC diagnoses may increase the likelihood of detection of concomitant comorbid conditions including MetS,60 we defined our exposures starting 91 days before the index dates.30 The similar strengths of associations for both non-metastatic and metastatic disease further supported the robustness of our findings. Furthermore, we have also adjusted for a list of variables associated with healthcare utilization such as outpatient visits, Charlson Comorbidity Index, and colonoscopies. Our stratified analyses according to outpatient visits lend strong support for the robustness of the findings.
Study limitations include using obesity as a proxy for central obesity, 31 as well as the known undercoding of obesity within claims data.61 Thus, we have likely underestimated the prevalence of MetS. Moreover, according to prior32,62 and the most recent harmonized (2009) definitions,63 MetS represents complex biochemical and arterial pressure alterations that precede cardiometabolic diseases. As such, cut points for abnormal blood pressure and levels of triglycerides, high-density lipoprotein cholesterol, and fasting glucose have been designed explicitly for men and women and vary by population. As lab values were not available in the MarketScan database, we relied on ICD codes for such identification, which could lead to further underestimation of MetS. To address this, we also leveraged medication data to define MetS. Collectively, these non-differential misclassifications are likely to bias the associations toward the null, but limit us from estimating the population attributable risk of MetS for early-onset CRC. Moreover, potential confounding could not be ruled out as lifestyle factors such as smoking and physical activity were not available, both of which have been linked to MetS49,64 and CRC65,66 in older populations. Since these lifestyle factors are relatively moderate risk factors for MetS and CRC, the potential impact of the confounding is expected to be modest. Understanding the association between MetS and early-onset CRC within each race/ethnicity group is critical. Minority populations have higher prevalence of MetS,67,68 comparable/ higher rates of early-onset CRC,69,70 but early-onset CRC rates have been relatively stable in the past 2 decades in sharp contrast to the steep increase among non-Hispanic whites.1,69,70 Unfortunately, the Marketscan database doesn’t include race/ethnicity information. Validation in diverse populations as well as studies elucidating the time lag between MetS onset and elevated risk of EOCRC will shed light on the etiology and preventative strategies. Finally, CRC diagnosed at 50–64 was not sufficiently representative of late-onset CRC as the median age of diagnosis is 66.1 Additional studies comparing the strengths of associations with a much older population are warranted.
In conclusion, findings from this large nested case-control study based on real-world claims data lend strong support to the role of MetS and metabolic comorbid conditions in early-onset CRC. Given the substantial increase in the prevalence of these conditions at the population level, our data indicate that metabolic dysregulation may at least in part contribute to the rising incidence of early-onset CRC. Prevention of MetS among younger adults should be further prioritized in the context of cancer prevention. Due to established care models for cardiometabolic diseases including MetS,71 MetS may also serve as a promising avenue for risk-based CRC screening among younger adults.
Supplementary Material
SUMMARY BOX.
What is already known about this subject?
Obesity and prolonged sitting are associated with increased risk of early-onset colorectal cancer (CRC), indicating that metabolic dysregulation may contribute to the rising incidence of early-onset CRC.
Metabolic syndrome (MetS) among young adults has been increasing worldwide in the past decades, but its association with early-onset CRC remains unexamined.
What are the new findings?
Leveraging real-world claims data that captured over 4,600 early-onset CRC, we found that MetS was associated with 25% increased risk of early-onset CRC, driven by proximal and distal colon cancers as compared to rectal cancer. The strength of this association was similar to that for CRC diagnosed between 50–64.
Number of metabolic comorbid conditions was positively associated with risk of early-onset CRC in a dose-dependent fashion, with a stronger linear relationship, compared to CRC diagnosed between 50–64.
How might it impact on clinical practice in the foreseeable future?
Metabolic dysregulation, as indicated by MetS and increased number of metabolic comorbid conditions, may in part contribute to the rising incidence of early-onset CRC.
MetS may be useful in tailoring clinical algorithms to assess risk of early-onset CRC among younger adults.
Prevention of MetS is likely critical for CRC prevention at all age.
Funding:
This work was supported by U.S. National Institutes of Health (NIH) grant P30CA091842, T32 DK007130, and K07 CA218377 (YC). The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the NIH and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ). HC is a self-funded visiting scholar without financial support from the First Affiliated Hospital of China Medical University. XBZ was supported by the International Program for PhD Candidates, Sun Yat-Sen University (Grant/award number: NA). ZL is a self-funded visiting scholar without financial support from National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Abbreviations:
- CI
confidence interval
- CRC
colorectal cancer
- HCUP CCS
Healthcare Cost and Utilization Project’s Clinical Classification Software
- HMO
Health Maintenance Organization
- IBD
inflammatory bowel diseases
- MetS
metabolic syndrome
- NSAID
nonsteroidal anti-inflammatory drug
- OR
odds ratio
- PPO
Preferred Provider Organization
- SD
standard deviation
Footnotes
Competing interests: All authors have no competing interests to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Sauer AG, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020:1–20. [Google Scholar]
- 2.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019;68(10):1820–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68(12):2179–85. [DOI] [PubMed] [Google Scholar]
- 4.Maur G, Sartore Bianch A, Russo AG, et al. Early onset colorectal cancer in young individuals. Molecular oncology 2019;13(2):109–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archambault AN, Su Y-R, Jeon J, et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants is More Strongly Associated With Early-onset vs Late-onset Cancer. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieu CH, Golemis EA, Serebriiskii IG, et al. Comprehensive genomic landscapes in early and later onset colorectal cancer. Clinical Cancer Research 2019;25(19):5852–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willauer AN, Liu Y, Pereira AA, et al. Clinical and molecular characterization of early, onset colorectal cancer. Cancer 2019;125(12):2002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holowatyj AN, Gigic B, Herpel E, et al. Distinct Molecular Phenotype of Sporadic Colorectal Cancers Among Young Patients Based on Multiomics Analysis. Gastroenterology 2020;158(4):1155–58. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P-H, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA oncology 2019;5(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen LH, Liu P-H, Zheng X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI cancer spectrum 2018;2(4):pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444(7121):881–87. [DOI] [PubMed] [Google Scholar]
- 12.Bankoski A, Harris TB, McClain JJ, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes care 2011;34(2):497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Jama 2001;285(19):2486. [DOI] [PubMed] [Google Scholar]
- 14.Jinjuvadia R, Lohia P, Jinjuvadia C, et al. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. Journal of clinical gastroenterology 2013;47(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trabulo D, Ribeiro S, Martins C, et al. Metabolic syndrome and colorectal neoplasms: An ominous association. World Journal of Gastroenterology: WJG 2015;21(17):5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YJ, Lee DH, Han K-D, et al. Abdominal obesity, glucose intolerance and decreased high-density lipoprotein cholesterol as components of the metabolic syndrome are associated with the development of colorectal cancer. European journal of epidemiology 2018;33(11):1077–85. [DOI] [PubMed] [Google Scholar]
- 17.Ulaganathan V, Kandiah M, Shariff ZM. A case-control study of the association between metabolic syndrome and colorectal cancer: a comparison of International Diabetes Federation, National Cholesterol Education Program Adults Treatment Panel III, and World Health Organization definitions. Journal of gastrointestinal oncology 2018;9(4):650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borch-Johnsen K The metabolic syndrome in a global perspective. The public health impact--secondary publication Dan Med Bull 2007;54(2):157–59. [PubMed] [Google Scholar]
- 19.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adults. Diabetes care 2004;27(10):2444–49. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama 2002;287(3):356–59. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar M, Bhuket T, Torres S, et al. Prevalence of the metabolic syndrome in the United States, 2003–2012. Jama 2015;313(19):1973–74. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. Jama 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. Jama 2010;303(20):2043–50. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. New England Journal of Medicine 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lascar N, Brown J, Pattison H, et al. Type 2 diabetes in adolescents and young adults. The Lancet Diabetes & Endocrinology 2018;6(1):69–80. [DOI] [PubMed] [Google Scholar]
- 26.Health IW. White paper: IBM MarketScan Research Databases for health services researchers. 2018. [Google Scholar]
- 27.Anaya DA, Becker NS, Richardson P, et al. Use of administrative data to identify colorectal liver metastasis. Journal of Surgical Research 2012;176(1):141–46. [DOI] [PubMed] [Google Scholar]
- 28.Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS) 2015. US Agency for Healthcare Research and Quality; 2015, 2014. [Google Scholar]
- 29.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology 2000;53(12):1258–67. [DOI] [PubMed] [Google Scholar]
- 30.Takvorian SU, Oganisian A, Mamtani R, et al. Association of Medicaid expansion under the Affordable Care Act with insurance status, cancer stage, and timely treatment among patients with breast, colon, and lung cancer. JAMA Network Open 2020;3(2):e1921653–e53. [DOI] [PubMed] [Google Scholar]
- 31.Japan ECoCfODi. New criteria for’obesity disease’in Japan. Circulation journal: official journal of the Japanese Circulation Society 2002;66(11):987. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Becker D, Clark LT, et al. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Circulation 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 33.Gillespie CD, Hurvitz KA, Control CfD, et al. Prevalence of hypertension and controlled hypertension—United States, 2007–2010. MMWR Surveill Summ 2013;62(Suppl 3):144–8. [PubMed] [Google Scholar]
- 34.Crawford AG, Cote C, Couto J, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity Electronic Medical Record database. Population health management 2010;13(3):151–61. [DOI] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 36.Siegel RL, Medhanie GA, Fedewa SA, et al. State variation in early-onset colorectal cancer in the United States, 1995–2015. JNCI: Journal of the National Cancer Institute 2019;111(10):1104–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97(22):1679–87. [DOI] [PubMed] [Google Scholar]
- 38.Milano A, Bianco MA, Buri L, et al. Metabolic syndrome is a risk factor for colorectal adenoma and cancer: a study in a White population using the harmonized criteria. Therapeutic advances in gastroenterology 2019;12:1756284819867839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong SN, Kim JH, Choe WH, et al. Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointestinal endoscopy 2010;72(3):480–89. [DOI] [PubMed] [Google Scholar]
- 40.Aleksandrova K, Boeing H, Jenab M, et al. Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer prevention research 2011;4(11):1873–83. [DOI] [PubMed] [Google Scholar]
- 41.Gurka MJ, Filipp SL, DeBoer MD. Geographical variation in the prevalence of obesity, metabolic syndrome, and diabetes among US adults. Nutrition & diabetes 2018;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovannucci E Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. The American journal of clinical nutrition 2007;86(3):836S–42S. [DOI] [PubMed] [Google Scholar]
- 43.Pollak M Insulin and insulin-like growth factor signalling in neoplasia. Nature Reviews Cancer 2008;8(12):915–28. [DOI] [PubMed] [Google Scholar]
- 44.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nature reviews Endocrinology 2019;15(3):139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirbel J, Pyl PT, Kartal E, et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nature medicine 2019;25(4):679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018;555(7695):210–15. [DOI] [PubMed] [Google Scholar]
- 47.Jia W, Xie G, Jia W. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nature reviews Gastroenterology & hepatology 2018;15(2):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best practice & research Clinical gastroenterology 2014;28(4):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford ES, Kohl HW III, Mokdad AH, et al. Sedentary behavior, physical activity, and the metabolic syndrome among US adults. Obesity research 2005;13(3):608–14. [DOI] [PubMed] [Google Scholar]
- 50.Zheng X, Nguyen LH, Liu P-H, et al. Comprehensive Assessment of Diet Quality and Risk of Early-Onset Colorectal Adenoma. DDW 2019. Gastroenterology 2019;156(6):S–208. [Google Scholar]
- 51.Wirfält E, Hedblad B, Gullberg B, et al. Food patterns and components of the metabolic syndrome in men and women: a cross-sectional study within the Malmö Diet and Cancer cohort. American journal of epidemiology 2001;154(12):1150–59. [DOI] [PubMed] [Google Scholar]
- 52.Kruk J, Aboul-Enein BH, Bernstein J, et al. Psychological Stress and Cellular Aging in Cancer: A Meta-Analysis. Oxid Med Cell Longev 2019;2019:1270397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitaliano PP, Scanlan JM, Zhang J, et al. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic medicine 2002;64(3):418–35. [DOI] [PubMed] [Google Scholar]
- 54.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308(5724):1043–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuhr L, El-Athman R, Scrima R, et al. The Circadian Clock Regulates Metabolic Phenotype Rewiring Via HKDC1 and Modulates Tumor Progression and Drug Response in Colorectal Cancer. EBioMedicine 2018;33:105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cornish AJ, Law PJ, Timofeeva M, et al. Modifiable pathways for colorectal cancer: a mendelian randomisation analysis. The lancet Gastroenterology & hepatology 2020;5(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holowatyj AN, Lewis MA, Pannier ST, et al. Clinicopathologic and racial/ethnic differences of colorectal cancer among adolescents and young adults. Clinical and translational gastroenterology 2019;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kasmari AJ, Welch A, Liu G, et al. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. The American journal of medicine 2017;130(6):746.e1–46.e7. [DOI] [PubMed] [Google Scholar]
- 59.Trabert B, Wentzensen N, Felix AS, et al. Metabolic syndrome and risk of endometrial cancer in the united states: a study in the SEER-medicare linked database. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2015;24(1):261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Bruijn KM, Ruiter R, de Keyser CE, et al. Detection bias may be the main cause of increased cancer incidence among diabetics: results from the Rotterdam Study. European journal of cancer 2014;50(14):2449–55. [DOI] [PubMed] [Google Scholar]
- 61.Golinvaux NS, Bohl DD, Basques BA, et al. Limitations of administrative databases in spine research: a study in obesity. The Spine Journal 2014;14(12):2923–28. [DOI] [PubMed] [Google Scholar]
- 62.Grundy SM. Metabolic syndrome scientific statement by the american heart association and the national heart, lung, and blood institute: Am Heart Assoc, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009;120(16):1640–45. [DOI] [PubMed] [Google Scholar]
- 64.Slagter SN, van Vliet-Ostaptchouk JV, Vonk JM, et al. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC medicine 2013;11(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsoi KK, Pau CY, Wu WK, et al. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clinical Gastroenterology and Hepatology 2009;7(6):682–88. e5. [DOI] [PubMed] [Google Scholar]
- 66.Samad A, Taylor R, Marshall T, et al. A meta, analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal disease 2005;7(3):204–13. [DOI] [PubMed] [Google Scholar]
- 67.Loucks EB, Rehkopf DH, Thurston RC, et al. Socioeconomic disparities in metabolic syndrome differ by gender: evidence from NHANES III. Annals of epidemiology 2007;17(1):19–26. [DOI] [PubMed] [Google Scholar]
- 68.Falkner B, Cossrow ND. Prevalence of metabolic syndrome and obesity-associated hypertension in the racial ethnic minorities of the United States. Current hypertension reports 2014;16(7):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holowatyj AN, Ruterbusch JJ, Rozek LS, et al. Racial/ethnic disparities in survival among patients with young-onset colorectal cancer. Journal of Clinical Oncology 2016;34(18):2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy CC, Wallace K, Sandler RS, et al. Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology 2019;156(4):958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sperling LS, Mechanick JI, Neeland IJ, et al. The CardioMetabolic Health Alliance. Working Toward a New Care Model for the Metabolic Syndrome 2015;66(9):1050–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


