Abstract
Despite the global effort to mitigate the spread, coronavirus disease 2019 (COVID-19) has become a pandemic that took more than 2 million lives. There are numerous ongoing clinical studies aiming to find treatment options and many are being published daily. Some effective treatment options, albeit of variable efficacy, have been discovered. Therefore, it is necessary to develop an evidence-based methodology, to continuously check for new evidence, and to update recommendations accordingly. Here we provide guidelines on pharmaceutical treatment for COVID-19 based on the latest evidence.
Keywords: COVID-19, Clinical practice guidelines, Korea
Background
Despite the preventive efforts being made globally against coronavirus disease (COVID-19), it is the most widespread infectious disease worldwide in 2020. No definitive cure has been developed, and vaccines are in the early stages of implementation. While Korea has successfully protected its citizens from large-scale COVID-19 outbreaks, healthcare providers on the frontline are in need of the continuously updated evidence-based clinical practice guidelines for treating patients with COVID-19 who developed pneumonia and COVID-19-related disease of other organs and body systems. Globally, many ongoing clinical studies aim to develop vaccines and therapeutics, and several reports of such clinical studies are being published daily. Therefore, it is necessary to develop an evidence-based methodology; continuously check for new evidence; and update recommendations accordingly. Since no standard antiviral treatment has been established, we aim to provide guidelines on antiviral and other drug treatment for COVID-19 based on the latest evidence.
Objectives
This study aimed to review the latest evidence on treatment for COVID-19 and outline evidence-based clinical practice guidelines for healthcare professionals. It also aimed to devise a guideline development methodology that can be rapidly applied in different settings.
Methods
Regarding the guideline development methodology, we used the adaptation method wherein the latest guidelines from major countries and organizations are reviewed within a short time. In addition, we applied the living guidelines development methodology in which guidelines are continuously updated using the latest evidence. The guideline development process is described in the following sections.
1. Search for practice guidelines
We selected the database, devised a search strategy, and developed the process of literature search with the help of literature search specialists. We decided to include published practice guidelines or regularly updated living guidelines between June 1 and December 9, 2020.
2. Search database
We searched international literature databases such as PubMed and EMBASE as well as major practice guideline databases and websites. We also searched the official websites of major government organizations, institutions, and academic societies because the data on these resources are updated in real-time.
3. Search strategy
We searched PubMed and EMBASE databases using filters to search for literatures regarding treatment and clinical guidelines. The search terms were combinations of index term and text words based on treatment terminologies from each clinical question: “coronavirus,” “novel coronavirus,” “novel coronavirus 2019,” “2019 nCoV,” “COVID-19,” “Wuhan coronavirus,” “Wuhan pneumonia,” “SARS-CoV-2,” “severe acute respiratory syndrome,” “treatment,” “therapy,” and “antiviral.”
4. Criteria for selection of guidelines
As studies released since June 2020 contain reliable information on the COVID-19 treatment, we decided to select the latest practice guidelines with high methodological quality (higher than 70 score in 3 domains out of 6 domains). We included guidelines developed by major countries and organizations that included recommendations for the selected clinical questions and are frequently updated. We excluded guidelines which are developed before June 2020, not including target treatments, review paper or consensus based guideline, not written in English or Korean.
5. Quality appraisal of guidelines
The quality of the practice guidelines was evaluated by three researchers using the AGREE (The Appraisal of Guidelines for Research and Evaluation) 2 tool [1]. To reduce the variation between the evaluators, the K-AGREE evaluation form developed by the Korean Medical Association was used. During the AGREE evaluation, to ensure the reproducibility and clarity of the result, the evaluators were asked to describe the reason for awarding a score in the comment section, share evaluation results, and, if necessary, correct errors or mistakes via a review. After quality evaluation of the practice guidelines, we decided to use the guidelines that received more than 70 score in the three areas of AGREE.
6. Searching and selection of the latest evidence
The references selected from the evidence table of existing practice guidelines were reviewed before being included. For additional literature search, we used three international databases (PubMed, EMBASE, and the Cochrane Database of Systematic Reviews [CDSR]) and one domestic database (KMBASE). Considering the rapid increase in the number of publications on the treatment of COVID-19, we initially planned to include two preprint databases, MedRxiv and bioRxiv. However, we decided to exclude them later because there was a sufficient amount of literature from our primary search. Search terms were devised for each clinical question based on the search strategy for practice guidelines.
For constantly updating evidence, a semi-automated software for systematic review, Covidence (Covidence, Melbourne, Australia), was purchased and used in the process of literature selection.
As with the guideline selection criteria, we decided to include human studies on COVID-19 treatment published from June 1 through December 9, 2020 and addressed the clinical questions of our guidelines. We included randomized clinical trials and observational studies with comparative designs after checking the amount of evidence for each clinical question.
7. Assessment of the risk of bias in the selected primary literature
The risk of bias of the references in the evidence table of existing practice guidelines was reviewed to determine if they met the quality criteria. For the quality evaluation of the additional publications, an appropriate tool was selected according to the research design. Two researchers evaluated each paper independently. If they did not reach an agreement, an additional researcher's consult was used to achieve consensus.
8. Managing conflicts of interest
We set-up the policy of managing conflicts of interest (COI) and developed a template for COI disclosure, including financial, intellectual, and other types of potential conflicts. All members of the guideline committee declared that they had no potential COI related to the results of the interventions assessed during the guideline development period.
Results
1. Selection and evaluation of practice guidelines
Seven out of eleven practice guidelines found by a manual search were screened using the AGREE evaluation. Four additional practice guidelines were identified from a systematic search and were evaluated using the AGREE. Finally, we selected four practice guidelines that are most up to date with a score of 70 or higher in three major areas of the AGREE evaluation. A table of recommendations from all 11 evaluated practice guidelines was prepared for comparison. The United States National Institutes of Health classification of the severity of illness was adopted after a review of similar classifications from various guidelines [2] (Table 1).
Table 1. Classification of severity of illness referred by National Institutes of Health.
| Severity of illness | Definition |
|---|---|
| 1. Asymptomatic | Individuals who test positive for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test or an antigen test), but who have no symptoms that are consistent with COVID-19. |
| 2. Mild | Individuals who have any of the various signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell) but who do not have shortness of breath, dyspnea, or abnormal chest imaging. |
| 3. Moderate | Individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have saturation of oxygen (SpO2) ≥94% on room air at sea level. |
| 4. Severe | Individuals who have SpO2 <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, respiratory frequency >30 breaths per minute, or lung infiltrates >50%. |
| 5. Critical | Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction. |
Source: NIH. Clinical Spectrum of SARS-CoV-2 Infection. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ [2].
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; SpO2, saturation of percutaneous oxygen; PaO2, partial pressure of oxygen; FiO2, fraction of Inspired oxygen.
2. Preparation of evidence table and review of recency
An evidence table of each clinical question with the selected literature was prepared according to the selection and exclusion criteria for the practice guidelines. The results of the quality evaluations of the existing guidelines were reviewed and reevaluated, if necessary. The studies retrieved on November 9 and December 9, 2020 were reviewed and added to the evidence tables.
3. Recommendations and external review
The committee in charge of clinical questions reviewed the summary of evidence and recommendations, prepared the first draft of recommendations, and decided on the final recommendations, certainty of evidence (Table 2), and grade of recommendation (Table 3) at a general meeting [3,4]. Then, external reviews from advisory and review committees were received in writing. The final draft of recommendations was completed after another discussion (Table 4).
Table 2. Certainty of evidence.
| Grade | Definition |
|---|---|
| High | We are very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate | We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low | Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. |
| Very Low | We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. |
Table 3. Definition of recommendation grading.
| Grade of recommendations | Explanation | |
|---|---|---|
| A | Strong recommendation | The intervention can be strongly recommended in most clinical practice, considering greater benefit than harm, evidence level, value and preference and resources. |
| B | Conditional recommendation | The intervention can be conditionally recommended in clinical practice considering balance of benefit and harm, evidence level, value and preference and resources. |
| C | Not recommended | The harm of the intervention maybe greater than the benefit. Also considering evidence level, value and preference and resources, the intervention should not be recommended. |
| I | Inconclusive | Considering of very low or insufficient evidence level, uncertain or variable in balancing of benefit and harm, value and preference, and resources, it is not possible to determine the strength and direction of recommendation It means that intervention cannot be recommended or opposed and the decision depends on clinician's judgement. |
| E | Expert consensus | There is not enough evidence to give an evidence-based recommendation but a consensus-based recommendation can be given based on clinical experiences and expert consensus methods under considering given the benefit and harm, preference and value, and resources. |
A, strong recommendation; B, conditional recommendation ; C, not recommended; I, inconclusive, E, expert Consensus.
Table 4. Summary of recommendations.
| Clinical Questions | Recommendation | Certainty of evidence | Grade of Recommendation |
|---|---|---|---|
| CQ1. Remdesivir | 1-1. Remdesivir is recommended for patients with COVID who require supplementary oxygen therapy, but who do not require invasive ventilation or ECMO. | Moderate | B |
| 1-2. There is insufficient evidence to recommend either for or against the use of remdesivir for patients who do not meet the condition in the Recommendation 1-1. | Moderate | I | |
| CQ2. HCQ ± azithromycin | HCQ monotherapy or HCQ + AZM combination therapy is not recommended for patients with COVID-19. | High | C |
| CQ3. LPV/r | Lopinavir/ritonavir is not recommended for patients with COVID-19. | High | C |
| CQ4. Other antiviral drugs | Administration of drugs known to have antiviral effects, such as favipiravir, ribavirin, umifenovir, and baloxavir marboxil, is not recommended for patients with COVID-19 regardless of the severity. | Low | C |
| CQ5. Steroids | 5-1. Steroid administration is recommended for patients in severe and critical stages of COVID-19. | Moderate | A |
| Clinical consideration: The steroid dose is 6 mg of dexamethasone for 7 – 10 days; it can be replaced with equivalent dose of other steroids (hydrocortisone, 150 – 200 mg; prednisone, 40 mg; methylprednisolone, 32 mg). | |||
| 5-2. Steroid administration is not recommended for patients with non-severe COVID-19. | Moderate | C | |
| CQ6. IL-6 inhibitors | 6-1. Interleukin-6 inhibitors can be administered, within the scope of clinical trials, to patients in severe or critical stages of COVID-19. | Moderate | B |
| 6-2. Administration of interleukin-6 inhibitors is not recommended for patients with mild COVID-19. | Moderate | C | |
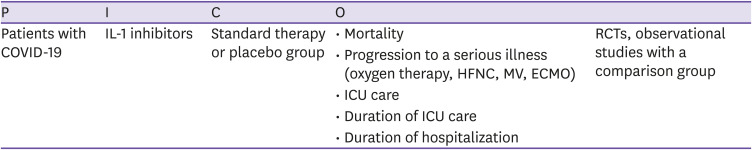
| CQ7. IL-1 inhibitors | There is insufficient evidence supporting the administration of interleukin-1 (IL-1) inhibitors for treating COVID-19. | Low | I |
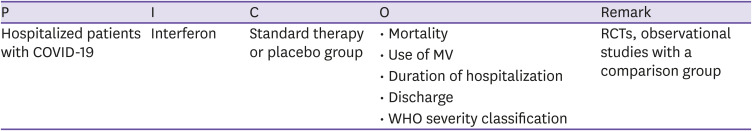
| CQ8. Interferon | Interferon can be used within the scope of clinical trials in patients with COVID-19. | Low | B |
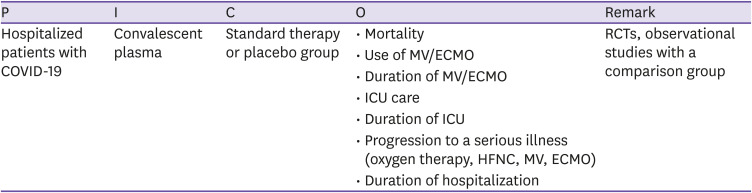
| CQ9. Convalescent plasma | There is insufficient evidence on the benefit of convalescent plasma therapy in patients with COVID-19 to make recommendations. | Low | I |
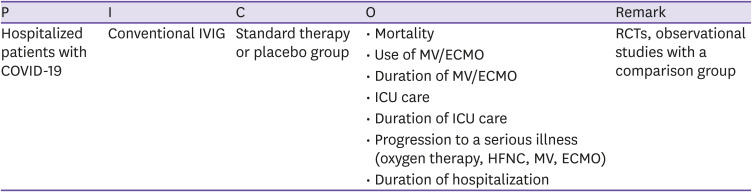
| CQ10. Conventional IVIG | Administration of conventional IVIG is not recommended for patients with COVID-19. However, IVIg therapy should not be excluded when indicated for treatment of complications of COVID-19. | Low | C |
COVID-19, coronavirus disease; ECMO, extracorporeal membrane oxygenation; HCQ, hydroxychloroquine; AZM, azithromycin; LPV/r, lopinavir and ritonavir; IL, interleukin; IVIG, intravenous immunoglobulin.
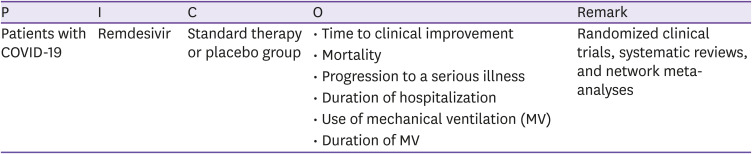
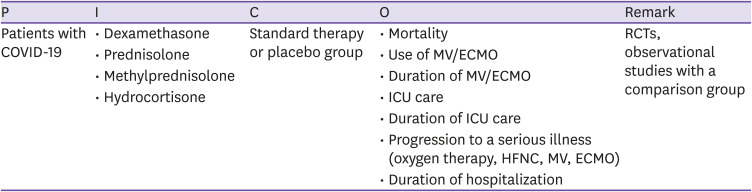
CQ1 Remdesivir
○ Clinical question
1. Is the administration of remdesivir recommended in patients hospitalized with COVID-19 regardless of clinical severity?
2. In patients hospitalized with COVID-19 who required oxygen therapy, is remdesivir treatment more effective than standard treatment?
○ PICO elements
○ Recommendations
1-1. Remdesivir is recommended for patients with COVID who require supplementary oxygen therapy, but who do not require invasive ventilation or extracorporeal membrane oxygenation (ECMO) (certainty of evidence: moderate, grade of recommendation: B).
1-2. There is insufficient evidence to recommend either for or against the use of remdesivir for patients who do not meet the condition in the Recommendation 1-1 (certainty of evidence: moderate, grade of recommendation: I [insufficient evidence]).
○ Basic information about remdesivir
Remdesivir (GS-5734) is a wide-range antiviral nucleotide (adenosine) analog monophosphoramidate pro-drug that acts as a ribonucleic acid (RNA)-dependent RNA polymerase inhibitor for various RNA viruses [5]. In in vitro experiments, potent antiviral effects of remdesivir have been observed against various RNA viruses (Ebola virus, Marburg virus, Middle East respiratory syndrome (MERS)-CoV [6], SARS-CoV [6], other coronaviruses, respiratory syncytial virus, Nipah virus, and Hendra virus). In in vivo experiments using the Rhesus macaque model, administration of remdesivir as prophylactic treatment 24 hours before MERS-CoV inoculation was found to prevent MERS-CoV infection, and remdesivir treatment at 12 hours after virus inoculation led to clinical improvement, inhibition of virus replication, and reduction of cell injury in the lungs [7]. Phase 1 safety and pharmacokinetic data in humans have been recorded, and remdesivir has also been administered to a 19-day-old newborn [8]. In a study involving patients infected with the Ebola virus, remdesivir was administered to 175 patients [9].
Based on the reports on in-vitro antiviral experiments and safety data of remdesivir in different clinical studies, remdesivir was administered to a patient with COVID-19 for compassionate use on January 26, 2020, and remdesivir treatment was successfully completed without adverse side effects [10].
The National Institute of Allergy and Infectious Diseases (NIAID) Adaptive COVID-19 Treatment Trial (ACTT-1) (NCT04280705) involving patients with mild/moderate and severe COVID-19 was started on February 21 [10]. As an interim report showed that remdesivir treatment reduced the clinical recovery time, on May 1, 2020, the Food and Drug Administration (FDA) issued an emergency use authorization (EUA) allowing the use of remdesivir (product name: Veklury®) for treating patients with COVID-19. On October 22, 2020, after the results of the ACTT-1 were reported, remdesivir was officially approved as the first drug for treating patients with COVID-19. However, on November 2, 2020, the World Health Organization (WHO) announced that remdesivir did not significantly reduce the number of deaths due to COVID-19, as per the SOLIDARITY trial results [11], and revised the guidelines to suggest against administering remdesivir in addition to standard care in hospitalized patients with COVID-19 (recommendation against [weak])[12]. Thus, the effects of remdesivir remain controversial.
○ Evidence summary
A total of four guidelines that included recommendations for remdesivir were selected (WHO living guideline, November 2, 2020; Australian Clinical Practice Guideline [ACPG], as of December 3, 2020 v.30.0; National Institute of Health [NIH] as of December 3, 2020; and Infectious Diseases Society of America [IDSA] updated November 11, 2020), based on the results of four randomized controlled trials (RCTs).
The guidelines were based on five studies: four RCTs, one systematic review, and a network meta-analysis study of the four RCTs included in the final evidence table. Clinical improvement was the primary endpoint in the three studies (Wang [13], Biegel [10], Spinner [14], and in-hospital mortality was the primary endpoint in the SOLIDARITY trial [11].
In the ACTT-1 clinical trial [10], which first reported the effects of remdesivir, 1,062 patients were enrolled: 159 patients with mild/moderate COVID-19 and 903 (85.0%) with severe, respectively. The primary endpoint, the time to recovery, was 10 median days in the remdesivir group and 15 days in the placebo group (recovery rate ratio [RR], 1.49; 95% confidence interval [CI], 1.12 – 1.49; P <0.001), and for patients with severe COVID-19 (957 patients) at the time of enrollment, the meantime to recovery was 11 days in the remdesivir group and 18 days in the placebo group (recovery RR, 1.31; 95% CI, 1.12 – 1.52; P <0.001). However, there was no difference between the two groups for those patients who received MV support or extracorporeal membrane oxygenation (ECMO) treatment at the time of enrollment. The mortality rate by day 15 was 6.7% in the remdesivir group and 11.9% in the placebo group (hazard ratio [HR], 0.55; 95% CI, 0.36 – 0.83), and by day 29, mortality rate was 11.4% vs. 15.2% (HR, 0.73; 95% CI, 0.52 – 1.03), respectively. The mortality rate varied depending on the severity of COVID-19 at the time of enrollment, and the administration of remdesivir decreased the mortality rate in those patients who required oxygen therapy.
In a clinical trial conducted in China [13], 237 patients with severe COVID-19 were enrolled: 158 and 79 patients received remdesivir and placebo, respectively. The primary endpoint was time to clinical recovery within 28 days, and clinical improvement was defined as a decrease of 2 points on a 6-point scale or discharge from the hospital. Intention-to-treat analysis showed that the time to clinical recovery was 21 days (median; interquartile range [IQR], 13.0 – 28.0) and 23 days (IQR, 15.0 – 28.0) in the remdesivir and placebo groups, respectively, indicating no difference between the two groups (HR, 1.23; 95% CI, 0.87 – 1.75). Although there was no statistical difference, the time to recovery was 18 days (median; IQR, 12.0 – 28.0) when remdesivir was administered within 10 days of symptom onset, which was slightly shorter than the 23 days (IQR, 15.0 – 28.0) in the placebo group (HR, 1.52; 95% CI, 0.95 – 2.43).
In a clinical trial involving patients with moderate pneumonia [14], 596 patients were enrolled, and 197, 199, and 200 patients were administered remdesivir for 10 days and 5 days and placebo, respectively. The primary endpoint was clinical status evaluated on a 7-point ordinal scale on the 11th day after enrollment; the clinical status of the group treated with remdesivir for 5 days had significantly improved compared to that of the placebo group (OR, 1.65; 95% CI, 1.09 – 2.48; p = 0.02). The 28-day mortality rate of the patients was 1% (95% CI, 0.0 – 2.6) in the 5-day treatment group, 2% in the 10-day treatment group (95% CI, 0.0 – 3.6%), and 2% in the placebo group (95% CI, 0.1 – 4.1%), and there was no statistically significant difference between the groups.
The interim report of the WHO SOLIDARITY trial consortium, which was conducted to evaluate repurposed antiviral drugs, was published as a MedRxiv version on October 5, 2020, and the interim findings were officially announced on December 2 [11]. A total of 405 hospitals from 30 countries participated in the study, and 11,266 adults were randomly assigned to the remdesivir group (n = 2,743) and standard treatment group (n = 2,708) and the results were compared. The 28-day mortality risk of the remdesivir group was 0.95 (0.81 – 1.11; P = 0.50; 301/2,743 vs. 303/2,708), similar to that of the standard treatment group.
Based on the data from the aforementioned four RCTs, systematic review, and meta-analysis [12], on November 2, 2020, the WHO made the following announcements: remdesivir did not have a significant effect on the mortality rate, use of MV, severe adverse reactions leading to discontinuation of drug administration, virus clearance rate at day 7, acute kidney injury, time to clinical improvement, duration of hospitalization, and duration of MV. Therefore, regardless of the severity of COVID-19 infection, the WHO suggested the conditional recommendation against administering remdesivir in addition to standard care. However, the certainty of the evidence was low to very low.
○ Considerations for recommendation
1. Certainty of evidence
The differences in the baseline conditions of the patients and characteristics of the control group were clearly described in the four RCTs, and the level of evidence was high. However, three RCTs evaluated the time to clinical recovery as the primary endpoint, while mortality rate was the endpoint in the other RCT and systematic analysis study. Thus, there were differences in the endpoints of the studies. Moreover, patients with varying disease severity were enrolled, and according to the evaluation index and disease severity, the therapeutic effects of remdesivir differed between studies. On evaluating the risk of bias in the main RCTs, there were concerns about certain aspects such as missing data; therefore, the level of evidence was moderate.
2. Benefit and harm
The RCTs reported that remdesivir shortened the time to recovery while other studies showed no effect on the recovery time or mortality rate; hence, the results were inconsistent. Based on the evidence so far, it is unlikely that remdesivir will reduce mortality in patients with severe COVID-19. However, remdesivir may shorten the clinical recovery time in some patients with severe COVID-19. In all the studies, there was no significant difference in the frequency of severe adverse reactions caused by antiviral drugs and the frequency of discontinuation of drugs between the remdesivir treatment and control groups.
3. Patient values and preferences
There is no study assessing the values and preferences for remdesivir among patients with COVID-19 in South Korea. However, the Korean Ministry of Food and Drug Safety has approved remdesivir, and it is provided free of charge to patients with severe COVID-19. There is no other antiviral drug that can replace remdesivir. Therefore, the preference for remdesivir is expected to be high.
4. Resources (including cost)
Remdesivir is offered free of charge to patients with severe COVID-19 by the Korean government.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
The WHO issued conditional recommendation guidelines against use of remdesivir in hospitalized patients with COVID-19; however, the level and grade of recommendation were not specified. In the Australian guidelines, conditional recommendations were made for inpatients who require oxygen therapy but not MV support, and a recommendation grade of 2 was assigned.
The US NIH does not recommend the use of remdesivir for patients with mild/moderate COVID-19 (AI). Administration of remdesivir is recommended for 5 days in those patients who require oxygen therapy but not high-flow oxygen, non-invasive/invasive MV support, or ECMO. No specific guidelines are provided for these patients, as there is no clinical evidence on the effectiveness of remdesivir for this population. The IDSA guidelines recommended the use of remdesivir rather than other antiviral drugs in hospitalized patients with severe COVID-19 (conditional recommendation, moderate certainty of evidence), and 5-day administration of remdesivir rather than 10-day administration was recommended for patients who require oxygen therapy but not MV support or ECMO (conditional recommendation, low certainty of evidence).
2) Evaluation of acceptance and applicability in Korea
The Korean evaluation tool in the National Evidence-based Healthcare Collaborating Agency (NECA) Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
The Korean Ministry of Food and Drug Safety decided to import remdesivir on June 3, 2020, and it was administered to patients with severe COVID-19 from July 1, 2020. On July 24, 2020, the Ministry approved Veklury®. However, this is only a measure taken to ensure a stable domestic supply of remdesivir considering the urgent COVID-19 situation. In a nationwide multicenter retrospective study, remdesivir group showed clinical and virologic benefit in terms of MV requirement and viral load reduction, supporting remdesivir treatment for severe COVID-19 [15].
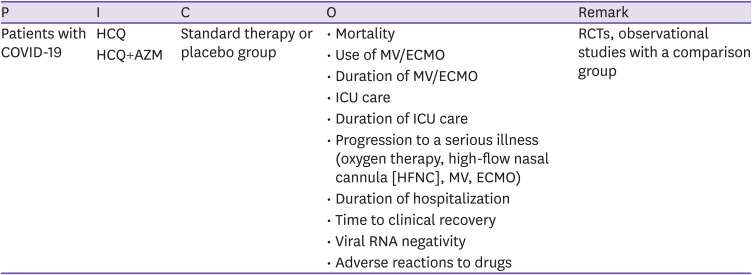
CQ2 Hydroxychloroquine ± Azithromycin
○ Clinical question
Is the administration of hydroxychloroquine (HCQ) or co-administration of HCQ and azithromycin (AZM) effective and safe for patients with COVID-19 compared to standard treatment or no treatment?
○ PICO elements
○ Recommendations
HCQ monotherapy or HCQ + AZM combination therapy is not recommended for patients with COVID-19 (certainty of evidence: high).
○ Basic information on antiviral agents
Chloroquine (CQ) has been used for a long time to treat malaria and intracellular bacterial infections caused by Coxiella burnetii and Tropheryma whipplei. As a heme polymerase inhibitor, CQ interferes with in vitro binding between the cell and virus by increasing the pH of the polyphagosomes and inhibiting glycosylation of the cellular receptors of SARS-CoV [16,17,18]. However, the demand for CQ in the clinics has decreased over the past years, leading to a decrease in its production and supply. Instead, HCQ, an analog formulation of CQ developed in 1946, has replaced CQ. HCQ has shown anti-SARS-CoV activity in in vitro experiments [19] and can be administered for a longer period and at higher doses than CQ; it has few drug interactions and can achieve higher concentration in tissues such as the lungs, liver, kidneys, and spleen than in the plasma. Thus, it is thought to be relatively safe compared to CQ [20,21]. Moreover, HCQ has significantly higher antiviral effects against SARS-CoV-2 than CQ [22]. In patients with severe COVID-19, cytokine storms, featuring high circulating cytokine levels, are observed in the plasma and are associated with disease severity [23]. Unlike other antiviral drugs, HCQ is a safe anti-inflammatory drug used for treating various autoimmune diseases. It can significantly reduce the production of various cytokines, especially pro-inflammatory cytokines, resulting in positive effects against COVID-19 [21]. However, HCQ can cause cardiotoxicity, including QT prolongation, renal dysfunction, and hepatic dysfunction. Thus, any underlying diseases related to these dysfunctions need to be identified before administration, and HCQ must be administered with care after assessing the interactions with other prescribed or previously administered medications [24]. AZM, a macrolide antibiotic, is an antibiotic drug against various bacterial diseases, and it has antiviral [25,26,27,28,29] and immune-modulatory [10,30,31] effects against some viruses (Zika, Ebola, Rhinovirus, Enterovirus, and Influenza), including SARS-CoV-2. Thus, HCQ and AZM combination therapy has been used for treating patients with COVID-19 [32,33,34,35,36,37,38,39,40,41,42,43,44].
○ Evidence summary
A total of four guidelines that included recommendations for HCQ monotherapy and HCQ + AZM combination therapy were selected; 47 studies, i.e., 13 RCTs and 34 observational studies, related to those guidelines were included in the final evidence table. The results are summarized below:
In nine out of the 13 RCTs on HCQ, improvement of clinical course or progression to severe period (oxygen administration, mechanical ventilation, ECMO, etc.) and time to improvement/deterioration were evaluated as additional endpoints. Among these studies, six studies assessed mortality rate as the primary endpoint. Negative conversion of the virus was evaluated as the primary endpoint in six RCTs, and adverse drug effect was measured as an additional endpoint in five out of the 13 RCTs.
The RECOVERY trial is an important study among those that assessed mortality and improvement in clinical course or progression to severe disease [29]. It is a randomized, open, clinical trial that evaluated the effects of HCQ in patients admitted to 176 hospitals in the UK; 1,561 and 3,155 were assigned to the HCQ and standard treatment groups, respectively. In the HCQ group, 412 patients (27%) died within 28 days while 790 patients (25%) died in the standard treatment group, suggesting no significant difference in the 28-day mortality rate between the two groups (RR, 1.09; 95% CI, 0.97 – 1.23; P = 0.15). The patients in the HCQ group had a longer duration of hospitalization (16 days vs. 13 days) and were less likely to survive for 28 days and be discharged (59.6% vs. 62.9%; RR, 0.90; 95% CI, 0.83 – 0.98) than those in the standard treatment group. In addition, among those patients who did not require MV support at the time of hospitalization, those who received HCQ showed a higher incidence of invasive MV support or death than those who received standard treatment (30.7% vs. 26.9%; RR, 1.14; 95% CI, 1.03 – 1.27) [29]. In a randomized, double-blinded clinical trial (242 and 237 patients in the HCQ group and untreated control group, respectively) involving 34 hospitals in the US, there was no significant difference between the groups on using the 7-point clinical ordinal scale on the 14th day (HCQ, 6 [4 – 7] vs. untreated control group, 6 [4 – 7]; aOR, 1.02 [95% CI, 0.73 – 1.42]), and there was no significant difference in the 28-day mortality rate between the two groups (HCQ, 10.4% (25/241) vs. 10.6% (25/236), (absolute difference, −0.2% [95% CI, −5.7% to 5.3%]; aOR, 1.07 [95% CI, 0.54 – 2.09]) [45]. In a randomized double-blinded clinical trial involving three hospitals in New York, 67 and 61 patients were assigned to the HCQ group and untreated control group (calcium citrate), respectively. There was no significant difference in the 30-day mortality rate between the two groups (HCQ: n = 7, 10.4% vs. control: n = 6, 9.8%, P = 1.0), and the mean duration of hospitalization was not significantly different between the two groups as well (HCQ, 9.75 [± 10.3] days vs. control, 6.80 [± 5.92], P = 0.053). The durations of antipyretic treatment and oxygen-free days were not significantly different between the two groups, and there was also no significant difference in the clinical severity score on day 14 between the two groups (P = 0.354) [46]. In a multi-center, randomized, open, clinical trial conducted in Brazil, the patients were assigned to standard treatment group (173 patients), standard treatment with HCQ group (159 patients), and standard treatment with HCQ + AZM group (172 patients);there was no significant difference in the results between the groups using a 7-point clinical ordinal scale on day 15 (HCQ + AZM vs. standard treatment: odds ratio [OR], 0.99; 95% CI, 0.57 – 1.73; P = 1.00; HCQ vs. standard treatment: OR, 1.21; 95% CI, 0.69 – 2.11; P = 1.00; HCQ + AZM vs. HCQ: OR, 0.82; 95% CI, 0.47 – 1.43; P = 1.00) [44]. In a multi-center randomized clinical trial conducted in Egypt, 97 patients were assigned to the HCQ group and standard treatment group, and the frequency of MV support was not significantly different between the two groups (HCQ: n = 4, 4.1% vs. standard treatment: n = 5, 5.2%; P = 0.75), and there was no significant difference in overall mortality between the two groups (HCQ: n = 6, 6.2% vs. standard treatment: n = 5, 5.2%; P = 0.77) [47]. In a randomized clinical trial conducted in a hospital in Shanghai, China, with 30 patients with moderate COVID-19 (15 patients in each of the HCQ group and standard treatment group), the time from hospitalization to the onset of fever was 1 day (0 – 2) in both groups, with no significant difference between the two groups. Chest computed tomography (CT) in 5 (33.3%) and 7 (46.7%) patients in the HCQ and standard treatment groups, respectively, showed worsening of pneumonia; however, follow-up CT showed recovery in all patients. Furthermore, the period from hospitalization to viral negativity was 4 days (1 – 9) and 2 days (1 – 4) in the HCQ and standard treatment groups, respectively, with no statistically significant difference (P >0.05). However, only a small number of patients was enrolled in the study; thus, the findings need to be confirmed in further studies with a large number of patients [48]. In the randomized SOLIDARITY trial conducted by the WHO, 954 and 906 hospitalized patients with COVID-19 were assigned to the HCQ group and standard treatment group, respectively. The overall mortality rate was not significantly different between the two groups (HCQ: 11% [104/947] vs. standard treatment: 9.3% [84/906]; risk ratio, 1.19; 95% CI, 0.89 – 1.59; P = 0.23), and there was no significant difference in the use of MV at trial entry (HCQ: 8.8% [75/862] vs. standard treatment: 8% [66/824]) and duration of hospitalization [11].
In a randomized, open, clinical trial involving moderate/severe COVID-19 patients hospitalized in Norway, viral negativity 5 days after the first onset of clinical symptoms, which started after a mean of 8 days after infection, in the HCQ group (27 patients) was not significantly different from that in the standard treatment group (26 patients) (HCQ group: 0.24 [95% CI, 0.03 – 0.46] log10 RNA copies/mL/24 h vs. standard treatment group: 0.14 [95% CI −0.10 to 0.37] log10 RNA copies/mL/24 h; reduction rate difference between the groups: 0.11 [95% CI −0.21 to 0.43] log10 RNA copies/mL/24 h) [49].
In a multi-center, randomized, open clinical trial (75 patients in each of the HCQ group and standard treatment group) conducted in China involving 150 hospitalized patients with COVID-19 (148 patients with mild/moderate disease; two with severe disease), the rate of negative conversion of the virus was not significantly different between the two groups until day 28 (HCQ: 85.4% [95% CI, 73.8 – 93.8%] vs. standard treatment: 81.3% [95% CI, 71.2 – 89.6%]) [50]. In a randomized, open, clinical trial of 48 patients hospitalized with moderate COVID-19 in Wuhan, China, 18, 18, and 12 patients were assigned to the CQ, HCQ, and standard treatment groups, respectively. The time from trial entering to clinical recovery was 5.5 (3.25 – 7.5) days in the CQ group, 6 (3 – 8) days in the HCQ group, and 7.5 (5 – 16.25) days in the standard treatment group, and the results were significant in both CQ (P = 0.019) and HCQ groups (P = 0.049) when compared to the standard treatment group. The time to viral negativity was 2.5 (2.0 – 3.8) days, 2 (2 – 3.5) days, and 7 (3 – 10) days in the CQ, HCQ, and standard treatment groups, respectively (P = 0.006). Both CQ and HCQ groups showed a reduction in the hospitalization period and increased clinical improvement on chest CT compared to the standard treatment group. In this study, CQ or HCQ was effective in treating patients with moderate COVID-19. However, the number of enrolled patients was small; thus, the findings need to be confirmed in further studies with an increased number of patients [51]. In a randomized, open clinical trial of 293 patients with mild COVID-19 within 5 days of symptom onset (136 and 157 in the HCQ and standard treatment groups, respectively), there were no significant differences in virus concentration at days 3 and 7 between the two groups (day 3, HCQ: -1.41 vs. standard treatment group, -1.41 log10 copies/mL; day 7, HCQ: -3.44 vs. standard treatment: -3.37 log10 copies/mL), and HCQ treatment did not reduce the risk of hospitalization (HCQ: 5.9% vs. standard treatment: 7.1%) and recovery period of clinical symptoms (HCQ: 10 days vs. standard treatment: 12 days). Therefore, HCQ treatment did not show meaningful results compared to standard treatment in patients with mild COVID-19 [52]. In a multi-center, randomized, double-blinded clinical trial of patients with mild COVID-19 who were not hospitalized within 4 days of symptom onset conducted in the US and Canada, 212 and 211 patients were included in the HCQ and untreated control groups, respectively (US: folic acid, Canada: lactose treatment). There was no significant difference in the severity of symptoms for 14 days between the two groups (difference in the severity of symptom: relative value, 12%; absolute value, -0.27 point [95% CI, -0.6 – 10.07 point]; P = 0.117), and on day 14, the symptoms were less persistent in the HCQ group (24%, 49/201) than in the untreated control group (30%, 59/194); however, there was no significant difference between the two groups (P = 0.21) [53]. In Taiwan, multi-center, randomized, controlled, open, and retrospective studies involving patients with mild/moderate COVID-19, admitted after 4 days of symptom onset, were conducted simultaneously. In a multi-center, open-label, RCT, 33 patients were enrolled: 21 and 12 patients in the HCQ and standard treatment groups, respectively. Among them, 1 (4.8%) patient in the HCQ group and 2 (16.7%) in the standard treatment group were simultaneously treated with AZM. From the start of the trial to 14 days in the hospital, the time to viral negativity was 5 (1 – 9) days and 10 (2 – 12) days in the HCQ and standard groups, respectively, with no significant difference (P = 0.4). On day 14, 81% (17/21) of the HCQ group and 75% (9/12) of the standard treatment group showed findings of viral negativity (P = 0.36). On day 14, clinical recovery was observed in 28.6% and 41.7% of the HCQ and standard treatment groups, respectively, with no significant difference between the two groups (P = 0.51). In the retrospective study, 37 patients were enrolled: 28 and nine patients in the HCQ and standard treatment groups, respectively. On day 14 of hospitalization, viral negativity was observed in 42.9% (12/28) and 55.6% (5/9) of the HCQ and standard treatment groups, respectively (P = 0.70) [54].
Observational studies have shown that HCQ monotherapy or HCQ + AZM combination therapy might reduce mortality due to COVID-19 or promote clinical improvement [43,55,56,57,58] and that co-administration of HCQ and steroids could reduce the risk of hospitalization by 50–60% among those who were in the early stages of COVID-19 and not yet been hospitalized [59]. However, other studies have shown that these treatments did not reduce the mortality rate and worsened arrhythmia [32,40,41,42,60,61,62,63,64,65]. Some studies have shown that HCQ [34] and combination therapy with HCQ + AZM could help reduce the time to viral negativity [36,38]. Other studies showed that lopinavir/ritonavir was more effective than HCQ in cases of negative viral conversion [66,67,68,69]; yet other studies reported that neither drug was effective [70].
○ Considerations for recommendation
1. Certainty of evidence
Based on published RCTs, observational studies, and literature reviews on HCQ monotherapy and HCA + AZM combination therapy, the certainty of evidence was high for the 28-day clinical recovery in those who required MV support. Since the number of patients was insufficient to fully address adverse reactions, and many studies were not double-blinded, the certainty of evidence for adverse reactions was moderate. Since the time to viral negativity and improvement in clinical symptoms were not assessed in large-scale studies and the results were inconsistent, the certainty of evidence for time to viral negativity was low. The overall certainty of evidence was high according to the core outcome indicators.
2. Benefit and harm
To date, no study has shown improved mortality, lack of progression to severe conditions, and reduced duration of hospitalization and of negative viral conversion with the use of HCQ monotherapy or combination therapy with AZM for treating COVID-19. However, the drug causes various side effects such as arrhythmia due to increased QT interval, so the harm is greater than the potential benefit.
3. Patient values and preferences
Currently, the choice of treatment for patients with COVID-19 is minimal, and there are hardly any drugs available with proven therapeutic effects. However, the preference is expected to be low, considering the side effects and lack of clinical effects.
4. Resources (including cost)
In Korea, HCQ is a commercially available drug used for treating malaria, rheumatoid arthritis, lupus, and delayed skin porphyria, and it is easily accessible. The price with insurance and general price are 130 and 570 won, respectively, for 100 mg of oral Oxiklorin® in Korea. AZM is also an antibiotic used to treat various bacterial diseases in Korea, and it can be easily supplied. The price with insurance and general price are 920 won and 2020 won for 250 mg of oral Zithromax®.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
The Australian guidelines do not recommend using HCQ for treating COVID-19, and the NIH guidelines recommend that inpatients do not undergo HCQ monotherapy or combination therapy with AZM. For non-hospitalized patients, HCQ monotherapy or combination therapy with AZM is not recommended other than in clinical trials. The IDSA guidelines also recommend that inpatients do not receive HCQ monotherapy or in combination with AZM. In the Chinese guidelines, there are no recommendations because of the lack of consistent data on the effects of CQ or HCQ, but combination therapy with HCQ and AZM is not recommended.
2) Evaluation of acceptance and applicability in Korea
The Korean acceptance and applicability evaluation tool in the NECA Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
On April 27, 2020, the US FDA issued an EUA allowing the use of HCQ to treat patients with COVID-19. However, on June 17, 2020, the WHO announced the interim results of the SOLIDARITY trial and RECOVERY trial, which showed that the mortality rate of inpatients did not improve and discontinued the SOLIDARITY trial. Additionally, the US FDA also repealed the EUA, as serious side effects such as arrhythmia and insufficient therapeutic effects were reported among patients with COVID-19.
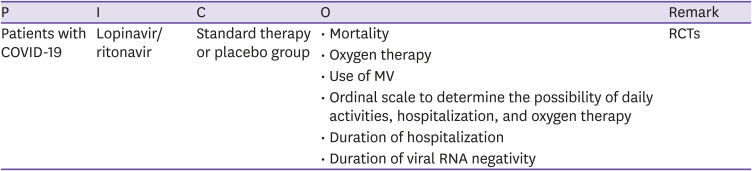
CQ3 Lopinavir/ritonavir
○ Clinical question
Is lopinavir/ritonavir effective for patients with COVID-19?
○ PICO elements
○ Recommendations
Lopinavir/ritonavir is not recommended for patients with COVID-19 (certainty of evidence: high, grade of recommendation: C).
○ Basic information about lopinavir/ritonavir
Lopinavir/ritonavir is a protease inhibitor that inhibits HIV replication and is composed of two antiviral components. After being approved for commercial purposes in 2000 in the US, it is currently distributed and used worldwide to treat HIV. Because it causes digestive discomfort such as diarrhea and vomiting, it is not more convenient to use than other HIV drugs.
Thus, it is not currently recommended as the primary treatment option. In 2002, studies on severe acute respiratory syndrome (SARS) showed the clinical effects of lopinavir/ritonavir on coronavirus [71], and in 2020, during the early days of the COVID-19 pandemic, it was reported that lopinavir/ritonavir could inhibit the action of SARS-CoV-2 [72].
○ Evidence summary
A total of six guidelines on recommendations for lopinavir/ritonavir in COVID-19 patients were selected. The guidelines were based on 17 studies. Among them, in one RCT, lopinavir/ritonavir was co-administered with other antiviral agents, and two RCTs consisted of only one control group that was administered other antiviral drugs; these RCTs were excluded along with 10 observational studies. Therefore, a total of four RCTs that involved a lopinavir/ritonavir treatment group and a control group that did not receive any other antiviral drugs were included in the final evidence table.
Among the selected clinical studies, the one with the highest number of subjects and the highest quality was the RECOVERY (randomized evaluation of COVID-19 therapy) study, conducted by the RECOVERY Collaborative Group [73]. In this open, non-blinded, researcher-led study involving 176 medical institutions in the UK, patients over the age of 18 who were hospitalized for COVID-19 infection were included; 1,616 and 3,424 patients in the lopinavir/ritonavir and non-antiviral control groups, respectively, were included in the final analysis. There were no differences in basic characteristics such as age, sex, race, underlying disease, duration of symptoms, and oxygen demand at hospitalization between the two groups. The 28-day mortality rate was not significantly different between the two groups at 23% and 22% (RR, 1.03; 95% CI, 0.91 – 1.17; P = 0.60), and the MV rates were 10% and 9% in the lopinavir/ritonavir and non-antiviral control groups, respectively, showing no significant difference [73].
A large-scale, open RCT on remdesivir, hydroxychloroquine, lopinavir/ritonavir, and interferon beta-1a was conducted by the WHO involving 405 hospitals in 30 countries around the world [11]. This study enrolled patients with moderate COVID-19 over 19 years who were hospitalized and required oxygen therapy. In the lopinavir/ritonavir study, a total of 2,791 patients were recruited: 1,399 and 1,372 patients in lopinavir/ritonavir and non-drug control groups, respectively; there was no difference in in-hospital mortality at 9.7% and 10.3% between the groups (RR, 1.00; 95% CI, 0.79 – 1.25; P = 0.97), and 9.8% and 9.6% of the patients required MV during treatment, respectively, with no difference between the two groups (RR, 0.97; 95% CI, 0.79 – 1.25; P = 0.97).
Another RCT assessed the effects of lopinavir/ritonavir in the early stages of the COVID-19 pandemic [74]. This study was conducted in a hospital in Wuhan, China, and enrolled patients with moderate COVID-19, aged over 18 years, who required oxygen therapy; 99 out of 199 patients received lopinavir/ritonavir while the remaining 100 patients received non-pharmaceutical treatment. Ordinal scales used to determine clinical improvement in previous studies of hospitalized patients with severe influenza (probability of daily activities, hospitalization, and need for oxygen supply) was used for this study. The risk ratio was 1.31 (95% CI, 0.95 – 1.80), which showed no improvement, and mortality rate at day 28 was 19.2% vs. 25.0% (95% CI, -17.3 – 5.7), which was not significantly different between the two groups. Additionally, virus excretion and duration of hospitalization were not significantly different between the two groups. However, in the lopinavir/ritonavir group, 13 patients (13.8%) discontinued treatment due to discomfort of the digestive system.
Another study was conducted with 86 patients, including 34 patients in the lopinavir/ritonavir group. As the sample size was small, the reliability of the results may be low. However, there were no differences in the virus excretion period and clinical symptom improvement indicators between the groups of patients with mild or moderate disease[75].
The results of the RCTs suggest that lopinavir/ritonavir treatment is ineffective in improving clinical symptoms, period of virus excretion, and mortality rate. The appropriate dose of lopinavir/ritonavir for patients with COVID-19 is still debated. However, a concentration of CE90 or higher is maintained in the lungs with the dose used for HIV patients; thus, it is thought that changing the dose will not yield any further clinical improvements [76]. Moreover, there may be pharmacokinetics changes in patients under MV support as the drug must be administered through the nasogastric tube. However, the RCTs reported that lopinavir/ritonavir was not effective in those who were not under MV support, and the drug had to be discontinued due to adverse reactions. Hence, lopinavir/ritonavir is not recommended for patients with COVID-19.
○ Considerations for recommendation
1. Certainty of evidence
All of the supporting studies were non-blinded studies. However, the number of patients was high, and the research methods were appropriate. The result indexes were clear, and consistent results were observed. Therefore, the certainty of evidence is high.
2. Benefit and harm
In a study on the safety of lopinavir/ritonavir, the rates of adverse reactions were 48.4% and 49.5% in the intervention and non-intervention groups, respectively, which were not significantly different. This finding is limited as it may have been confounded by new symptoms, changes in blood test results, and adverse reactions caused by co-administered drugs. However, it is worth noting that 13.8% of the patients in the lopinavir/ritonavir group discontinued the drug due to digestive discomfort, and there were no therapeutic benefits; therefore, the risk may outweigh the benefit.
3. Patient values and preferences
If lopinavir/ritonavir does not lead to clinical improvement, it will not be a preferred drug.
4. Resources (including cost)
Lopinavir/ritonavir is distributed for patients infected with HIV, so it is highly accessible. However, it is not cost-effective.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
The WHO guideline, Centers for Disease Control and Prevention guideline, IDSA guideline, BC Centre for Disease Control: Treatments guideline, Australian clinical practice guideline, and NSW Health interim guidance on the use of antiviral and immunomodulation therapy in COVID-19 do not recommend the use of lopinavir/ritonavir for treatment and prevention purposes.
2) Evaluation of acceptance and applicability in Korea
The Korean acceptance and applicability evaluation tool in the NECA Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
None.
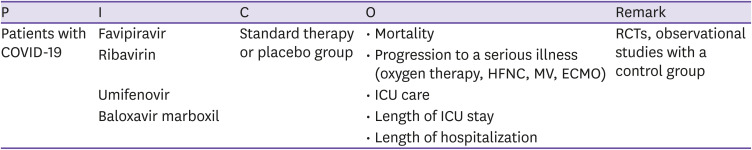
CQ4 Favipiravir, ribavirin, umifenovir, and baloxavir marboxil
○ Clinical question
Are drugs that are known to have antiviral effects, such as favipiravir, ribavirin, umifenovir, and baloxavir marboxil, more effective and safe for patients with COVID-19 compared to standard treatment or no treatment?
○ PICO elements
○ Recommendations
Administration of drugs known to have antiviral effects, such as favipiravir, ribavirin, umifenovir, and baloxavir marboxil, is not recommended for patients with COVID-19 regardless of the severity.
(Certainty of evidence: low, grade of recommendation: C)
○ Basic information about antiviral agents
Favipiravir (Avigan®, T-705) is effective against various RNA viruses. The mechanism of its action is not clearly established; however, it is thought to inhibit the action of the viral RNA-dependent RNA polymerases by acting as a purine analog [77,78]. In one study, it was reported to inhibit SARS-CoV-2 at a relatively high concentration (half-maximal effective concentration, EC50 = 61.88 μM, selectivity index >6.46) [79].
Ribavirin inhibited SARS-CoV and MERS-CoV at high concentrations in several in vitro studies [80,81,82,83]. However, it does not inhibit SARS-CoV or MERS-CoV at the general concentrations achieved in clinical practice; its inhibitory effects were only observed at toxic concentrations. In in vitro studies on SARS-CoV, combination therapy with ribavirin and interferon showed synergistic effects that allowed a lower concentration of ribavirin [84,85,86].
Umifenovir (Arbidol®) is an antiviral drug developed in Russia that is approved for the prevention and treatment of influenza in Russia and China. As an indole derivative, it is known to have a wide range of antiviral effects through various mechanisms, including the inhibition of membrane fusion between virus and host cells [87]. In experiments using Vero E6 cells, umifenovir inhibited SARS-CoV-2 infection by interfering with viral adhesion and release from the endolysosome [88].
Baloxavir, a metabolite of Baloxavir marboxil (Xofluza®), is an inhibitor of the polymerase acidic (PA) protein subunit in the polymerase complex of the influenza virus. It inhibits cap-dependent endonuclease activity and prevents replication of the influenza virus [89]. In experiments using Vero E6 cell line, baloxavir exhibited antiviral effects against SARS-CoV-2 [90], but another study reported contrasting results [88].
○ Evidence summary
A total of six guidelines on recommendations for favipiravir, ribavirin, umifenovir, and baloxavir marboxil were selected. Fifteen studies were identified from those guidelines, and 5 more studies from the literature search. Finally, 20 studies (nine RCTs and 11 observational studies) were included in the final evidence table.
Six RCTs examined favipiravir and umifenovir. Only one RCT used clinical improvement as the primary endpoint. In this study, 240 patients were randomly assigned to receive favipiravir or umifenovir, and clinical improvement was compared on day 7. In all, 61.2% and 51.7% of the patients in the favipiravir and umifenovir groups, respectively, showed clinical improvement, defined as the absence of fever, tachypnea, and hypoxia for more than 72 hours; no significant difference was observed between the two groups (P = 0.140). Additionally, there was no significant difference in the rate of progression to severe disease requiring respiratory support beyond supplementary oxygen [91]. In the other three RCTs on favipiravir that selected negative conversion of polymerase chain reaction (PCR) as the primary endpoint, there was no difference in the time to negative conversion between the group who received favipiravir and groups who received other or no antiviral drugs or favipiravir later in the course [90,92,93]. In some studies, time to resolution of fever was the clinical endpoint; however, important clinical endpoints such as death and progression to severe disease were not assessed as the primary endpoint, and there was no difference between the two groups in death and progression to severe disease assessed as secondary endpoints. Two observational studies compared favipiravir and lopinavir/ritonavir (both drugs were combined with inhaled interferon-α) and reported the superior effect of favipiravir in terms of time to negative conversion of PCR and improvement of chest CT. However, no clinical endpoints were evaluated [94]. In a study that combined favipiravir and HCQ, there was no difference in mortality between the two treatment groups. The length of ICU stay was shorter in the favipiravir combination group; however, the two groups were not contemporaneous, and the primary drug was changed to favipiravir in the later stages of the COVID-19 pandemic [95].
Two RCTs on umifenovir were exploratory trials, and there were no significant differences in the negative conversion of PCR, resolution of fever, improvement of respiratory symptoms, and progression to severe disease [75,96]. There were six observational studies on umifenovir, among which PCR negative conversion was the primary endpoint in four. In studies that used lopinavir/ritonavir- or Chinese medicine-treated control group, the time to negative conversion was earlier in the favipiravir group; however, there was no significant difference between the groups in studies that did not treat the control group with antiviral drugs [97,98,99,100]. Clearance of fever and cough were assessed even when clinical endpoints were selected; more meaningful outcome of mortality, progression to severe disease, or hospital discharge was not reported [101,102].
There were three RCTs on ribavirin. In a study that compared combination treatment with ribavirin, interferon-β1b, and lopinavir/ritonavir against lopinavir/ritonavir alone, significant improvements were observed in time to PCR negative conversion, clinical improvement (symptoms, National Early Warning Score 2, or sequential organ failure assessment [SOFA]), and length of hospital stay. However, the rate of disease progression beyond supplementary oxygen was not different between the two groups, and no patient died in both groups [103]. A study compared ribavirin + interferon-α, lopinavir/ritonavir + interferon-α, and ribavirin + lopinavir/ritonavir + interferon-α, there was no significant difference in PCR-negative conversion time, and gastrointestinal side effects were more common in patients treated with triple regimen [104]. In a small-scale RCT that compared ribavirin + sofosbuvir/daclatasvir therapy against no antiviral treatment, there were no significant differences in the duration of hospitalization, admission to the ICU, and mortality. However, there were differences in the baseline characteristics between the two groups, and the number of patients was small to draw firm conclusions [105]. A total of three studies were conducted on ribavirin. In two retrospective studies, ribavirin treatment was compared to no antiviral treatment, and lopinavir/ritonavir + interferon-α was compared to ribavirin + lopinavir/ritonavir + interferon-α treatment. There were no significant differences in time to negative conversion of PCR, duration of hospitalization, and mortality [106,107]. In a prospective observational study, ribavirin or sofosbuvir/daclatasvir were added to lopinavir/ritonavir + HCQ. As compared to the ribavirin group, the sofosbuvir/daclatasvir group showed a reduction in the duration of hospitalization, duration of ICU stay, and mortality. However, the control group with no antiviral drugs was absent; thus, the effects of ribavirin could not be assessed [108].
Only one RCT on baloxavir marboxil was identified. In this study, baloxavir and favipiravir were also administered to patients treated with lopinavir/ritonavir + interferon-α and darunavir/cobicistat + interferon-α. There were no significant differences in time to negative PCR conversion and rate of clinical improvement [90].
○ Considerations for recommendation
1. Certainty of evidence
RCTs and observational studies on the effects of favipiravir, ribavirin, umifenovir, and baloxavir marboxil all had significant limitations, such as co-administration of several drugs, differences in control groups and baseline characteristics, and small size. Few studies assessed mortality, progression to severe disease, and discharge after recovery as primary endpoints. No statistically significant benefit was observed in the important clinical outcomes, sample sizes were not optimal, and the risk of bias was present. Therefore, the certainty of evidence is low.
2. Benefit and harm
A few RCTs on the effects of favipiravir, ribavirin, umifenovir, and baloxavir marboxil have been reported, but only a small number of studies assessed important clinical indicators such as mortality, progression to severe disease, and discharge after recovery as primary endpoints. No consistent benefit was observed in the studies that evaluated clinical indicators, and even the results on non-clinical indicators such as PCR negative conversion or improvement on CT scans were inconsistent. Therefore, based on the evidence, it is unlikely that these drugs will reduce mortality, progression to severe disease, or length of hospital stay for patients with COVID-19.
In most studies, there was no significant difference in the frequency of severe adverse events associated with antiviral drugs or discontinuation of drugs. However, in a dose-finding study of favipiravir, approximately 5% of the patients discontinued the drug due to side effects. In other studies, hyperuricemia and elevated serum levels of triglycerides and alanine aminotransferase (ALT) were observed [92,93]. In an RCT on umifenovir, the incidence of nausea/vomiting was high (14.3%) [75]; however, in observational studies, the frequency of nausea/vomiting was not significantly higher in the umifenovir-treated group than that in the control group [99,101]. It is difficult to assess the safety of ribavirin as there were rarely any studies in which ribavirin was administered alone. However, in an observational study with a control group of patients who did not receive any antiviral drugs, frequency of laboratory-reported abnormalities were not higher in the ribavirin-treated group and there was no early discontinuation due to side effects [107]. In contrast, the frequency of side effects was high in the ribavirin + lopinavir/ritonavir + interferon-α-treated group in another RCT, and hemolytic anemia and leukopenia are well-known side effects of ribavirin. Therefore, ribavirin should be used with caution.
3. Patient values and preferences
There are no studies in Korea that assessed the values and preferences for therapeutic drugs in patients with COVID-19. However, while the clinical effects of the above drugs may be absent or small, those drugs are either not approved in Korea (favipiravir and umifenovir) or approved for unrelated indications (ribavirin). Baloxavir marboxil is not yet commonly used in Korea. Therefore, the preference for these drugs is expected to be low.
4. Resources (including cost)
Favipiravir and umifenovir are not approved in Korea and can be used only in clinical trials. Ribavirin is approved, and the price is relatively low compared to the total cost of hospitalization. Baloxavir marboxil is not covered by insurance and was administered 2 – 3 times a day in studies on COVID-19. Therefore, the cost burden of this drug is relatively high.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
The WHO guidelines explicitly recommend that favipiravir and umifenovir not be used for COVID-19 and recommend comprehensively against other agents with potential antiviral effects. The Australian guidelines recommend that baloxavir, favipiravir, and umifenovir should not be administered outside clinical trials.
2) Evaluation of acceptance and applicability in Korea
The Korean acceptance and applicability evaluation tool in the NECA Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
Favipiravir and umifenovir have not been approved for use in Korea as of December 2020. Baloxavir marboxil is approved for the treatment of influenza A or B infections in adults and adolescents over 12 years. Oral ribavirin is approved for co-administration with interferon alpha-2b or peginterferon alpha-2a and -2b injection in patients with chronic hepatitis C.
CQ5 Corticosteroids
○ Clinical question
Is the administration of steroids effective and safe for COVID-19 patients compared to standard treatment or no treatment?
○ PICO elements
○ Recommendations
○ Basic information on steroids
Steroids have potent anti-inflammatory, immunosuppressive, and anti-neoplastic effects and play a vital role in the treatment of various conditions, including autoimmune diseases, allergic reactions, asthma exacerbations, chronic obstructive pulmonary disease, and malignant tumors [109]. In cases of severe infections such as sepsis, steroids may improve the immune response [110]. Acute respiratory distress syndrome, which has a 40 – 60% mortality rate, is the most lethal form of respiratory failure associated with systematic inflammatory responses [111], and several circulating and proinflammatory cytokines are involved in the onset and exacerbation of acute respiratory distress syndrome due to sepsis. The dysregulated inflammation due to the loss of autoregulatory function of the cytokines is the early pathophysiological cause of acute respiratory distress syndrome [112,113]. Steroids are the final effectors of the hypothalamic-pituitary-adrenal axis that controls the cytokines, and they can suppress severe systematic inflammation, e.g., acute respiratory distress syndrome [114]. Steroids are used to treat patients with severe sepsis, septic shock, and acute respiratory distress syndrome. In clinical studies of patients infected with SARS-CoV or MERS-CoV prior to the COVID-19 pandemic, steroid administration did not improve mortality and side effects such as avascular necrosis, diabetes, and psychosis were observed [84]]. Moreover, prolonged viral shedding from the lower respiratory tract was observed [115], therefore, steroids were not recommended. Recently, it was reported that patients with severe sepsis and septic shock who received steroids recovered from shock faster and showed lower mortality rates than those who did not receive steroids [116,117]. Some studies also reported that steroids reduced the mortality rate and the number of days under MV support among patients with acute respiratory distress syndrome [118,119].
○ Evidence summary
A total of four guidelines that included recommendations for steroids were selected. The guidelines were based on 26 studies. A total of 10 studies, i.e., five RCTs and five observational studies, were included in the final evidence table. The results are summarized below.
In the Randomized Evaluation of COVID-19 Therapy (RECOVERY) study, which was a large-scale RCT, the 28-day mortality was significantly reduced among those who received 6 mg of dexamethasone up to 10 times a day compared to among those who did not receive dexamethasone (482/2104 [22.9%] vs. 1110/4321 [25.7%]; age-adjusted RR, 0.83; 95% CI, 0.75 – 0.93; P <0.001). Improved mortality rate was observed among those requiring MV support (29.3% vs. 41.4%; RR, 0.64; 95% CI, 0.72 – 0.94) and oxygen therapy (23.3% vs. 26.2%; RR, 0.82; 95% CI, 0.72 – 0.94); however, steroid administration did not improve mortality in those who did not need oxygen therapy (17.8% vs. 14.0%; RR, 1.19; 95% CI, 0.62 – 0.95) [120]. In the COVID-19 Dexamethasone (CoDEX) RCT involving 41 ICUs in Brazil, a total of 299 COVID-19 patients with moderate-to-severe acute respiratory distress syndrome were randomly assigned in a 1:1 ratio to the treatment group (20 mg of intravenous dexamethasone a day for 5 days and then 10 mg for 5 days or until transfer from the ICU) and control group. The primary endpoint in this study was the number of ventilator-free days for the first 28 days, which was significantly different between the groups at 6.6 days vs. 4.0 days (difference, 2.26; 95% CI, 0.2 – 4.38; P = 0.04). On day 7, the SOFA score was 6.1 and 7.5 for the treatment and control group, respectively (P = 0.004), with better improvement in the treatment group. However, there was no difference in other endpoints such as mortality rate, ICU-free days, and duration of MV support after 28 days between the two groups. Further, 21.9% (33/151) and 29.1% (43/148) of the patients in the treatment and control groups, respectively, experienced secondary infections, and 31.1% and 28.3%, respectively, required insulin to control the blood glucose level [121]. In the Community-Acquired Pneumonia: Evaluation of Corticosteroids in Coronavirus Disease (CAPE-COVID) study conducted in France, patients with severe COVID-19 who were being treated in the ICU were randomly assigned in a 1:1 ratio to the control group and treatment group (200 mg/d of hydrocortisone for 7 days, followed by 100 mg/d for 4 days, and 50 mg/d for 3 days); treatment failure on day 21 was assessed as the primary endpoint. On day 21, the treatment failure rate had not reduced in the low-dose hydrocortisone group compared to in the control group (42.1% [32/76] vs. 50.7% [37/73], P = 0.29). However, the CAPE-COVID study was discontinued after studies reported positive results on dexamethasone. Therefore, it may be a statistically underpowered study with only 149 patients, as it did not reach the intended sample size of 290 patients. However, the low mortality rate observed in the hydrocortisone-treated group is consistent with that observed in dexamethasone-treated group [122]. In the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) study, patients were randomly assigned in a 1:1:1 ratio to two hydrocortisone groups (200 mg/d of hydrocortisone for 7 days [2 patients received 400 mg/d] and 200 mg/d of hydrocortisone only when shock was evident [discontinued after recovering from shock or stopping vasopressor agents for 24 hours] and a control group. The primary endpoint was days free of treatment within 21 days. Compared to the control group, the probabilities of superiority were 93% in the 7-day hydrocortisone group and 80% in the cortisone group. However, this may not be a definitive conclusion as the study was discontinued early [123]. Meanwhile, in the Metcovid study conducted in Brazil, inpatients were randomly assigned in a 1:1 ratio to a high-dose methylprednisolone group (0.5 mg/kg twice a day for 5 days) and control group. The 28-day mortality was not improved; however, in subgroup analysis of those over the age of 60, the mortality rate was significantly lower in the high-dose methylprednisolone group than in the control group (46.6% vs. 61.9%) [124]. In three observational studies including various patient groups, type of steroid, and duration of administration, steroid administration improved the mortality rate, ICU transfer rate, and airway intubation index [125,126,127]. However, in a retrospective cohort study of COVID-19 patients with mild pneumonia, short-term administration of low-dose steroid for 3 – 5 days was associated with clinical deterioration [128].
In the Rapid Evidence Appraisal for COVID-19 Therapies (REACT) study by the WHO, a meta-analysis of seven RCTs, including currently ongoing RCTs involving 1,703 patients with severe COVID-19, the 28-day mortality rate was lower in the steroid group than in the control group [129].
○ Considerations for recommendation
1. Certainty of evidence
In the RCTs, observational studies, and meta-analysis on the effects of steroids, improved mortality, a key clinical indicator, was evaluated; however, the results varied depending on the dose and duration of administration and severity of COVID-19. The overall certainty of evidence for this CQ is moderate, with inconsistent risk of bias.
2. Benefit and harm
Steroid treatment can improve mortality in cases of severe COVID-19 patients who receive oxygen therapy or MV support. Although previous studies reported that steroids could increase the frequency of avascular necrosis, diabetes, delayed virus clearance, hyperglycemia, hypernatremia, and hypokalemia [84,130,131], the certainty of evidence is low. Considering the improved mortality rates reported in patients with COVID-19, the benefit of steroids outweighs the potential harm.
3. Patient values and preferences
There is no study on the values and preferences for the use of steroids in patients with COVID-19. However, the preference will not be low as steroids are used to treat symptomatic sepsis and acute respiratory distress syndrome.
4. Resources (including cost)
Steroid use is approved, and the cost is expected to be as low as that of other treatment options.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
The WHO and IDSA guidelines recommend the use of systemic steroids in severe or critical cases of COVID-19 but not non-severe cases. The Australian guidelines recommend intravenous or oral administration of dexamethasone at 6 mg (or acceptable alternative steroids) for up to 10 days in patients with COVID-19 who receive oxygen therapy, including MV; the NIH guidelines also recommend the administration of dexamethasone and other steroids.
2) Evaluation of acceptance and applicability in Korea
The Korean acceptance and applicability evaluation tool in the NECA Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
None.
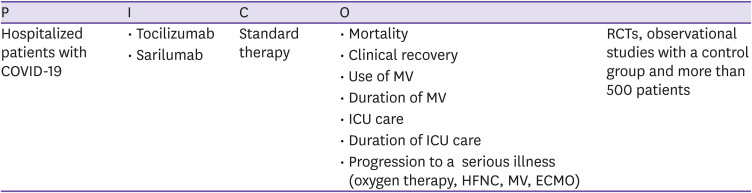
CQ6 Interleukin-6 inhibitor (tocilizumab, sarilumab)
○ Clinical question
Is the administration of interleukin-6 inhibitors such as tocilizumab and sarilumab effective and safe compared to standard treatment in patients with COVID-19?
○ PICO elements
○ Recommendations
6-1. Interleukin-6 inhibitors can be administered, within the scope of clinical trials, to patients in severe or critical stages of COVID-19 (certainty of evidence: moderate, grade of recommendation: B).
6-2. Administration of interleukin-6 inhibitors is not recommended for patients with mild COVID-19 (certainty of evidence: low, grade of recommendation: C).
○ Basic information on interleukin-6 inhibitors
Hyper-inflammatory syndrome or cytokine storm is a severe complication of infectious disease treatment, inflammatory immune diseases, and malignant diseases. It is caused by dysregulated synthesis of cytokines, leading to pathological activity of the innate and adaptive immune system (Th1 and Th17 mediated immunity). In particular, interleukin-6 (IL-6), a differentiation factor for B-cells, is an important pro-inflammatory mediator in immune defense and immunomodulatory diseases; it mediates multiple pleiotropic functions in immune response, with anti-inflammatory effects [132,133]. Currently, there are four pharmacological inhibitors (blockers) of IL-6 that are available for clinical use: tocilizumab, sarilumab, and satralizumab, which are anti-IL-6 receptor monoclonal antibodies, and siltuximab, an anti-IL-6 monoclonal antibody.
Tocilizumab is a humanized immunoglobulin G subclass 1 (IgG1) monoclonal antibody against the IL-6 receptor; it binds to cell membrane-bound or free IL-6 receptors and inhibits their inflammatory action. Sarilumab is a human recombinant IgG1 monoclonal antibody. Similar to tocilizumab, it inhibits the binding of cytokines to the receptors and inhibits their action. Siltuximab is a chimeric anti-Il-6 monoclonal antibody that directly inhibits Il-6, and satralizumab is a humanized monoclonal antibody that binds to the IL-6 receptor and inhibits inflammation mediated by the IL-6 signaling pathway.
Significantly elevated levels of inflammatory markers (D-dimer, ferritin, C-reactive protein) and pro-inflammatory cytokines (IL-6) are observed in cases of severe COVID-19, and it is hypothesized that regulation of the inflammatory pathways helps prevent disease exacerbation [134,135,136,137,138]. IL-6 inhibitors have not yet been approved for treating COVID-19, and studies on the effects and safety of IL-6 pathway-related drugs in patients with COVID-19 are being conducted.
○ Evidence review
A total of three guidelines that included recommendations for IL-6 inhibitors were selected. The guidelines were based on 16 studies, i.e., six RCTs, six (retrospective or prospective) cohort studies, two case-control studies, and two case series, that were included in the final evidence table. The results are summarized below.
According to a multi-center cohort study by Gupta et al. involving 4,485 patients with COVID-19 admitted to the ICU, the mortality rate was lower among those who were treated with intravenous or subcutaneous tocilizumab within 2 days of hospitalization than in those who were not (125/433 [28.9%] vs. 1,419/3,491 [40.6%]; unadjusted HR, 0.64; 95% CI, 0.54 – 0.77) [139]. Similarly, in a prospective multi-center study, administration of tocilizumab within 6 days of hospitalization to patients with severe COVID-19 significantly increased the survival rate (HR, 2.2; 95% CI, 1.3 – 6.7; P <0.05) [140]. In a retrospective cohort study by Rosi et al., administration of tocilizumab (400 mg intravenous or 324 mg subcutaneously) within 2 days of hospitalization to patients with COVID-19 who experienced respiratory failure and received HCQ or lopinavir/ritonavir, significantly reduced the mortality rate (34/68 [50%] vs. 7/90 [7.7%]; multivariate HR, 0.057; 95% CI, 0.017 – 0.187; P <0.001), but the side effects of tocilizumab were not reported [141]. They suggested that early administration of tocilizumab improves the treatment outcomes in patients with severe COVID-19. However, the confounding variables were not controlled in this study. On the other hand, single-center retrospective studies have reported that administration of tocilizumab did not significantly contribute to improved mortality or clinical improvement in patients with severe COVID-19 [142,143].
In a study by Guaraldi et al. involving 544 patients hospitalized for severe COVID-19 (tocilizumab-treated group: 179 patients) at tertiary medical institutions in Italy, multivariate analysis showed that tocilizumab administration significantly reduced the rate of invasive MV support and mortality (HR, 0.61; 95% CI, 0.40 – 0.92) [144]. In a multi-center cohort study by Rodriguez-Bano et al., regression analysis showed that the mortality rate was significantly decreased at day 21 in the tocilizumab-treated group compared to that of the control group (OR, 0.07; 95% CI, 0.02 – 0.17; P <0.001) [145]. Propensity score-matching analysis in another multi-center observational study showed that tocilizumab significantly reduced the mortality rate of patients with severe COVID-19 (HR, 0.64; 95% CI, 0.47 – 0.87; P = 0.004) [146]. In a single-center retrospective cohort study that included propensity score matching analysis, the incidence rates of tracheal intubation and mortality were low in patients with moderate-to-severe COVID-19 who received tocilizumab compared to in non-treated patients (HR, 0.40; 95% CI, 0.20 – 0.77) [147]. Another single-center retrospective cohort study also used regression analysis to show that administration of tocilizumab in patients with severe COVID-19 pneumonia could significantly reduce the incidence of invasive ventilation and death (adjusted HR, 0.61; 95% CI, 0.40 – 0.92; P = 0.020) [144].
On the other hand, in five RCTs, the mortality rate was not significantly reduced in the tocilizumab-treated group as compared to in the control group. In a randomized, double-blinded, placebo-controlled study conducted by Stone et al., 243 patients with severe COVID-19 who did not undergo tracheal intubation and showed evidence of pro-inflammatory status (high levels of C-reactive protein, ferritin, D-dimer, or lactate dehydrogenase) were included. There was no significant reduction in tracheal intubation and 28-day mortality rates in the tocilizumab-treated group compared to in the placebo group (17/161 [10.6%] vs. 10/80 [12.5%]; HR, 0.83; 95% CI, 0.38 – 1.81) [148]. In a global multi-center, double-blinded, placebo-controlled study (COVACTA study) by Rosas et al., 452 patients with severe COVID-19 pneumonia were included. There was no significant difference in clinical improvement (P = 0.36) or mortality (P = 0.94) at day 28 between the treatment group (n = 294) and control group (n = 144). However, the duration of hospitalization and ICU care were shortened by 8 days (20.0 vs. 28.0 days; P = 0.037) and 5.5 days (9.8 vs. 15.5 days; P = 0.045) in the treatment group [149]. This finding is similar to that of a randomized, double-blinded, placebo-controlled study (EMPACTA study) by Roche pharmaceuticals, which showed that administration of tocilizumab did not significantly reduce 28-day mortality in patients with COVID-19 [150].
Salvarani et al. conducted a prospective, non-blinded, randomized clinical trial of 126 patients with findings of COVID-19 pneumonia on imaging tests, PaO2/FiO2 = 200 – 300 mmHg, high fever, and high levels of C-reactive protein. Clinical deterioration within 14 days after randomization was not significantly different between the two groups (tocilizumab group: 17/60 [28.3%] vs. standard treatment group: 17/63 [27.0%]; RR, 1.05; 95% CI, 0.59 – 1.86; P = 0.87]. Additionally, admission to the ICU within 14 days after randomization (10% vs. 7.9%; RR, 1.26; 95% CI, 0.41 – 3.91) and 30-day mortality rate (3.3% vs. 1.6%; RR, 2.10; 95% CI, 0.20 – 22.6) were not significantly different between the two groups as well. Regarding severe adverse effects, gastrointestinal bleeding was observed in one patient of the tocilizumab-treated group and in two patients of the standard treatment group [151]. Hermine et al. conducted a multi-center, non-blinded, randomized study of 131 severe COVID-19 patients admitted to the ICU, were not under MV support, and required oxygen therapy at 3 L/min. Compared to standard treatment, tocilizumab treatment did not reduce the 28-day mortality rate (tocilizumab group: 7/64 [10.9%] vs. standard treatment group: 8/67 [11.9%]; HR, 0.92; 95% CI, 0.33 – 2.53); however, it significantly reduced the rates of non-invasive/invasive MV and mortality on day 14 of treatment (tocilizumab group: 24% vs. standard treatment group: 36%; HR, 0.58; 90% CI, 0.33 – 1.00). There was no significant difference in the frequency of severe adverse events between the two groups (tocilizumab group: 32% vs. standard treatment group: 43%; P = 0.21) [152]. The findings of RCTs and observational studies were different, so the effects of confounding variables, which were not assessed in observational studies, need to be considered. In a multicenter, blind, randomized clinical trial by Wang et al., 400 mg tocilizumab was first administered intravenously on the first day of treatment to 33 out of 65 patients. A second dose was administered when fever was observed within 24 hours of the first dose. Treatment did not improve the outcomes (tocilizumab group: 94.12% vs. standard treatment group: 87.10%; P = 0.41) but exacerbation of hypoxia was significantly decreased in the tocilizumab group (P = 0.021). No serious adverse effects were observed in those treated with tocilizumab; however, the frequencies of adverse effects were 59% (20/34) and 13% (4/31) in the tocilizumab-treated and control groups, respectively. The most common adverse effects were liver dysfunction (18%), leukocyte reduction (15%), and neutrophil reduction (9%), all of which improved after conservative treatment [153].
A multicenter cohort observational study of patients hospitalized for COVID-19 by Martinex-Sanz et al. provided insights on selecting those patients who could benefit the most from tocilizumab. C-reactive protein >150 mg/L at the beginning of treatment significantly reduced the rate of mortality (adjusted HR, 0.34; 95% CI, 0.17 – 0.71; p = 0.005) and intensive care (aHR, 1.77; 95% CI, 1.41 – 2.22; p < 0.001). On the other hand, the treatment benefits could not be observed in those with C-reactive protein level ≤150 mg/L [154].
In six recently published systematic reviews and meta-analyses, the effects of tocilizumab on mortality in patients with severe COVID-19 were not consistent. In four studies, tocilizumab treatment significantly reduced the mortality rate [155,156,157,158], while opposite findings were observed in two other studies [159,160].
Studies on the therapeutic effects of sarilumab in patients with COVID-19 are limited. In a single-center case study, 10 out of 15 patients with COVID-19 who received sarilumab showed improved symptoms, and rapid decrease in C-reactive protein level was associated with clinical improvement [161].
○ Considerations for recommendation
1. Certainty of evidence
Studies on the effect of tocilizumab were mainly involved patients with severe COVID-19; however, the criteria for severity varied between different studies, and patients in the standard treatment group received different drugs. Among the RCTs, only one study was double-blinded, and the number of enrolled patients in the study was limited. In patient-control group studies, there were differences in age, underlying diseases, inflammatory markers, and disease severity between the two groups. The tocilizumab-treated group was simultaneously treated with other drugs, which made it difficult to assess the effects of tocilizumab. Additionally, in studies that assessed clinical MV support as a measure of clinical improvement, provision of MV support was based on the subjective judgments of medical staff, which led to concerns of bias in determining clinical improvement.
The effective dose and route of tocilizumab administration for treating COVID-19 are not clear, therefore, differences in treatment effects according to the administration time need to be identified. In particular, the optimal patient group needs to be determined for the maximal treatment effect. The small number of patients made it challenging to observe statistical significance, although in some studies, statistical techniques were used to adjust for confounding variables in the treatment and control groups. Therefore, based on these results, the certainty of evidence is moderate.
2. Benefit and harm
The findings of RCTs and cohort studies did not consistently show that tocilizumab improves treatment outcomes in COVID-19 patients. However, some studies reported that tocilizumab treatment improved the mortality rates and reduced the frequency of MV support and ICU treatment with a low frequency of side effects. Therefore, large-scale, double-blinded RCTs are required to identify the optimal patient group and to assess the dosage and regimen of tocilizumab.
In most studies, the frequency of severe side effects was not significantly different between the tocilizumab-treated and control groups. Adverse effects related to tocilizumab generally include nasopharyngitis, headache, upper respiratory tract infection, gastritis, rash, joint pain, muscle pain, fatigue and nausea, skin and soft tissue infections, and gastrointestinal perforation, which is the most common adverse effect. Abnormal findings observed in blood tests include neutropenia, thrombocytopenia, dyslipidemia, and elevated liver enzymes levels. There is no clear evidence that tocilizumab treatment significantly increases reactivation of infections in patients with malignant and autoimmune diseases or of tuberculosis in those with rheumatoid arthritis [162]. In a retrospective cohort study by Guaraldi et al., the rate of new infections had significantly increased in those who received tocilizumab compared to in those who did not (24/179 [13%] vs. 14/365 [4%]; P <0.0001); however, the increase in the rate of infections was not observed in other studies [144,148,151,152].
3. Patient values and preferences
Although there are no results of standardized studies on patient values and preferences, IL-6 inhibitors approved for the treatment of rheumatoid arthritis or juvenile idiopathic arthritis are being prescribed for patients with COVID-19 without FDA approval and are not eligible for coverage. Moreover, pharmaceutical companies such as Roche and Sanofi/Regenerondo that manufacture IL-6 inhibitors reported that clinical studies on IL-6 inhibitors did not show improvements in symptoms or mortality from COVID-19. Foreign medical guidelines also do not recommend the use of IL-6 inhibitors to treat COVID-19. Therefore, patient preference is expected to be low.
4. Resources (including cost)
IL-6 inhibitors, Actemra® (tocilizumab) and Kevzara® (sarilumab), were approved in Korea in 2012 and are currently used for the treatment of rheumatoid arthritis and juvenile idiopathic arthritis, and they can be easily accessed. However, there are no studies on the cost-effectiveness of IL-6 inhibitors for patients with COVID-19. IL-6 inhibitors can only be used in clinical trials on COVID-19. Therefore, they are not covered by insurance, and the cost burden is relatively high. It would be difficult to select certain patients for clinical trials, and the patients themselves would need to pay for the treatment. Therefore, there may be inequalities related to access to treatment.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
The ACPG and NIH guidelines do not recommend using tocilizumab for the treatment of COVID-19 outside of RCTs, with appropriate approval of an institutional review board (IRB). The IDSA guidelines also prohibit the routine use of tocilizumab in hospitalized patients with COVID-19.
2) Evaluation of acceptance and applicability in Korea
The dynamically changing epidemiological situation of the COVID-19 pandemic and medical resources could affect the acceptance of this recommendation. Compared with the ACPG, IDSA, and NIH guidelines, it is difficult to find significant differences according to the characteristics of the populations or the values and preferences of patients concerning our recommendations. Overall, high preference is expected, with individual differences, and the benefits of this recommendation are expected to be similar to those of other guidelines.
IL-6 inhibitors are specialized drugs approved for autoimmune diseases and are already distributed and used in clinics in Korea. However, they have not been approved for treatment of COVID-19. Therefore, they must be used with the approval of an appropriate IRB. Considering legal and institutional requirements, our recommendations are appropriate.
6. Other considerations
None.
CQ7 Interleukin-1 inhibitor (anakinra)
○ Clinical question
Is the administration of IL-1 inhibitors beneficial for patients with COVID-19?
○ PICO elements
○ Recommendations
There is insufficient evidence supporting the administration of interleukin-1 (IL-1) inhibitors for treating COVID-19 (certainty of evidence: low, grade of recommendation: I).
○ Basic information on IL-1 inhibitors
The production of cytokines such as interleukin-1 (IL-1) increases in patients with severe COVID-19 [163]. IL-1 inhibitors (i.e., anakinra) are approved for use in patients with rheumatoid arthritis and cryopyrin-associated periodic syndrome (especially neonatal multi-organ inflammatory syndrome). In addition, IL-1 inhibitors are used to treat severe cytokine release syndrome caused by chimeric antigen receptor T cell (CAR T-cell), macrophage activation syndrome, and hemophagocytic lymphohistiocytosis. In COVID-19 patients with severe inflammatory reactions due to excessive cytokine secretion, anakinra was found to alleviate cytokine storms and facilitate recovery.
○ Evidence summary
There are no RCTs that compared IL-1 inhibitor-treated and untreated control groups of patients with COVID-19. All of the following studies on IL-1 inhibitors used anakinra.
In March 2020, COVID-19 patients over 18 years with moderate to severe respiratory distress syndrome (ARDS) and hyperinflammation who were receiving non-invasive MV treatment outside the ICU were included in a retrospective cohort study in Italy [164]. The treatment group was treated with 5 mg/kg of intravenous anakinra (high dose) twice a day (29 patients), as compared to the control group (16 patients). On day 21, 72% of the treatment group showed decreased levels of CRP and improved respiratory function, while 17% and 6% of the patients required MV and died, respectively. In the control group, 50% of the patients showed improvements while 6% and 44% required MV and died, respectively. Bacteremia was observed in 14% of the anakinra-treated group and 13% of the control group.
A study involving patients over the age of 18 who had severe COVID-19 with bilateral pneumonia was conducted in France between March and April 2020 [165]. In this study, data from historical cohort control groups were compared with data collected prospectively from patients treated with anakinra. Patients in the anakinra group received 100 mg of subcutaneous anakinra twice a day for 3 days, after which the frequency was reduced to once a day for 7 days. There were 55 and 44 patients in the anakinra group and control group, respectively. ICU care with MV and death were observed in 25% of the anakinra group compared to in 73% of the control group (HR, 0.22; 95% CI, 0.11 - 0.41; P <0.0001). Statistical significance was also observed in multivariate analysis (HR, 0.22; 95% CI, 0.10 - 0.49; P = 0.0002). The liver enzyme levels had increased by 13% and 9% in the anakinra and control groups, respectively.
A retrospective cohort study of patients with COVID-19 treated in California, US, compared patients treated with tocilizumab and anakinra between March and April 2020 [166]; 52 patients received tocilizumab in the first half, and 41 patients received anakinra in the second half of the study period. The mortality rate was lower in those who received 100 mg of subcutaneous anakinra 4 times a day compared to those who received tocilizumab (22% vs. 46%). However, statistical significance was not observed after correcting the difference in disease severity at the beginning of treatment (propensity score-adjusted HR, 0.46; 95% CI, 0.18–1.20).
In France, COVID-19 patients received intravenous administration of anakinra 300 mg once a day for 5 days, followed by 200 mg once a day for 2 additional days, and 100 mg on the last day, and the data were retrospectively analyzed [167]. A total of 12 and 10 patients were included in the anakinra and control groups. Significant clinical improvement was observed in those who received anakinra treatment compared to the control group (oxygen demand, number of days free of MV), and no death was observed. In the control group, one death was observed.
Electronic medical records of 5,776 patients treated for COVID-19 at 12 hospitals were reviewed in the eastern US between March and April 2020 [168]. The survival data of patients who were treated with immunomodulatory drugs to control the cytokine storm caused by COVID-19 were compared. The mortality rates of the steroid monotherapy (HR, 0.66; 95% CI, 0.57 - 0.76; P <0.0001), steroid-tocilizumab combination therapy (HR, 0.44; 95% CI, 0.35 - 0.55; P <0.0001), and steroid-anakinra combination therapy (HR, 0.68; 95% CI, 0.57 - 0.81; P <0.0001) groups were lower than those of the standard treatment group. However, the mortality rates of the tocilizumab monotherapy and anakinra monotherapy groups were not different from those of the standard treatment group.
○ Considerations for recommendation
1. Certainty of evidence
There are no RCTs on IL-1 inhibitor treatment in COVID-19 patients. Only retrospective cohort studies are available. In addition, the dose, regimen, and treatment duration of anakinra vary in different studies. Some studies reported improved recovery, MV requirements, and mortality rates in the anakinra-treated group; however, some studies reported that the statistical significance disappeared after adjusting for severity of COVID-19 at the beginning of treatment. Moreover, since many patients received combination therapy with steroids and anakinra even in the anakinra-treated groups, interpreting the results is difficult. In the US-based study that analyzed the largest number of patients, anakinra monotherapy did not show any advantage in terms of survival compared to steroid monotherapy or steroid/anakinra combination therapy. Therefore, the overall certainty of evidence for the treatment effects of anakinra is low.
2. Benefit and harm
In patients with severe COVID-19, anakinra is expected to reduce excessive inflammatory reactions. However, it needs to be confirmed whether the risk of bacterial and fungal infections or additional complications is not high and has a superior effect on clinical recovery than steroids.
3. Patient values and preferences
There is no study on the values and preferences of patients with COVID-19. As the information on cytokine storms, which leads to rapid deterioration in COVID-19, is widely known among the general public, preference for anakinra will not be low.
4. Resources (including cost)
A bottle of anakinra (100 mg/0.67 mL) costs 76,392 won in general. On the other hand, steroids are cheaper than anakinra. Therefore, the cost of anakinra needs to be considered before use.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
There are no guidelines in other countries that recommend the use of anakinra in patients with COVID-19.
2) Evaluation of acceptance and applicability in Korea
The Korean acceptance and applicability evaluation tool in the NECA Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
There are no established standards for dosage and regimen. Systematic data on the use of IL-1 inhibitors in patients with complications of and resistance to steroid treatment are required.
CQ8 Interferon
○ Clinical question
Is interferon treatment effective and safe for patients with COVID-19 compared to standard treatment or no treatment?
○ PICO elements
○ Recommendations
Interferon can be used within the scope of clinical trials in patients with COVID-19 (certainty of evidence: low, grade of recommendation: B).
○ Basic information
Interferon is an immunomodulatory molecule that exhibits antiviral properties, and it has been used as a therapeutic agent in cases of SARS-CoV-1 and MERS-CoV in the past. In in-vitro experiments, interfeon inhibited the viral proliferation of SARS-CoV-2 [169]. In addition, type-1 interfeon secretion is decreased in patients with severe COVID-19, and autoantibodies that neutralize interfeon are a risk factor associated with severe COVID-19 progression [170,171].
○ Evidence summary
A total of three guidelines that included recommendations for interfeon were selected. The guidelines were based on six studies, i.e., four RCTs and two observational studies, that were included in the final evidence table. The results are summarized as follows.
Studies on the use of interfeon in patients with COVID-19 mostly compared the effects of interfeon-lopinavir/ritonavir combination therapy with standard lopinavir/ritonavir therapy. The largest of the four RCTs was the SOLIDARITY trial conducted in 30 countries, commissioned by the WHO. Interfeon β-1a is one of the four drugs tested in the SOLIDARITY trial, and approximately 4,1000 patients hospitalized for COVID-19 were included in this RCT. Those who received subcutaneous interfeon β-1a three times for 6 days with standard therapy were compared to those who only received standard therapy. The mortality ratio during hospitalization between the two groups was 1.16 (95% CI, 0.96 – 1.39), suggesting that interfeon β-1a combination therapy did not reduce the mortality rate compared to standard therapy [11]. In this study, interfeon β-1a was also not effective in reducing the duration of MV support and hospitalization. The other three RCTs on interfeon were small-scale studies with less than 100 patients. In one study on inhaled interfeon β-1a, improvements were observed, using the WHO clinical symptom ordinal scale, at 2 weeks after use of interfeon β-1a compared to the use of placebo (OR, 2.32; 95% CI, 1.07 – 5.04) [172]. In a study that treated patients with interfeon β-1a + ribavirin + lopinavir/ritonavir and lopinavir/ritonavir only, the duration of virus excretion was shortened in the combination therapy group treated with interfeon β-1a + ribavirin + lopinavir/ritonavir [103]. Another study compared the effect of interfeon β-1a combined with lopinavir/ritonavir and HCQ to that of standard treatment with lopinavir/ritonavir and HCQ, interfeon β-1a combination therapy increased the rate of discharge on day 14 and decreased the 28-day mortality rate compared to standard treatment [173].
○ Considerations for recommendation
1. Certainty of evidence
The interpretation of the results of the RCTs and observational studies on the effects of interfeon treatment is limited due to co-administration of drugs and differences in route and timing of administration, disease severity, and sample size. The SOLIDARITY trial is a large-scale RCT study of more than 4,000 patients; however, confounding variables such as combination therapy with steroids, disease severity before administration, and route of administration limit the interpretation of the study results. Currently, some clinical trials on subcutaneous injection of interfeon β-1a are ongoing, and further studies on the effects of interfeon treatment are needed [174].
2. Benefit and harm
In the SOLIDARITY trial, interfeon combined with standard therapy did not affect mortality, need for MV, and duration of hospitalization. However, in other small-scale RCTs, inhaled or transdermal interfeon β-1a effectively improved clinical symptoms and discharge and mortality rates. It is difficult to recommend interfeon for COVID-19 based on the results of published studies. However, interfeon can be used within the scope of clinical trials.
The main side effects of interfeon α, which has been used to treat chronic hepatitis, are neuropsychiatric symptoms such as fever, muscle pain, fatigue, hemocytopenia, depression, and suicidal ideation [175]. Interfeon β has fewer side effects than interfeon α, and the frequency of side effects of interfeon β in patients with COVID-19 vary depending on studies [172]. In one study, the frequency of fever, chills, headache, and fatigue related to interfeon administration, which was not observed in the standard treatment group, and was 19.0% in those who received subcutaneous interfeon. Additionally, the frequency of neuropsychiatric problems such as anxiety was 9.5% [173]. However, in another study, there was no difference in the frequency of side effects between interfeon combination treatment and standard treatment groups [103]. On the other hand, the frequency of treatment-related side effects such as cough was higher in the interfeon inhalation group (15%) than in the placebo group (4%) in a study on inhaled interfeon [176].
3. Patient values and preferences
Currently, the choice of treatment for COVID-19 patients is highly limited, and very few drugs have shown proven therapeutic effects. At the beginning of the COVID-19 pandemic, when information on treatment was scarce, interfeon was covered by insurance when co-administered with other antiviral drugs to COVID-19 patients based on experiences with SARS-CoV-1 and MERS-CoV patients in the past. However, currently, it is mainly used within the scope of clinical trials.
4. Resources (including cost)
Transdermal interfeon β-1a is available in South Korea as Rebif®, which has been used to treat multiple sclerosis. Inhaled interfeon β-1a was developed to treat patients with viral infections of the lower respiratory tract; however, it has not been approved in Korea yet for treating COVID-19.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
The clinical practice guidelines by the WHO, NIH, and Australian authorities do not recommend the use of interfeon for patients with COVID-19 outside the scope of clinical trials.
2) Evaluation of acceptance and applicability in South Korea
The Korean acceptance and applicability evaluation tool in the NECA Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
Several clinical trials on interfeon combination therapy for COVID-19 are being conducted in Korea and internationally.
CQ9 Convalescent plasma
○ Clinical question
Is convalescent plasma therapy effective and safe for patients with COVID-19 compared to standard treatment or no treatment?
○ PICO elements
○ Recommendations
There is insufficient evidence on the benefit of convalescent plasma therapy in patients with COVID-19 to make recommendations (certainty of evidence: low, grade of recommendation: I).
○ Basic information
Convalescent plasma may contain virus-specific neutralizing antibodies and has been considered as a treatment option for infectious diseases through immune responses such as antibody-dependent cytotoxicity, complement activation, and phagocytosis. Although convalescent plasma showed encouraging effects against Ebola virus, SARS, and MERS, the sample sizes were small, and the studies were not randomized [177,178,179].
○ Evidence summary
Four guidelines included in the recommendations for convalescent plasma were selected. The guidelines were based on 13 studies, i.e., five RCTs, three (retrospective or prospective) cohort studies, four case-control studies, and one descriptive study, which were included in the final evidence table. Two of the five RCTs were not peer-reviewed. The results are summarized as follows.
Li et al. found no differences in the 28-day clinical outcomes (HR, 1.40; 95% CI, 0.79 – 2.49; P = 0.26) and mortality (treatment group: 16% vs. non-treatment group: 24%, P = 0.3) between the convalescent plasma treatment (n = 52) and non-treatment (n = 51) groups. In this study, plasma with a specific IgG titer of 1:640 or higher was administered, and the period from the onset of symptoms to participation in the study was relatively long (27 days for the treatment group and 30 days for the non-treatment group). Additionally, it was not a blinded study [180]. In the RCT that compared convalescent plasma treatment group (n = 235) and non-treatment group (n = 239), the 28-day mortality was 19% and 18%, respectively (risk difference, 0.008; 95% CI, -0.062 – 0.078; risk ratio, 1.04; 95% CI, 0.71 – 1.54); this was not a blinded study, and neutralizing antibody titers were not measured [181].
There were two RCTs that were not peer-reviewed. In one study, 38 patients who underwent convalescent plasma treatment did not require MV support or died during the study. In contrast, 14% of 43 patients who did not receive convalescent plasma therapy showed clinical deterioration and 9.3% were died, respectively, suggesting that convalescent plasma treatment was effective [182]. However, this study was not blinded. In another study, there was no significant difference in disease progression and mortality rate between convalescent plasma treatment (n = 43) and non-treatment (n = 43) groups [183].
In a blinded study that randomized patients with severe pneumonia in a 2:1 ratio to convalescent plasma treatment (n = 228) and non-treatment (n = 105) groups, the mortality rate was 10.95% and 11.43%, respectively, which was not significantly different (OR, 0.83; 95% CI, 0.52 – 1.35; P = 0.46) [184].
○ Considerations for recommendation
1. Certainty of evidence
The RCTs that assessed the effects of convalescent plasma therapy had small sample sizes, and most studies were not blinded. Moreover, antibody titers and time points for plasma administration were different for each study. Therefore, the overall certainty of evidence is low due to inaccuracy and inconsistency.
2. Benefit and harm
There are little RCTs with a small number of patients on the effects of convalescent plasma therapy, and this therapy did not show clinical improvement or reduced mortality in patients with COVID-19, except in one study. The criteria for selection of plasma and the timing of administration are also different for each study, and it is difficult to determine the benefits of convalescent plasma administration based on the evidence so far. In a safety study of 200 patients, only 78 cases of side effects related to blood transfusion were observed, which was less than 1% [185]; in all, 63 (0.3%) deaths were reported, and of them, 13 were associated with blood transfusion.
3. Patient values and preferences
There are no studies in Korea that assessed the value and preference for this therapy in patients with COVID-19. There is a case report in South Korea of two patients with acute respiratory failure from COVID-19 pneumonia who had a good prognosis after steroid and plasma treatment [186].
4. Resources (including cost)
It is important to set the criteria for donor selection as the effects of the neutralizing antibodies may differ according to the plasma of the donors.
5. Acceptability and Applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
In the IDSA guidelines, convalescent plasma treatment is recommended only for clinical trials of hospitalized patients with COVID-19, and the ACPG does not recommend trials other than ethically approved RCTs. The NIH and Chinese guidelines report that there is insufficient evidence on the use of convalescent plasma treatment.
2) Evaluation of acceptance and applicability in Korea
The Korean acceptance and applicability evaluation tool in the NECA Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
Convalescent plasma treatment has not been approved for use in patients with COVID-19 by the FDA.
CQ10 Conventional intravenous immunoglobulin (IVIG)
○ Clinical question
Is the administration of conventional intravenous immunoglobulin (IVIG) effective and safe for patients with COVID-19 compared to standard treatment or no treatment?
○ PICO elements
○ Recommendations
Administration of conventional IVIG is not recommended for patients with COVID-19.
However, IVIg therapy should not be excluded when indicated for treatment of complications of COVID-19 (certainty of evidence: low, grade of recommendation: C).
○ Basic information related to other antiviral agents
Theoretically, immunoglobulins that are produced by the antibodies in the plasma can suppress viruses and regulate the inflammatory response. For example, cytomegalovirus immunoglobulins for preventing cytomegalovirus infection are safe and effective. However, there is no information on the effectiveness of immunoglobulins, including specific antibodies, in patients with COVID-19. Commonly used immunoglobulins may benefit the immune system; however, this is not clear for patients with COVID-19.
○ Evidence summary
The guidelines that included recommendations for immunoglobulins were selected. The guidelines were based on three studies, two RCTs and one (retrospective or prospective) cohort study, which were included in the final evidence table. The results are summarized as follows.
In one randomized, double-blinded study by Gharenbaghi et al. involving patients with moderate COVID-19 who did not respond to initial treatment, the mortality rate was significantly lower in the immunoglobulin the treatment group (n = 30) than in the control group (n = 29) (6 [20.0%] vs. 14 [48.3%], respectively; P = 0.022) [187]. In the RCT that was not peer-reviewed, the rate of clinical deterioration (2/14 vs. 7/12, P = 0.038) and duration of ICU care (2.5 days vs. 12.5 days, P = 0.006) were reduced in those who were treated with immunoglobulins and steroids (n = 16) compared to in those who received standard treatment (n = 17) [188]. In the retrospective cohort study by Shao et al., with 174 and 151 patients who did and did not receive immunoglobulin therapy, respectively, the duration of hospitalization was longer in the treatment group. However, the study was not randomized, and the immunoglobulin treatment group was older and had a higher number of cardiovascular diseases than the control group [189].
○ Considerations for recommendation
1. Certainty of evidence
There were only two RCTs on immunoglobulins, with a small number of patients, and the dose and point of immunoglobulin administration were different between the two studies. Therefore, the overall certainty of evidence is low due to inaccuracy and inconsistency.
2. Benefit and harm
There are no studies on SARS-CoV-2-specific immunoglobulins. In the two RCTs, the immunoglobulin treatment group showed good prognosis; however, immunoglobulin was administered alone in only one study. The studies also had a small sample size. Therefore, the benefit of immunoglobulins in patients with COVID-19 is not clear. Side effects of immunoglobulin administration include symptoms of common cold, skin reactions, arrhythmia, hypotension, and lung damage related to blood transfusion [190].
3. Patient values and preferences
No study in Korea has assessed the values and preferences for immunoglobin therapy in patients with COVID-19.
4. Resources (including cost)
There is no study on the cost-effectiveness of immunoglobulins in patients with COVID-19. In Korea, general human immunoglobulins are available, and administration of 5 g a day for 7 days for severe infections is covered by insurance. Additional benefits are offered in cases of sepsis or acute respiratory distress syndrome in cases of MERS and COVID-19.
5. Acceptability and applicability
1) Comparison of recommendations with other countries' clinical practice guidelines
(Refer to the table comparing different recommendations)
The NIH guidelines do not have sufficient data on the effects of SARS-CoV-2 immunoglobulins, and it is recommended that non-specific immunoglobulins should not be used in clinical practice, except in clinical studies. Moreover, the NIH guidelines state that immunoglobulins should not be excluded when indicated for the treatment of complications of COVID-19. The ACPG does not recommend the use of immunoglobulin and steroid combination therapy for patients with COVID-9 patients, except in clinical studies.
2) Evaluation of acceptance and applicability in South Korea
The Korean acceptance and applicability evaluation tool in the NECA Clinical Practice Guidelines Handbook (2015) was used to evaluate the acceptance and applicability of the treatment guidelines.
6. Other considerations
In Korea, clinical studies on hyper-immunoglobulin therapy are currently being conducted.
Future perspectives
These recommendations were developed during October and December 2020. There exist certain limitations, such as lack of meta-analysis and multidisciplinary review, due to the time constraint.
Regular review of updated literature and emerging issues are planned to keep the guidelines up to date. Recommendations regarding monoclonal antibody are currently being reviewed. The Korean Academy of Medical Sciences and the NECA are planning for a comprehensive, multidisciplinary guideline on the medical care of COVID-19, which will cover pharmaceutical treatments as well as critical care, children and pregnant women, and people with special conditions. The KSID will continue to contribute to this joint effort.
ACKNOWLEDGMENTS
We are grateful to all the medical personnel in the fight against SARS-CoV-2 in the Korea and worldwide. We are also grateful to other task force members of the Emerging Infectious Diseases of the Korean Society of Infectious Diseases (KSID) for their advice on increasing the credibility of the paper: Kyong Ran Peck (chairman), Sang Il Kim (vice-chairman), Cheol-In Kang, Nam Joong Kim, Sung Han Kim, Min Jae Kim, Jin Yong Kim, Sung Ran Kim, Su Hyun Kim, Shin-Woo Kim, Yeon-Sook Kim, Yeon-Jae Kim, Tae Hyong Kim, Taek soo Kim, Tark Kim, Hong Bin Kim, Hong Jae Kim, So Yeon Park, Jin Hwi Baek, Jang Wook Sohn, Young Goo Song, Joon Young Song, Hye-Jin Shi, Myoung Jin Shin, Jong Gyun Ahn, Joong Sik Eom, So-Yeon Yoo, Jacob Lee, Sun Hee Lee, Jin Seo Lee, Seung Kwan Lim, Hong Sang Oh, Heeyoung Lee, Sook-In Jung, Seong-Ho Choi, Jae-Phil Choi, Ji-youn Choi, Sang Hoon Han, Su Ha Han.
Footnotes
Funding: This research was supported by the National Evidence-based Collaborating Agency (NECA-P-20-002) and KSID (Korean Society of Infection Disease).
Conflict of Interest: No conflict of interest.
- Conceptualization: SBK, WSC, JSY, KRP, MYC, DAP.
- Data curation: MYC, DAP, SYY, HJL, JMK, YJ, JEP, SER.
- Formal analysis: SYY, HJL, JMK, YJ, JEP, SER.
- Funding acquisition: MYC.
- Investigation: MYC, DAP, SYY, HJL, JMK, YJ, JEP, SER, SBK, KMH, JYH, JEJ, YJK, WSC, YJK, YKY, SJJ, KRP, JSY.
- Methodology: MYC, DAP, SYY, HJL, JMK, YJ, JEP, SER.
- Project administration: SBK, KMH, JYH, JEJ, YJK, WSC, YJK, YKY, SJJ.
- Resources: YBS, MYC, DAP.
- Software: SBK, KMH, JYH, JEJ, YJK, WSC, YJK, YKY, SJJ.
- Supervision: YJK, JSY, KRP, MYC.
- Validation: YJK, JSY, KRP, MYC.
- Visualization: MYC, DAP, SYY, HJL, JMK, YJ, JEP, SER.
- Writing - original draft: SBK, KMH, JYH, JEJ, YJK, WSC, YJK, YKY, SJJ, MYC.
- Writing - review & editing: SBK, KMH, JYH, JEJ, YJK, WSC, YJK, YKY, SJJ, MYC, SER, KRP, JSY.
SUPPLEMENTARY MATERIAL
Guideline Korean version.
References
- 1.Lee YK, Shin ES, Shim JY, Min KJ, Kim JM, Lee SH. Developing a scoring guide for the Appraisal of Guidelines for Research and Evaluation II instrument in Korea: a modified Delphi consensus process. J Korean Med Sci. 2013;28:190–194. doi: 10.3346/jkms.2013.28.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health (NIH) Clinical spectrum of SARS-CoV-2 infection. [Accessed 17 December 2020]. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 3.Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, Atkins D, Kunz R, Montori V, Jaeschke R, Rind D, Dahm P, Akl EA, Meerpohl J, Vist G, Berliner E, Norris S, Falck-Ytter Y, Schünemann HJ. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151–157. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook. [Accssed 8 December 2020]. Available at: https://gdt.gradepro.org/app/handbook/handbook.html.
- 5.Brown AJ, Won JJ, Graham RL, Dinnon KH, 3rd, Sims AC, Feng JY, Cihlar T, Denison MR, Baric RS, Sheahan TP. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dörnemann J, Burzio C, Ronsse A, Sprecher A, De Clerck H, Van Herp M, Kolié MC, Yosifiva V, Caluwaerts S, McElroy AK, Antierens A. First Newborn Baby to Receive Experimental Therapies Survives Ebola Virus Disease. J Infect Dis. 2017;215:171–174. doi: 10.1093/infdis/jiw493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulangu S, Dodd LE, Davey RT, Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum JJ, Sivahera B, Camara M, Kojan R, Walker R, Dighero-Kemp B, Cao H, Mukumbayi P, Mbala-Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallée D, Nordwall J PALM Consortium Study Team. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Solidarity Trial Consortium. Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed antiviral drugs for Covid-19 - Interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) Therapeutics and COVID-19: living guideline. [Accessed 8 December 2020]. Available at: https://www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline.
- 13.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, Ogbuagu O, Malhotra P, Mullane KM, Castagna A, Chai LYA, Roestenberg M, Tsang OTY, Bernasconi E, Le Turnier P, Chang SC, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wang H, Gaggar A, Brainard DM, McPhail MJ, Bhagani S, Ahn MY, Sanyal AJ, Huhn G, Marty FM GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joo EJ, Ko JH, Kim SE, Kang SJ, Baek JH, Heo EY, Shi HJ, Eom JS, Choe PG, Bae S, Ra SH, Kim DY, Kim BN, Kang YM, Kim JY, Chung JW, Chang HH, Bae S, Cheon S, Park Y, Choi H, Lee E, Lee BY, Park JW, Sohn Y, Heo JY, Kim SH, Peck KR. Clinical and virologic effectiveness of remdesivir treatment for severe coronavirus disease 2019 (COVID-19) in Korea: a nationwide multicenter retrospective cohort study. J Korean Med Sci. 2021;36:e83. doi: 10.3346/jkms.2021.36.e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L, Chan PK, Sidwell RW. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother. 2006;17:275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- 19.Biot C, Daher W, Chavain N, Fandeur T, Khalife J, Dive D, De Clercq E. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49:2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- 20.McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983;75:11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weniger H. Review of side effects and toxicity of chloroquine. Geneva: WHO; 1979. [Google Scholar]
- 25.Danesi R, Lupetti A, Barbara C, Ghelardi E, Chella A, Malizia T, Senesi S, Angeletti CA, Del Tacca M, Campa M. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J Antimicrob Chemother. 2003;51:939–945. doi: 10.1093/jac/dkg138. [DOI] [PubMed] [Google Scholar]
- 26.Tran DH, Sugamata R, Hirose T, Suzuki S, Noguchi Y, Sugawara A, Ito F, Yamamoto T, Kawachi S, Akagawa KS, Ōmura S, Sunazuka T, Ito N, Mimaki M, Suzuki K. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J Antibiot (Tokyo) 2019;72:759–768. doi: 10.1038/s41429-019-0204-x. [DOI] [PubMed] [Google Scholar]
- 27.Iannetta M, Ippolito G, Nicastri E. Azithromycin shows anti-Zika virus activity in human glial cells. Antimicrob Agents Chemother. 2017;61:e01152–17. doi: 10.1128/AAC.01152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Zu S, Deng YQ, Li D, Parvatiyar K, Quanquin N, Shang J, Sun N, Su J, Liu Z, Wang M, Aliyari SR, Li XF, Wu A, Ma F, Shi Y, Nielsevn-Saines K, Jung JU, Qin FX, Qin CF, Cheng G. Azithromycin protects against Zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob Agents Chemother. 2019;63:e00394–19. doi: 10.1128/AAC.00394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RECOVERY Collaborative Group. Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse T, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell LC, Faust SN, Jaki T, Jeffery K, Lim WS, Montgomery A, Rowan K, Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, Wurtz N, Rolain JM, Colson P, La Scola B, Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, Weinberg P, Kirkwood J, Muse A, DeHovitz J, Blog DS, Hutton B, Holtgrave DR, Zucker HA. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, Mailhe M, Doudier B, Aubry C, Amrane S, Seng P, Hocquart M, Eldin C, Finance J, Vieira VE, Tissot-Dupont HT, Honoré S, Stein A, Million M, Colson P, La Scola B, Veit V, Jacquier A, Deharo JC, Drancourt M, Fournier PE, Rolain JM, Brouqui P, Raoult D. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonny A, Talle MA, Ngantcha M, Tayebjee MH. Conflicting evidence on the efficacy of hydroxychloroquine and azithromycin as the early treatment of COVID-19. Comment on “Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France”. Travel Med Infect Dis. 2020;37:101861. doi: 10.1016/j.tmaid.2020.101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guérin V, Lévy P, Thomas JL, Lardenois T, Lacrosse P, Sarrazin E, de Andreis NR, Wonner M. Azithromycin and hydroxychloroquine accelerate recovery of outpatients with mild/moderate COVID-19. Asian J Med Health. 2020;18:45–55. [Google Scholar]
- 38.Esper RB, da Silva RS, Oikawa FTC, Castro MM, Razuk-Filho A, Batista PB, Lotze SM, da Rocha CN, de Sá Cunha Filho R, de Oliveira SEB, Ribeiro PL, Martins VCV, Bueno FSB, Esper PLG, Parrillo EF. Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine. [Accessed 8 December 2020]. Available at: https://pgibertie.files.wordpress.com/2020/04/2020.04.15-journal-manuscript-final.pdf.
- 39.Risch HA. Early outpatient treatment of symptomatic, high-risk COVID-19 patients that should be ramped-up immediately as key to the pandemic crisis. Am J Epidemiol. 2020;189:1218–1226. doi: 10.1093/aje/kwaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahévas M, Tran VT, Roumier M, Chabrol A, Paule R, Guillaud C, Fois E, Lepeule R, Szwebel TA, Lescure FX, Schlemmer F, Matignon M, Khellaf M, Crickx E, Terrier B, Morbieu C, Legendre P, Dang J, Schoindre Y, Pawlotsky JM, Michel M, Perrodeau E, Carlier N, Roche N, de Lastours V, Ourghanlian C, Kerneis S, Ménager P, Mouthon L, Audureau E, Ravaud P, Godeau B, Gallien S, Costedoat-Chalumeau N. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magagnoli J, Narendran S, Pereira F, Cummings TH, Hardin JW, Sutton SS, Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med (N Y) 2020;1:114–27.e3. doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, Brar I, Alangaden GJ, Ramesh MS, McKinnon JE, O'Neill W, Zervos M Henry Ford COVID-19 Task Force. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Self WH, Semler MW, Leither LM, Casey JD, Angus DC, Brower RG, Chang SY, Collins SP, Eppensteiner JC, Filbin MR, Files DC, Gibbs KW, Ginde AA, Gong MN, Harrell FE, Jr, Hayden DL, Hough CL, Johnson NJ, Khan A, Lindsell CJ, Matthay MA, Moss M, Park PK, Rice TW, Robinson BRH, Schoenfeld DA, Shapiro NI, Steingrub JS, Ulysse CA, Weissman A, Yealy DM, Thompson BT, Brown SM National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Steingrub J, Smithline H, Tiru B, Tidswell M, Kozikowski L, Thornton-Thompson S, De Souza L, Hou P, Baron R, Massaro A, Aisiku I, Fredenburgh L, Seethala R, Johnsky L, Riker R, Seder D, May T, Baumann M, Eldridge A, Lord C, Shapiro N, Talmor D, O'Mara T, Kirk C, Harrison K, Kurt L, Schermerhorn M, Banner-Goodspeed V, Boyle K, Dubosh N, Filbin M, Hibbert K, Parry B, Lavin-Parsons K, Pulido N, Lilley B, Lodenstein C, Margolin J, Brait K, Jones A, Galbraith J, Peacock R, Nandi U, Wachs T, Matthay M, Liu K, Kangelaris K, Wang R, Calfee C, Yee K, Hendey G, Chang S, Lim G, Qadir N, Tam A, Beutler R, Levitt J, Wilson J, Rogers A, Vojnik R, Roque J, Albertson T, Chenoweth J, Adams J, Pearson S, Juarez M, Almasri E, Fayed M, Hughes A, Hillard S, Huebinger R, Wang H, Vidales E, Patel B, Ginde A, Moss M, Baduashvili A, McKeehan J, Finck L, Higgins C, Howell M, Douglas I, Haukoos J, Hiller T, Lyle C, Cupelo A, Caruso E, Camacho C, Gravitz S, Finigan J, Griesmer C, Park P, Hyzy R, Nelson K, McDonough K, Olbrich N, Williams M, Kapoor R, Nash J, Willig M, Ford H, Gardner-Gray J, Ramesh M, Moses M, Ng Gong M, Aboodi M, Asghar A, Amosu O, Torres M, Kaur S, Chen JT, Hope A, Lopez B, Rosales K, Young You J, Mosier J, Hypes C, Natt B, Borg B, Salvagio Campbell E, Hite RD, Hudock K, Cresie A, Alhasan F, Gomez-Arroyo J, Duggal A, Mehkri O, Hastings A, Sahoo D, Abi Fadel F, Gole S, Shaner V, Wimer A, Meli Y, King A, Terndrup T, Exline M, Pannu S, Robart E, Karow S, Hough C, Robinson B, Johnson N, Henning D, Campo M, Gundel S, Seghal S, Katsandres S, Dean S, Khan A, Krol O, Jouzestani M, Huynh P, Weissman A, Yealy D, Scholl D, Adams P, McVerry B, Huang D, Angus D, Schooler J, Moore S, Files C, Miller C, Gibbs K, LaRose M, Flores L, Koehler L, Morse C, Sanders J, Langford C, Nanney K, MdalaGausi M, Yeboah P, Morris P, Sturgill J, Seif S, Cassity E, Dhar S, de Wit M, Mason J, Goodwin A, Hall G, Grady A, Chamberlain A, Brown S, Bledsoe J, Leither L, Peltan I, Starr N, Fergus M, Aston V, Montgomery Q, Smith R, Merrill M, Brown K, Armbruster B, Harris E, Middleton E, Paine R, Johnson S, Barrios M, Eppensteiner J, Limkakeng A, McGowan L, Porter T, Bouffler A, Leahy JC, deBoisblanc B, Lammi M, Happel K, Lauto P, Self W, Casey J, Semler M, Collins S, Harrell F, Lindsell C, Rice T, Stubblefield W, Gray C, Johnson J, Roth M, Hays M, Torr D, Zakaria A, Schoenfeld D, Thompson T, Hayden D, Ringwood N, Oldmixon C, Ulysse C, Morse R, Muzikansky A, Fitzgerald L, Whitaker S, Lagakos A, Brower R, Reineck L, Aggarwal N, Bienstock K, Freemer M, Maclawiw M, Weinmann G, Morrison L, Gillespie M, Kryscio R, Brodie D, Zareba W, Rompalo A, Boeckh M, Parsons P, Christie J, Hall J, Horton N, Zoloth L, Dickert N, Diercks D. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulrich RJ, Troxel AB, Carmody E, Eapen J, Bäcker M, DeHovitz JA, Prasad PJ, Li Y, Delgado C, Jrada M, Robbins GA, Henderson B, Hrycko A, Delpachitra D, Raabe V, Austrian JS, Dubrovskaya Y, Mulligan MJ. Treating COVID-19 with hydroxychloroquine (TEACH): a multicenter, double-blind randomized controlled trial in hospitalized patients. Open Forum Infect Dis. 2020;7:ofaa446. doi: 10.1093/ofid/ofaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abd-Elsalam S, Esmail ES, Khalaf M, Abdo EF, Medhat MA, Abd El Ghafar MS, Ahmed OA, Soliman S, Serangawy GN, Alboraie M. Hydroxychloroquine in the treatment of COVID-19: a multicenter randomized controlled study. Am J Trop Med Hyg. 2020;103:1635–1639. doi: 10.4269/ajtmh.20-0873. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, Ling Y, Huang D, Song S, Zhang D, Qian Z, Li T, Shen Y, Lu H. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyngbakken MN, Berdal JE, Eskesen A, Kvale D, Olsen IC, Rueegg CS, Rangberg A, Jonassen CM, Omland T, Røsjø H, Dalgard O. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun. 2020;11:5284. doi: 10.1038/s41467-020-19056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, Chen W, Wang X, Yang J, Lin J, Zhao Q, Yan Y, Xie Z, Li D, Yang Y, Liu L, Qu J, Ning G, Shi G, Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Zhang ZY, Fu JG, Feng ZP, Zhang SZ, Han QY, Zhang XB, Xiao X, Chen HM, Liu LL, Chen XL, Lan YP, Zhong DJ, Hu L, Wang JH, Yu XH, She DY, Zhu YH, Yin ZY. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study. MedRxiv. 2020 [Epub ahead of print] [Google Scholar]
- 52.Mitjà O, Corbacho-Monné M, Ubals M, Tebe C, Peñafiel J, Tobias A, Ballana E, Alemany A, Riera-Martí N, Pérez CA, Suñer C, Laporte P, Admella P, Mitjà J, Clua M, Bertran L, Sarquella M, Gavilán S, Ara J, Argimon JM, Casabona J, Cuatrecasas G, Cañadas P, Elizalde-Torrent A, Fabregat R, Farré M, Forcada A, Flores-Mateo G, Muntada E, Nadal N, Narejos S, Gil-Ortega AN, Prat N, Puig J, Quiñones C, Reyes-Ureña J, Ramírez-Viaplana F, Ruiz L, Riveira-Muñoz E, Sierra A, Velasco C, Vivanco-Hidalgo RM, Sentís A BCN PEP-CoV-2 RESEARCH GROUP. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis. 2020:ciaa1009. doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, Williams DA, Okafor EC, Pullen MF, Nicol MR, Nascene AA, Hullsiek KH, Cheng MP, Luke D, Lother SA, MacKenzie LJ, Drobot G, Kelly LE, Schwartz IS, Zarychanski R, McDonald EG, Lee TC, Rajasingham R, Boulware DR. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized Trial. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen CP, Lin YC, Chen TC, Tseng TY, Wong HL, Kuo CY, Lin WP, Huang SR, Wang WY, Liao JH, Liao CS, Hung YP, Lin TH, Chang TY, Hsiao CF, Huang YW, Chung WS, Cheng CY, Cheng SH Taiwan HCQ Study Group. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19) PLoS One. 2020;15:e0242763. doi: 10.1371/journal.pone.0242763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mikami T, Miyashita H, Yamada T, Harrington M, Steinberg D, Dunn A, Siau E. Risk factors for mortality in patients with COVID-19 in New York city. J Gen Intern Med. 2021;36:17–26. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catteau L, Dauby N, Montourcy M, Bottieau E, Hautekiet J, Goetghebeur E, van Ierssel S, Duysburgh E, Van Oyen H, Wyndham-Thomas C, Van Beckhoven D Belgian Collaborative Group on COVID-19 Hospital Surveillance. Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants. Int J Antimicrob Agents. 2020;56:106144. doi: 10.1016/j.ijantimicag.2020.106144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The COVID-19 RISK and Treatments (CORIST) Collaboration. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur J Intern Med. 2020;82:38–47. doi: 10.1016/j.ejim.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu B, Li C, Chen P, Zhou N, Wang L, Li J, Jiang H, Wang DW. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci China Life Sci. 2020;63:1515–1521. doi: 10.1007/s11427-020-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szente Fonseca SN, de Queiroz Sousa A, Wolkoff AG, Moreira MS, Pinto BC, Valente Takeda CF, Rebouças E, Vasconcellos Abdon AP, Nascimento ALA, Risch HA. Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: Comparative analysis. Travel Med Infect Dis. 2020;38:101906. doi: 10.1016/j.tmaid.2020.101906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang HD, Jneid H, Aziz M, Ravi V, Sharma PS, Larsen T, Chatterjee N, Saour B, Aziz Z, Nayak H, Trohman RG, Krishnan K. Safety and effectiveness of hydroxychloroquine and azithromycin combination therapy for treatment of hospitalized patients with COVID-19: a propensity-matched study. Cardiol Ther. 2020;9:523–534. doi: 10.1007/s40119-020-00201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Annie FH, Sirbu C, Frazier KR, Broce M, Lucas BD., Jr Hydroxychloroquine in hospitalized patients with COVID-19: real-world experience assessing mortality. Pharmacotherapy. 2020;40:1072–1081. doi: 10.1002/phar.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalligeros M, Shehadeh F, Atalla E, Mylona EK, Aung S, Pandita A, Larkin J, Sanchez M, Touzard-Romo F, Brotherton A, Shah R, Cunha CB, Mylonakis E. Hydroxychloroquine use in hospitalised patients with COVID-19: an observational matched cohort study. J Glob Antimicrob Resist. 2020;22:842–844. doi: 10.1016/j.jgar.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paccoud O, Tubach F, Baptiste A, Bleibtreu A, Hajage D, Monsel G, Tebano G, Boutolleau D, Klement E, Godefroy N, Palich R, Itani O, Fayssal A, Valantin MA, Tubiana R, Burrel S, Calvez V, Caumes E, Marcelin AG, Pourcher V. Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe Covid-19 in a French university hospital. Clin Infect Dis. 2020:ciaa791. doi: 10.1093/cid/ciaa791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Satlin MJ, Goyal P, Magleby R, Maldarelli GA, Pham K, Kondo M, Schenck EJ, Rennert H, Westblade LF, Choi JJ, Safford MM, Gulick RM. Safety, tolerability, and clinical outcomes of hydroxychloroquine for hospitalized patients with coronavirus 2019 disease. PLoS One. 2020;15:e0236778. doi: 10.1371/journal.pone.0236778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furtado RHM, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR, Lapa MG, Zampieri FG, Veiga VC, Azevedo LCP, Rosa RG, Lopes RD, Avezum A, Manoel ALO, Piza FMT, Martins PA, Lisboa TC, Pereira AJ, Olivato GB, Dantas VCS, Milan EP, Gebara OCE, Amazonas RB, Oliveira MB, Soares RVP, Moia DDF, Piano LPA, Castilho K, Momesso RGRAP, Schettino GPP, Rizzo LV, Neto AS, Machado FR, Cavalcanti AB COALITION COVID-19 Brazil II Investigators. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Faíco-Filho KS, Conte DD, de Souza Luna LK, Carvalho JMA, Perosa AHS, Bellei N. No benefit of hydroxychloroquine on SARS-CoV-2 viral load reduction in non-critical hospitalized patients with COVID-19. Braz J Microbiol. 2020;51:1765–1769. doi: 10.1007/s42770-020-00395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karolyi M, Pawelka E, Mader T, Omid S, Kelani H, Ely S, Jilma B, Baumgartner S, Laferl H, Ott C, Traugott M, Turner M, Seitz T, Wenisch C, Zoufaly A. Hydroxychloroquine versus lopinavir/ritonavir in severe COVID-19 patients : Results from a real-life patient cohort. Wien Klin Wochenschr. 2020:1–8. doi: 10.1007/s00508-020-01720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JW, Kim EJ, Kwon HH, Jung CY, Kim KC, Choe JY, Hong HL. Lopinavir-ritonavir versus hydroxychloroquine for viral clearance and clinical improvement in patients with mild to moderate coronavirus disease 2019. Korean J Intern Med. 2021;36(Suppl 1):S253–S263. doi: 10.3904/kjim.2020.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lecronier M, Beurton A, Burrel S, Haudebourg L, Deleris R, Le Marec J, Virolle S, Nemlaghi S, Bureau C, Mora P, De Sarcus M, Clovet O, Duceau B, Grisot PH, Pari MH, Arzoine J, Clarac U, Boutolleau D, Raux M, Delemazure J, Faure M, Decavele M, Morawiec E, Mayaux J, Demoule A, Dres M. Comparison of hydroxychloroquine, lopinavir/ritonavir, and standard of care in critically ill patients with SARS-CoV-2 pneumonia: an opportunistic retrospective analysis. Crit Care. 2020;24:418. doi: 10.1186/s13054-020-03117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MM, Tse MW, Que TL, Peiris JS, Sung J, Wong VC, Yuen KY. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 72.Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, Peiris M, Yen HL. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y, Mo X, Wang J, Wang Y, Peng P, Chen X, Hong W, Xiao G, Liu J, Zhang L, Hu F, Li F, Zhang F, Deng X, Li L. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med (N Y) 2020;1:105–13.e4. doi: 10.1016/j.medj.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arshad U, Pertinez H, Box H, Tatham L, Rajoli RKR, Curley P, Neary M, Sharp J, Liptrott NJ, Valentijn A, David C, Rannard SP, O'Neill PM, Aljayyoussi G, Pennington SH, Ward SA, Hill A, Back DJ, Khoo SH, Bray PG, Biagini GA, Owen A. Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin Pharmacol Ther. 2020;108:775–790. doi: 10.1002/cpt.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209:107512. doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, Jones SM, Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon- alpha. J Infect Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan EL, Ooi EE, Lin CY, Tan HC, Ling AE, Lim B, Stanton LW. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, Li PT, Dai J, Mok FK, Chen H, Hayden FG, Yuen KY. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J., Jr Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326:905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H, Li Y, Zhao L, Li W, Sun X, Yang X, Shi Z, Deng F, Hu Z, Zhong W, Wang M. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6:28. doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shirley M. Baloxavir marboxil: a review in acute uncomplicated influenza. Drugs. 2020;80:1109–1118. doi: 10.1007/s40265-020-01350-8. [DOI] [PubMed] [Google Scholar]
- 90.Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, Luo R, Yang X, He L, Lu X, Zhao Q, Liang T, Qiu Y. Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial. Eur J Pharm Sci. 2021;157:105631. doi: 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Chen S, Zhang Y, Chem B, Lu M, Luo Y, Ju L, Zhang J, Wang X. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020:03.17.20037432 [Google Scholar]
- 92.Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, Gordeev IG, Ilin AP, Karapetian RN, Kravchenko DV, Lomakin NV, Merkulova EA, Papazova NA, Pavlikova EP, Savchuk NP, Simakina EN, Sitdekov TA, Smolyarchuk EA, Tikhomolova EG, Yakubova EV, Ivachtchenko AV. AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2020:ciaa1176. doi: 10.1093/cid/ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M, Mutoh Y, Homma Y, Terada M, Ogawa T, Kashizaki F, Yokoyama T, Koba H, Kasahara H, Yokota K, Kato H, Yoshida J, Kita T, Kato Y, Kamio T, Kodama N, Uchida Y, Ikeda N, Shinoda M, Nakagawa A, Nakatsumi H, Horiguchi T, Iwata M, Matsuyama A, Banno S, Koseki T, Teramachi M, Miyata M, Tajima S, Maeki T, Nakayama E, Taniguchi S, Lim CK, Saijo M, Imai T, Yoshida H, Kabata D, Shintani A, Yuzawa Y, Kondo M. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64:e01897–20. doi: 10.1128/AAC.01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C, Li X, Peng L, Huang D, Zhang J, Zhang S, Wang F, Liu J, Chen L, Chen S, Wang Z, Zhang Z, Cao R, Zhong W, Liu Y, Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020;6:1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kocayiğit H, Özmen Süner K, Tomak Y, Demir G, Yaylacı S, Dheir H, Güçlü E, Erdem AF. Observational study of the effects of favipiravir vs lopinavir/ritonavir on clinical outcomes in critically Ill patients with COVID-19. J Clin Pharm Ther. 2021;46:454–459. doi: 10.1111/jcpt.13305. [DOI] [PubMed] [Google Scholar]
- 96.Yethindra V, Tagaev T, Uulu MS, Parihar Y. Efficacy of umifenovir in the treatment of mild and moderate COVID-19 patients. Int J Pharm Sci Res. 2020;11:506–509. [Google Scholar]
- 97.Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, Lu J, Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81:e21–3. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, Hong Z, Xia J. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81:e1–5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang J, Li H, Du W, Yu P, Guan YY, Ma SY, Liu D, Chen W, Shi GC, Bian XL. Efficacy of early combination therapy with lianhuaqingwen and arbidol in moderate and severe COVID-19 patients: a retrospective cohort study. Front Pharmacol. 2020;11:560209. doi: 10.3389/fphar.2020.560209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen W, Yao M, Fang Z, Lv X, Deng M, Wu Z. A study on clinical effect of Arbidol combined with adjuvant therapy on COVID-19. J Med Virol. 2020;92:2702–2708. doi: 10.1002/jmv.26142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao W, Chen S, Wang K, Chen R, Guo Q, Lu J, Wu X, He Y, Yan Q, Wang S, Wang F, Jin L, Hua J, Li Q. Clinical features and efficacy of antiviral drug, Arbidol in 220 nonemergency COVID-19 patients from East-West-Lake Shelter Hospital in Wuhan: a retrospective case series. Virol J. 2020;17:162. doi: 10.1186/s12985-020-01428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, Ng YY, Lo J, Chan J, Tam AR, Shum HP, Chan V, Wu AK, Sin KM, Leung WS, Law WL, Lung DC, Sin S, Yeung P, Yip CC, Zhang RR, Fung AY, Yan EY, Leung KH, Ip JD, Chu AW, Chan WM, Ng AC, Lee R, Fung K, Yeung A, Wu TC, Chan JW, Yan WW, Chan WM, Chan JF, Lie AK, Tsang OT, Cheng VC, Que TL, Lau CS, Chan KH, To KK, Yuen KY. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang YQ, Tang SQ, Xu XL, Zeng YM, He XQ, Li Y, Harypursat V, Lu YQ, Wan Y, Zhang L, Sun QZ, Sun NN, Wang GX, Yang ZP, Chen YK. No statistically apparent difference in antiviral effectiveness observed among ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate coronavirus disease 2019: results of a randomized, open-labeled prospective study. Front Pharmacol. 2020;11:1071. doi: 10.3389/fphar.2020.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abbaspour Kasgari H, Moradi S, Shabani AM, Babamahmoodi F, Davoudi Badabi AR, Davoudi L, Alikhani A, Hedayatizadeh Omran A, Saeedi M, Merat S, Wentzel H, Garratt A, Levi J, Simmons B, Hill A, Tirgar Fakheri H. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother. 2020;75:3373–3378. doi: 10.1093/jac/dkaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X, Liang Y, Ding X, Tan G, Tang S, Liu L, Liu Y, Pan Y, Wang Z. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020;69:599–606. doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tong S, Su Y, Yu Y, Wu C, Chen J, Wang S, Jiang J. Ribavirin therapy for severe COVID-19: a retrospective cohort study. Int J Antimicrob Agents. 2020;56:106114. doi: 10.1016/j.ijantimicag.2020.106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eslami G, Mousaviasl S, Radmanesh E, Jelvay S, Bitaraf S, Simmons B, Wentzel H, Hill A, Sadeghi A, Freeman J, Salmanzadeh S, Esmaeilian H, Mobarak M, Tabibi R, Jafari Kashi AH, Lotfi Z, Talebzadeh SM, Wickramatillake A, Momtazan M, Hajizadeh Farsani M, Marjani S, Mobarak S. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. J Antimicrob Chemother. 2020;75:3366–3372. doi: 10.1093/jac/dkaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Chambers DC, Yusen RD, Stehlik J International Society for Heart and Lung Transplantation. The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36:1037–1046. doi: 10.1016/j.healun.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 110.Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G. Glucocorticoids and inflammation revisited: the state of the art. NIH clinical staff conference. Neuroimmunomodulation. 2002-2003;10:247–260. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- 111.Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald RD, Koc D, Schneider B, Hammerle AF, Steltzer H. The acute respiratory distress syndrome: definitions, severity and clinical outcome. An analysis of 101 clinical investigations. Intensive Care Med. 1996;22:519–529. doi: 10.1007/BF01708091. [DOI] [PubMed] [Google Scholar]
- 112.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 113.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 114.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 115.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA Saudi critical care trial group. Saudi critical care trial group. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 116.Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D'Aragon F, Duan E, English S, Gossack-Keenan K, Alghuroba M, Szczeklik W, Menon K, Alhazzani W, Sevransky J, Vandvik PO, Annane D, Guyatt G. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Crit Care Med. 2018;46:1411–1420. doi: 10.1097/CCM.0000000000003262. [DOI] [PubMed] [Google Scholar]
- 117.Lian XJ, Huang DZ, Cao YS, Wei YX, Lian ZZ, Qin TH, He PC, Liu YH, Wang SH. Reevaluating the role of corticosteroids in septic shock: an updated meta-analysis of randomized controlled trials. BioMed Res Int. 2019;2019:3175047. doi: 10.1155/2019/3175047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, Martín-Rodríguez C, Díaz-Domínguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Añón JM, Fernández RL, González-Martín JM dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 119.Lewis SR, Pritchard MW, Thomas CM, Smith AF. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7:CD004477. doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP COALITION COVID-19 Brazil iII investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, François B, Aubron C, Ricard JD, Ehrmann S, Jouan Y, Guillon A, Leclerc M, Coffre C, Bourgoin H, Lengellé C, Caille-Fénérol C, Tavernier E, Zohar S, Giraudeau B, Annane D, Le Gouge A CAPE COVID trial group and the CRICS-TriGGERSep network. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients With COVID-19: a randomized clinical trial. JAMA. 2020;324:1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, van Bentum-Puijk W, Berry L, Bhimani Z, Bonten M, Bradbury C, Brunkhorst F, Buxton M, Buzgau A, Cheng AC, de Jong M , Detry M, Estcourt L, Fitzgerald M, Goossens H, Green C, Haniffa R, Higgins AM, Horvat C, Hullegie SJ, Kruger P, Lamontagne F, Lawler PR, Linstrum K, Litton E, Lorenzi E, Marshall J, McAuley D, McGlothin A, McGuinness S, McVerry B, Montgomery S, Mouncey P, Murthy S, Nichol A, Parke R, Parker J, Rowan K, Sanil A, Santos M, Saunders C, Seymour C, Turner A, van de Veerdonk E, Venkatesh B, Zarychanski R, Berry S, Lewis RJ, McArthur C, Webb SA, Gordon AC, Al-Beidh F, Angus D, Annane D, Arabi Y, van Bentum-Puijk W, Berry S, Beane A, Bhimani Z, Bonten M, Bradbury C, Brunkhorst F, Buxton M, Cheng A, De Jong M, Derde L, Estcourt L, Goossens H, Gordon A, Green C, Haniffa R, Lamontagne F, Lawler P, Litton E, Marshall J, McArthur C, McAuley D, McGuinness S, McVerry B, Montgomery S, Mouncey P, Murthy S, Nichol A, Parke R, Rowan K, Seymour C, Turner A, van de Veerdonk F, Webb S, Zarychanski R, Campbell L, Forbes A, Gattas D, Heritier S, Higgins L, Kruger P, Peake S, Presneill J, Seppelt I, Trapani T, Young P, Bagshaw S, Daneman N, Ferguson N, Misak C, Santos M, Hullegie S, Pletz M, Rohde G, Rowan K, Alexander B, Basile K, Girard T, Horvat C, Huang D, Linstrum K, Vates J, Beasley R, Fowler R, McGloughlin S, Morpeth S, Paterson D, Venkatesh B, Uyeki T, Baillie K, Duffy E, Fowler R, Hills T, Orr K, Patanwala A, Tong S, Netea M, Bihari S, Carrier M, Fergusson D, Goligher E, Haidar G, Hunt B, Kumar A, Laffan M, Lawless P, Lother S, McCallum P, Middeldopr S, McQuilten Z, Neal M, Pasi J, Schutgens R, Stanworth S, Turgeon A, Weissman A, Adhikari N, Anstey M, Brant E, de Man A, Lamonagne F, Masse MH, Udy A, Arnold D, Begin P, Charlewood R, Chasse M, Coyne M, Cooper J, Daly J, Gosbell I, Harvala-Simmonds H, Hills T, MacLennan S, Menon D, McDyer J, Pridee N, Roberts D, Shankar-Hari M, Thomas H, Tinmouth A, Triulzi D, Walsh T, Wood E, Calfee C, O'Kane C, Shyamsundar M, Sinha P, Thompson T, Young I, Bihari S, Hodgson C, Laffey J, McAuley D, Orford N, Neto A, Detry M, Fitzgerald M, Lewis R, McGlothlin A, Sanil A, Saunders C, Berry L, Lorenzi E, Miller E, Singh V, Zammit C, van Bentum Puijk W, Bouwman W, Mangindaan Y, Parker L, Peters S, Rietveld I, Raymakers K, Ganpat R, Brillinger N, Markgraf R, Ainscough K, Brickell K, Anjum A, Lane JB, Richards-Belle A, Saull M, Wiley D, Bion J, Connor J, Gates S, Manax V, van der, Reynolds J, van Beurden M, Effelaar E, Schotsman J, Boyd C, Harland C, Shearer A, Wren J, Clermont G, Garrard W, Kalchthaler K, King A, Ricketts D, Malakoutis S, Marroquin O, Music E, Quinn K, Cate H, Pearson K, Collins J, Hanson J, Williams P, Jackson S, Asghar A, Dyas S, Sutu M, Murphy S, Williamson D, Mguni N, Potter A, Porter D, Goodwin J, Rook C, Harrison S, Williams H, Campbell H, Lomme K, Williamson J, Sheffield J, van't Hoff W, McCracken P, Young M, Board J, Mart E, Knott C, Smith J, Boschert C, Affleck J, Ramanan M, D'Souza R, Pateman K, Shakih A, Cheung W, Kol M, Wong H, Shah A, Wagh A, Simpson J, Duke G, Chan P, Cartner B, Hunter S, Laver R, Shrestha T, Regli A, Pellicano A, McCullough J, Tallott M, Kumar N, Panwar R, Brinkerhoff G, Koppen C, Cazzola F, Brain M, Mineall S, Fischer R, Biradar V, Soar N, White H, Estensen K, Morrison L, Smith J, Cooper M, Health M, Shehabi Y, Al-Bassam W, Hulley A, Whitehead C, Lowrey J, Gresha R, Walsham J, Meyer J, Harward M, Venz E, Williams P, Kurenda C, Smith K, Smith M, Garcia R, Barge D, Byrne D, Byrne K, Driscoll A, Fortune L, Janin P, Yarad E, Hammond N, Bass F, Ashelford A, Waterson S, Wedd S, McNamara R, Buhr H, Coles J, Schweikert S, Wibrow B, Rauniyar R, Myers E, Fysh E, Dawda A, Mevavala B, Litton E, Ferrier J, Nair P, Buscher H, Reynolds C, Santamaria J, Barbazza L, Homes J, Smith R, Murray L, Brailsford J, Forbes L, Maguire T, Mariappa V, Smith J, Simpson S, Maiden M, Bone A, Horton M, Salerno T, Sterba M, Geng W, Depuydt P, De Waele J, De Bus L, Fierens J, Bracke S, Reeve B, Dechert W, Chassé M, Carrier FM, Boumahni D, Benettaib F, Ghamraoui A, Bellemare D, Cloutier È, Francoeur C, Lamontagne F, D'Aragon F, Carbonneau E, Leblond J, Vazquez-Grande G, Marten N, Wilson M, Albert M, Serri K, Cavayas A, Duplaix M, Williams V, Rochwerg B, Karachi T, Oczkowski S, Centofanti J, Millen T, Duan E, Tsang J, Patterson L, English S, Watpool I, Porteous R, Miezitis S, McIntyre L, Brochard L, Burns K, Sandhu G, Khalid I, Binnie A, Powell E, McMillan A, Luk T, Aref N, Andric Z, Cviljevic S, Ðimoti R, Zapalac M, Mirković G, Baršić B, Kutleša M, Kotarski V, Vujaklija Brajković A, Babel J, Sever H, Dragija L, Kušan I, Vaara S, Pettilä L, Heinonen J, Kuitunen A, Karlsson S, Vahtera A, Kiiski H, Ristimäki S, Azaiz A, Charron C, Godement M, Geri G, Vieillard-Baron A, Pourcine F, Monchi M, Luis D, Mercier R, Sagnier A, Verrier N, Caplin C, Siami S, Aparicio C, Vautier S, Jeblaoui A, Fartoukh M, Courtin L, Labbe V, Leparco C, Muller G, Nay MA, Kamel T, Benzekri D, Jacquier S, Mercier E, Chartier D, Salmon C, Dequin P, Schneider F, Morel G, L’Hotellier S, Badie J, Berdaguer FD, Malfroy S, Mezher C, Bourgoin C, Megarbane B, Voicu S, Deye N, Malissin I, Sutterlin L, Guitton C, Darreau C, Landais M, Chudeau N, Robert A, Moine P, Heming N, Maxime V, Bossard I, Nicholier TB, Colin G, Zinzoni V, Maquigneau N, Finn A, Kreß G, Hoff U, Friedrich Hinrichs C, Nee J, Pletz M, Hagel S, Ankert J, Kolanos S, Bloos F, Petros S, Pasieka B, Kunz K, Appelt P, Schütze B, Kluge S, Nierhaus A, Jarczak D, Roedl K, Weismann D, Frey A, Klinikum Neukölln V, Reill L, Distler M, Maselli A, Bélteczki J, Magyar I, Fazekas Á, Kovács S, Szőke V, Szigligeti G, Leszkoven J, Collins D, Breen P, Frohlich S, Whelan R, McNicholas B, Scully M, Casey S, Kernan M, Doran P, O'Dywer M, Smyth M, Hayes L, Hoiting O, Peters M, Rengers E, Evers M, Prinssen A, Bosch Ziekenhuis J, Simons K, Rozendaal W, Polderman F, de Jager P, Moviat M, Paling A, Salet A, Rademaker E, Peters AL, de Jonge E, Wigbers J, Guilder E, Butler M, Cowdrey KA, Newby L, Chen Y, Simmonds C, McConnochie R, Ritzema Carter J, Henderson S, Van Der Heyden K, Mehrtens J, Williams T, Kazemi A, Song R, Lai V, Girijadevi D, Everitt R, Russell R, Hacking D, Buehner U, Williams E, Browne T, Grimwade K, Goodson J, Keet O, Callender O, Martynoga R, Trask K, Butler A, Schischka L, Young C, Lesona E, Olatunji S, Robertson Y, José N, Amaro dos Santos Catorze T, de Lima Pereira TNA, Neves Pessoa LM, Castro Ferreira RM, Pereira Sousa Bastos JM, Aysel Florescu S, Stanciu D, Zaharia MF, Kosa AG, Codreanu D, Marabi Y, Al Qasim E, Moneer Hagazy M, Al Swaidan L, Arishi H, Muñoz-Bermúdez R, Marin-Corral J, Salazar Degracia A, Parrilla Gómez F, Mateo López MI, Rodriguez Fernandez J, Cárcel Fernández S, Carmona Flores R, León López R, de la Fuente Martos C, Allan A, Polgarova P, Farahi N, McWilliam S, Hawcutt D, Rad L, O'Malley L, Whitbread J, Kelsall O, Wild L, Thrush J, Wood H, Austin K, Donnelly A, Kelly M, O'Kane S, McClintock D, Warnock M, Johnston P, Gallagher LJ, Mc Goldrick C, Mc Master M, Strzelecka A, Jha R, Kalogirou M, Ellis C, Krishnamurthy V, Deelchand V, Silversides J, McGuigan P, Ward K, O'Neill A, Finn S, Phillips B, Mullan D, Oritz-Ruiz de Gordoa L, Thomas M, Sweet K, Grimmer L, Johnson R, Pinnell J, Robinson M, Gledhill L, Wood T, Morgan M, Cole J, Hill H, Davies M, Antcliffe D, Templeton M, Rojo R, Coghlan P, Smee J, Mackay E, Cort J, Whileman A, Spencer T, Spittle N, Kasipandian V, Patel A, Allibone S, Genetu RM, Ramali M, Ghosh A, Bamford P, London E, Cawley K, Faulkner M, Jeffrey H, Smith T, Brewer C, Gregory J, Limb J, Cowton A, O'Brien J, Nikitas N, Wells C, Lankester L, Pulletz M, Williams P, Birch J, Wiseman S, Horton S, Alegria A, Turki S, Elsefi T, Crisp N, Allen L, McCullagh I, Robinson P, Hays C, Babio-Galan M, Stevenson H, Khare D, Pinder M, Selvamoni S, Gopinath A, Pugh R, Menzies D, Mackay C, Allan E, Davies G, Puxty K, McCue C, Cathcart S, Hickey N, Ireland J, Yusuff H, Isgro G, Brightling C, Bourne M, Craner M, Watters M, Prout R, Davies L, Pegler S, Kyeremeh L, Arbane G, Wilson K, Gomm L, Francia F, Brett S, Sousa Arias S, Elin Hall R, Budd J, Small C, Birch J, Collins E, Henning J, Bonner S, Hugill K, Cirstea E, Wilkinson D, Karlikowski M, Sutherland H, Wilhelmsen E, Woods J, North J, Sundaran D, Hollos L, Coburn S, Walsh J, Turns M, Hopkins P, Smith J, Noble H, Depante MT, Clarey E, Laha S, Verlander M, Williams A, Huckle A, Hall A, Cooke J, Gardiner-Hill C, Maloney C, Qureshi H, Flint N, Nicholson S, Southin S, Nicholson A, Borgatta B, Turner-Bone I, Reddy A, Wilding L, Chamara Warnapura L, Agno Sathianathan R, Golden D, Hart C, Jones J, Bannard-Smith J, Henry J, Birchall K, Pomeroy F, Quayle R, Makowski A, Misztal B, Ahmed I, KyereDiabour T, Naiker K, Stewart R, Mwaura E, Mew L, Wren L, Willams F, Innes R, Doble P, Hutter J, Shovelton C, Plumb B, Szakmany T, Hamlyn V, Hawkins N, Lewis S, Dell A, Gopal S, Ganguly S, Smallwood A, Harris N, Metherell S, Lazaro JM, Newman T, Fletcher S, Nortje J, Fottrell-Gould D, Randell G, Zaman M, Elmahi E, Jones A, Hall K, Mills G, Ryalls K, Bowler H, Sall J, Bourne R, Borrill Z, Duncan T, Lamb T, Shaw J, Fox C, Moreno Cuesta J, Xavier K, Purohit D, Elhassan M, Bakthavatsalam D, Rowland M, Hutton P, Bashyal A, Davidson N, Hird C, Chhablani M, Phalod G, Kirkby A, Archer S, Netherton K, Reschreiter H, Camsooksai J, Patch S, Jenkins S, Pogson D, Rose S, Daly Z, Brimfield L, Claridge H, Parekh D, Bergin C, Bates M, Dasgin J, McGhee C, Sim M, Hay SK, Henderson S, Phull MK, Zaidi A, Pogreban T, Rosaroso LP, Harvey D, Lowe B, Meredith M, Ryan L, Hormis A, Walker R, Collier D, Kimpton S, Oakley S, Rooney K, Rodden N, Hughes E, Thomson N, McGlynn D, Walden A, Jacques N, Coles H, Tilney E, Vowell E, Schuster-Bruce M, Pitts S, Miln R, Purandare L, Vamplew L, Spivey M, Bean S, Burt K, Moore L, Day C, Gibson C, Gordon E, Zitter L, Keenan S, Baker E, Cherian S, Cutler S, Roynon-Reed A, Harrington K, Raithatha A, Bauchmuller K, Ahmad N, Grecu I, Trodd D, Martin J, Wrey Brown C, Arias AM, Craven T, Hope D, Singleton J, Clark S, Rae N, Welters I, Hamilton DO, Williams K, Waugh V, Shaw D, Puthucheary Z, Martin T, Santos F, Uddin R, Somerville A, Tatham KC, Jhanji S, Black E, Dela Rosa A, Howle R, Tully R, Drummond A, Dearden J, Philbin J, Munt S, Vuylsteke A, Chan C, Victor S, Matsa R, Gellamucho M, Creagh-Brown B, Tooley J, Montague L, De Beaux F, Bullman L, Kersiake I, Demetriou C, Mitchard S, Ramos L, White K, Donnison P, Johns M, Casey R, Mattocks L, Salisbury S, Dark P, Claxton A, McLachlan D, Slevin K, Lee S, Hulme J, Joseph S, Kinney F, Senya HJ, Oborska A, Kayani A, Hadebe B, Orath Prabakaran R, Nichols L, Thomas M, Worner R, Faulkner B, Gendall E, Hayes K, Hamilton-Davies C, Chan C, Mfuko C, Abbass H, Mandadapu V, Leaver S, Forton D, Patel K, Paramasivam E, Powell M, Gould R, Wilby E, Howcroft C, Banach D, Fernández de, Cabreros L, White I, Croft M, Holland N, Pereira R, Zaki A, Johnson D, Jackson M, Garrard H, Juhaz V, Roy A, Rostron A, Woods L, Cornell S, Pillai S, Harford R, Rees T, Ivatt H, Sundara Raman A, Davey M, Lee K, Barber R, Chablani M, Brohi F, Jagannathan V, Clark M, Purvis S, Wetherill B, Dushianthan A, Cusack R, de Courcy-Golder K, Smith S, Jackson S, Attwood B, Parsons P, Page V, Zhao XB, Oza D, Rhodes J, Anderson T, Morris S, Xia Le, Thomas A, Keen A, Digby S, Cowley N, Wild L, Southern D, Reddy H, Campbell A, Watkins C, Smuts S, Touma O, Barnes N, Alexander P, Felton T, Ferguson S, Sellers K, Bradley-Potts J, Yates D, Birkinshaw I, Kell K, Marshall N, Carr-Knott L, Summers C. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Safe IP, Borba MGS, Abreu-Netto RL, Maciel ABS, Neto JRS, Oliveira LB, Figueiredo EFG, Dinelly KMO, Rodrigues MGA, Brito M, Mourão MPG, Pivoto João GA, Hajjar LA, Bassat Q, Romero GAS, Naveca FG, Vasconcelos HL, Tavares MA, Brito-Sousa JD, Costa FTM, Nogueira ML, Baía-da-Silva D, Xavier MS, Monteiro WM, Lacerda MVG for the Metcovid Team. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020:ciaa1177. doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruiz-Irastorza G, Pijoan JI, Bereciartua E, Dunder S, Dominguez J, Garcia-Escudero P, Rodrigo A, Gomez-Carballo C, Varona J, Guio L, Ibarrola M, Ugarte A, Martinez-Berriotxoa A Cruces COVID Study Group. Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: an observational comparative study using routine care data. PLoS One. 2020;15:e0239401. doi: 10.1371/journal.pone.0239401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Majmundar M, Kansara T, Lenik JM, Park H, Ghosh K, Doshi R, Shah P, Kumar A, Amin H, Chaudhari S, Habtes I, Pancaldi L, Tedeschi S, Giannella M, Rinaldi M, Bussini L, Valentini I, Ferravante AF, Potalivo A, Marchionni E, Fornaro G, Pascale R, Pasquini Z, Puoti M, Merli M, Barchiesi F, Volpato F, Rubin A, Saracino A, Tonetti T, Gaibani P, Ranieri VM, Viale P, Cristini F PREDICO Study Group. Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York Metropolitan region. PLoS One. 2020;15:e0238827. doi: 10.1371/journal.pone.0238827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bartoletti M, Marconi L, Scudeller L. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin Microbiol Infect. 2021;27:105–111. doi: 10.1016/j.cmi.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Q, Li W, Jin Y, Xu W, Huang C, Li L, Huang Y, Fu Q, Chen L. Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 pneumonia: a retrospective cohort study. Infect Dis Ther. 2020;9:823–836. doi: 10.1007/s40121-020-00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.World Health Organization (WHO) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. WHO: 2020. [Google Scholar]
- 131.Lamontagne F, Rochwerg B, Lytvyn L, Guyatt GH, Møller MH, Annane D, Kho ME, Adhikari NKJ, Machado F, Vandvik PO, Dodek P, Leboeuf R, Briel M, Hashmi M, Camsooksai J, Shankar-Hari M, Baraki MK, Fugate K, Chua S, Marti C, Cohen D, Botton E, Agoritsas T, Siemieniuk RAC. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ. 2018;362:k3284. doi: 10.1136/bmj.k3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 133.Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16:335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J, Bansal A, Srivastava A, Zhou Y, Finkel D, Green A, Mallappallil M, Faugno AJ, Zhang J, Velez JCQ, Shaefi S, Parikh CR, Charytan DM, Athavale AM, Friedman AN, Redfern RE, Short SAP, Correa S, Pokharel KK, Admon AJ, Donnelly JP, Gershengorn HB, Douin DJ, Semler MW, Hernán MA, Leaf DE STOP-COVID Investigators. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sciascia S, Aprà F, Baffa A, Baldovino S, Boaro D, Boero R, Bonora S, Calcagno A, Cecchi I, Cinnirella G, Converso M, Cozzi M, Crosasso P, De Iaco F, Di Perri G, Eandi M, Fenoglio R, Giusti M, Imperiale D, Imperiale G, Livigni S, Manno E, Massara C, Milone V, Natale G, Navarra M, Oddone V, Osella S, Piccioni P, Radin M, Roccatello D, Rossi D. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 141.De Rossi N, Scarpazza C, Filippini C, Cordioli C, Rasia S, Mancinelli CR, Rizzoni D, Romanelli G, Cossi S, Vettoretto N, Bove S, Manfredini S, Beindorf EA, Mosca C, Scipione V, Flamminio G, Albini EA, Giansiracusa P, Capra R Montichiari COVID-19 Study Group. Early use of low dose tocilizumab in patients with COVID-19: a retrospective cohort study with a complete follow-up. EClinicalMedicine. 2020;25:100459. doi: 10.1016/j.eclinm.2020.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A, Baldissera E, Rovere-Querini P, Ruggeri A, Monti G, De Cobelli F, Zangrillo A, Tresoldi M, Castagna A, Dagna L TOCI-RAF Study Group. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kewan T, Covut F, Al-Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID-19: A retrospective cohort study. EClinicalMedicine. 2020;24:100418. doi: 10.1016/j.eclinm.2020.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo G, Orlando G, Borghi V, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Corradi L, Fantini R, Castaniere I, Tabbì L, Girardis M, Tedeschi S, Giannella M, Bartoletti M, Pascale R, Dolci G, Brugioni L, Pietrangelo A, Cossarizza A, Pea F, Clini E, Salvarani C, Massari M, Viale PL, Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodríguez-Baño J, Pachón J, Carratalà J, Ryan P, Jarrín I, Yllescas M, Arribas JR, Berenguer J SAM-COVID Study Group. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin Microbiol Infect. 2021;27:244–252. doi: 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, Sinclaire BA, Bednarz U, Marafelias M, Hansen E, Siegel DS, Goy AH, Pecora AL, Sawczuk IS, Koniaris LS, Simwenyi M, Varga DW, Tank LK, Stein AA, Allusson V, Lin GS, Oser WF, Tuma RA, Reichman J, Brusco L, Jr, Carpenter KL, Costanzo EJ, Vivona V, Goldberg SL. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chilimuri S, Sun H, Alemam A, Kang KS, Lao P, Mantri N, Schiller L, Sharabun M, Shehi E, Tejada J, Yugay A, Nayudu SK. Tocilizumab use in patients with moderate to severe COVID-19: a retrospective cohort study. J Clin Pharm Ther. 2021;46:440–446. doi: 10.1111/jcpt.13303. [DOI] [PubMed] [Google Scholar]
- 148.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK BACC bay tocilizumab trial investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, Savic S, Youngstein T, Del Sorbo L, Cubillo Gracian A, De La Zerda DJ, Ustianowski A, Bao M, Dimonaco S, Graham E, Matharu B, Spotswood H, Tsai L, Malhotra A. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021:NEJMoa2028700. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roche. Roche's phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia. [Accessed 8 December 2020]. Available at: https://www.roche.com/media/releases/med-cor-2020-09-18.htm.
- 151.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, Bruzzi P, Boni F, Braglia L, Turrà C, Ballerini PF, Sciascia R, Zammarchi L, Para O, Scotton PG, Inojosa WO, Ravagnani V, Salerno ND, Sainaghi PP, Brignone A, Codeluppi M, Teopompi E, Milesi M, Bertomoro P, Claudio N, Salio M, Falcone M, Cenderello G, Donghi L, Del Bono V, Colombelli PL, Angheben A, Passaro A, Secondo G, Pascale R, Piazza I, Facciolongo N, Costantini M RCT-TCZ-COVID-19 Study Group. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P CORIMUNO-19 collaborative group. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang D, Fu B, Peng Z, Yang D, Han M, Li M, Yang Y, Yang T, Sun L, Li W, Shi W, Yao X, Ma Y, Xu F, Wang X, Chen J, Xia D, Sun Y, Dong L, Wang J, Zhu X, Zhang M, Zhou Y, Pan A, Hu X, Mei X, Wei H, Xu X. Tocilizumab in patients with moderate or severe COVID-19: a randomized, controlled, open-label, multicenter trial. Front Med. 2021 doi: 10.1007/s11684-020-0824-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martínez-Sanz J, Muriel A, Ron R, Herrera S, Pérez-Molina JA, Moreno S, Serrano-Villar S. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicentre cohort study. Clin Microbiol Infect. 2021;27:238–243. doi: 10.1016/j.cmi.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boregowda U, Perisetti A, Nanjappa A, Gajendran M, Kutti Sridharan G, Goyal H. Addition of tocilizumab to the standard of care reduces mortality in severe COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2020;7:586221. doi: 10.3389/fmed.2020.586221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aziz M, Haghbin H, Abu Sitta E, Nawras Y, Fatima R, Sharma S, Lee-Smith W, Duggan J, Kammeyer JA, Hanrahan J, Assaly R. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93:1620–1630. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 157.Berardicurti O, Ruscitti P, Ursini F, D'Andrea S, Ciaffi J, Meliconi R, Iagnocco A, Cipriani P, Giacomelli R. Mortality in tocilizumab-treated patients with COVID-19: a systematic review and meta-analysis. Clin Exp Rheumatol. 2020;38:1247–1254. [PubMed] [Google Scholar]
- 158.Kotak S, Khatri M, Malik M, Malik M, Hassan W, Amjad A, Malik F, Hassan H, Ahmed J, Zafar M. Use of tocilizumab in COVID-19: a systematic review and meta-analysis of current evidence. Cureus. 2020;12:e10869. doi: 10.7759/cureus.10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, Altannir Y, Al-Tannir M, Tleyjeh R, Hassett L, Kashour T. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:215–227. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Montesarchio V, Parrela R, Iommelli C, Bianco A, Manzillo E, Fraganza F, Palumbo C, Rea G, Murino P, De Rosa R, Atripaldi L, D'Abbraccio M, Curvietto M, Mallardo D, Celentano E, Grimaldi AM, Palla M, Trojaniello C, Vitale MG, Million-Weaver SL, Ascierto PA. Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. J Immunother Cancer. 2020;8:e001089. doi: 10.1136/jitc-2020-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Campbell L, Chen C, Bhagat SS, Parker RA, Östör AJ. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2011;50:552–562. doi: 10.1093/rheumatology/keq343. [DOI] [PubMed] [Google Scholar]
- 163.Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, Dudley RA, Tignanelli CJ. Immunomodulation in COVID-19. Lancet Respir Med. 2020;8:544–546. doi: 10.1016/S2213-2600(20)30226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan Din C, Boffini N, Tomelleri A, Farina N, Ruggeri A, Rovere-Querini P, Di Lucca G, Martinenghi S, Scotti R, Tresoldi M, Ciceri F, Landoni G, Zangrillo A, Scarpellini P, Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–31. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, Sacco E, Naccache JM, Bézie Y, Laplanche S, Le Berre A, Le Pavec J, Salmeron S, Emmerich J, Mourad JJ, Chatellier G, Hayem G. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Langer-Gould A, Smith JB, Gonzales EG, Castillo RD, Figueroa JG, Ramanathan A, Li BH, Gould MK. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis. 2020;99:291–297. doi: 10.1016/j.ijid.2020.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cauchois R, Koubi M, Delarbre D, Manet C, Carvelli J, Blasco VB, Jean R, Fouche L, Bornet C, Pauly V, Mazodier K, Pestre V, Jarrot PA, Dinarello CA, Kaplanski G. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A. 2020;117:18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, Malhotra P, Bloom O, Liu A, Lesser ML, Hajizadeh N Northwell COVID-19 research consortium. Comparative survival analysis of immunomodulatory therapy for coronavirus disease 2019 cytokine storm. Chest. 2021;159:933–948. doi: 10.1016/j.chest.2020.09.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clementi N, Ferrarese R, Criscuolo E, Diotti RA, Castelli M, Scagnolari C, Burioni R, Antonelli G, Clementi M, Mancini N. Interferon-β-1a inhibition of severe acute respiratory syndrome-coronavirus 2 in vitro when administered after virus infection. J Infect Dis. 2020;222:722–725. doi: 10.1093/infdis/jiaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Philippot Q, Rosain J, Béziat V, Manry J, Shaw E, Haljasmägi L, Peterson P, Lorenzo L, Bizien L, Trouillet-Assant S, Dobbs K, de Jesus AA, Belot A, Kallaste A, Catherinot E, Tandjaoui-Lambiotte Y, Le Pen J, Kerner G, Bigio B, Seeleuthner Y, Yang R, Bolze A, Spaan AN, Delmonte OM, Abers MS, Aiuti A, Casari G, Lampasona V, Piemonti L, Ciceri F, Bilguvar K, Lifton RP, Vasse M, Smadja DM, Migaud M, Hadjadj J, Terrier B, Duffy D, Quintana-Murci L, van de, Roussel L, Vinh DC, Tangye SG, Haerynck F, Dalmau D, Martinez-Picado J, Brodin P, Nussenzweig MC, Boisson-Dupuis S, Rodríguez-Gallego C, Vogt G, Mogensen TH, Oler AJ, Gu J, Burbelo PD, Cohen JI, Biondi A, Bettini LR, D'Angio M, Bonfanti P, Rossignol P, Mayaux J, Rieux-Laucat F, Husebye ES, Fusco F, Ursini MV, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Castagnoli R, Montagna D, Licari A, Marseglia GL, Duval X, Ghosn J HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort. Tsang JS, Goldbach-Mansky R, Kisand K, Lionakis MS, Puel A, Zhang SY, Holland SM, Gorochov G, Jouanguy E, Rice CM, Cobat A, Notarangelo LD, Abel L, Su HC, Casanova JL. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, Ogishi M, Sabli IKD, Hodeib S, Korol C, Rosain J, Bilguvar K, Ye J, Bolze A, Bigio B, Yang R, Arias AA, Zhou Q, Zhang Y, Onodi F, Korniotis S, Karpf L, Philippot Q, Chbihi M, Bonnet-Madin L, Dorgham K, Smith N, Schneider WM, Razooky BS, Hoffmann HH, Michailidis E, Moens L, Han JE, Lorenzo L, Bizien L, Meade P, Neehus AL, Ugurbil AC, Corneau A, Kerner G, Zhang P, Rapaport F, Seeleuthner Y, Manry J, Masson C, Schmitt Y, Schlüter A, Le Voyer T, Khan T, Li J, Fellay J, Roussel L, Shahrooei M, Alosaimi MF, Mansouri D, Al-Saud H, Al-Mulla F, Almourfi F, Al-Muhsen SZ, Alsohime F, Al Turki S, Hasanato R, van de Beek B, Biondi A, Bettini LR, D'Angio M, Bonfanti P, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Oler AJ, Tompkins MF, Alba C, Vandernoot I, Goffard JC, Smits G, Migeotte I, Haerynck F, Soler-Palacin P, Martin-Nalda A, Colobran R, Morange PE, Keles S, Çölkesen F, Ozcelik T, Yasar KK, Senoglu S, Karabela ŞN, Rodríguez-Gallego C, Novelli G, Hraiech S, Tandjaoui-Lambiotte Y, Duval X, Laouénan C COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group. Snow AL, Dalgard CL, Milner JD, Vinh DC, Mogensen TH, Marr N, Spaan AN, Boisson B, Boisson-Dupuis S, Bustamante J, Puel A, Ciancanelli MJ, Meyts I, Maniatis T, Soumelis V, Amara A, Nussenzweig M, García-Sastre A, Krammer F, Pujol A, Duffy D, Lifton RP, Zhang SY, Gorochov G, Béziat V, Jouanguy E, Sancho-Shimizu V, Rice CM, Abel L, Notarangelo LD, Cobat A, Su HC, Casanova JL. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Food and Drug Administration (FDA) Interferon alpha-2b (Intron® A) [Accessed 8 December 2020]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103132Orig1s5199lbl.pdf.
- 173.Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, Kazemzadeh H, Yekaninejad MS. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64:e01061–20. doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.National Institutes of Health (NIH) Ongoing clinical trials for interferon and COVI-19. [Accessed 8 December 2020]. Available at: https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/interferons/
- 175.Food and Drug Administration (FDA) Interferon alpha-2b (Intron® A) [Accessed 8 December 2020]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103132Orig1s5199lbl.pdf.
- 176.Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, Gabbay FJ, Davies DE, Holgate ST, Ho LP, Clark T, Djukanovic R, Wilkinson TMA Inhaled Interferon Beta COVID-19 Study Group. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9:196–206. doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mo Y, Fisher D. A review of treatment modalities for Middle East respiratory syndrome. J Antimicrob Chemother. 2016;71:3340–3350. doi: 10.1093/jac/dkw338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sahr F, Ansumana R, Massaquoi TA, Idriss BR, Sesay FR, Lamin JM, Baker S, Nicol S, Conton B, Johnson W, Abiri OT, Kargbo O, Kamara P, Goba A, Russell JB, Gevao SM. Evaluation of convalescent whole blood for treating ebola virus disease in freetown, Sierra Leone. J Infect. 2017;74:302–309. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Kong Y, Ren L, Wei Q, Mei H, Hu C, Tao C, Yang R, Wang J, Yu Y, Guo Y, Wu X, Xu Z, Zeng L, Xiong N, Chen L, Wang J, Man N, Liu Y, Xu H, Deng E, Zhang X, Li C, Wang C, Su S, Zhang L, Wang J, Wu Y, Liu Z. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P PLACID Trial Collaborators. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Avendaño-Solà C, Ramos-Martínez A, Muñez-Rubio E, Ruiz-Antorán B, Malo de, Torres F, Fernández-Cruz A, Callejas-Díaz A, Calderón J, Payares-Herrera C, Salcedo I, Romera I, Lora-Tamayo J, Mancheño-Losa M, Paciello ML, Villegas C, Estrada V, Saez-Serrano I, Porras-Leal ML, Jarilla-Fernández MC, Paño-Pardo JR, Moreno-Chulilla JA, Arrieta-Aldea I, Bosch A, Belhassen-Garcia M, López-Villar O, Ramos-Garrido A, Blanco L, Madrigal-Sánchez ME, Contreras E, Muñiz-Díaz E, Domingo-Morera JM, Casas-Flecha I, Pérez-Olmeda M, Garcia-Pérez J, Alcamí J, Bueno JL, Duarte RF for the ConPlas-19 Study Group. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. MedRxiv. 2020 [Epub ahead of print] [Google Scholar]
- 183.Gharbharan A, Jordans CC, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FP, Stalenhoef JE, Dofferhoff A, Ludwig I, Koster A, Hassing RJ, Bos JC, van Pottelberge GR, Vlasveld IN, Ammerlaan HSM, van Leeuwen-Segarceanu EM, Miedema J, van der Eerden M, Papageorgiou G, te Boekhorst P, Swaneveld FH, Katsikis PD, Mueller Y, Okba NMA, Koopmans MPG, Haagmans BHG, Rokx C, Rijnders BJA. Convalescent plasma for COVID-19. A randomized clinical trial. MEDRxiv. 2020 [Epub ahead of print] [Google Scholar]
- 184.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, Savoy N, Giunta DH, Pérez LG, Sánchez MDL, Gamarnik AV, Ojeda DS, Santoro DM, Camino PJ, Antelo S, Rainero K, Vidiella GP, Miyazaki EA, Cornistein W, Trabadelo OA, Ross FM, Spotti M, Funtowicz G, Scordo WE, Losso MH, Ferniot I, Pardo PE, Rodriguez E, Rucci P, Pasquali J, Fuentes NA, Esperatti M, Speroni GA, Nannini EC, Matteaccio A, Michelangelo HG, Follmann D, Lane HC, Belloso WH PlasmAr Study Group. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, Wiggins CC, Senefeld JW, Klompas AM, Hodge DO, Shepherd JRA, Rea RF, Whelan ER, Clayburn AJ, Spiegel MR, Baker SE, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Herasevich V, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, van Buskirk CM, Winters JL, Stubbs JR, van Helmond N, Butterfield BP, Sexton MA, Diaz Soto JC, Paneth NS, Verdun NC, Marks P, Casadevall A, Fairweather D, Carter RE, Wright RS. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95:1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, Jeong SJ, Kim JH, Ku NS, Yeom JS, Roh J, Ahn MY, Chin BS, Kim YS, Lee H, Yong D, Kim HO, Kim S, Choi JY. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci. 2020;35:e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. Correction to: The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20:895. doi: 10.1186/s12879-020-05507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sakoulas G, Geriak M, Kullar R, Greenwood KL, Habib M, Vyas A, Ghafourian M, Dintyala VNK, Haddad F. Intravenous immunoglobulin (IVIG) significantly reduces respiratory morbidity in COVID-19 pneumonia: a prospective randomized trial. medRxiv. 2020 doi: 10.1097/CCE.0000000000000280. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shao Z, Feng Y, Zhong L, Xie Q, Lei M, Liu Z, Wang C, Ji J, Liu H, Gu Z, Hu Z, Su L, Wu M, Liu Z. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9:e1192. doi: 10.1002/cti2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;9:1299. doi: 10.3389/fimmu.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Guideline Korean version.