Abstract
Background
Inflammatory biomarkers have been reported to be associated with anticancer drug efficacy in various cancers. This study aimed to investigate the associations between baseline inflammatory biomarkers or dynamics of neutrophil-to-lymphocyte ratio (NLR) and treatment outcomes of lenvatinib in ATC.
Methods
Twenty ATC patients whose complete blood count were available were included in this study. Patients characteristics, overall survival (OS), and the associations between baseline inflammatory biomarkers or dynamics of NLR and treatment outcomes of lenvatinib were investigated.
Results
All 20 patients had a median baseline NLR of 4.5 (range, 1.4–19.7), a median platelet-to-lymphocyte ratio (PLR) of 169.9 (range, 66.8–671.1), and a median lymphocyte-to-monocyte ratio (LMR) of 2.6 (range, 0.5–5.5). The median OS was 4.2 (95% CI: 1.1–10.3) months in patients with baseline NLR ≤4.5 and 3.1 (95% CI: 1.1–8.3) months in patients with baseline NLR >4.5 (P=0.681). The median OS was 4.2 (95% CI: 1.1–7.8) months in patients with baseline PLR ≤169.9 and 3.9 (95% CI: 0.6–8.3) months in patients with baseline PLR >169.9 (P=0.822). The median OS was 3.7 (95% CI: 1.1–9.8) months in patients with baseline LMR ≤2.6 and 4.2 (95% CI: 0.6–5.4) months in patients with baseline LMR >2.6 (P=0.421). NLR was increased more than the standard deviation of the baseline NLR after lenvatinib initiation in two of 16 patients with follow-up NLR data available. The median OS was 2.0 (95% CI: 1.1– not estimable) months in the increased group but was 5.3 (95% CI: 3.1–9.8) months in the non-increased group (P=0.003).
Conclusions
There was seemed to be no association between prognosis or treatment efficacy of lenvatinib and baseline inflammatory biomarker values in our cases with ATC. However, we possibly estimate prognosis for ATC during lenvatinib treatment by observing the dynamics of NLR.
Keywords: Anaplastic thyroid carcinoma (ATC), inflammation, lenvatinib
Introduction
Anaplastic thyroid carcinoma (ATC), which is composed of 1–2% of all thyroid carcinomas, has very poor prognosis and, there is no established effective treatment strategy (1,2).
Lenvatinib inhibits various signal receptors, and showed a good efficacy in global phase III study for differentiated thyroid carcinoma (DTC) patients (3). Additionally, lenvatinib is also used for ATC patients in Japan since it had a significantly antitumor effect (4). We previously reported that patients with ATC treated with lenvatinib had a good response, disease control rate, and overall survival (OS) rate (5).
Inflammatory reactions have been reported to be associated with tumor progression, and also affects immune surveillance (6). The neutrophil-to-lymphocyte ratio (NLR), which is considered to be as a systemic marker of inflammation, and the dynamics of NLR have been reported to be associate with prognosis or treatment efficacy in various cancers (7-11). Other inflammatory biomarkers, such as platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR), have been also reported to be associated with cancer prognosis (10,12). Yamazaki et al. revealed that the dynamics of NLR have association of prognosis in ATC patients (13). Additionally, Fukuda et al. reported that the baseline NLR value is a potential prognostic marker (14).
We conducted this study to investigate the associations between baseline inflammatory values and dynamics of NLR, and treatment outcomes of lenvatinib for ATC in our institution. We present the following article in accordance with STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-871).
Methods
Subjects
The medical records of ATC patients treated with lenvatinib from May 2015 to December 2019 were retrospectively reviewed. Baseline patient characteristics, blood test, efficacy of lenvatinib, and survival were subsequently collected for analysis. This study included patients with age of 18 or older, confirmed ATC by histopathological examination, whose baseline complete blood count (CBC) data were available. The exclusion criterion was double primary carcinoma at diagnosis. Four ATC patients were excluded because of unavailability of baseline CBC data. Ultimately, 20 patients with ATC were included in this study. All patients had no comorbidities such as autoimmune disease, and did not take a medicine such as steroid and other immunosuppressant. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol of this retrospective study was approved by the Institutional Review Board of Kanagawa Cancer Center (IRB approval no. 2019-146) and the need for informed consent was waived due to the retrospective nature of this study.
Definitions
The cancer staging used the latest American Joint Committee on Cancer tumor-node-metastasis staging system (15). Baseline CBC data were obtained while the patients were in a stable condition, which had no other inflammatory factor such as infection, before undergoing surgery or core needle biopsy for ATC. The Response Evaluation Criteria in Solid Tumors, version 1.1 classification scheme was used for assessing the efficacy of lenvatinib (16). NLR, PLR, and LMR was calculated like previous study (13). OS was calculated as the duration from the point of starting lenvatinib to the date of death from any cause.
Outcomes
Baseline inflammatory biomarkers and OS
The median baseline NLR was 4.5, PLR was 169.9, and LMR was 2.6 in all 20 patients. We subclassified patients into the two groups based on a median baseline value of each inflammatory markers, and compared OS between high and low value groups.
Dynamics of NLR after treatment with lenvatinib and OS
In 16 patients with ATC with follow-up NLR data available, we collected NLR data of the first two months after initiation of lenvatinib, because first image assessment timing was two months after initiation of lenvatinib previous trial (3). We investigated the dynamics of NLR between baseline and after treatment of lenvatinib. The post-treatment NLR minus NLR at baseline was defined as the difference in NLR. Increased NLR was defined as an NLR increase of more than the standard deviation of the baseline NLR (17). With this in mind, we subclassified the 16 patients with ATC into an increased group and a non-increased group, then compared OS between the two.
Statistical analysis
All statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (18). Continuous variables were compared using the Mann-Whitney U test. An OS curve was constructed using the Kaplan-Meier method. A P value of less than 0.05 was considered to be statistically significant. None of the patients were lost to follow-up.
Results
Baseline patient characteristics
Table 1 shows patient characteristics. This study included nine men and 11 women with an average age of 73.6±9.0 years. All patients had a mean baseline NLR of 6.1±5.3 with a median of 4.5 (range, 1.4–19.7), mean baseline PLR of 215.6±142.6 with a median of 169.9 (range, 66.8–671.1), and mean baseline LMR of 2.7±1.2 with a median of 2.6 (range, 0.5–5.5). No significant differences in baseline inflammatory biomarkers were observed between ATC patients whose primary thyroid tumor was resectable (n=6) and unresectable (n=14) [median NLR: resectable, 4.9 (range, 1.8–19.7) vs. unresectable, 4.4 (range, 1.4–16.7); P=0.509, median PLR: resectable, 230.3 (range, 66.–671.1) vs. unresectable, 164.4 (range, 68.2–377.6); P=0.547, median LMR: resectable, 2.7 (range, 0.5–4.1) vs. unresectable, 2.6 (range, 1.6–5.5); P=0.901].
Table 1. Baseline characteristics of the study participants.
| Characteristics | Total (n=20) |
|---|---|
| Sex | |
| Male | 9 |
| Female | 11 |
| Age (years), mean ± SD | 73.6±9.0 |
| Performance status | |
| 0, 1 | |
| ≥2 | |
| WBC count (/μL), mean ± SD | 11,090±5,067 |
| Neutrophil (%), mean ± SD | 71.0±11.1 |
| Lymphocyte (%), mean ± SD | 18.0±9.4 |
| Monocyte (%), mean ± SD | 6.7±2.0 |
| Platelet (×104/μL), mean ± SD | 29.7±10.0 |
| NLR | |
| Mean ± SD | 6.1±5.3 |
| Median (range) | 4.5 (1.4–19.7) |
| PLR | |
| Mean ± SD | 215.6±142.6 |
| Median (range) | 169.9 (66.8–671.1) |
| LMR | |
| Mean ± SD | 2.7±1.2 |
| Median (range) | 2.6 (0.5–5.5) |
| Initial dose (mg) | |
| 24 | 12 |
| ≤20 | 8 |
| Best clinical response | |
| Complete response | 0 |
| Partial response | 2 |
| Stable disease | 7 |
| Progressive disease | 7 |
| Not evaluable | 4 |
LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SD, standard deviation; WBC, white blood cell.
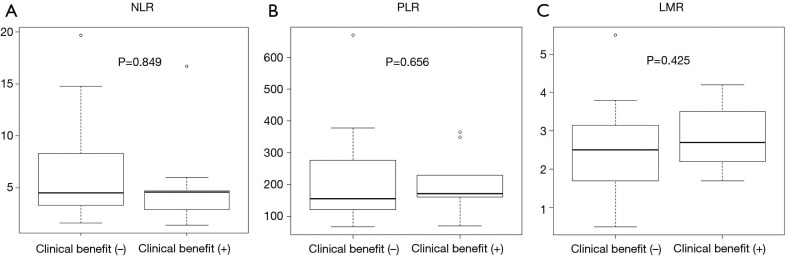
The patients with or without clinical benefit of lenvatinib (complete response, partial response, or stable disease) showed median OS lengths of 7.6 (95% CI: 2.3–10.3) and 2.8 (95% CI: 1.1–4.7) months, respectively (P=0.042) (Figure 1). Although patients who received the clinical benefit of lenvatinib achieved better OS than those who did not, baseline inflammatory markers were not significantly different between the two populations (Figure 2).
Figure 1.
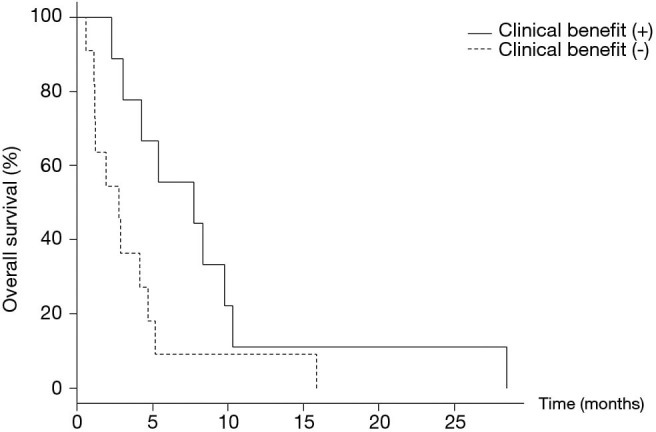
Overall survival based on the clinical benefit of lenvatinib. The study participants with or without a clinical benefit of lenvatinib (stable disease or partial response) had a median overall survival of 7.6 [95% confidence interval (CI): 2.3–10.3] and 2.8 [95% CI: 1.1–4.7] months, respectively (P=0.042).
Figure 2.
Association between baseline inflammatory biomarker values and clinical benefit of lenvatinib. No significant differences in baseline neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) were observed between study participants with and those without a clinical benefit (complete response, partial response, and stable disease) of lenvatinib [(A) median NLR: with clinical benefit: 4.6 (range, 1.4–16.7) vs. without clinical benefit: 4.5 (range, 1.6–19.7), P=0.849; (B) median PLR: with clinical benefit: 171.7 (range, 69.6–365.1) vs. without clinical benefit: 155.7 (range, 66.8–671.1), P=0.656; (C) median LMR: with clinical benefit: 2.7 (range, 1.7–4.2) vs. without clinical benefit: 2.5 (range, 0.5–5.5), P=0.425].
Baseline inflammatory markers and OS
Baseline NLR and OS
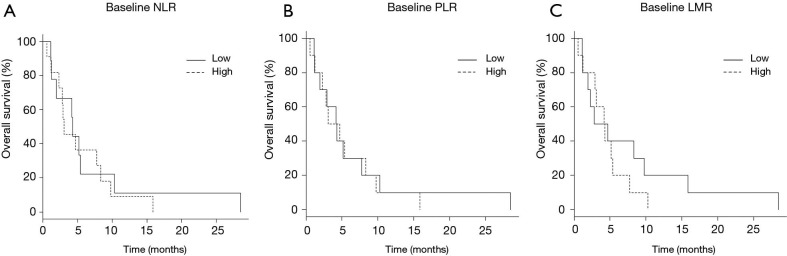
The median OS was 4.2 (95% CI: 1.1–10.3) months in patients with baseline NLR ≤4.5 and 3.1 (95% CI: 1.1–8.3) months in patients with baseline NLR >4.5, respectively (P=0.681) (Figure 3A). The median OS was 4.2 (95% CI: 1.1–7.8) months in patients with baseline PLR ≤169.9 and 3.9 (95% CI: 0.6–8.3) months in patients with baseline PLR >169.9, respectively, (P=0.822) (Figure 3B). Finally, the median OS was 3.7 (95% CI: 1.1–9.8) months in patients with baseline LMR ≤2.6 and 4.2 (95% CI: 0.6–5.4) months in patients with baseline NLR >2.6, respectively (P=0.421) (Figure 3C). There were no significant differences in OS between each group by baseline inflammatory biomarker value.
Figure 3.
Overall survival based on the baseline inflammatory biomarker values. (A) The median overall survival (OS) was 4.2 [95% confidence interval (CI): 1.1–10.3] months in patients with baseline neutrophil-to-lymphocyte ratio (NLR) ≤4.5 and 3.1 (95% CI: 1.1–8.3) months in patients with baseline NLR >4.5, respectively (P=0.681). (B) The median OS was 4.2 (95% CI: 1.1–7.8) months in patients with baseline platelet-to-lymphocyte ratio (PLR) ≤169.9 and 3.9 (95% CI: 0.6–8.3) months in patients with baseline PLR >169.9, respectively, (P=0.822) [with baseline NLR >2.6, respectively (P=0.421) (C)].
Dynamics of NLR after treatment of lenvatinib and OS
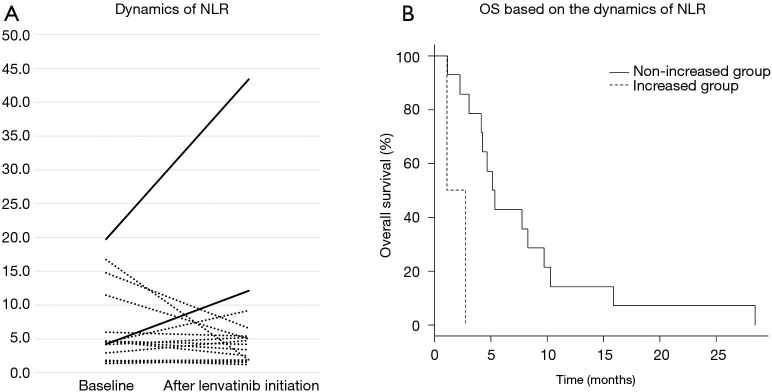
Table 2 shows CBC and inflammatory biomarkers after initiation of lenvatinib. Separately, Figure 4A displays the dynamics of NLR between baseline and after the initiation of lenvatinib, where NLR was increased more than the standard deviation of the baseline NLR after lenvatinib initiation in two of 16 patients with ATC. The median OS was 2.0 (95% CI: 1.1– not estimable) months in the increased group but was 5.3 (95% CI: 3.1–9.8) months in the non-increased group (P=0.003) (Figure 4B).
Table 2. Complete blood count and inflammatory biomarkers data after initiation of lenvatinib.
| Total (n=16) | |
|---|---|
| WBC count (/μL), mean ± SD | 8,868±4,561 |
| Neutrophil (%), mean ± SD | 67.8±13.0 |
| Lymphocyte (%), mean ± SD | 19.4±10.8 |
| Monocyte (%), mean ± SD | 6.1±2.6 |
| Platelet (×104/μL), mean ± SD | 18.7±14.3 |
| NLR | |
| Mean ± SD | 6.9±10.2 |
| Median (range) | 4.5 (1.2–43.4) |
| PLR | |
| Mean ± SD | 152.6±114.7 |
| Median (range) | 118.1 (43.0–430.3) |
| LMR | |
| Mean ± SD | 3.5±1.7 |
| Median (range) | 3.6 (1.0–7.0) |
LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SD, standard deviation; WBC, white blood cell.
Figure 4.
Dynamics of neutrophil-to-lymphocyte ratio after lenvatinib initiation and overall survival. (A) The solid lines show patients with increased neutrophil-to-lymphocyte (NLR) values and the dotted lines show patients with non-increased NLR values after the initiation of lenvatinib. Increased NLR was defined as an NLR increase of more than the standard deviation of the baseline NLR. (B) The median overall survival (OS) was 2.0 [95% confidence interval (CI): 1.1– not estimable] months in the increased group but was 5.3 (95% CI: 3.1–9.8) months in the non-increased group (P=0.003).
Discussion
This study investigated the association between inflammatory biomarkers and treatment outcomes of lenvatinib in ATC. There were no significant differences in OS by baseline inflammatory biomarker values recorded during lenvatinib treatment for ATC. However, patients whose NLR values increased during lenvatinib treatment experienced a worse prognosis.
It has been reported that a median NLR in DTC patients is from 1.57 to 2.44 (19-21). Meanwhile, poorly differentiated thyroid carcinoma and ATC patients probably have higher NLR than DTC (19). The patients with ATC included in this study had a mean and median NLR values of 6.1 and 4.5, respectively. These values were as high as previous reports (4,19). In DTC and medullary thyroid carcinoma, NLR or PLR have been reported to be associated with advanced stage, recurrence, treatment efficacy, and metastases (7,22-24). However, there were no reports on association between PLR and lenvatinib efficacy in ATC, and this is the first study.
Elsewhere, high baseline NLR has been reported to be associated with poor response rate in anticancer drug therapy (25-27). Similarly, higher baseline NLR was reported to be associated with poor prognosis in lenvatinib treatment for ATC (14). However, our study did not show a significant association between baseline inflammatory biomarkers, including NLR, and treatment outcome of lenvatinib. We think that the different cutoff value caused these results. In previous report, the NLR cutoff value was determined by using receiver operating characteristic curve analysis of the disease control rate. On the other hand, we chose the median NLR for cutoff value in this study. Further case accumulation is needed to determine properly cutoff value of NLR for ATC.
In a phase II open-label study of lenvatinib for ATC, the median PFS was 7.4 months, the median OS was 10.6 months, and the objective response rate was 24% (28). In our study, the response rate was 10%, and the patients who received the clinical benefit of lenvatinib tended to show better OS. However, there was no significant difference in baseline inflammatory biomarker values between patients with and without a resulting clinical benefit of lenvatinib. Therefore, at least in this study, it seemed to be difficult to accurately predict the association of OS or treatment efficacy of lenvatinib in patients with ATC using baseline inflammatory biomarker values. We have previously examined the association between the treatment efficacy of lenvatinib and the expression of target receptors in ATC tissues, and reported that the fibroblast growth factor receptor 4 expression was associated with treatment efficacy (29,30). Additionally, the same association has also been shown in hepatocellular carcinoma (HCC) (31). Therefore, the response to lenvatinib might be influenced not only by baseline inflammatory biomarkers but also the expression of target receptors. In this study, there was no association between baseline PLR value and treatment efficacy though lenvatinib inhibits platelet-derived growth factor receptor α (PDGFRα). One possible reason is that lenvatinib shows antitumor effect by mainly inhibiting vascular endothelial growth factor 2 (29). Another possible reason is that the expression of PDGFRα in tumor tissue may be more correlated with efficacy than PLR value. There is no report on the expression of PDGFRα and lenvatinib treatment, and further study is needed to investigate this association.
Not only baseline NLR but also the dynamics of NLR have been reported to be associated with treatment efficacy. Moschetta et al. postulated that increased NLR after immunotherapy had a negative effect on PFS in clinical trials studying various cancers (9). In advanced biliary tract cancer, high NLR with increased NLR after chemotherapy was associated with worse OS and PFS, and it was suggested that systemic inflammation represented by NLR might predict treatment outcomes in such patients (10). In non-small cell lung cancer, during nivolumab therapy, the early dynamics of NLR were considered to be associated with the late efficacy of subsequent salvage chemotherapy (11). In this study, NLR was increased more than the standard deviation of the baseline NLR after lenvatinib initiation in two of 16 patients, and the median OS of the increased group was worse than that of the non-increased group. Therefore, NLR dynamics in the treatment of lenvatinib for ATC may be associated with efficacy as well as other carcinomas or other anticancer drug therapies. Nakano et al. evaluated the changes in the NLR before and one month after molecular-targeted treatment which included lenvatinib and reported that HCC patients with decreased neutrophil-to-lymphocyte ratio survived significantly longer than patients with increased neutrophil-to-lymphocyte ratio (32). In our study, we collected NLR data of the first two months after initiation of lenvatinib based on previous trial, but the trial included only DTCs. Since ATCs progress rapidly compared with DTC, we may need to evaluate the change of NLR at shorter interval like HCC. Further prospective study is needed to determine the appropriate evaluation interval.
In DTC, thyroglobulin can be a marker for tumor progression, and it is indicated that the reduction of thyroglobulin may be a marker of response in lenvatinib therapy (33,34). However, ATC has no useful marker for assessing tumor progression like DTC. Our findings suggest that NLR was increased in two of 16 patients, and these patients had tended to have worse OS like previous report (13). Therefore, there was the possibility that NLR increase reflects treatment resistance to lenvatinib in ATCs. If the dynamics of NLR are associated with disease progression in ATC, NLR may be clinically useful for conducting follow-up during lenvatinib treatment because CBC can be routinely performed in clinical practice.
The present study has some limitations worth noting. First, this was a single-center retrospective investigation that included a small number of patients. Second, there was a degree of selection bias in this study because we excluded patients with ATC whose baseline inflammatory biomarker values were not calculated. Finally, the initial doses of lenvatinib were different in some patients, and this might have affected the treatment efficacy and NLR dynamics. Despite these limitations, we believe that the results of this study are important given that this, to our knowledge, is the largest study to investigate the association between inflammatory biomarker values and treatment outcomes of lenvatinib in ATC.
In conclusion, there was seemed to be no association between prognosis or treatment efficacy of lenvatinib and baseline inflammatory biomarker values in our cases with ATC. However, we possibly estimate prognosis for ATC during lenvatinib treatment by observing the dynamics of NLR. Further large-scale prospective studies are needed to clarify the association between inflammatory biomarker values and treatment outcomes of lenvatinib in patients with ATC.
Acknowledgments
We thank Enago (https://www.enago.jp/) for editing this manuscript.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We received written informed consent for the publication of patient images from the patients themselves. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol of this retrospective study was approved by the Institutional Review Board of Kanagawa Cancer Center (IRB approval no. 2019-146), which waived the requirement for informed consent of clinicopathologic data due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-871
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-871
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-871
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-871). The authors have no conflicts of interest to declare.
References
- 1.Sugitani I, Miyauchi A, Sugino K, et al. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg 2012;36:1247-54. 10.1007/s00268-012-1437-z [DOI] [PubMed] [Google Scholar]
- 2.Tiedje V, Stuschke M, Weber F, et al. Anaplastic thyroid carcinoma: review of treatment protocols. Endocr Relat Cancer 2018;25:R153-61. 10.1530/ERC-17-0435 [DOI] [PubMed] [Google Scholar]
- 3.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. 10.1056/NEJMoa1406470 [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Kiyota N, Yamazaki T, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol 2019;15:717-26. 10.2217/fon-2018-0557 [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki H, Yamazaki H, Takasaki H, et al. Lenvatinib as a novel treatment for anaplastic thyroid cancer: A retrospective study. Oncol Lett 2018;16:7271-7. 10.3892/ol.2018.9553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu N, Jian Y, Wang Y, et al. Evaluation of neutrophil-to-lymphocyte ratio and calcitonin concentration for predicting lymph node metastasis and distant metastasis in patients with medullary thyroid cancer. Mol Clin Oncol 2018;9:629-34. 10.3892/mco.2018.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 9.Moschetta M, Uccello M, Kasenda B, et al. Dynamics of Neutrophils-to-Lymphocyte Ratio Predict Outcomes of PD-1/PD-L1 Blockade. Biomed Res Int 2017;2017:1506824. 10.1155/2017/1506824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho KM, Park H, Oh DY, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget 2017;8:2329-41. 10.18632/oncotarget.13731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soda H, Ogawara D, Fukuda Y, et al. Dynamics of blood neutrophil-related indices during nivolumab treatment may be associated with response to salvage chemotherapy for non-small cell lung cancer: A hypothesis-generating study. Thorac Cancer 2019;10:341-6. 10.1111/1759-7714.12952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn J, Song E, Oh HS, et al. Low Lymphocyte-to-Monocyte Ratios Are Associated with Poor Overall Survival in Anaplastic Thyroid Carcinoma Patients. Thyroid 2019;29:824-9. 10.1089/thy.2018.0684 [DOI] [PubMed] [Google Scholar]
- 13.Yamazaki H, Sugino K, Matsuzu K, et al. Inflammatory biomarkers and dynamics of neutrophil-to-lymphocyte ratio in anaplastic thyroid carcinoma. Endocrine 2020;70:115-22. 10.1007/s12020-020-02313-5 [DOI] [PubMed] [Google Scholar]
- 14.Fukuda N, Toda K, Fujiwara YU, et al. Neutrophil-to-Lymphocyte Ratio as a Prognostic Marker for Anaplastic Thyroid Cancer Treated With Lenvatinib. In Vivo 2020;34:2859-64. 10.21873/invivo.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017;27:751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Ding H, Wu Q, et al. Neutrophil-lymphocyte ratio dynamics are useful for distinguishing between recurrence and pseudoprogression in high-grade gliomas. Cancer Manag Res 2019;11:6003-9. 10.2147/CMAR.S202546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho JS, Park MH, Ryu YJ, et al. The neutrophil to lymphocyte ratio can discriminate anaplastic thyroid cancer against poorly or well differentiated cancer. Ann Surg Treat Res 2015;88:187-92. 10.4174/astr.2015.88.4.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manatakis DK, Tseleni-Balafouta S, Balalis D, et al. Association of baseline neutrophil-to-lymphocyte ratio with clinicopathological characteristics of papillary thyroid carcinoma. Int J Endocrinol 2017;2017:8471235. 10.1155/2017/8471235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceylan Y, Kumanlıoğlu K, Oral A, et al. The correlation of clinicopathological findings and neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in papillary thyroid carcinoma. Mol Imaging Radionucl Ther 2019;28:15-20. 10.4274/mirt.galenos.2018.60490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee F, Yang PS, Chien MN, et al. An Increased Neutrophil-to-Lymphocyte Ratio Predicts Incomplete Response to Therapy in Differentiated Thyroid Cancer. Int J Med Sci 2018;15:1757-63. 10.7150/ijms.28498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SM, Kim EH, Kim BH, et al. Association of the Preoperative Neutrophil-to-ymphocyte Count Ratio and Platelet-to-Lymphocyte Count Ratio with Clinicopathological Characteristics in Patients with Papillary Thyroid Cancer. Endocrinol Metab (Seoul) 2015;30:494-501. 10.3803/EnM.2015.30.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang K, Lei J, Chen W, et al. Association of the preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios with lymph node metastasis and recurrence in patients with medullary thyroid carcinoma. Medicine (Baltimore) 2016;95:e5079. 10.1097/MD.0000000000005079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Shi Y, Shen H, et al. The prognostic value of platelet-lymphocyte ratio and neutrophil-lymphocyte ratio in the treatment response and survival of patients with peripheral T-cell lymphoma. Leuk Lymphoma 2020;61:623-30. 10.1080/10428194.2019.1700244 [DOI] [PubMed] [Google Scholar]
- 26.lack AJ, Zargar H, Zargar-Shoshtari K, et al. The prognostic value of the neutrophil-to-lymphocyte ratio in patients with muscle-invasive bladder cancer treated with neoadjuvant chemotherapy and radical cystectomy. Urol Oncol 2020;38:3.e17-3.e27. [DOI] [PubMed]
- 27.Kim HY, Kim TH, Yoon HK, et al. The Role of Neutrophil-lymphocyte Ratio and Platelet-lymphocyte Ratio in Predicting Neoadjuvant Chemotherapy Response in Breast Cancer. J Breast Cancer 2019;22:425-38. 10.4048/jbc.2019.22.e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahara M, Kiyota N, Yamazaki T, et al. Lenvatinib for Anaplastic Thyroid Cancer. Front Oncol 2017;7:25. 10.3389/fonc.2017.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki H, Yokose T, Hayashi H, et al. Expression of vascular endothelial growth factor receptor 2 and clinical response to lenvatinib in patients with anaplastic thyroid cancer. Cancer Chemother Pharmacol 2018;82:649-54. 10.1007/s00280-018-3657-x [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki H, Yokose T, Hayashi H, et al. Expression of fibroblast growth factor receptor 4 and clinical response to lenvatinib in patients with anaplastic thyroid carcinoma: a pilot study. Eur J Clin Pharmacol 2020;76:703-9. 10.1007/s00228-020-02842-y [DOI] [PubMed] [Google Scholar]
- 31.Yamauchi M, Ono A, Ishikawa A, et al. Tumor Fibroblast Growth Factor Receptor 4 Level Predicts the Efficacy of Lenvatinib in Patients With Advanced Hepatocellular Carcinoma. Clin Transl Gastroenterol 2020;11:e00179. 10.14309/ctg.0000000000000179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano M, Kuromatsu R, Niizeki T, et al. Immunological inflammatory biomarkers as prognostic predictors for advanced hepatocellular carcinoma. ESMO Open 2021;6:100020. 10.1016/j.esmoop.2020.100020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner RA, Lückerath K, Schmid JS, et al. Thyroglobulin fluctuations in patients with iodine-refractory differentiated thyroid carcinoma on lenvatinib treatment-initial experience. Sci Rep 2016;6:28081. 10.1038/srep28081 [DOI] [PMC free article] [PubMed] [Google Scholar]