Abstract
Background
Umbilical cord mesenchymal stem cells (UC-MSCs), which possess potent immunomodulatory effects and low immunogenicity, are considered to be a promising stem cell-based therapy for sepsis. In the current study, we aimed to investigate whether the combined use of UC-MSCs and imipenem has a better effect than imipenem alone in treating Escherichia coli (E. coli)-induced sepsis and to explore the mechanism by which UC-MSCs exert their therapeutic effect in septic mice.
Methods
We randomly divided mice into five groups with 10 mice in each group: the normal control group (control group), the sepsis group (vehicle group), the MSCs treatment group (MSCs group), the imipenem treatment group (imipenem group), and the imipenem plus MSCs treatment group (imipenem + MSCs group). We monitored the survival rate in each group every 12 h for 3 days. After observing the survival rate, another 50 mice were also randomly divided into five groups, and the mice were sacrificed after 24 h. Bacterial colonies from the blood and peritoneal lavage fluid were counted in a blinded manner. Organ injury was analyzed by hematoxylin and eosin (HE) staining. Frequencies of myeloid-derived suppressor cells (MDSCs) in the blood, spleen, and bone marrow (BM) were determined by flow cytometry. Plasma levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, and IL-10 were detected by enzyme-linked immunosorbent assay (ELISA).
Results
Compared with imipenem treatment, the co-administration of UC-MSCs and imipenem dramatically improved the survival rate, decreased the bacterial load, and ameliorated organ injury. Furthermore, UC-MSCs treatment, either alone or in combination with imipenem, significantly increased plasma levels of IL-10 and the percentage of MDSCs by inducing arginase-1 in septic mice.
Conclusions
Our results indicated that UC-MSCs protect mice against sepsis by acting on MDSCs. Combination therapy of UC-MSCs and imipenem may be a new approach for the future clinical treatment of sepsis.
Keywords: Sepsis, umbilical cord mesenchymal stem cells (UC-MSCs), imipenem, myeloid-derived suppressor cells (MDSCs)
Introduction
Sepsis is a complicated inflammatory syndrome caused by dysregulated host response to severe infection, which leads to life-threatening organ dysfunction (1). Acute lung injury (ALI) and multiple organ dysfunction syndrome, complications that occur in most septic patients, are important causes of mortality in critical patients (2). Despite the improvement in intensive care and antibiotic therapy, the mortality rate remains at 25–30% (3). Therefore, novel strategies are necessary to advance the treatment of sepsis.
Mesenchymal stem cells (MSCs) are fibroblast-like multipotent cells that can be isolated from various tissues or organs (4,5). Our previous studies revealed that MSCs exert immunomodulatory effects on various immune cells, including B cells, T cells, macrophages, and dendritic cells (6-9). Because of their regenerative and immunomodulatory properties, MSCs are extensively used to treat a variety of human diseases (10). Recently, studies suggested that combination therapy of antibiotics and menstrual-derived MSCs (MenSCs) or adipose-derived MSCs can reduce organ damage and improve survival in the animal model of sepsis (11,12). However, whether the co-administration of UC-MSCs and antibiotics shows the same effect during sepsis development remains to be elucidated.
Myeloid-derived suppressor cells (MDSCs), which are defined as a heterogeneous population of myeloid progenitors, can potently suppress T cell responses (13). The MDSCs phenotype differs between mice and humans. Mouse MDSCs are characterized as CD11b+Gr-1+ cells and can be further divided into CD11b+Ly6GlowLy6Chigh monocytic MDSCs (M-MDSCs) and CD11b+Ly6GhighLy6Clow granulocytic MDSCs (G-MDSCs) (14). Human MDSCs are CD11b+CD33+HLA-DR– cells (15). Previous studies have shown that MDSCs dramatically expand in both septic patients and mice (16,17). It has been found that the numbers of CD11b+Gr-1+ cells dramatically increase in the spleen, lymph nodes, and bone marrow (BM) during polymicrobial sepsis, which can contribute to sepsis-induced T cell suppression and preferential Th2 polarization (18). Hence, exploring the mechanism of MDSCs generation and regulation may shed new light on the treatment of sepsis.
In the present study, we aimed to investigate the hypothesis that umbilical cord MSCs (UC-MSCs) combined with imipenem is superior to imipenem monotherapy in reducing bacterial sepsis-induced mortality and organ damage. Moreover, we explored the mechanism by which UC-MSCs exert their therapeutic effect in septic mice. Our results showed that the co-administration of UC-MSCs and imipenem significantly improved the survival rates of Escherichia coli (E. coli)-induced septic mice, decreased the bacterial load, and increased the percentage of MDSCs. These findings indicate that combination therapy of UC-MSCs and imipenem may be a new approach for the future clinical treatment of sepsis.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6371).
Methods
Bacterial strains
E. coli strains were kindly provided by the Medical Laboratory Center of Zhong da Hospital (Nanjing, Jiangsu, China). They were isolated from human clinical specimens and prepared in Luria-Bertani medium.
Isolation and culture of UC-MSCs
UC-MSCs were obtained and isolated as previously reported (19,20). Briefly, umbilical cords were collected from term infants immediately after birth. The cords were rinsed twice in phosphate-buffered saline (PBS) with 100 units/mL penicillin and streptomycin, and the cord blood vessels were carefully removed. Wharton’s jelly was cut into 1–2 mm3 pieces and resuspended in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 containing 10% fetal bovine serum (FBS) (all from Gibco, Life Technologies). After culture for 2 days, the medium containing non-adherent cells was discarded, and the medium was replaced twice a week thereafter. MSCs between passages 4 to 6 were used for subsequent experiments.
Mice
One hundred male ICR mice (30±2 g, 6 weeks old) were purchased from SPF (Beijing) Biotechnology Co., Ltd. (Beijing, China) and kept in specific pathogen-free conditions at the animal center of the Affiliated Drum Tower Hospital of Nanjing University Medical School. To observe the survival rate, 50 mice were divided into five groups: the normal control group, the vehicle group, the MSCs group, the imipenem group, and the imipenem + MSCs group; E. coli-induced sepsis was induced as previously described (21). E. coli were harvested, washed, and resuspended in PBS in a total volume of 200 μL (1×108 CFU/mouse). Then, 0.2 mL bacterial suspension was intraperitoneally injected into the mice. Four hours after E. coli injection, UC-MSCs (1×106 cells in a volume of 300 μL) were intravenously infused via the tail vein (22), and imipenem was intraperitoneally injected in a volume of 200 μL (25 mg/kg) (23,24). Mice in the control group received the same volume of PBS. The survival rate was monitored every 12 h for 3 days. In a separate experiment, another 50 mice were also divided into those five groups (n=10), and the mice were sacrificed after 24 h. Whole blood and peritoneal lavage fluid were collected, and the number of colonies was calculated and is expressed as the log10 (CFU/mL). Experiments were performed under a project license (No. 2020AE01033) granted by the ethics board of the Affiliated Drum Tower Hospital of Nanjing University Medical School, in compliance with the institutional guidelines for the care and use of animals.
MDSCs isolation and coculture with BM-derived macrophages (BMDMs)
Splenic MDSCs were isolated by using Myeloid-Derived Suppressor Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions. To generate BMDMs, BM cells from normal control ICR mice were cultured in RPMI 1640 medium supplemented with 10% FBS, 1% penicillin/streptomycin and 50 ng/mL M-CSF (PEPROTECH, Rocky Hill, USA). The cell medium was refreshed on day 3, and BMDMs were harvested on day 5. BMDMs were replated in 12-well plates (5×105 cells/well) and cocultured with the same number of MDSCs in the presence of 100 ng/mL lipopolysaccharide (LPS) for 24 h. The supernatants were collected for enzyme-linked immunosorbent assay (ELISA).
ELISA
Concentrations of plasma and supernatant tumor necrosis factor-α (TNF-α), IL-6, IL-1β, and IL-10 were determined by ELISA according to the manufacturer’s instructions (Bio-Legend, San Diego, CA, USA).
Flow cytometry
Before staining, spleens were mashed on ice to obtain single cell suspensions. BM cells were flushed from femurs and tibias. Then, BM cells and splenocytes were separately passed through a 200-mesh sieve. Single-cell suspensions were stained at 4 °C with a Fc receptor block (clone 93) for 20 min. Total MDSCs were labeled with APC anti-mouse CD11b (clone M1/70) and PE anti-mouse Gr-1 (clone RB6-8C5) antibodies. M-MDSCs and G-MDSCs were detected with APC anti-mouse CD11b (clone M1/70), FITC anti-mouse Ly6-G (clone RB6-8C5) and PE anti-mouse Ly6C (clone HK1.4) antibodies. All antibodies were purchased from eBioscience. Data were collected with a fluorescence activated cell sorting (FACS) Calibur flow cytometer (BD Biosciences) and analyzed with FlowJo software.
Histopathological examination
After mice were sacrificed, lungs and livers were collected and fixed in 4% paraformaldehyde immediately. Paraffin-embedded lungs and livers were cut into 4-μm-thick sections and subsequently stained with hematoxylin and eosin (HE) for morphologic analysis according to previous studies (25,26) by a blinded observer.
Real-time PCR
Total RNA was extracted from isolated splenic MDSCs using TRIzol Reagent (Invitrogen). Real-time PCR assays were then performed with SYBR Green Master Mix (Invitrogen) on a Step One sequence detection system (Applied Biosystems Waltham, MA, USA). The relative abundance of genes was calculated by using the 2–ΔΔCT method with GAPDH as an internal control. The primers used for real-time PCR are listed in Table S1.
Statistical analysis
Data are expressed as the mean ± SEM, and data analysis was performed using GraphPad Prism 8 software. The survival rate was analyzed with Kaplan-Meier survival curves. One-way analysis of variance (ANOVA) followed by Tukey’s post-test was used to analyze multiple groups. P values less than 0.05 were considered statistically significant.
Results
UC-MSCs improve the survival rate of imipenem monotherapy and ameliorate organ injury in E. coli-induced septic mice
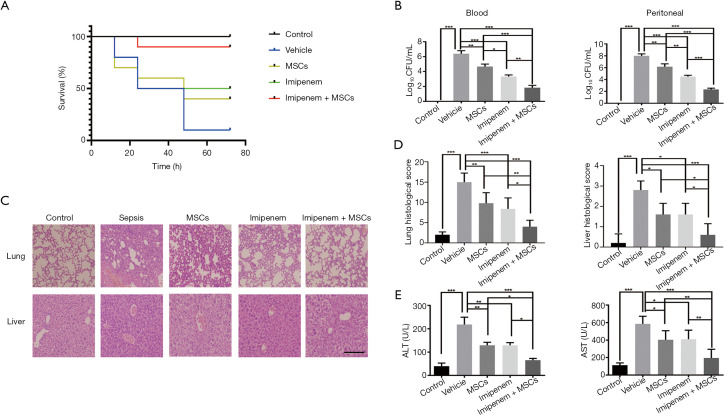
To observe the effect of combined treatment on acute bacterial sepsis, we established a mouse sepsis model by intraperitoneal injection of 1×108 CFU E. coli per mouse. At 72 h after different treatments, the survival rate reached by the imipenem+ UC-MSCs group was 90%, while the survival rates of the vehicle, UC-MSCs, and imipenem groups were 10%, 40%, and 50%, respectively (Figure 1A). Compared to the vehicle group, the number of bacterial colonies from the blood and peritoneal cavity were decreased in both the MSCs group and imipenem group, while a greater inhibition of bacterial growth was observed in response to combination treatment than MSCs or imipenem treatment alone at 24 h (Figure 1B). Both morphological damage and injury scores were ameliorated in the cotreatment group (Figure 1C,D). Additionally, the serum concentration of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which are biochemical indicators of liver function, were markedly decreased in the combined treatment group 24 h after E. coli injection (Figure 1E). These results suggest that UC-MSCs therapy in combination with imipenem provides a more protective effect against E. coli-induced sepsis than imipenem or UC-MSCs treatment alone.
Figure 1.
UC-MSCs improve the survival rate of imipenem monotherapy and ameliorate organ injury in E. coli-induced septic mice. Septic mice were treated with UC-MSCs and imipenem together or separately 4 h after E. coli infection (1×108 CFU/mouse). Mice in the control group were treated with PBS. The survival rate of each group was monitored every 12 h for 3 days. (A) Kaplan-Meier curves were used to analyze survival rates (n=10). (B) In a separate experiment, mice were treated as in (A) except they were anesthetized and killed 24 h after E. coli infection. Blood and peritoneal fluid were collected and plated for 16 h. Statistical analysis of the CFU number is shown (n=5). (C) Lung and liver tissues were analyzed using HE staining (original magnification, ×20); the scale bar represents 150 µm. (D) Injury scores of lungs and livers in different experimental groups (n=5). (E) Serum concentration of ALT and AST were measured (n=5). The data are presented as the mean ± SEM. The experimental data shown in this figure are representative of three independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001. UC-MSC, umbilical cord mesenchymal stem cell; E. coli, Escherichia coli; PBS, phosphate-buffered saline; HE, hematoxylin and eosin; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Effect of UC-MSCs treatment on the inflammatory cytokine response
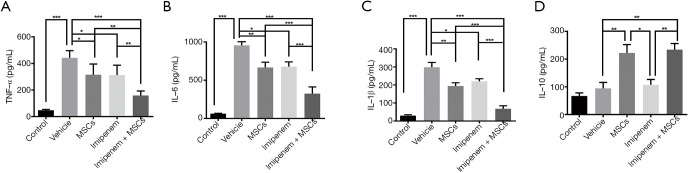
To elucidate the anti-inflammatory effect of UC-MSCs treatment, we evaluated plasma levels of several inflammatory cytokines at 24 h post-infection. We found that TNF-α, IL-6, and IL-1β levels in the plasma collected from septic mice significantly decreased after UC-MSCs plus imipenem treatment (Figure 2A,B,C). Additionally, the level of IL-10 significantly increased in the UC-MSCs group and the combined treatment group (Figure 2D). All these data indicated that co-administration of UC-MSCs and imipenem may bring immune responses back into balance.
Figure 2.
Effect of UC-MSCs treatment on the inflammatory cytokine response. (A,B,C,D) Plasma levels of TNF-α, IL-6, IL-1β, and IL-10 in each group were determined by ELISA. The data are presented as the mean ± SEM (n=5). The experimental data shown in this figure are representative of three independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001. UC-MSC, umbilical cord mesenchymal stem cell; TNF-α, tumor necrosis factor-α; IL, interleukin; ELISA, enzyme-linked immunosorbent assay.
UC-MSCs treatment increased the percentage of MDSCs in septic mice
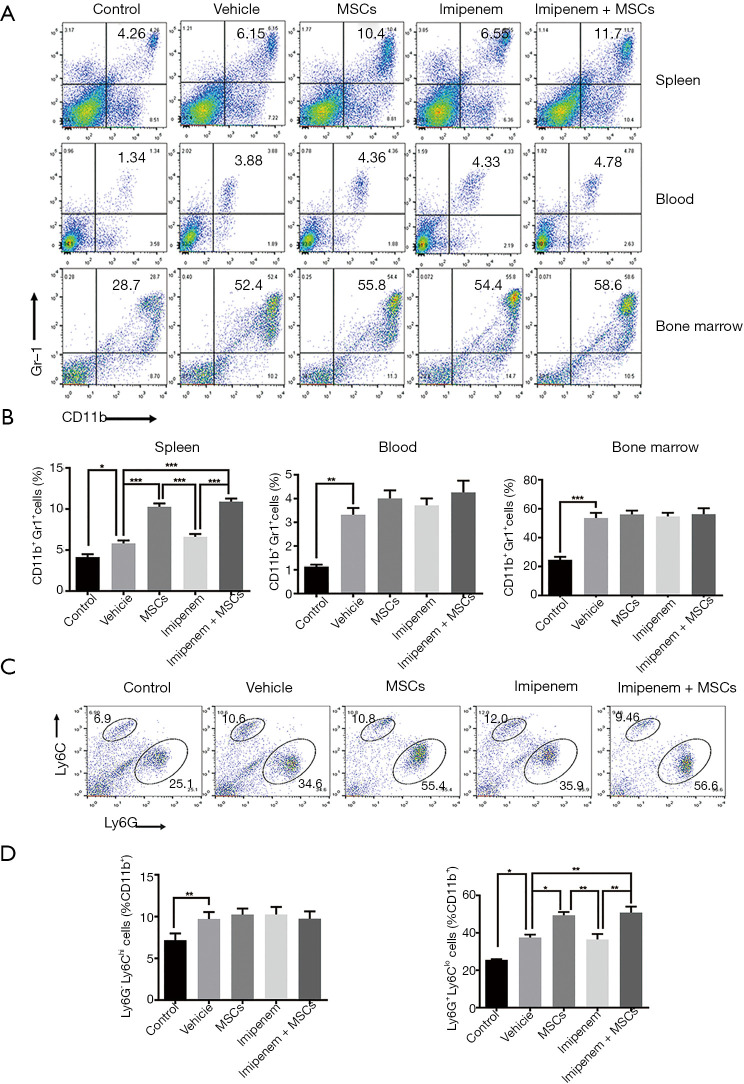
It has been reported that the percentage of MDSCs increases in different tissues of septic mice, including the blood, spleen, and BM (18,27). In the present study, we found that the proportion of MDSCs significantly increased in the blood, spleen, and BM of E. coli-induced septic mice at 24 h. Compared with the other treatments, UC-MSCs treatment and cotreatment further increased the frequency of MDSCs in the spleen but not the blood and BM (Figure 3A,B). In addition, UC-MSCs treatment and cotreatment preferentially increased G-MDSCs in the spleen of septic mice (Figure 3C,D). These data revealed that the therapeutic effect of UC-MSCs treatment may be associated with the elevation of MDSCs in septic mice.
Figure 3.
UC-MSCs treatment increased the percentage of MDSCs in septic mice. (A) Flow cytometry was used to determine the percentage of MDSCs in the blood, BM, and spleens of mice treated with or without MSCs and imipenem 24 h after E. coli infection. (B) Statistical analysis of the percentage of MDSCs in different tissues. (C) The percentage of G-MDSCs and M-MDSCs in the spleens of mice. (D) Statistical analysis of the percentage of G-MDSCs and M-MDSCs in the spleens of mice. The data are presented as the mean ± SEM (n=5). The data shown in this figure are representative of three independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001. UC-MSC, umbilical cord mesenchymal stem cell; MDSC, myeloid-derived suppressor cell; BM, bone marrow; E. coli, Escherichia coli; G-MDSC, granulocytic MDSC; M-MDSC, monocytic MDSC.
UC-MSCs significantly increased arginase-1 expression in MDSCs
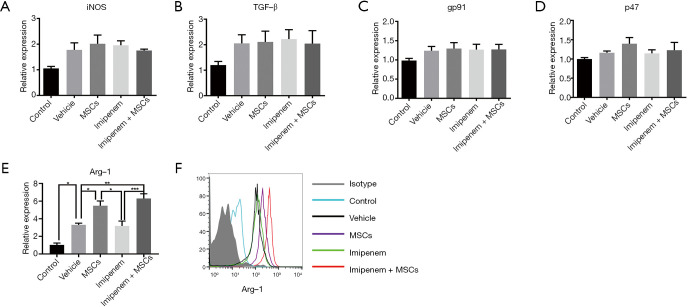
MDSCs suppress the activity of immune cells through various functional molecules, including arginase-1, transforming growth factor-β (TGF-β), reactive nitrogen species (ROS), and inducible nitric oxide synthase (iNOS) (28). Thus, we explored whether UC-MSCs treatment exerted its function through these molecules. We found that UC-MSCs treatment and combination treatment significantly enhanced the mRNA level of arginase-1 but did not affect iNOS, TGF-β, gp91-phox or p47-phox expression in splenic MDSCs (Figure 4A,B,C,D,E). The protein level of arginase-1 also significantly increased in splenic MDSCs in the UC-MSCs and combination treatment groups compared with the other groups (Figure 4F).
Figure 4.
UC-MSCs significantly increase arginase-1 expression in MDSCs. (A,B,C,D,E) The mRNA levels of iNOS, TGF-β, gp91-phox, p47-phox, and Arg-1 were determined by real-time PCR. (F) The protein level of arginase-1 was detected by flow cytometry. The data are presented as the mean ± SEM (n=5). The data shown in this figure are representative of three independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001. UC-MSC, umbilical cord mesenchymal stem cell; MDSC, myeloid-derived suppressor cell; iNOS, inducible nitric oxide synthase; TGF-β, transforming growth factor-β; gp91, gp91-phox; p47, p47-phox; Arg-1, arginase-1.
UC-MSCs enhance the immunosuppressive function of splenic MDSCs through arginase-1
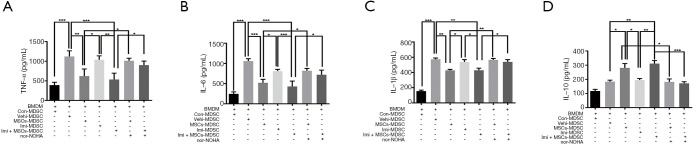
Considering that MDSCs may interact with macrophages and influence inflammatory cytokine secretion, splenic MDSCs were cocultured with LPS-stimulated BMDMs. Cytokine levels in the supernatant were assessed. As shown in Figure 5A,B,C,D, MDSCs isolated from the spleens of mice in the UC-MSCs treatment and the combination treatment groups exhibited suppressed proinflammatory TNF-α, IL-6, and IL-1β production but significantly increased production of the anti-inflammatory cytokine IL-10. The presence of nor-NOHA (500 μM, an arginase inhibitor) blocked these effects. These results suggest that UC-MSCs treatment enhances the immunosuppressive activity of splenic MDSCs mainly through arginase-1, which may contribute to re-balanced cytokine production in septic mice.
Figure 5.
UC-MSCs enhance the function of MDSCs via arginase-1. MDSCs isolated from the spleens of control mice, septic mice, MSCs-treated septic mice, imipenem-treated septic mice, and MSCs + imipenem-treated septic mice were cocultured with equal numbers of LPS (10 ng/mL)-stimulated BMDMs (5×105 cells/well) for 24 h. (A,B,C,D) The levels of TNF-α, IL-6, IL-1β, and IL-10 in the supernatant were determined by ELISA. The data are presented as the mean ± SEM (n=5). The data shown in this figure are representative of three independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001. UC-MSC, umbilical cord mesenchymal stem cell; MDSC, myeloid-derived suppressor cell; LPS, lipopolysaccharide; BMDM, bone marrow-derived macrophage; TNF-α, tumor necrosis factor-α; IL, interleukin; ELISA, enzyme-linked immunosorbent assay.
Discussion
In the present study, we found that combination therapy of UC-MSCs and imipenem significantly reduced the bacterial load in both peritoneal lavage fluid and peripheral blood in septic mice and improved the survival rate in mice with lethal E. coli infection. Additionally, levels of TNF-α, IL-6, and IL-1β in the plasma collected from septic mice significantly decreased after combination treatment.
Sepsis initiates an overwhelming proinflammatory response in the first few hours of onset (29), and if not treated early, the patient’s condition will deteriorate rapidly. Studies have revealed that MSCs might be favourable in sepsis therapy when infused at the early stage (11,30). As UC-MSCs can be easily isolated from the umbilical cord and expanded in vitro, they are considered a promising tool for treating sepsis. He et al. showed that intravenous infusion of UC-MSCs was safe and well-tolerated in patients with severe sepsis (31). The mechanism, however, is still unknown.
Previous studies revealed that MDSCs play an important role in the pathogenesis of infectious diseases along with sepsis (18,32,33). Despite MDSCs have been considered to be deleterious in patients with cancer (34), the role of MDSCs in sepsis is complex. In the early stages, MDSCs may be beneficial by limiting excessive inflammation, thus protecting against organ dysfunction. Derive et al. demonstrated that adoptive transfer of day 10 MDSCs into septic mice decreased peritoneal cytokine production and enhanced the survival rate (17). Sander et al. showed that septic mice lacking gp130 were unable to expand their MDSCs population and exhibited markedly higher mortality associated with increased inflammatory cytokine production (27). However, overzealous MDSCs proliferation may lead to persistent immunosuppression through inhibition of T cell proliferation and elaboration of anti-inflammatory cytokines in the late stage of sepsis. Mathias et al. suggested that persistently increased circulating MDSCs are associated with adverse long-term outcomes (35).
Until now, whether UC-MSCs can exert their immunomodulatory properties via MDSCs during early sepsis has not been reported. In this study, we focused on the amount and function of MDSCs in the acute phase, and the results revealed that UC-MSCs treatment, either alone or in combination with imipenem, significantly increased levels of the inhibitory cytokine IL-10 and the number of splenic MDSCs other than in the blood and BM. More importantly, we also demonstrated that the suppressive activity may be related to the upregulation of arginase-1 in MDSCs in vivo and in vitro. Therefore, we speculated that UC-MSCs inhibit excessive inflammation in the early stage of sepsis by regulating the number and function of MDSCs. The detailed mechanism, however, of how UC-MSCs regulate MDSCs needs further exploration. In addition, further studies are also needed to elucidate whether UC-MSCs can promote the differentiation and maturation of MDSCs, reduce the number of MDSCs in the late stage, and thus restore the immune response during sepsis.
More recently, Alcayaga and colleagues reported beneficial effects of MenSCs and antibiotics in sepsis and proposed a mechanism involving the antimicrobial and immunomodulatory properties of MenSCs (11). Although we observed comparable reductions in experimental sepsis-induced mortality, the antimicrobial effect of UC-MSCs in vitro, which will be our next research focus, was less involved in this study. Altogether, these data suggested that MSCs derived from different sources combined with antibiotics can improve the survival rate of septic mice, and the mechanism may be related to multiple targets. Collectively, our work could partly explain the noteworthy decrease in inflammation and mortality in animals with sepsis after receiving combination treatment. To our knowledge, this is the first report on the therapeutic effect of UC-MSCs combined with imipenem in mice with sepsis.
Conclusions
In summary, our data showed that the co-administration of UC-MSCs and imipenem significantly improved the survival rates of E. coli-induced septic mice, decreased the bacterial load, and increased the percentage of MDSCs. These findings highlighted the therapeutic potential of utilizing a combination of UC-MSCs and imipenem for the treatment of sepsis, especially in the early stage.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by grants from the Major International (Regional) Joint Research Project of China (81720108020) and the National Natural Science Foundation of China (81901672).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2020AE01033) granted by the ethics board of the Affiliated Drum Tower Hospital of Nanjing University Medical School, in compliance with the institutional guidelines for the care and use of animals.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6371
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6371
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6371). Dr. LW serves as an unpaid section editor of Annals of Translational Medicine from Oct 2019 to Sep 2020. The other authors have no conflicts of interest to declare.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801-10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujishima S. Organ dysfunction as a new standard for defining sepsis. Inflamm Regen 2016;36:24. 10.1186/s41232-016-0029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016;193:259-72. 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 4.Keating A. Mesenchymal stromal cells: new directions. Cell stem cell 2012;10:709-16. 10.1016/j.stem.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 5.Tang X, Li W, Wen X, et al. Transplantation of dental tissue-derived mesenchymal stem cells ameliorates nephritis in lupus mice. Ann Transl Med 2019;7:132. 10.21037/atm.2019.02.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Che N, Li X, Zhang L, et al. Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol 2014;193:5306-14. 10.4049/jimmunol.1400036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Wang Q, Feng X, et al. Umbilical cord-derived mesenchymal stem cells suppress autophagy of t cells in patients with systemic lupus erythematosus via transfer of mitochondria. Stem Cells Int 2016;2016:4062789. 10.1155/2016/4062789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Niu L, Tang X, et al. Mesenchymal stem cells prevent podocyte injury in lupus-prone B6.MRL-Faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrol Dial Transplant 2018;33:2069. 10.1093/ndt/gfy280 [DOI] [PubMed] [Google Scholar]
- 9.Yuan X, Qin X, Wang D, et al. Mesenchymal stem cell therapy induces FLT3L and CD1c(+) dendritic cells in systemic lupus erythematosus patients. Nat Commun 2019;10:2498. 10.1038/s41467-019-10491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei X, Yang X, Han ZP, et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacologica Sinica 2013;34:747-54. 10.1038/aps.2013.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcayaga-Miranda F, Cuenca J, Martin A, et al. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther 2015;6:199. 10.1186/s13287-015-0192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung PH, Chiang HJ, Chen CH, et al. Combined therapy with adipose-derived mesenchymal stem cells and ciprofloxacin against acute urogenital organ damage in rat sepsis syndrome induced by intrapelvic injection of cecal bacteria. Stem Cells Transl Med 2016;5:782-92. 10.5966/sctm.2015-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trikha P, Carson WE, 3rd. Signaling pathways involved in MDSC regulation. Biochim Biophys Acta 2014;1846:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi J, Tang X, Li W, et al. Mesenchymal stem cells inhibited the differentiation of MDSCs via COX2/PGE2 in ex-perimental sialadenitis. Stem Cell Res Ther 2020;11:325. 10.1186/s13287-020-01837-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian J, Rui K, Hong Y, et al. Increased GITRL impairs the function of myeloid-derived suppressor cells and exacerbates primary Sjogren syndrome. J Immunol 2019;202:1693-703. 10.4049/jimmunol.1801051 [DOI] [PubMed] [Google Scholar]
- 16.Uhel F, Azzaoui I, Grégoire M, et al. Early expansion of circulating granulocytic myeloid-derived suppressor cells predicts development of nosocomial infections in patients with sepsis. Am J Respir Crit Care Med 2017;196:315-27. 10.1164/rccm.201606-1143OC [DOI] [PubMed] [Google Scholar]
- 17.Derive M, Bouazza Y, Alauzet C, et al. Myeloid-derived suppressor cells control microbial sepsis. Intensive Care Med 2012;38:1040-9. 10.1007/s00134-012-2574-4 [DOI] [PubMed] [Google Scholar]
- 18.Delano MJ, Scumpia PO, Weinstein JS, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 2007;204:1463-74. 10.1084/jem.20062602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Wang D, Liu D, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood 2012;120:3142-51. 10.1182/blood-2011-11-391144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Feng X, Lu L, et al. A CD8 T cell/indoleamine 2,3-dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus. Arthritis Rheumatol 2014;66:2234-45. 10.1002/art.38674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Y, Wang J, Xue Y, et al. GSKJ4 protects mice against early sepsis via reducing proinflammatory factors and up-regulating mir-146a. Front Immunol 2018;9:2272. 10.3389/fimmu.2018.02272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang S, Wu S, Zhang Z, et al. Mesenchymal stem cells induced CD4+ T cell apoptosis in treatment of lupus mice. Biochem Biophys Res Commun 2018;507:30-5. 10.1016/j.bbrc.2018.10.133 [DOI] [PubMed] [Google Scholar]
- 23.Vyas D, Javadi P, Dipasco PJ, et al. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol 2005;289:R1048-53. 10.1152/ajpregu.00312.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhury S, Kandasamy K, Maruti BS, et al. Atorvastatin along with imipenem attenuates acute lung injury in sepsis through decrease in inflammatory mediators and bacterial load. Eur J Pharmacol 2015;765:447-56. 10.1016/j.ejphar.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 25.Li GM, Ji MH, Sun XJ, et al. Effects of hydrogen-rich saline treatment on polymicrobial sepsis. J Surg Res 2013;181:279-86. 10.1016/j.jss.2012.06.058 [DOI] [PubMed] [Google Scholar]
- 26.Renckens R, Roelofs JJ, Bonta PI, et al. Plasminogen activator inhibitor type 1 is protective during severe Gram-negative pneumonia. Blood 2007;109:1593-601. 10.1182/blood-2006-05-025197 [DOI] [PubMed] [Google Scholar]
- 27.Sander LE, Sackett SD, Dierssen U, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med 2010;207:1453-64. 10.1084/jem.20091474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin S, Yang Z, Hao X, et al. Roles of HMGB1 in regulating myeloid-derived suppressor cells in the tumor micro-environment. Biomark Res 2020;8:21. 10.1186/s40364-020-00201-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osuchowski MF, Welch K, Siddiqui J, et al. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol 2006;177:1967-74. 10.4049/jimmunol.177.3.1967 [DOI] [PubMed] [Google Scholar]
- 30.Wu KH, Wu HP, Chao WR, et al. Time-series expression of toll-like receptor 4 signaling in septic mice treated with mesenchymal stem cells. Shock 2016;45:634-40. 10.1097/SHK.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 31.He X, Ai S, Guo W, et al. Umbilical cord-derived mesenchymal stem (stromal) cells for treatment of severe sepsis: aphase 1 clinical trial. Transl Res 2018;199:52-61. 10.1016/j.trsl.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 32.Pereira WF, Ribeiro-Gomes FL, Guillermo LV, et al. Myeloid-derived suppressor cells help protective immunity to Leishmania major infection despite suppressed T cell responses. J Leukoc Biol 2011;90:1191-7. 10.1189/jlb.1110608 [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Akbar SM, Abe M, et al. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol 2011;166:134-42. 10.1111/j.1365-2249.2011.04445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao P, Wan X, Cui B, et al. Interleukin 33 in tumor microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. Oncoimmunology 2015;5:e1063772. 10.1080/2162402X.2015.1063772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathias B, Delmas AL, Ozrazgat-Baslanti T, et al. Human Myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg 2017;265:827-34. 10.1097/SLA.0000000000001783 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as