Abstract
Background:
Ichthyoses are rare, debilitating disorders associated with generalized scaling, erythema, and epidermal barrier impairment. Pathogenesis-based therapy is largely lacking, since the underlying molecular basis is poorly understood.
Objective:
To characterize molecularly cutaneous inflammation and its correlation with clinical and barrier characteristics.
Methods:
We analyzed biopsies from 21 genotyped ichthyosis patients (congenital ichthyosiform erythroderma, lamellar ichthyosis, epidermolytic ichthyosis, and Netherton syndrome) by immunohistochemistry and RT-PCR and compared them with healthy controls, and with atopic dermatitis (AD) and psoriasis patients. Clinical measures included a severity score for ichthyosis (IASI), which integrates erythema (IASI-E) and scaling (IASI-S), transepidermal water loss (TEWL), and pruritus.
Results:
Ichthyosis samples showed increased epidermal hyperplasia (increased thickness and K16 expression) and T-cell and dendritic-cell infiltrates. Increases of general inflammatory (IL-2), innate (IL-1β), and some Th1/IFN (IFNγ) markers in ichthyosis were comparable to psoriasis or AD. TNFα levels in ichthyosis were elevated only in Netherton syndrome, but were much lower than in psoriasis and AD. Expression of Th2 cytokines (IL-13, IL-31) in ichthyoses was similar to controls. Most notable in ichthyosis was the striking induction of IL-17-related genes or markers synergistically induced by IL-17 and TNFα (IL-17A/C, IL-19, CXCL1, PI3, CCL20, IL36G; p<0.05), similar to psoriasis. IASI and IASI-E strongly correlated with IL-17A (r=0.74, p<0.001) and IL-17/TNF-synergistic/additive genes. These markers also significantly correlated with TEWL, suggesting a link between functional barrier defects and inflammation in ichthyosis.
Conclusion:
Our data associates the shared ichthyosis immune fingerprinting to Th17/IL-23 polarization, and raises the possibility of IL-17-targeting strategies for the ichthyoses.
Clinical Implications:
The link between Th17 pathway activation and clinical disease severity raises the possibility of a new therapeutic paradigm of targeted IL-17/IL-23 intervention for ichthyosis patients.
Capsule Summary:
CIE, LI, EI and NS subtypes of ichthyosis are Th17-skewed. IL-17/TNF-synergistic/additive genes are most dominantly activated and significantly correlated with disease severity scores and functional barrier abnormalities (TEWL).
Keywords: Ichthyosis, inflammation, autosomal recessive congenital ichthyosis, congenital ichthyosiform erythroderma, lamellar ichthyosis, Netherton syndrome, epidermolytic ichthyosis, skin, IL-17, TNFα
Introduction
Ichthyoses are rare, genetically and clinically heterogeneous disorders with generalized skin scaling, thickening, and erythema.1–6 Affected individuals have an extremely compromised quality of life because of disfigurement and accompanying itching, pain, and functional limitation.7,8 The epidermal barrier is abnormal with defects in lipids, differentiation, and transepidermal water loss/TEWL.9–11
Treatment for ichthyosis is largely supportive and unsatisfactory. For more severely affected individuals, oral retinoids, vitamin A analogues, are often administered to improve the hyperkeratosis.12–14 However, retinoids can worsen skin inflammation and pruritus, and have deleterious effects (hypertriglyceridemia, teratogenicity, hyperostosis),15 limiting their use. Topical anti-inflammatory medications (i.e., steroids, calcineurin inhibitors) are often ineffective and easily absorbed systemically, restricting chronic use.16,17 Thus, a huge unmet need exists for safe, more effective treatments which ideally will also target the erythema/inflammation.
Despite an improved understanding of the genetic basis,1 the molecular mechanisms for various ichthyosis forms are poorly understood and predominantly based on culture and animal models.18–28 These model systems chiefly focus on abnormal barrier function and lipid homeostasis, with attention paid to immune disturbances.6,29,30 The limited data from ichthyosis patients primarily involves small numbers of individuals with Netherton syndrome/NS or the lamellar ichthyosis/LI form of autosomal recessive congenital ichthyosis/ARCI, and has explored a few cytokines in blood with little study in human skin.31–37 Blood analyses found inconsistent Th2 skewing38 and increases in pro-inflammatory cytokines (tumor necrosis factor-alpha/TNFα, IL-1β, IL-2, IL-18).39–41 Skin studies showed increased expression of TNFα and IL-1β in ARCI-LI,34 and of protease-activated receptor 2/PAR2,31 thymic stromal lymphopoietin/TSLP, TNFα, IL-8,42 and the Th2 cytokine IL-33 in NS,37 often coupled with increased expression of terminal differentiation products (i.e filaggrin/FLG, loricrin/LOR, involucrin/IVL), and lipid impairements.31,34,36,37 A few human studies investigated changes in a limited array of barrier or immune markers with systemic treatments, including retinoids (n=11), anti-TNF (n=1), and oral corticosteroids combined with omalizumab (n=1) in ARCI-LI and NS patients, respectively.32–34 Therapy-induced decreases in IL-1β, IL-8, TSLP, IL-5, and IL-17A were found in NS, while IL-1α and TNFα were decreased (non-significantly) in ARCI-LI.
To elucidate the basis for the cutaneous inflammation in ichthyosis and its correlation with clinical characteristics, we analyzed skin from 21 individuals with three of the more prevalent orphan ichthyoses: ARCI (LI and congenital ichthyosiform erythroderma/CIE forms), epidermolytic ichthyosis/EI, and NS. All subtypes showed Th17-skewing in skin, which correlated with disease severity. This Th17 profile most closely resembled that of psoriasis, in which IL-17 antagonism is highly effective in reversing the inflammation and epidermal pathology.43–46 These data may lead to a new treatment paradigm targeting the Th17/IL-23 pathway in ichthyosis.
Methods
Patient characteristics
21 patients (ages 10–57 years) with ichthyosis and known mutations were enrolled (Tables 1–E1, Supplementary Materials in this journal’s online repository/OR). Written IRB-approved consent was provided by subjects (≥12 years) and parents (<18 years). Demographic information, medical history, physical examination, clinical severity scores, pruritus (5-D Itch Scale and Itch Numerical Rating Scale/NRS), photography, and TEWL were captured. Few scoring instruments have been used for ichthyosis severity, and the only validated one (the Congenital Ichthyoses Severity Index/CISI) includes erythema and hyperkeratosis/scaling, but also alopecia (found in the minority of patients), fails to score potential differences in body regions, and is geared towards patient-reported assessment.47 As a result we quantified severity using a tool that modifies the 5-point Likert CISI scale, eliminating alopecia and prorating based on body region and extent to create a composite score, similar to the Psoriasis Area and Severity Index/PASI.48 This Ichthyosis Area and Severity Index/IASI measures severity of the erythema/IASI-E and scaling/IASI-S, adding them together to a total IASI score (Tables 1, E3; Supplementary Methods in OR). 4mm biopsies were collected and compared with normal, AD and psoriasis tissues previously published by our group.49–53 Four samples of healthy adolescents were also included (Table E2) for appropriate comparisons with the younger ichthyosis cohort. Patient characteristics are presented in Tables 1, E1–E2.
Table 1.
Patient demographics and clinical severity scores.
| A. Characteristics of subjects with different ichthyosis subtypes. | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Parameter | CIE (n = 6) | LI (n = 7) | EI (n = 5) | NS (n = 3) | P-value |
| Age (years) | Mean (±SD) | 26.4 ± 14.7 | 30.4 ± 14.8 | 27.6 ± 19.2 | 18.2 ± 5.3 | 0.718 |
| Median [age range] | 23.2 [10.8 – 45] | 28.0 [10.8 – 57] | 17.9 [11.6 – 55] | 19.2 [12.5 – 23] | ||
| Gender | Female | 3 | 6 | 3 | 1 | 0.511 |
| Male | 3 | 1 | 2 | 2 | ||
| Race | White | 5 | 5 | 5 | 2 | 0.695 |
| Black | 0 | 1 | 0 | 1 | ||
| Asian | 1 | 0 | 0 | 0 | ||
| Hispanic | 0 | 1 | 0 | 0 | ||
| Disease Severity Scores | IASI (±SD) | 24.5 ± 11.1 | 29.7 ± 8.0 | 33.6 ± 6.3 | 27.3 ± 12.3 | 0.437 |
| IASI-E (±SD) | 13.9 ± 8.9 | 10.3 ± 6.4 | 15.1 ± 6.7 | 16.2 ± 11.0 | 0.652 | |
| IASI-S (±SD) | 10.6 ± 4.7 | 19.4 ± 5.5 | 18.5 ± 4.9 | 11.1 ± 1.3 | 0.017 | |
| TEWL (g/m2/hr, ±SD) | 22.1 ± 5.4 | 16.2 ± 4.5 | 15.2 ± 6.8 | 28.1 ± 0.0 | 0.04 | |
| 5-D Itch Scale (±SD) | 11.2 ± 3.1 | 13.8 ± 5.8 | 10.2 ± 3.1 | 17.0 ± 7.1 | 0.268 | |
AD: atopic dermatitis; CIE: congenital ichthyosiform erythroderma; EI: erythrodermic ichthyosis; NS: Netherton syndrome; IASI: ichthyosis area severity index (with E: erythema; S: scaling); LI: lamellar ichthyosis; LS: lesionai; N/A: not applicable. NL: non- lesional; PASI: psoriasis area severity index; PSO: psoriasis; SCORAD: scoring atopic dermatitis; SD: standard deviation; TEWL: transepidermal water loss.
Quantitative RT-PCR
RT-PCR was performed as described.54,55 Expression values were normalized to human acidic ribosomal protein/hARP.
Immunohistochemistry
IHC was performed on frozen sections as described.56 Antibodies are listed in Table E7. IHC data are shown in Table E8.
Statistical Analyses
hARP normalized RT-PCR expression values under the limit of detection/LOD were imputed as 20% of the minimum observed values (over LOD) and log2-transformed prior to analysis. No other missing value imputation method was performed and all available observations were included in analyses, which were performed using the statistical language R (www.R-project.org) and its available packages. Differences in expression values (in log2-scale), cell-counts, and clinical variables were assessed using linear models.
Unsupervised hierarchical clustering of variables or samples/patients was performed using Pearson correlation coefficient as a distance metric with the Mcquiity agglomeration algorithm. The results are represented as a heatmap with a dendrogram, and a tree or phylogram (using R package ape) (see extended statistics in OR).
Results
Demographics and clinical characteristics of ichthyosis subjects
21 individuals with 3 ichthyosis subtypes were included: 13 patients with two forms of ARCI (CIE, n=6; and LI, n=7), EI (n=5), or NS (n=3), ages ≥10 years, with known genetic mutations (Tables 1 and E1). ARCI patients are typically born as “collodion babies” (Fig. 1A),57,58 and have the eventual phenotype ranging from large plate-like scales overlying variable erythema (LI) (Fig. 1B) to fine flaky scale and intense erythema (CIE) (Fig. 1C).1,3 All LI patients had mutations in TGM1, encoding transglutaminase, which enables stratum corneum crosslinking;59 CIE subjects had a range of mutated genes (Table E1), particularly encoding proteins of the hepoxilin pathway.1,60,61 EI patients display erythema under warty scale (Fig. 1D); all our patients had KRT10 mutations. NS, resulting from mutations in SPINK5,62,63 encoding a protease inhibitor, ranges from milder erythema with unique scaling (ichthyosis linearis circumflexa) to generalized erythroderma (Fig. 1E–F, respectively). Normal skin from healthy individuals (≥10 years; n=16; Table E2) as well as lesional and non-lesional skin from moderate-to-severe adults with two common skin disorders, atopic dermatitis (AD, n=16; Fig. 1G) and psoriasis (n=10; Fig. 1H), were also included for appropriate comparisons with all polar cytokine pathways (Table 1).49–53 Due to age differences between groups, with ichthyosis being the youngest cohort (p<0.001), all analyses were age-adjusted (detailed Statistics in OR; Tables 1, E1).
Figure 1.
Representative clinical pictures of the collodion-baby phenotype (A), lamellar ichthyosis/LI (B), congenital ichthyosis erythroderma/CIE (C), epidermolytic ichthyosis/EI (D), and Netherton syndrome/NS (E-F). They share varying degrees of erythema and scaling, as in two common inflammatory skin diseases, atopic dermatitis/AD (G) and psoriasis (H). (I-M) Clinical severity scores by subtypes of ichthyosis. +p<0.1, *p<0.05, **p<0.01.
There were no significant differences in IASI or IASI-E scores among subtypes (Fig. 1I–K). However, LI and EI showed greater IASI-S in comparison with CIE (p<0.01 for LI, p<0.05 for EI) and NS (p<0.05 for LI) (Fig. 1J). Two pruritus scores were measured, the Itch Numerical Rating Scale/NRS and the 5-D Itch Scale.64 Since the two itch scales were highly correlated (Fig. E1), the 5-D Itch Scale was used for correlations. The 5-D Itch Scale was significantly higher (p<0.05) for NS compared to EI and CIE (Fig. 1L). The mean TEWL, a measure of barrier function, was also significantly greater in NS compared to EI and LI, and in CIE compared to EI subtype (p<0.05) (Table 1, Fig. 1M).
Increased hyperplasia and cellular infiltration characterize ichthyotic skin
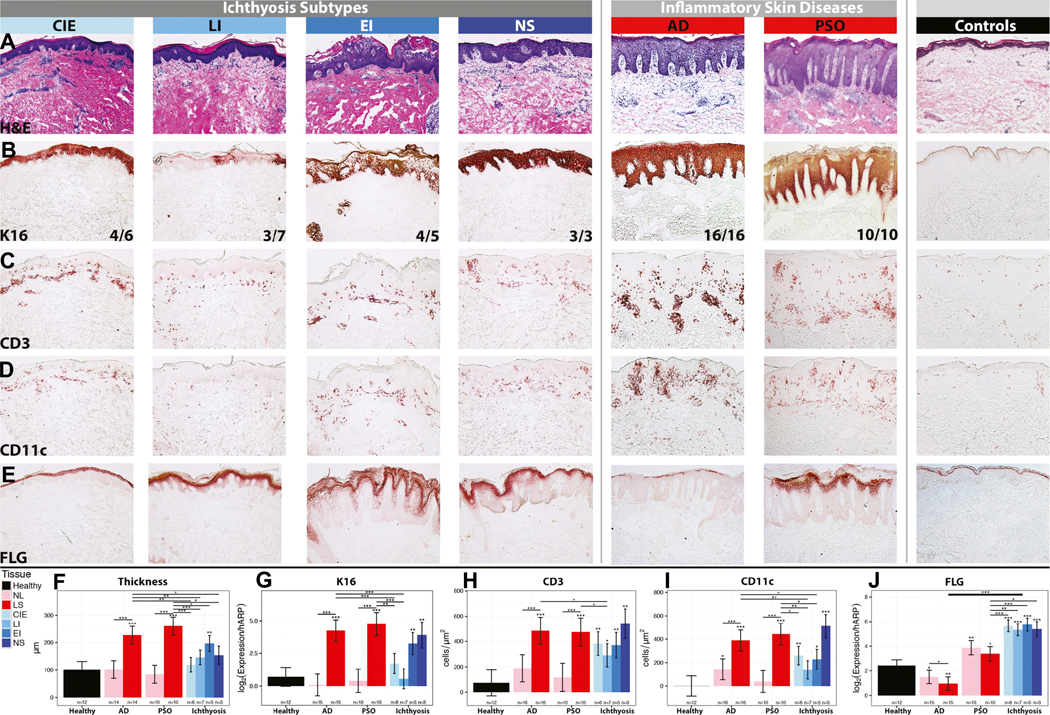
Epidermal hyperplasia (as measured by epidermal thickness, mRNA and protein expressions of keratin 16/K16, a marker of epidermal proliferation) 65,66 was seen in all ichthyosis subtypes compared to controls, with the greatest increases observed in NS (Fig. 2A). K16 staining patterns in ichthyosis were comparable to those of lesional AD and psoriasis, characterized by widespread K16 staining (16/16 and 10/10, respectively) (except LI, with only 3/7 K16+; Fig. 2B). The highest increases in epidermal thickness and K16 mRNA were seen in EI and NS (Fig. 2A–B, 2F–G). Significant increases in CD3+ T-cell and CD11c+ myeloid dendritic cells/DCs, DC-LAMP+ DCs, and neutrophil elastase+ neutrophils characterized all ichthyosis subtypes compared to controls, with greatest increases observed in NS (Fig. 2C–D, 2H–I, Fig. E2). The infiltrates in ichthyosis were comparable to those of highly inflammatory lesions from AD and psoriasis patients. In fact, NS had similar increases in neutrophils (vs. control skin) to psoriasis, considered a highly neutrophilic disease (Fig. E2).53 Unlike AD and psoriasis, no significant increases in CD1a+ Langerhans cells/LC (Fig. E2).
Figure 2.
Representative staining in ichthyoses, AD, psoriasis/PSO and controls using (A) hematoxylin-eosin/H&E, (B) K16 with fractions of positive samples, (C) CD3+ T-cells, (D) CD11c+ DC, and (E) filaggrin/FLG. Quantification of (F) epidermal thickness, (G) K16 mRNA, (H-I) CD3+ and CD11c+ cells and (J) filaggrin/FLG mRNA. mRNA-log2 values were adjusted to hARP. Mean±SEM. Controls comparisons: stars above bars. *p<0.05, **p<0.01, ***p<0.001. LS: lesional; NL: non-lesional.
We also analyzed protein and mRNA expression of the keratinocyte differentiation markers (filaggrin/FLG, loricrin/LOR, periplakin/PPL), which are largely down-regulated in AD.10,67–69 Unlike the continuous, clear expression of FLG in normal skin, AD lesions showed skipped and faint FLG expression in the upper layers of the epidermis, including the stratum corneum (Fig. 2E). Similar to psoriasis, most ichthyosis tissues, and particularly NS, showed increased and more intense expression of FLG, in the spinous and granular layers, compared with control skin. This was paralleled by significantly increased mRNA expression of FLG, LOR, and PPL, which was even higher in ichthyosis than psoriasis, but largely suppressed in AD compared to controls, as reported,68,70 and consistent with reduced FLG immunostaining (Figs 2J, Fig. E3).
Ichthyotic skin shows a Th17-centered inflammation
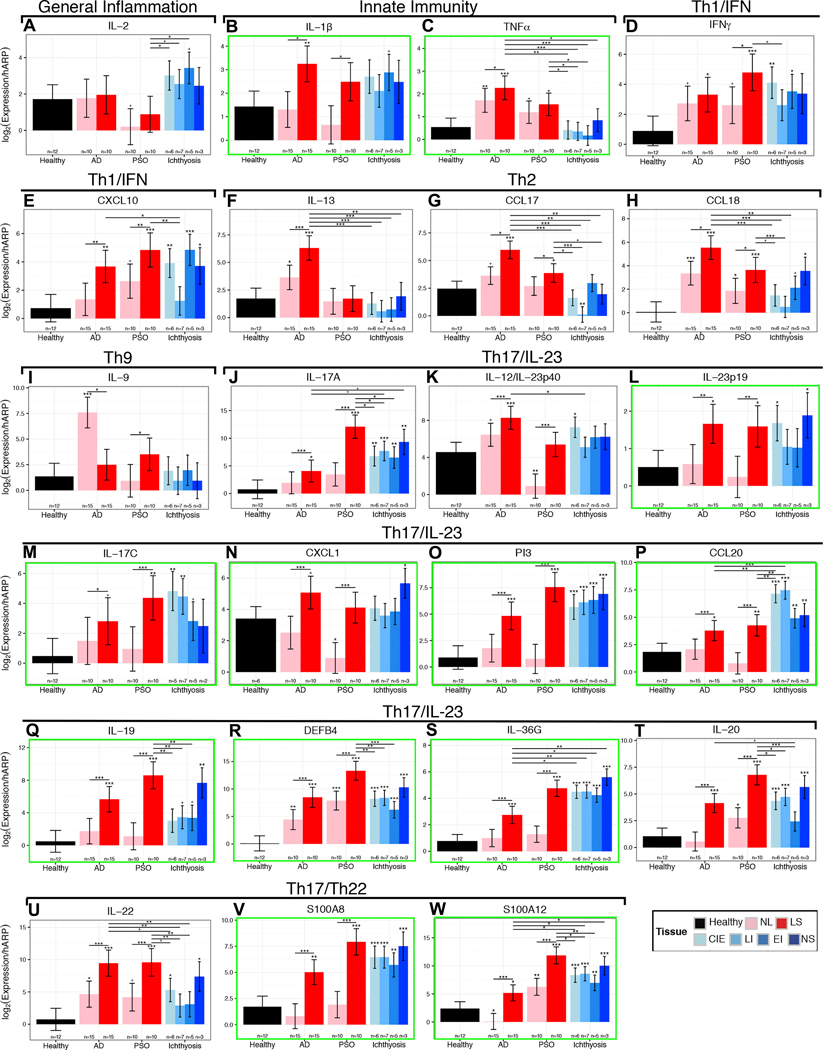
To evaluate primary Th1, Th2, Th9, Th17, Th22 cytokines and some epidermal markers, which are often below detection levels on gene-arrays,71 we performed qRT-PCR. We observed large increases in expression of general inflammatory (IL-2, IL-15) and some innate immune (IL-1β, IL-8) markers in ichthyosis compared with control skin (Figs. 3–4A, Fig. E3). These increases were comparable and even higher than those in AD and psoriasis. Interestingly, TNFα was up-regulated in AD and psoriasis compared to controls, but not in ichthyosis, although higher levels were seen in NS, as reported (Fig. 3–4A).6,32,42 Expression of Th1-related markers (IFNγ, CXCL10, CXCL9) was also increased in ichthyosis compared to controls (Figs. 3–4A, Fig. E3). The expression of Th2 cytokines (IL-13, IL-31, IL-5, CCL17) was lower in ichthyosis than in AD, and largely similar to controls (Fig. 3–4A, Fig. E3). Some Th2 markers (CCL18, IL-10, CCL22) showed increases in NS, but much smaller than in AD and comparable or even lower than in psoriasis (Figs. 3–4A, Fig. E3). IL-9/Th9 cytokine was not increased in ichthyosis compared with controls (Fig. 3).
Figure 3.
Comparison of immune markers in ichthyosis subtypes, AD, psoriasis and controls using RT-PCR (A-W). mRNA log2 values were adjusted to hARP expression levels. Stars without bars denote comparison to controls. Stars above bars denote p-values with comparators defined by the bar. LSmean (log2 expression/hARP) ± SEM. +p<0.1, *p<0.05, **p<0.01, ***p<0.001.
Figure 4.
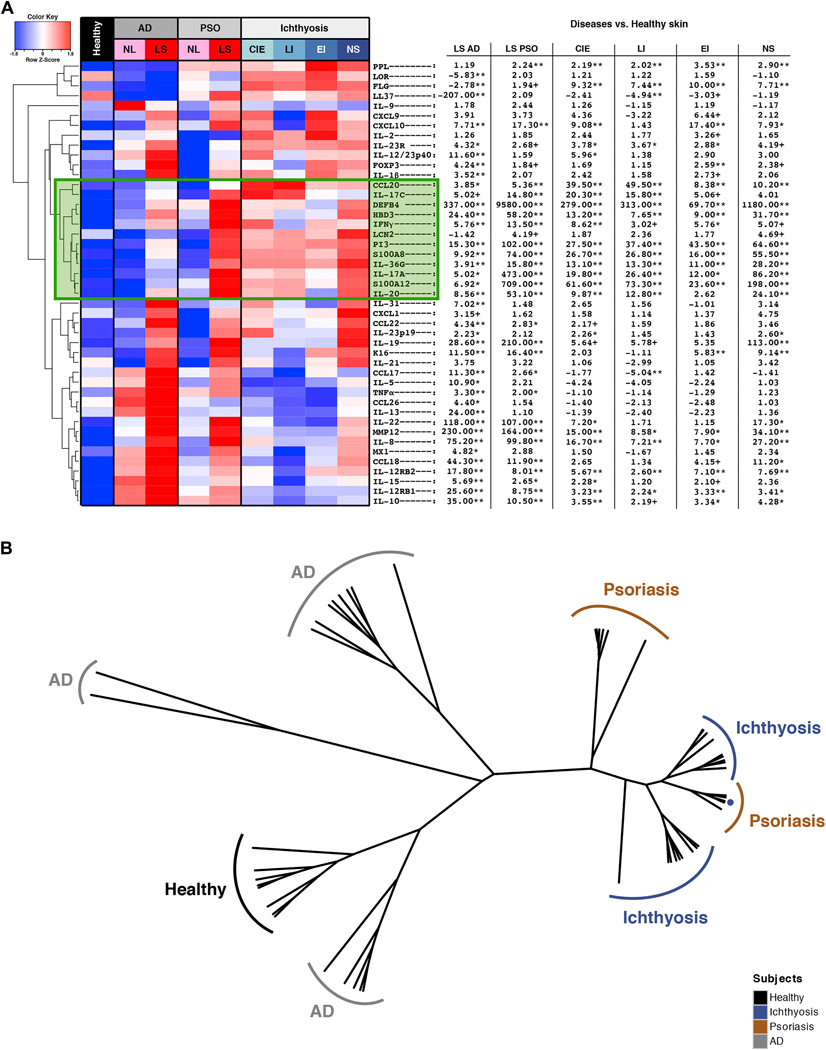
Unsupervised hierarchical clustering of mRNA expression in AD, psoriasis, ichthyosis, and controls (A) as a heatmap with fold changes/FCHs between diseases and healthy skin. Green box: Cluster of upregulated IL-17-related genes in ichthyosis and psoriasis. +p<0.1, *p<0.05, **p<0.01. red, up-regulation; blue, down-regulation. (B) Unsupervised clustering of samples (phylogenetic tree) based on expression profiles of 45 immune/barrier markers; Distance: Pearson correlation, agglomeration: average.
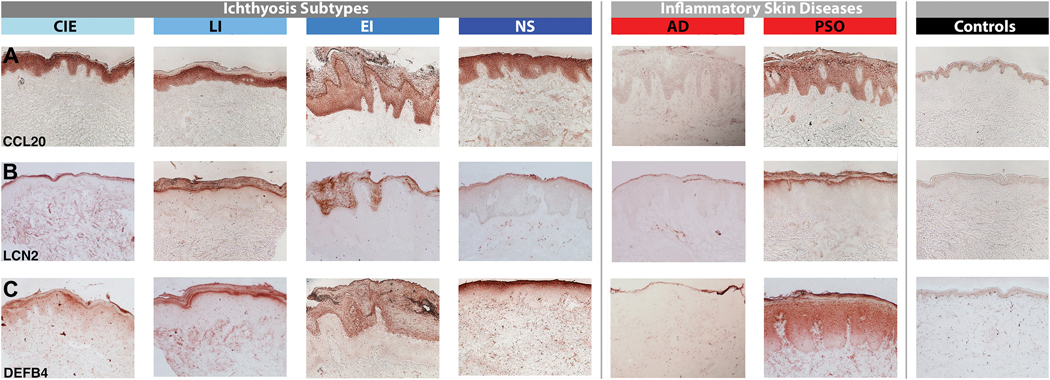
In contrast, Th17/IL-23 pathway genes were significantly induced, including those previously reported as synergistically or additively regulated by IL-17 and TNFα (highlighted by green boxes in Figs. 3–4A, and E3).72 IL-17A, p19 and p40 IL-23 subunits, IL-20, IL-23R, and IL-17-induced chemokines (i.e., HBD3) were significantly elevated, and up-regulation of IL-17/TNFα-synergistic/additive genes (IL-19, IL-17C, IL-36G/IL1F9, PI3, CCL20, DEFB4, S100A9) was particularly striking. NS showed the highest induction of Th17 pathway genes among ichthyosis subgroups, including the largest expression of IL-19, which is induced by both Th17 and Th2 cytokines, and in turn amplifies the IL-17 effects in keratinocytes (Figs. 3–4A).73–77 Although not directly induced by TNFα, IL-19 is synergistically induced by IL-17 and TNFα.72 Many IL-17-related factors, including those displaying a synergistic/additive effect with TNFα, showed comparable or even higher (IL-17C, CCL20, IL-36G) up-regulation in ichthyosis compared to lesional psoriasis (Figs. 3–4A). While IL-22/Th22 was only mildly elevated in NS and CIE, the S100As (S100A8/9/12), induced by both IL-17 and IL-22,78 showed significant increases in ichthyosis versus controls. A cluster of IL-17-related and IL-17/TNFα-synergistic/additive genes (IL-17A/C, lipocalin-2/LCN2, S100A8/12, IL-36G, IL-20, PI3) was up-regulated in ichthyosis to a similar extent as in psoriasis (and much higher than AD; highlighted green box in Fig. 4A). All RT-PCR values and comparisons are listed in Table E4.
To further evaluate how ichthyosis profiles relate phenotypically to psoriasis, we performed an unsupervised hierarchical clustering of ichthyosis, AD, psoriasis, and control skin using expression profiles of all markers evaluated by qRT-PCR. Results are represented as a phylogenetic tree (Fig. 4B) showing the tight clustering of ichthyosis and psoriasis lesions, while controls and AD are much farther. Of note, ichthyosis tissues did not sub-cluster by subtype.
Erythema and disease severity highly correlate with IL-17 activation in ichthyosis
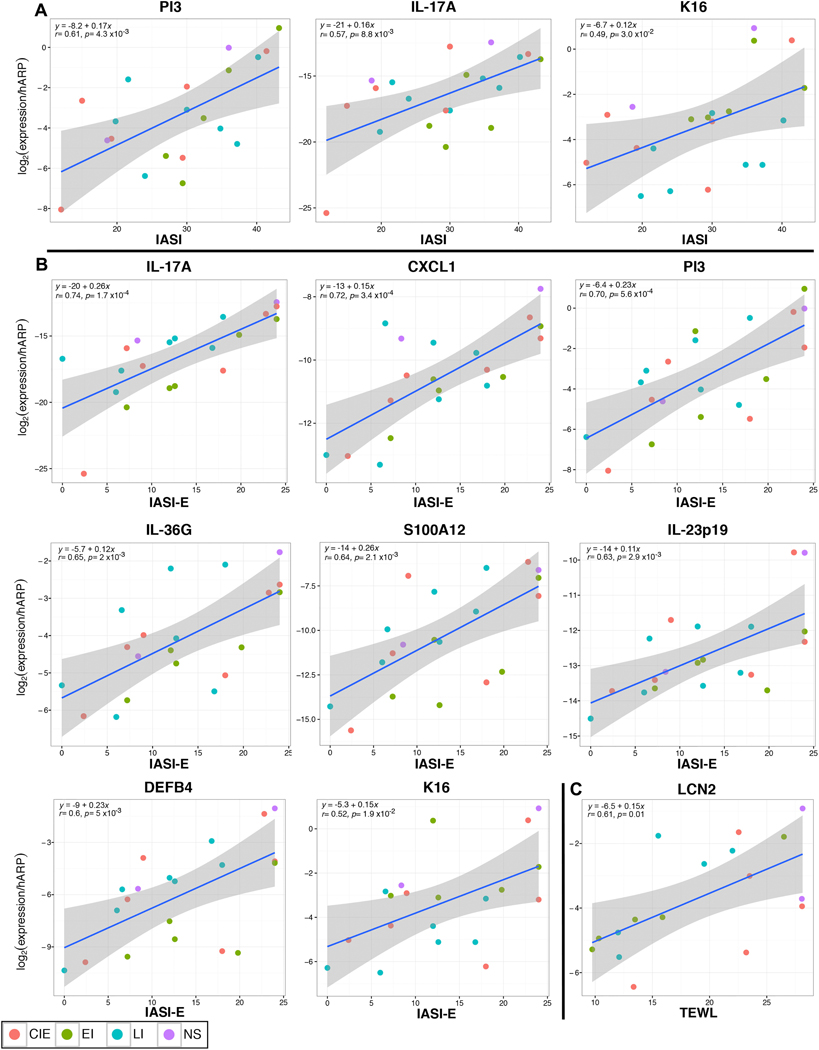
To determine how clinical severity, as measured by total IASI and its sub-scores, IASI-E (erythema/inflammation) and IASI-S (scaling), is linked to individual cellular or molecular markers, we used Pearson correlation-coefficients. Markers showing the highest correlations with total IASI score included IASI-E (r=0.74), IL-17A (r=0.57), IL-17-related markers (i.e., PI3, r=0.61) and the proliferation marker K16 (r=0.49) (p<0.03; Fig. 5A, Table E5). Highly significant correlations were found between IASI-E and IL-17A (r=0.74) and IL-17-related or IL-17/TNF-synergistic genes (CXCL1, PI3, IL-36G, S100As; IL-23p19, DEFB4, LCN2; P<0.005). Significant correlations were also noted between IASI-E and K16 and other immune (IL-1β) or cellular (DC-LAMP+) markers (Fig. 5B, Table E5). IASI-S was significantly correlated only with IASI. The 5-D Itch scale showed few, non-significant correlations. TEWL showed significant correlations with many IL-17-related markers (i.e IL-17A/IL-17-C, LCN2, CXCL1) and with TNFα (Fig. 5C and Table E5).
Figure 5.
Pearson correlation plots of the mRNA gene expression that correlated highest with (A) overall clinical severity score (IASI), (B) erythema severity subscore (IASI-E), and (C) TEWL in ichthyosis subtypes. r = Pearson correlation-coefficient with associated p value (p). y = equation for linear regression (blue line) with its confidence interval (smoothed confidence interval in grey).
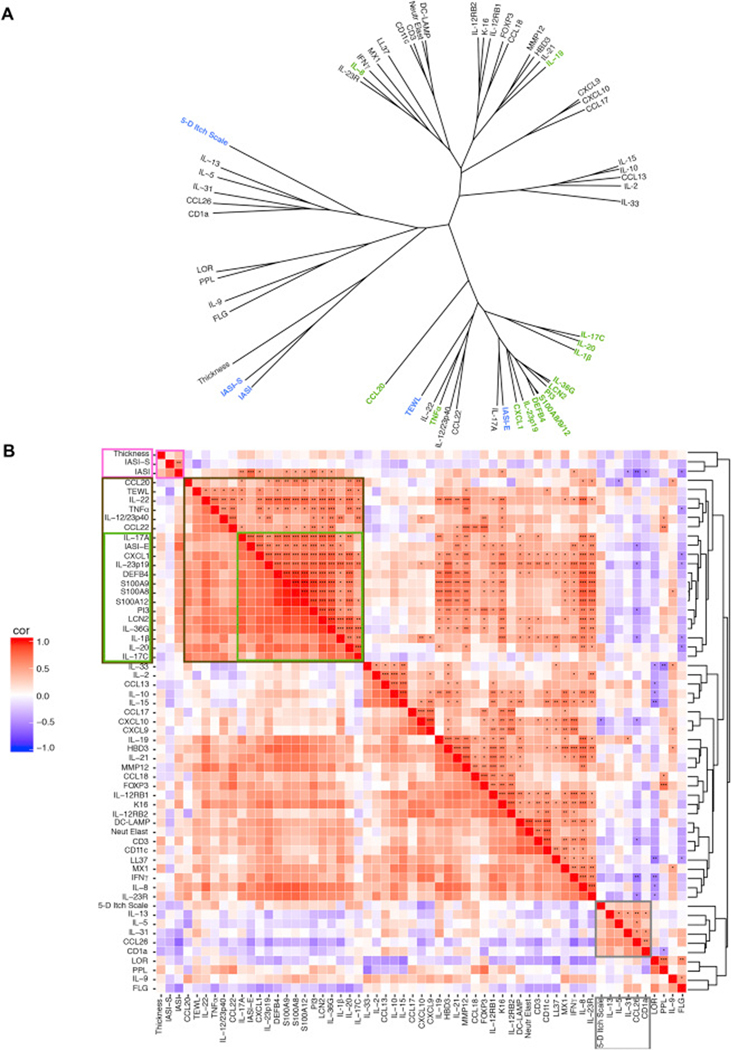
To evaluate how different clinical scores (IASI, IASI-E, IASI-S, pruritus, TEWL) relate to biomarkers, we performed unsupervised hierarchical clustering of clinical scores (blue), cell counts, thickness measurements, and mRNA expression (black; and green: IL-17/TNF-synergistic/additive genes) for all ichthyosis subtypes using Pearson correlation as a similarity metric and Mcquitty as an agglomeration algorithm. A graphic representation of the distance between variables is presented as a phylogenetic tree, with closer distances reflecting higher correlations (Fig. 6A). A tight cluster was found between IASI-E and IL-17-induced or IL-17/TNFα-synergistically modulated markers (IL-17A, CXCL1, DEFB4, PI3, etc), supporting the link between IL-17 activation and ichthyosis erythema. In proximity to this cluster are two clusters of IL-17/IL-23/TNFα-related genes (IL-22, IL-12/23p40 and IL-17C, IL-20; Fig. 6A). TEWL clustered with IL-22, TNFα and close to IL-17 markers, and the thickness measure clustered with IASI-S and close to terminal differentiation markers (LOR, LOR, PPL), reflecting a possible link between barrier and immune measures. The 5-D Itch Scale closely clustered with Th2 markers, including IL-13, IL-5, the itch cytokine IL-31,79,80 and CCL26. Markers of T-cells (CD3+), DCs (CD11c+, DC-LAMP+), and neutrophils clustered together, and in proximity to a large cluster of IL-17/IL-23-related and other immune genes (Fig. 6A).
Figure 6.
Correlation matrix of all ichthyosis measurements. (A) Unsupervised hierarchical clustering of clinical (blue), with barrier/immune markers (black), including IL-17-synergistic/additive genes (green). Distance: Pearson correlation, algorithm: Mcquitty agglomeration. (B) Correlation heatmap. Pink box: correlations with IASI score. Brown box: the most significant cluster of IL-17- synergistic/additive genes, with green box highlighting the IASI-E sub-cluster. Grey box: pruritus correlations. red, positive correlations; blue, negative correlations. *p<0.05, **p<0.01, ***p<0.001.
These data are also presented as a heatmap showing positive (red) or negative (blue) correlations of all molecular, cellular measures, and clinical measures in ichthyosis patients (Fig. 6B), with color intensity reflecting the correlation’s strength. A green box shows the associations of IASI-E with IL-17A and other IL-17-related genes (that clustered together in the phylogenetic tree). Associations with scaling/thickness, TEWL, and 5-D Itch Scale are highlighted by pink, brown and grey boxes, respectively. Individual correlations with clinical scores are in Table E5.
Discussion
The ichthyoses are rare life-altering genetic disorders, characterized by scaling, epidermal thickening, and erythema.1,5 Available treatments for ichthyosis (primarily oral retinoids) are unsatisfactory, lack specificity, and are associated with potential side effects. These treatments are primarily focused on reducing thickening and scaling, without addressing the erythema or inflammatory component.12,15
Few studies have evaluated the role of immune dysregulation in ichthyosis patients32–34 with the underlying molecular basis predominantly based on limited data from in vitro and animal models.6,18–30 These models observed pro-inflammatory signals, with increases in cytokines (IL-1, TNFα) and chemokines (S100As, CXCL1, TSLP, PAR2), and parallel epidermal hyperplasia (increases in K16, K6B) and abnormalities in differentiation (LOR, FLG) and lipid genes.21,23,29,30,81 Mouse models of NS showed diverse cytokine activation with increases in innate, Th2, Th17 and Th22 cytokines (IL-1β, TNFα, IL-4, Il-13, IL-17, IL-22) and corresponding chemokines (TSLP, CCL17, CXCL1, CCL20, S100A8/9).19 Moreover, inhibition of inflammation in model systems, using IL-37b overexpression and IL-1 blockade, considerably improved the epidermal phenotype, including the hyperplasia and aberrant differentiation.21,30 The few investigations with human ichthyotic skin focused primarily on barrier alterations (hyperplasia, premature expression of terminal differentiation products and lipid defects).31–37 Selected polar cytokines (i.e IFNγ, TNFα, IL-17, IL-8, IL-23, IL-9, IL-4, IL-5) have been found to be increased in keratinocytes and/or peripheral blood from ichthyosis patients38–41 and immune-modulators (retinoids, biologics, oral prednisone) decreased the expression of some of these cytokines (i.e IL-17, TNFα).32–34
The erythema, hyperkeratosis, and compromised barrier of ichthyosis are shared features with two common skin disorders, psoriasis and AD. In these diseases, inflammatory responses play an important role in disease progression.44,46,50–52,82–86 Advances in understanding pathogenesis have translated into rapid development of cytokine-targeted therapeutics, which reverse the clinical inflammation but also the epidermal disease phenotype.82,84–94 Pathogenesis-based therapies are available for psoriasis based on its Th17/IL-23-centered activation. TNFα has been functionally linked to the Th17/IL-23 pathway, and TNF antagonists are highly effective for psoriasis.72,95 Furthermore, psoriasis treatment with etanercept, an anti-TNF, suppresses genes that are synergistically induced by IL-17 and TNF to a greater extent than TNFα-regulated genes alone.72
This is the first comprehensive molecular profiling of ichthyosis subtypes. Since ichthyoses share clinical and histological characteristics with AD and psoriasis (i.e., inflammation, epidermal hyperplasia and compromised barrier), we also compared the cutaneous signatures of major ichthyosis subgroups (ARCI-CIE, ARCI-LI, EI, and NS) with lesional and non-lesional skin from moderate-to-severe AD and psoriasis patients, as well as skin from healthy volunteers. This approach allowed determination of cytokine pathway up-regulation in ichthyosis and comparison with AD (primarily Th2-driven) and psoriasis (primarily Th17–23 driven). Our data show that all ichthyoses share impressive Th17/IL-23-skewing in skin. Similar to psoriasis, particularly large increases were observed in IL-17/TNF-synergistic/additive markers (IL-19, IL-17C, IL-36G, PI3, S100A12, CCL20), despite non-significant TNFα modulation. IL-36G has been reported to amplify TNFα and IL-17 pathways in psoriasis, and to accurately differentiate psoriasis from AD lesions.96 The induction of genes modulated by IL-17 alone or IL-17 and TNFα together was largely comparable to psoriasis,97,98 perhaps leading to tight clustering of ichthyoses and psoriasis samples. This was unexpected, given the pruritic nature of ichthyoses (especially CIE, NS) and their clinical resemblance to AD.99 However, mRNAs of Th2 markers (e.g., IL-5, IL-13, IL-31, CCL17, CCL26) in all ichthyosis subsets were surprisingly low. The only exception was NS, in which some Th2 markers (CCL18, CCL22) were elevated. The concomitant increases of Th17- and Th2-related markers in NS might also contribute to the large increases in IL-19 in this subtype, given that Th17, but also Th2 cytokines can induce IL-19.73–77 IL-19 induces epidermal hyperplasia and S100As,73–77 which were highest in NS. Interestingly, although mouse NS models demonstrate increased Th2/Th17 responses,6,19,20 Th2 inhibition through PAR2/TSLP suppression did not improve cutaneous inflammation.29 Expression of Th1 markers varied in different ichthyoses, but was mostly lower than in AD and psoriasis. Increases in innate markers (IL-1β, IL-8) were also seen in ichthyoses and largely comparable with those in AD and psoriasis.
Importantly, the significant elevations in IL-17- and IL-17/TNF-modulated genes are strongly correlated with clinical severity. IASI, and particularly the erythema subscore/IASI-E was highly correlated with IL-17A and IL-17/TNF-regulated genes (CXCL1, IL-36G). Significant correlations were also found between IASI and IASI-E and epidermal hyperplasia, as measured by K16.65,100 While in AD and psoriasis, epidermal hyperplasia is linked to IL-22, IL-22 activation was far lower in ichthyosis than AD or psoriasis. Other hyperplasia-inducing IL-20 family cytokines (i.e., IL-19) might contribute to increased epidermal thickness in ichthyosis.101–104
The ichthyoses are recognized as having significant epidermal abnormalities,66,70,105,106 including epidermal hyperplasia, higher TEWL, and lipid and differentiation abnormalities.1,3 Hyperplasia and differentiation abnormalities, particularly in ARCI and NS subtypes, are similar to psoriasis, with hyperplastic epidermis and largely increased expression of differentiation proteins (LOR, FLG, PPL) in the upper epidermis.31,42,107 We too found higher expression of these markers in ichthyosis and psoriasis, but much reduced expression in AD. The significant correlations between TEWL with IL-17A and IL-17/TNF-regulated genes (IL-17C, CXCL1, LCN2, IL-36G) may link the immune activation and functional barrier abnormalities in ichthyosis. Finally, similar to psoriasis, ichthyosis patients are able to mount significant IL-17-induced antimicrobial peptide/AMP responses, as shown by high expression of LL37, DEFB4B, HBD3, and CCL20. AMPs were recently shown108,109 to up-regulate tight junctions and keratinocyte differentiation, and may further explain increases in these products in ichthyosis and psoriasis vs. AD patients. Indeed, although Staphylococcus aureus infections occur more often in ichthyosis than in psoriasis, their frequency is considerably less than in severe AD.97,110
Targeting the Th17/IL23 pathway in psoriasis and Th2 pathway in AD has not only suppressed lesional inflammation, but has also improved the epidermal pathology.44,50,52,68,83–85,92,111,112 Future studies using IL-17/IL-23 or TNF-targeting strategies are needed to determine the contribution of immune activation in ichthyosis, and whether the barrier abnormalities can be ameliorated when inflammation is reduced. Interestingly, pruritus, although described in >70% of subjects, was not linked to IL-17A-related markers, and clustered with Th2 cytokines, including IL-31, a cytokine correlated with itch in AD.79,113,114 Whether anti-IL-17/IL-23/TNF-targeting strategies could also reduce itch also remains a question.
Limitations of our data include the small sample size, reflecting the rarity of ichthyoses. Nevertheless, a large and significant effect was observed for IL-17-modulated or IL-17/TNF-synergistically regulated markers and their association with disease severity. Also, although 25% of controls were children ≥10 years old, the age difference between ichthyosis and healthy subjects was statistically significant. However, IL-17 expression increases with age in healthy skin,115,116 suggesting that our results might actually underestimate the increased Th17 activation in ichthyosis. Furthermore, our AD and psoriasis samples were obtained from individuals ≥18 years old, although 8/21 of ichthyosis subjects were <18yo. Although the adolescent skin phenotype in AD and psoriasis is commonly considered close to adults, there are no studies comparing the two. Thus, for proper comparisons, all our analyses were age-adjusted. Future studies should address the effect of age on the observed differences.
Our data links Th17/IL-23 pathway and IL-17/TNF synergistic interactions with ichthyosis severity and inflammation, providing evidence that ichthyosis more closely resembles psoriasis in its immune profile. The linkage between immune alterations and functional barrier abnormalities in ichthyoses potentially suggests a similar model to psoriasis and AD, in which increased cytokine production perpetuates the barrier alterations. These results imply that psoriasis therapeutics might be applicable for ichthyosis patients. One NS patient demonstrated clinical improvement and reduction in levels of IL-17 after administration of infliximab (anti-TNFα used to treat psoriasis).32,117,118 IL-17/IL-23-targeting strategies,92,111,119,120 have been shown to be more effective than TNFα inhibitors in psoriasis, dramatically improving PASI scores.90,121 We propose that specific IL-17/IL-23-targeting might establish a novel shared treatment paradigm for the ichthyoses, and will further elucidate the underlying molecular basis and role of IL-17 activation.
Supplementary Material
Table 2.
Comparison of ichthyosis patients vs. healthy controls and subjects with AD or psoriasis.
| Comparison of ichthyosis patients vs. healthy controls and subjects with AD or psoriasis. | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Parameter | Controls (n=16) | Ichthyosis (n=21; LS=21) | P-value Controls vs. Ichthyosis | AD (n=16; LS=16; NL=16) | PSO (n=10; LS=10; NL=10) | P-value All groups |
| Age (years) | Mean (±SD) | 38.7 ± 17.1 | 26.8 ± 14.6 | 0.033 | 52.8 ± 13.1 | 51.3 ± 11.0 | < 0.001 |
| Median [age range] | 44.5 [10.6–57] | 23.0 [10.8–57] | 49.5 [33–73] | 54.0 [30–64] | |||
| Gender | Female | 7 | 13 | 0.444 | 8 | 4 | 0.609 |
| Male | 9 | 8 | 8 | 6 | |||
| Race | White | 13 | 17 | 0.269 | 16 | 10 | 0.201 |
| Black | 0 | 2 | 0 | 0 | |||
| Asian | 0 | 1 | 0 | 0 | |||
| Hispanic | 3 | 1 | 0 | 0 | |||
| Disease Severity Scores | IASI | SCORAD | PASI | ||||
| Mean (±SD) | N/A | 28.9 ± 9.0 | 56.6 ± 10.7 | 20.3 ± 15.4 | |||
| Median [IQR] | N/A | 29.7 [21.2 – 36.0] | 55.0 [51.5 – 63.0] | 14.2 [12.1 – 21.2] | |||
AD: atopic dermatitis; CIE: congenital ichthyosiform erythroderma; EI: erythrodermic ichthyosis; NS: Netherton syndrome; IASI: ichthyosis area severity index (with E: erythema; S: scaling); LI: lamellar ichthyosis; LS: lesionai; N/A: not applicable. NL: non- lesional; PASI: psoriasis area severity index; PSO: psoriasis; SCORAD: scoring atopic dermatitis; SD: standard deviation; TEWL: transepidermal water loss.
Acknowledgements:
We thank Drs. Adam Berry, Jayla Gray, Isabel Haugh, Anjali Shroff, and Robalee Wanderman for helping with patient enrollment. This research was supported by the Foglia Family Foundation Endowment and the National Psoriasis Foundation (RF fellowship). We are grateful to the Foundation for Ichthyosis and Related Skin Types for allowing this research to be performed in part at its Family Conference in 2014. We acknowledge Core resources provided by the Northwestern University (NU) Skin Disease Research Center (NIAMS P30AR057216).
Funding: Supported by the Foglia Family Foundation Endowment and the National Psoriasis Foundation (RF fellowship).
Disclosures: JGK has received research support (grants paid to his institution) and/or personal fees from Pfizer, Amgen, Janssen, Lilly, Merck, Novartis, Kadmon, Dermira, Boehringer, Innovaderm, Kyowa, BMS, Serono, BiogenIdec, Delenex, AbbVie, Sanofi, Baxter, Paraxel, Xenoport, and Kineta. EGY is a board member for Sanofi Aventis, Regeneron, Stiefel/GlaxoSmithKline, MedImmune, Celgene, Anacor, AnaptysBio, Celsus, Dermira, Galderma, Glenmark, Novartis, Pfizer, Vitae and Leo Pharma; has received consultancy fees from Regeneron, Sanofi, MedImmune, Celgene, Stiefel/GlaxoSmithKline, Celsus, BMS, Amgen, Drais, AbbVie, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, LEO Pharma, Novartis, Pfizer, Vitae, Mitsubishi Tanabe and Eli Lilly; and has received research support from Janssen, Regeneron, Celgene, BMS, Novartis, Merck, LEO Pharma and Dermira. MSF has received research support from Pfizer and Quorum Consulting. ASP is an investigator for AbbVie, Anacor, Astellas, Celgene and LEO, and a consultant for Amgen, Celgene, Anacor, Galderma, GSK-Stiefel, Lilly, Novartis, Regeneron, and Vitae Pharmaceuticals. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations:
- AD
Atopic dermatitis
- AMP
Antimicrobial peptides
- ARCI
Autosomal recessive congenital ichthyosis
- CIE
Congenital ichthyosis erythroderma
- CISI
Congenital ichthyoses severity index
- DC
Dendritic cell
- EI
Epidermolytic ichthyosis
- FCH
Fold change
- FLG
Filaggrin
- hARP
Human acidic ribosomal protein
- IASI
Ichthyosis area severity index
- IASI-E
Ichthyosis area severity index-Erythema
- IASI-S
Ichthyosis area severity index-Scaling
- IHC
Immunohistochemistry
- K16
Keratin 16
- LC
Langerhans cells
- LCN2
lipocalin-2
- LEKI
Lympho-epithelial Kazal-type-related inhibitor
- LI
Lamellar ichthyosis
- LOD
Limit of detection
- LOR
Loricrin
- NRS
Numerical Rating Scale
- NS
Netherton syndrome
- PAR2
Protease-activated receptor 2
- PASI
Psoriasis area and severity index
- PPL
Periplakin
- qRT-PCR
Quantitative real-time PCR
- TEWL
Transepidermal water loss
- TSLP
Thymic stromal lymphopoietin
References
- 1.Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Soreze 2009. J Am Acad Dermatol 2010; 63:607–41. [DOI] [PubMed] [Google Scholar]
- 2.DiGiovanna JJ, Robinson-Bostom L. Ichthyosis: etiology, diagnosis, and management. Am J Clin Dermatol 2003; 4:81–95. [DOI] [PubMed] [Google Scholar]
- 3.Oji V, Traupe H. Ichthyoses: differential diagnosis and molecular genetics. Eur J Dermatol 2006; 16:349–59. [PubMed] [Google Scholar]
- 4.Craig WY, Roberson M, Palomaki GE, Shackleton CH, Marcos J, Haddow JE. Prevalence of steroid sulfatase deficiency in California according to race and ethnicity. Prenat Diagn 2010; 30:893–8. [DOI] [PubMed] [Google Scholar]
- 5.Traupe H, Fischer J, Oji V. Nonsyndromic types of ichthyoses - an update. J Dtsch Dermatol Ges 2014; 12:109–21. [DOI] [PubMed] [Google Scholar]
- 6.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med 2009; 206:1135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganemo A, Lindholm C, Lindberg M, Sjoden PO, Vahlquist A. Quality of life in adults with congenital ichthyosis. J Adv Nurs 2003; 44:412–9. [DOI] [PubMed] [Google Scholar]
- 8.Dreyfus I, Pauwels C, Bourrat E, Bursztejn AC, Maruani A, Chiaverini C, et al. Burden of inherited ichthyosis: a French national survey. Acta Derm Venereol 2015; 95:326–8. [DOI] [PubMed] [Google Scholar]
- 9.Williams ML. Ichthyosis: mechanisms of disease. Pediatr Dermatol 1992; 9:365–8. [DOI] [PubMed] [Google Scholar]
- 10.Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res 2008; 49:697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckl KM, Weindl G, Ackermann K, Kuchler S, Casper R, Radowski MR, et al. Increased cutaneous absorption reflects impaired barrier function of reconstructed skin models mimicking keratinisation disorders. Exp Dermatol 2014; 23:286–8. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Martin A, Aranegui B, Martin-Santiago A, Garcia-Doval I. A systematic review of clinical trials of treatments for the congenital ichthyoses, excluding ichthyosis vulgaris. J Am Acad Dermatol 2013; 69:544–9 e8. [DOI] [PubMed] [Google Scholar]
- 13.Vahlquist A, Ganemo A, Virtanen M. Congenital ichthyosis: an overview of current and emerging therapies. Acta Derm Venereol 2008; 88:4–14. [DOI] [PubMed] [Google Scholar]
- 14.Fleckman P Management of the ichthyoses. Skin Therapy Lett 2003; 8:3–7. [PubMed] [Google Scholar]
- 15.Digiovanna JJ, Mauro T, Milstone LM, Schmuth M, Toro JR. Systemic retinoids in the management of ichthyoses and related skin types. Dermatol Ther 2013; 26:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halverstam CP, Vachharajani A, Mallory SB. Cushing syndrome from percutaneous absorption of 1% hydrocortisone ointment in Netherton syndrome. Pediatr Dermatol 2007; 24:42–5. [DOI] [PubMed] [Google Scholar]
- 17.Allen A, Siegfried E, Silverman R, Williams ML, Elias PM, Szabo SK, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol 2001; 137:747–50. [PubMed] [Google Scholar]
- 18.Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet 2005; 37:56–65. [DOI] [PubMed] [Google Scholar]
- 19.Furio L, de Veer S, Jaillet M, Briot A, Robin A, Deraison C, et al. Transgenic kallikrein 5 mice reproduce major cutaneous and systemic hallmarks of Netherton syndrome. J Exp Med 2014; 211:499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furio L, Pampalakis G, Michael IP, Nagy A, Sotiropoulou G, Hovnanian A. KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome. PLoS Genet 2015; 11:e1005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Shaughnessy RF, Choudhary I, Harper JI. Interleukin-1 alpha blockade prevents hyperkeratosis in an in vitro model of lamellar ichthyosis. Hum Mol Genet 2010; 19:2594–605. [DOI] [PubMed] [Google Scholar]
- 22.Jensen JM, Schutze S, Neumann C, Proksch E. Impaired cutaneous permeability barrier function, skin hydration, and sphingomyelinase activity in keratin 10 deficient mice. J Invest Dermatol 2000; 115:708–13. [DOI] [PubMed] [Google Scholar]
- 23.Roth W, Kumar V, Beer HD, Richter M, Wohlenberg C, Reuter U, et al. Keratin 1 maintains skin integrity and participates in an inflammatory network in skin through interleukin-18. J Cell Sci 2012; 125:5269–79. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberger S, Dick A, Latzko S, Hausser I, Stark HJ, Rauh M, et al. A mouse organotypic tissue culture model for autosomal recessive congenital ichthyosis. Br J Dermatol 2014; 171:1347–57. [DOI] [PubMed] [Google Scholar]
- 25.Krieg P, Rosenberger S, de Juanes S, Latzko S, Hou J, Dick A, et al. Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J Invest Dermatol 2013; 133:172–80. [DOI] [PubMed] [Google Scholar]
- 26.Zuo Y, Zhuang DZ, Han R, Isaac G, Tobin JJ, McKee M, et al. ABCA12 maintains the epidermal lipid permeability barrier by facilitating formation of ceramide linoleic esters. J Biol Chem 2008; 283:36624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyth I, Hacking DF, Hilton AA, Mukhamedova N, Meikle PJ, Ellis S, et al. A mouse model of harlequin ichthyosis delineates a key role for Abca12 in lipid homeostasis. PLoS Genet 2008; 4:e1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aufenvenne K, Rice RH, Hausser I, Oji V, Hennies HC, Rio MD, et al. Long-term faithful recapitulation of transglutaminase 1-deficient lamellar ichthyosis in a skin-humanized mouse model, and insights from proteomic studies. J Invest Dermatol 2012; 132:1918–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briot A, Lacroix M, Robin A, Steinhoff M, Deraison C, Hovnanian A. Par2 inactivation inhibits early production of TSLP, but not cutaneous inflammation, in Netherton syndrome adult mouse model. J Invest Dermatol 2010; 130:2736–42. [DOI] [PubMed] [Google Scholar]
- 30.Cottle DL, Ursino GM, Ip SC, Jones LK, Ditommaso T, Hacking DF, et al. Fetal inhibition of inflammation improves disease phenotypes in harlequin ichthyosis. Hum Mol Genet 2015; 24:436–49. [DOI] [PubMed] [Google Scholar]
- 31.Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, D’Alessio M, et al. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J Invest Dermatol 2006; 126:1622–32. [DOI] [PubMed] [Google Scholar]
- 32.Fontao L, Laffitte E, Briot A, Kaya G, Roux-Lombard P, Fraitag S, et al. Infliximab infusions for Netherton syndrome: sustained clinical improvement correlates with a reduction of thymic stromal lymphopoietin levels in the skin. J Invest Dermatol 2011; 131:1947–50. [DOI] [PubMed] [Google Scholar]
- 33.Yalcin AD. A case of netherton syndrome: successful treatment with omalizumab and pulse prednisolone and its effects on cytokines and immunoglobulin levels. Immunopharmacol Immunotoxicol 2016; 38:162–6. [DOI] [PubMed] [Google Scholar]
- 34.Pavez Lorie E, Ganemo A, Borgers M, Wouters L, Blockhuys S, van de Plassche L, et al. Expression of retinoid-regulated genes in lamellar ichthyosis vs. healthy control epidermis: changes after oral treatment with liarozole. Acta Derm Venereol 2009; 89:12–20. [DOI] [PubMed] [Google Scholar]
- 35.Hannula-Jouppi K, Laasanen SL, Ilander M, Furio L, Tuomiranta M, Marttila R, et al. Intrafamily and Interfamilial Phenotype Variation and Immature Immunity in Patients With Netherton Syndrome and Finnish SPINK5 Founder Mutation. JAMA Dermatol 2016; 152:435–42. [DOI] [PubMed] [Google Scholar]
- 36.de Oliveira GV, Hawkins HK, Guedes AC, Pinto LF, Oliveras G, Kitten GT, et al. Comel-Netherton syndrome in brothers and expression of cytokeratins. J Am Acad Dermatol 2005; 52:725–6. [DOI] [PubMed] [Google Scholar]
- 37.Konishi T, Tsuda T, Sakaguchi Y, Imai Y, Ito T, Hirota S, et al. Upregulation of interleukin-33 in the epidermis of two Japanese patients with Netherton syndrome. J Dermatol 2014; 41:258–61. [DOI] [PubMed] [Google Scholar]
- 38.Van Gysel D, Koning H, Baert MR, Savelkoul HF, Neijens HJ, Oranje AP. Clinico-immunological heterogeneity in Comel-Netherton syndrome. Dermatology 2001; 202:99–107. [DOI] [PubMed] [Google Scholar]
- 39.Renner ED, Hartl D, Rylaarsdam S, Young ML, Monaco-Shawver L, Kleiner G, et al. Comel-Netherton syndrome defined as primary immunodeficiency. J Allergy Clin Immunol 2009; 124:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosomi N, Fukai K, Nakanishi T, Funaki S, Ishii M. Caspase-1 activity of stratum corneum and serum interleukin-18 level are increased in patients with Netherton syndrome. Br J Dermatol 2008; 159:744–6. [DOI] [PubMed] [Google Scholar]
- 41.Akagi A, Kitoh A, Moniaga CS, Fujimoto A, Fujikawa H, Shimomura Y, et al. Case of Netherton syndrome with an elevated serum thymus and activation-regulated chemokine level. J Dermatol 2013; 40:752–3. [DOI] [PubMed] [Google Scholar]
- 42.Lacroix M, Lacaze-Buzy L, Furio L, Tron E, Valari M, Van der Wier G, et al. Clinical expression and new SPINK5 splicing defects in Netherton syndrome: unmasking a frequent founder synonymous mutation and unconventional intronic mutations. J Invest Dermatol 2012; 132:575–82. [DOI] [PubMed] [Google Scholar]
- 43.Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol 2013; 34:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol 2015; 135:324–36. [DOI] [PubMed] [Google Scholar]
- 45.Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013; 133:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang CQ, Suarez-Farinas M, Nograles KE, Mimoso CA, Shrom D, Dow ER, et al. IL-17 induces inflammation-associated gene products in blood monocytes, and treatment with ixekizumab reduces their expression in psoriasis patient blood. J Invest Dermatol 2014; 134:2990–3. [DOI] [PubMed] [Google Scholar]
- 47.Kamalpour L, Rice ZP, Pavlis M, Veledar E, Chen SC. Reliable methods to evaluate the clinical severity of ichthyosis. Pediatr Dermatol 2010; 27:148–53. [DOI] [PubMed] [Google Scholar]
- 48.Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica 1978; 157:238–44. [DOI] [PubMed] [Google Scholar]
- 49.Noda S, Suarez-Farinas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol 2015; 136:1254–64. [DOI] [PubMed] [Google Scholar]
- 50.Khattri S, Shemer A, Rozenblit M, Dhingra N, Czarnowicki T, Finney R, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol 2014; 133:1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson-Huang LM, Suarez-Farinas M, Sullivan-Whalen M, Gilleaudeau P, Krueger JG, Lowes MA. Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J Invest Dermatol 2010; 130:2654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tintle S, Shemer A, Suarez-Farinas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol 2011; 128:583–93 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhingra N, Suarez-Farinas M, Fuentes-Duculan J, Gittler JK, Shemer A, Raz A, et al. Attenuated neutrophil axis in atopic dermatitis compared to psoriasis reflects TH17 pathway differences between these diseases. J Allergy Clin Immunol 2013; 132:498–501 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esaki H, Ewald DA, Ungar B, Rozenblit M, Zheng X, Xu H, et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J Allergy Clin Immunol 2015; 135:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suarez-Farinas M, Ungar B, Correa da Rosa J, Ewald DA, Rozenblit M, Gonzalez J, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allergy Clin Immunol 2015; 135:1218–27. [DOI] [PubMed] [Google Scholar]
- 56.Czarnowicki T, Gonzalez J, Bonifacio KM, Shemer A, Xiangyu P, Kunjravia N, et al. Diverse activation and differentiation of multiple B-cell subsets in patients with atopic dermatitis but not in patients with psoriasis. J Allergy Clin Immunol 2016; 137:118–129 e5. [DOI] [PubMed] [Google Scholar]
- 57.Taieb A, Labreze C. Collodion baby: what’s new. J Eur Acad Dermatol Venereol 2002; 16:436–7. [DOI] [PubMed] [Google Scholar]
- 58.Van Gysel D, Lijnen RL, Moekti SS, de Laat PC, Oranje AP. Collodion baby: a follow-up study of 17 cases. J Eur Acad Dermatol Venereol 2002; 16:472–5. [DOI] [PubMed] [Google Scholar]
- 59.Russell LJ, DiGiovanna JJ, Rogers GR, Steinert PM, Hashem N, Compton JG, et al. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet 1995; 9:279–83. [DOI] [PubMed] [Google Scholar]
- 60.Eckl KM, de Juanes S, Kurtenbach J, Natebus M, Lugassy J, Oji V, et al. Molecular analysis of 250 patients with autosomal recessive congenital ichthyosis: evidence for mutation hotspots in ALOXE3 and allelic heterogeneity in ALOX12B. J Invest Dermatol 2009; 129:1421–8. [DOI] [PubMed] [Google Scholar]
- 61.Fischer J Autosomal recessive congenital ichthyosis. J Invest Dermatol 2009; 129:1319–21. [DOI] [PubMed] [Google Scholar]
- 62.Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet 2000; 25:141–2. [DOI] [PubMed] [Google Scholar]
- 63.Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, Jayakumar A, et al. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol 2006; 126:1609–21. [DOI] [PubMed] [Google Scholar]
- 64.Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol 2010; 162:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mansouri Y, Guttman-Yassky E. Immune Pathways in Atopic Dermatitis, and Definition of Biomarkers through Broad and Targeted Therapeutics. J Clin Med 2015; 4:858–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suarez-Farinas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 2011; 127:954–64 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rice RH, Bradshaw KM, Durbin-Johnson BP, Rocke DM, Eigenheer RA, Phinney BS, et al. Distinguishing ichthyoses by protein profiling. PLoS One 2013; 8:e75355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol 2014; 134:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol Biol Cell 2007; 18:3607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guttman-Yassky E, Suarez-Farinas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol 2009; 124:1235–44 e58. [DOI] [PubMed] [Google Scholar]
- 71.Suarez-Farinas M, Lowes MA, Zaba LC, Krueger JG. Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA). PLoS One 2010; 5:e10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 2011; 131:677–87. [DOI] [PubMed] [Google Scholar]
- 73.Witte E, Kokolakis G, Witte K, Philipp S, Doecke WD, Babel N, et al. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol 2014; 134:2757–67. [DOI] [PubMed] [Google Scholar]
- 74.Liao SC, Cheng YC, Wang YC, Wang CW, Yang SM, Yu CK, et al. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol 2004; 173:6712–8. [DOI] [PubMed] [Google Scholar]
- 75.Hsing CH, Hsu CC, Chen WY, Chang LY, Hwang JC, Chang MS. Expression of IL-19 correlates with Th2 cytokines in uraemic patients. Nephrol Dial Transplant 2007; 22:2230–8. [DOI] [PubMed] [Google Scholar]
- 76.Gallagher G, Eskdale J, Jordan W, Peat J, Campbell J, Boniotto M, et al. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol 2004; 4:615–26. [DOI] [PubMed] [Google Scholar]
- 77.Huang F, Wachi S, Thai P, Loukoianov A, Tan KH, Forteza RM, et al. Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma. J Allergy Clin Immunol 2008; 121:1415–21, 1421 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 2008; 159:1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006; 117:411–7. [DOI] [PubMed] [Google Scholar]
- 80.Cedeno-Laurent F, Singer EM, Wysocka M, Benoit BM, Vittorio CC, Kim EJ, et al. Improved pruritus correlates with lower levels of IL-31 in CTCL patients under different therapeutic modalities. Clin Immunol 2015; 158:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hewett DR, Simons AL, Mangan NE, Jolin HE, Green SM, Fallon PG, et al. Lethal, neonatal ichthyosis with increased proteolytic processing of filaggrin in a mouse model of Netherton syndrome. Hum Mol Genet 2005; 14:335–46. [DOI] [PubMed] [Google Scholar]
- 82.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–84. [DOI] [PubMed] [Google Scholar]
- 83.Chiricozzi A, Saraceno R, Chimenti MS, Guttman-Yassky E, Krueger JG. Role of IL-23 in the pathogenesis of psoriasis: a novel potential therapeutic target? Expert Opin Ther Targets 2014; 18:513–25. [DOI] [PubMed] [Google Scholar]
- 84.Hamilton JD, Suarez-Farinas M, Dhingra N, Cardinale I, Li X, Kostic A, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2014; 134:1293–300. [DOI] [PubMed] [Google Scholar]
- 85.Hamilton JD, Ungar B, Guttman-Yassky E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy 2015; 7:1043–58. [DOI] [PubMed] [Google Scholar]
- 86.Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014; 371:130–9. [DOI] [PubMed] [Google Scholar]
- 87.Thaci D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016; 387:40–52. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Ferrer A, Vilarrasa E, Puig L. Secukinumab (AIN457) for the treatment of psoriasis. Expert Rev Clin Immunol 2015; 11:1177–88. [DOI] [PubMed] [Google Scholar]
- 89.Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015; 386:541–51. [DOI] [PubMed] [Google Scholar]
- 90.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med 2014; 371:326–38. [DOI] [PubMed] [Google Scholar]
- 91.Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005; 152:1304–12. [DOI] [PubMed] [Google Scholar]
- 92.Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2015; 136:116–24 e7. [DOI] [PubMed] [Google Scholar]
- 93.Lebwohl M Psoriasis. Lancet 2003; 361:1197–204. [DOI] [PubMed] [Google Scholar]
- 94.Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol 2012; 132:304–14. [DOI] [PubMed] [Google Scholar]
- 95.Zaba LC, Suarez-Farinas M, Fuentes-Duculan J, Nograles KE, Guttman-Yassky E, Cardinale I, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol 2009; 124:1022–10 e1–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Erme AM, Wilsmann-Theis D, Wagenpfeil J, Holzel M, Ferring-Schmitt S, Sternberg S, et al. IL-36gamma (IL-1F9) is a biomarker for psoriasis skin lesions. J Invest Dermatol 2015; 135:1025–32. [DOI] [PubMed] [Google Scholar]
- 97.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol 2008; 181:7420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiricozzi A, Nograles KE, Johnson-Huang LM, Fuentes-Duculan J, Cardinale I, Bonifacio KM, et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One 2014; 9:e90284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samuelov L, Sprecher E. Peeling off the genetics of atopic dermatitis-like congenital disorders. J Allergy Clin Immunol 2014; 134:808–15. [DOI] [PubMed] [Google Scholar]
- 100.de Mare S, van Erp PE, Ramaekers FC, van de Kerkhof PC. Flow cytometric quantification of human epidermal cells expressing keratin 16 in vivo after standardized trauma. Arch Dermatol Res 1990; 282:126–30. [DOI] [PubMed] [Google Scholar]
- 101.He M, Liang P. IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis. J Immunol 2010; 184:1793–8. [DOI] [PubMed] [Google Scholar]
- 102.Wang F, Lee E, Lowes MA, Haider AS, Fuentes-Duculan J, Abello MV, et al. Prominent production of IL-20 by CD68+/CD11c+ myeloid-derived cells in psoriasis: Gene regulation and cellular effects. J Invest Dermatol 2006; 126:1590–9. [DOI] [PubMed] [Google Scholar]
- 103.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005; 174:3695–702. [DOI] [PubMed] [Google Scholar]
- 104.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 2007; 178:2229–40. [DOI] [PubMed] [Google Scholar]
- 105.Ye L, Lv C, Man G, Song S, Elias PM, Man MQ. Abnormal epidermal barrier recovery in uninvolved skin supports the notion of an epidermal pathogenesis of psoriasis. J Invest Dermatol 2014; 134:2843–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takahashi H, Tsuji H, Minami-Hori M, Miyauchi Y, Iizuka H. Defective barrier function accompanied by structural changes of psoriatic stratum corneum. J Dermatol 2014; 41:144–8. [DOI] [PubMed] [Google Scholar]
- 107.Kawashima J, Akiyama M, Takizawa Y, Takahashi S, Matsuo I, Shimizu H. Structural, enzymatic and molecular studies in a series of nonbullous congenital ichthyosiform erythroderma patients. Clin Exp Dermatol 2005; 30:429–31. [DOI] [PubMed] [Google Scholar]
- 108.Akiyama T, Niyonsaba F, Kiatsurayanon C, Nguyen TT, Ushio H, Fujimura T, et al. The human cathelicidin LL-37 host defense peptide upregulates tight junction-related proteins and increases human epidermal keratinocyte barrier function. J Innate Immun 2014; 6:739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kiatsurayanon C, Niyonsaba F, Smithrithee R, Akiyama T, Ushio H, Hara M, et al. Host defense (Antimicrobial) peptide, human beta-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J Invest Dermatol 2014; 134:2163–73. [DOI] [PubMed] [Google Scholar]
- 110.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002; 347:1151–60. [DOI] [PubMed] [Google Scholar]
- 111.Sofen H, Smith S, Matheson RT, Leonardi CL, Calderon C, Brodmerkel C, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol 2014; 133:1032–40. [DOI] [PubMed] [Google Scholar]
- 112.Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol 2005; 175:2721–9. [DOI] [PubMed] [Google Scholar]
- 113.Rabenhorst A, Hartmann K. Interleukin-31: a novel diagnostic marker of allergic diseases. Curr Allergy Asthma Rep 2014; 14:423. [DOI] [PubMed] [Google Scholar]
- 114.Sokolowska-Wojdylo M, Glen J, Zablotna M, Rebala K, Sikorska M, Florek A, et al. Association of distinct IL-31 polymorphisms with pruritus and severity of atopic dermatitis. J Eur Acad Dermatol Venereol 2013; 27:662–4. [DOI] [PubMed] [Google Scholar]
- 115.Dunbar GC, Kuchibhatla RV, Lee G, Group T-ACS. A randomized double-blind study comparing 25 and 50 mg TC-1734 (AZD3480) with placebo, in older subjects with age-associated memory impairment. J Psychopharmacol 2011; 25:1020–9. [DOI] [PubMed] [Google Scholar]
- 116.Lee JS, Lee WW, Kim SH, Kang Y, Lee N, Shin MS, et al. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol 2011; 140:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001; 357:1842–7. [DOI] [PubMed] [Google Scholar]
- 118.Schopf RE, Aust H, Knop J. Treatment of psoriasis with the chimeric monoclonal antibody against tumor necrosis factor alpha, infliximab. J Am Acad Dermatol 2002; 46:886–91. [DOI] [PubMed] [Google Scholar]
- 119.Russell CB, Rand H, Bigler J, Kerkof K, Timour M, Bautista E, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol 2014; 192:3828–36. [DOI] [PubMed] [Google Scholar]
- 120.Chiricozzi A, Krueger JG. IL-17 targeted therapies for psoriasis. Expert Opin Investig Drugs 2013; 22:993–1005. [DOI] [PubMed] [Google Scholar]
- 121.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010; 362:118–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.