Abstract
Mechanical ventilation in recent years has benefited from the development of new techniques and interfaces. These developments allowed clinicians to offer increasingly personalised therapies with the combination of different complementary techniques for treating respiratory insufficiency in patients with neuromuscular diseases. The mouthpiece ventilation, intermittent abdominal pressure ventilator and the negative pressure ventilation can offer many patients alternative therapy options when ventilation is required for many hours a day. In this non-systematic review, we will highlight the use of alternative methods to non-invasive mechanical ventilation at positive pressure in neuromuscular patients, to ensure the optimal interface for each patient.
Key words: mouth-piece ventilation, negative pressure ventilation, daytime non-invasive ventilatory support
Introduction
The birth of non-invasive mechanical ventilation (NIV) occurred in the late 1920s, following the poliomyelitis epidemic that was notable for respiratory muscle paralysis and subsequent death in many children. Mechanical ventilation was in the form of the iron lung, a non-invasive negative-pressure respirator developed by Philip Drinker and Charles McKhann 1. Motley et al. investigated the use of intermittent positive pressure ventilation in the form of expiratory positive airway pressure (EPAP) and continuous positive airway pressure (CPAP) via a rubber mask for the treatment of acute pulmonary edema, pneumonia, Guillain-Barre syndrome, near-drowning, drug overdose, and acute asthma 2,3. In 1980, Sullivan et al. described the successful use of CPAP via nasal mask in the management of obstructive sleep apnea 4. Subsequently, its use was extended to the chronic respiratory failure from neuromuscular disease (NMD) and symptomatic worsening nocturnal hypoventilation 5. During the 1990s, the Consensus Conference recognized non-invasive ventilation as a valuable and essential strategy in the managing of subjects with acute respiratory failure 6-8.
Acute respiratory failure is a frequent life-threatening problem of acute onset NMD and may exacerbate chronic hypoventilation in patients with NMD or chest wall disorders 9,10.
Respiratory care is of high importance because it is a main determinant of quality of life and survival 11. NIV is one of the limited modalities that has shown a survival benefit in the NMD patient population. Newer modes with smart technologies are being developed to assist in better ventilation 12. These developments allowed clinicians to offer increasingly personalised therapies, with the combination of different complementary techniques for treating respiratory insufficiency in these patients, who often require 24 hours non-invasive mechanical ventilation or tracheostomy 9-10.
MouthPiece Ventilation (MPV) 13, Intermittent Abdominal Pressure Ventilator (IAPV) 14 and Negative Pressure Ventilation (NPV) 15 can offer many patients the option of an alternative therapy when ventilation is required for many hours a day. The ability to alternate complementary techniques to NIV, may be a viable alternative to tracheotomy.
Various conditions such as claustrophobia, skin lesions induced by the mask, rhinitis, or no tolerance to the face’s pressure may be responsible for the failure of NIV, therefore alternative NIV techniques should be considered in highly dependent ventilator patients, besides the traditional ventilation with a mask.
The aim of this non-systematic review is to highlight the use of alternative methods of non-invasive respiratory support to positive pressure NIV in neuro-muscular patients.
Mouthpiece ventilation
MPV is a type of non-invasive ventilation delivered – as the name implies – via a mouthpiece. It is used for many years, and there is already evidence in literature documenting the effectiveness of the treatment and greater patient compliance 13.
The use of the mouthpiece was first described in 1953 in patients with polio, and to date many cases have been documented in the literature of successful treatment. However only one center has documented 500 cases of long-term survival for daytime use in patients requiring 24-hour ventilator support up to 1993 16,17. Surprisingly, this technology is still not commonly used. There were no evidence-based guidelines for this technique, that is applied on the basis of the experience of few centers until 2020, when the European Neuromuscular Centre (ENMC) Respiratory Therapy Consortium, during the 252nd ENMC International Workshop developed the “best practice guidelines for management of mouthpiece ventilation in neuromuscular disorders” 18.
The mouthpiece ventilation is used with single non-vented circuit ventilators in pressure-controlled or, more frequently, in volume-controlled mode to allow air stacking 19. The patient can achieve mouthpiece ventilation, breathe passively using the backup rate set on the ventilator, or actively trigger the breath, retain a part or all, of the delivered volume. Different types of triggers are available. In addition to the traditional flow or pressure trigger, normally used for NIV, the “Kiss trigger” is available on a portable ventilator (Trilogy, Philips Respironics, Murrysville, PA, USA). Such a dedicated MPV trigger allows for activation of inspiration when the patient’s lips touch the mouthpiece. It is possible to use a simple single-tube circuit or a circuit with a valve 20. The valve is preferred for patients who cannot disconnect to exhale outside the circuit and in this way can remain connected for a long time in succession, avoiding the rebreathing of carbon dioxide. Dedicated MPV mode has been introduced on many portable devices; it is possible to set the type of circuit selected and then select the pressure or volume mode, and the parameters chosen for the patient. In this way, the patient is able to independently remove the mouthpiece to speak, eat, cough, or call a family member. Its use presents no risk of skin breakdown, conjunctivitis, does not induce claustrophobia while causing a lower probability of gastric distension 21.
Despite these obvious advantages, this modality of ventilation is not commonly used. Mouthpieces for daytime use may cause salivation and more rarely vomiting while prolonged use can cause orthodontic deformities after 20 years 22.
However, the same problem was found with the traditional interface in pediatric patients. Nasal pledges or nose clips can prevent air leak through the nares for patients using lip cover interfaces for the NIV mouthpiece while sleeping 13. During the nighttime sleep, most patients use a mask because the mouthpiece requires collaboration and is uncomfortable. Moreover, though rarely, the air can also be ingested causing gastric distension 19.
Different angled replacement mouthpiece 22 and 15 mm, and MPV straw kit are available 20. Mouthpiece and nasal NIV are open systems of ventilator support; the low-pressure alarms of ventilators not having mouthpiece NIV modes can often be inactivate. Backpressure from a 15 mm angled mouthpiece is sufficient to prevent a low-pressure alarm set at 2 cmH2O.
Carlucci et al. studied how to set different types of the ventilator when using the mouthpiece 12. They found that a proper alarm setting, and a combination of VT and TI would allow most ventilators to be used for mouthpiece ventilation without the alarm activation 21.
The patient triggers the breath by placing lip on the mouthpiece and generating a small negative pressure in the circuit, by tasting or inspiring. The mouthpieces are very useful as additional daytime ventilation in patients with neuromuscular diseases, who do not have the capacity to preserve acceptable diurnal blood gas without frequent intermittent periods of care 16-18,22-24.
Some authors report that patients that used MPV were satisfied and preferred the mouthpiece to the nasal mask 22. Though this aspect can favour NIV adherence, however, it exposes the patient to the risk of underventilation because of frequent disconnection from the mouthpiece 14. Underventilation with hypoxemia and hypercapnia can be tolerated by the patient for a short time, for which he himself feels the need to reconnect. The mouthpiece allows support ventilation with the possibility of consecutive detachments, for speaking or eating. Desaturations during MPV are possible, as well as for mechanical mask ventilations, due to increased resistance (secretions) and excessive system leaks. For example, MPV-dedicated mode without backup respiratory rate may be beneficial in less-dependent patients (frequent disconnections), while severe ventilator-dependent patients may take greater advantage of a more reactive ventilator, with greater rapidity in adjusting tidal volume and setting back up rate 25.
Just like masked NIV, the patient should be monitored periodically to identify any progression of the disease and the need for therapeutic changes. The time of interruption is probably the major limitation of this approach to NIV. It has been documented that the periods of disconnection are associated with > 5 mmHg paCO2 increase and > 2% spO2 decrease, but no medical complication occurred before or after the monitoring time. Few patients accepted prolonged disconnections without developing hypercapnia 23.
The most common type of asynchrony was an ineffective effort, suggesting a need to improve trigger sensitivity. The newly introduced MPV software that allows the insufflation to be triggered only by positioning the patient’s lips appears to be a useful option for patients with severe muscle weakness 23. The most commonly used ventilation mode is assisted volume- and pressure-controlled with no expiratory positive airway pressure, with the low-pressure alarm set to apnea minimum and maximum duration 23-25.
The MPV characteristics, such as the intermittent disconnection of the patient and the presence of continuous leaks, may represent a challenge for turbine-based home ventilators. There are considerable differences in the ability of the different life-support ventilators to cope with the rapidly evolving respiratory load features that characterise MPV, which can be further accentuated by choice of ventilator settings. It is always needed to carefully monitor the patient during the adaption phase as MPV requires a real patient’s collaboration. Not all ventilators guarantee a rapid adaption to the patient’s breaths 25.
The physician should also evaluate the patient’s ability to synchronise with the mouthpiece held in the mouth, and whether or not to exhale outside the mouthpiece. Depending on the ability to turn the neck, the subject can uninterruptedly keep the mouthpiece between lips or leave it for a variable time 18. Patient’s limiting factors include inability to close one’s mouth to seal the interface, inability to move the neck, impaired bulbar function, non-acceptance to try MPV, lack of available interfaces / equipment, absence of caregivers who can guarantee the change with NIV if necessary (Tab. I). For these reasons, and because of its specific features and drawbacks such as air leaks, MPV must be managed by expert hands, and well-monitored (Tab. II).
Table I.
Indication and contraindication of MPV use.
| Indication | Contraindication |
|---|---|
| Diurnal respiratory support needed | Inability to close mouth to seal the interface |
| Dyspnoea persistent | Inability to move the neck |
| Weight loss | Impaired bulbar function |
| Adaptation to any NIV | Non-acceptance to try MPV |
| Daytime fatigue or hypercapnia | Poor compliance |
| Weaning from invasive mechanical ventilation | Lack of available interfaces/equipment |
| Request for autonomy by the patient | absence of caregivers who can change with NIV |
Table II.
Mode and setting of MPV.
| Pressure mode | Volume mode |
|---|---|
| ST, PSV | ACV |
| With dedicated mode | With or without dedicated mode |
| Pressure 10-14 cmH2O | VC 700-1500 ml |
| EPAP 0 | EPAP 0 |
| Back up frequency (as needed) | Back up frequency (as needed) |
| Inspiratory time 0.8-1.3 sec | Inspiratory time 0.8-1.3 sec |
Some authors described the use of mouthpiece in a cohort of patients affected by kyphoscoliosis and acute respiratory failure. They showed an improvement in clinical symptoms, blood gases and nocturnal ventilation, sleep related parameters, and HRQL scores. These improvements were accompanied by a significant increase in lung volumes and respiratory muscle function following diurnal ventilation via angled mouthpiece, alternated with nocturnal ventilation via mask 26.
Applications in clinical practice
Amyotrophic lateral sclerosis (ALS)
ALS is a progressive neuromuscular disease characterised by lower motor neuron and upper motor neuron dysfunction. Although clinical presentations can differ, there is no therapy for ALS, and the disease is generally terminal, with most patients dying of respiratory problems. Patients die within 3 to 5 years of diagnosis, unless they choose to undergo tracheostomy, in which case, they may live, on average, 2 additional years 27,28.
Data in literature confirmed the useful of MPV in ALS 29. Bach et al. 30 reported that mouthpiece ventilation was an effective alternative to tracheostomy in patients with adequate bulbar muscle function. In patients using NIV many hours a day or showing low NIV tolerance with oronasal and nasal masks, or skin lesions, eye irritation, or gastric distention, mouthpiece ventilation should be taken into account 31. Patients using ventilation even during the night can alternate between daytime MPV and a sleeping interface. Use of mouthpiece in ALS patients may be limited by the involvement of bulbar muscles, or by deterioration of cognitive status; furthermore, disease progression may render MPV ineffective 32. However it has been reported that MPV, while having no impact on survival, improves the quality of life of the patient with ALS 33.
Duchenne muscular dystrophy
Duchenne muscular dystrophy is a rare genetic neuromuscular disorder, due to mutations in the DMD gene, that affects skeletal and heart muscles causing muscle wasting and cardiomyopathy. Chronic respiratory failure is a constant feature in patients with DMD 34, who often require continuous ventilation and need respiratory support 24h a day. McKim et al. 35 argue that 24h NIV should be considered a safe alternative to tracheostomy in these patients, especially in those presenting skin lesions, gastric distension, or eye irritation. They examined the impact of diurnal mouthpiece intermittent positive pressure ventilation and concluded that it is safe, stabilises vital capacity and improves survival. The mouthpiece can be very valuable, in patients who use NIV many hours a day, alternating between nasal masks and full-face masks. It is also useful to promote adherence to NIV. 24
Myotonic dystrophy type 1
Myotonic dystrophy type 1 (DM1) or Steinert disease is the most common type of muscular dystrophy in adults, and presents multiple organ symptoms, including respiratory dysfunction. As a cause of respiratory dysfunction in DM1, a restrictive ventilatory pattern due to respiratory muscle weakness and central nervous system’s involvement has been reported, requiring non-invasive mechanical ventilation 36.
There are few data on the use of MPV in patients with Steinert disease. It could be useful for patients who previously refused NIV for tightness, claustrophobia, and poor compliance interface. MPV was successfully used in our practice in patients who yet refused nasal, oral or oro-nasal interface 37.
Other neuromuscular diseases
Bach et al. reported a large number of patients with neuromuscular diseases, long managed with 24hours NIV 30. They describe non-invasive acute and long-term management of patients with quadriplegia due to high spinal cord lesions. This includes full-setting, continuous ventilatory support by non-invasive intermittent positive pressure ventilation to sustenance inspiratory muscles and mechanically assisted coughing to support inspiratory and expiratory muscles. Even patients previously ventilated 24h/24h via tracheostomy were converted to non-invasive mechanical ventilation with MPV 30,35. Bilateral diaphragmatic paralysis (BDP) is usually associated with dyspnoea that worsens when the patient is recumbent, increasing breathing and exercise intolerance. With the BDP progression, there is an increase in ventilatory failure with hypoxaemia and hypercapnia, which can further worsen due to atelectasis and ventilation-perfusion mismatch. Reports are showing that MPV is a clinically beneficial treatment to improve exercise tolerance and exercise-induced dyspnoea in patients with BDP 38. MPV may also be useful for weaning from orotracheal tube or tracheostomy (Fig. 1).
Figure 1.
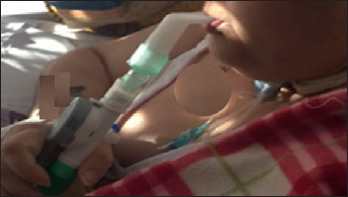
MPV for weaning from tracheostomy.
Intermittent abdominal pressure ventilator (IAPV)
Intermittent abdominal pressure ventilator was first described in 1935 by R.W. Paul for adults and young patients who require continuous respiratory support 39. In 1938 it was described for the treatment of post-diphtheritic respiratory paralysis or respiratory paralysis due to anterior poliomyelitis 40. Over the years, an alternative approach to NIV with IAPV was described in patients with spinal cord injury 41. Later Bach, in 1991, described the long-term use of IAPV in 209 patients diagnosed with myopathy, Duchenne dystrophy and spinal cord injury 14.
This approach was used in several types of neuromuscular patients: ventilator-dependent traumatic quadriplegic patients, spinal cord injured, non-Duchenne myopathy, Duchenne muscular dystrophy, myelopathy, polymyositis and Friedreich’s ataxia for long-term respiratory support 14,42-45. The Authors conclude that, in general, patients with traumatic high level spinal cord injury are the best candidates to benefit from these techniques because of their youth, intact mental status and bulbar musculature, absence of obstructive lung disease.
The new IAPV (LunaBelt, Dima, Italia) consists of a corset with an elastic inflatable bladder that fits over the abdomen. A hose attaches the bladder to a ventilator that gives up to 2.5 liter of air to the bladder and the abdominal wall (Fig. 2). This raises the diaphragm to cause expiration below the functional residual capacity. The new models that prevent clothing taking on the corset buckles, are more comfortable, lightweight, suitable, easy to make and put on and use Velcro for fastening 42. The following IAPV parameters can be set: Pressure inside the bladder, Tinsp (real inspiratory time when the diaphragm moves down), Frequency (respiratory rate), and Rise Time (time to inflate the bladder). The IAPV only works efficiently when patients are in sitting position, at an angle of 30° or greater with the optimum at 75°. No guidelines are available on the use of IAPV and on the parameters to be set, the indications usually derive from case reports and experience (Tab. III).
Figure 2.
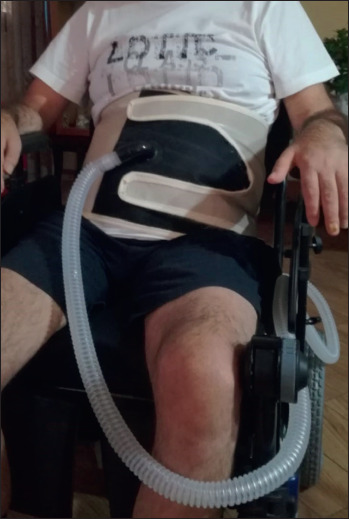
Patient during IAPV.
Table III.
IAPV indications and contraindications.
| Indications | Contraindications |
|---|---|
| Daytime respiratory support needed | Inability to posture trunk of at least 30° |
| Adaptation to any NIV Diaphragmatic weakness |
Intolerance of corset Severe sacral decubitus |
| Weaning from invasive mechanical ventilation Request of autonomy by patient |
Hiatal hernia with regurgitation during meals Recent abdominal surgery |
Applications in clinical practice
The use of IAPV is reported with success in patients with a post-ischemic cervical myelopathy 42 and in ALS patients with tracheostomy by De Mattia et al. 44 IPAV permitted optimal speech, efficient diurnal ventilatory pattern, good pulmonary gas exchange without dyspnoea, and a significant improvement in the management of salivary secretions, with a reduction in the number of tracheal aspirations. Furthermore, the Authors reported the resumption of the spontaneous respiratory activity, which demonstrates an improvement in the patient’s respiratory condition 42-44. IAPV facilitates diaphragmatic motion and may be particularly useful in patients with bilateral diaphragmatic weakness or paralysis, and allows for plugging of the tracheostomy tube with the cuff deflating for several hours during the day, thus preventing tracheal damage.
Pierucci et al. described the case of a young patient with late onset Pompe disease who was successfully treated with nocturnal NIV and daytime IAPV 45.
IAPV can also be used in patients who require NIV many hours a day. Patients with gastric distension may benefit from the abdominal compression exerted by the device during the exhalation phase 42. Disadvantages can be food regurgitation during meals (rarely), locking of clothing on straps and Velcro fasteners, redness of bony prominences, and inability to shower or bathe while using it 46,47. Indications and contraindications are described in Table IV. Furthermore, regular follow-up is required as it can become less effective over time 42,47.
Table IV.
NPV indications and contraindications.
| Indications | Contraindications |
|---|---|
| Severe facial decubitus | Sleep-apnoea syndrome |
| Mask intolerance | Severe obesity |
| Facial deformity | Severe kyphoscoliosis |
| Inability to fit mask | Rib fractures |
| Severe hypercapnic encephalopathy | Recent abdominal surgery |
| Severe respiratory acidosis | Claustrophobia or poor compliance |
IAVP can be less effective for the appearance of gastric complications, the worsening of respiratory function due to the evolution of the disease, and the need for invasive support.
Negative pressure ventilation
Negative pressure ventilation (NPV) has played a crucial role in the history of ventilatory support for patients with neuromuscular diseases and respiratory failure. A full-body type ventilator was the first description of a negative-pressure ventilator. The first “tank ventilator” was described by Dalziel in 1838. It was an airtight box, where the patient remained in a sitting position 48.
A pinnacle of negative-pressure ventilation appears with the development of the iron lung, originally designed and built by Drinker and Shaw 49, but manufactured and sold by Emerson during polio epidemics around the world, from 1930 to 1960. Numerous other types were developed over time, such as the “raincoat” and the “chest cuirass”. However, due to several factors, in the 1960s, there was a movement away from negative-pressure ventilation (excessive leaks; difficult time to maintain effective ventilation, inability to sustain high airway pressure or establish EPAP, limited access to the patients) 50.
This technique has some strengths as it is able to guarantee a breathing completely analogous to the natural one, consisting of an inspiratory phase followed by the expiratory phase. Both phases are applied by means of a negative pressure ventilator and some accessories connected to it, such as a cuirass or a poncho. The ventilator first applies a negative pressure forcing the movement of the diaphragm downwards while the rib muscles tend to enlarge the thorax: this process generates lung expansion by generating a lower intrathoracic pressure than the external one; subsequently, the ventilator exerts positive pressure forcing the air inside the chamber, to compress the chest and empty the lungs 51. The cuirass negative pressure ventilators were primarily beneficial in children with neuromuscular disorders. Children had their own cuirass built from a plaster prototype of the chest and abdomen. This was important when there was a severe thoracic scoliosis. The cuirass is a plastic model of the front and sides of the trunk, the edges are padded with airtight material and the cuirass attached to the patient with a back strap. Cuirass pressure injuries are also possible. Cuirass ventilators are easy to put on and suitable for home use (Fig. 3).
Figure 3.
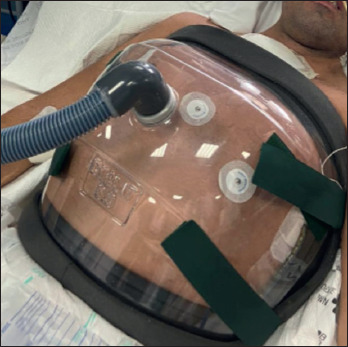
Negative pressure ventilation.
The last new soft cuirass (Dima Italia, Negavent - Pegaso Vent) is an accessory for negative ventilation, designed to ensure a good quality of life and normal daily activities. It is a structure that creates a ventilation chamber on the chest. On the edges, it is covered with a soft gasket to ensure patient comfort and low pressure losses. It is available in various sizes and the choice of size depends on the size of the chest, body structure, weight, and height of the patient, and of any deformities of the chest such as scoliosis. Generally new cuirasses are necessary as the patient grows 52.
Kavanagh et al. hypothesized that, compared with positive pressure ventilation, negative pressure translates in a greater functional residual capacity at the same transpulmonary pressure, and results in a greater oxygenation with the same residual capacity. NPV may distend lungs fundamentally differently to positive pressure, resulting in more homogeneous ventilation, less injury, and superior oxygenation 51,53.
The data showed that negative-pressure ventilation produces superior oxygenation unrelated to lung perfusion which may be explained by more effective lung volume inflation during both inspiration and expiration 53.
NPV may preserve physiological functions, such as speech, cough, swallowing and feeding and its major advantage is the prevention of endotracheal intubation and its related problems.
A limitation is the lack of upper airway protection, particularly in comatose and/or neurological patients, which may result in aspiration, considering the described impact of NPV on the lower esophageal sphincter 50,54. Upper airway obstruction can occur in unconscious patients, in patients with neurologic disorders with bulbar dysfunction, and in those with sleep apnea syndrome. This event can be prevented by using concomitant nasal continuous positive airway pressure, although switching to non-invasive positive pressure ventilation may be more helpful in this situation.
Those who cannot tolerate a facial mask due to facial deformity, claustrophobia or excessive airway secretion, or young children, and in particular in children undergoing complex cardiac reconstructive surgery considering the beneficial effects on the cardiopulmonary circulation, and patients in whom excessive airway secretion or difficulty in wearing a mask limits the application of NIV are the best candidates for this type of ventilatory support 54.
The choice of the best negative pressure mechanical ventilation device depends on the indications and contraindications and varies among subjects. The main indications and contraindications are listed in Table V. There are no guidelines on the use of NPV nor on the parameters to be set (Tab. VI).
Table V.
IAPV parameters; we suggest starting: Pbelt 0-70 Hpa (at the beginning 30-40 Hpa); select desired Ti (during the Ti setted, the PBAir will be deflated, while the patient will be able to inhale); back up rate as desidered; rise time usually 1.0s; Expiratory time (abdominal compression) will be linked to the back up rate and inspiratory time setted. For example: setted inspiratory time 1.5 sec, Fr 15 bpm, derivative expiratory time will be 2.5 sec.
| Intermittent abdominal pressure ventilator (LunaBelt) | ||
|---|---|---|
| Mode | Timed | Spontaneous/timed |
| Pression belt | 0-70 hPa | 0-70 hPa |
| Time inspiratory | 0.3-5.0 sec | na |
| Time inspiratory minimum | na | 0.3-3.0 sec |
| Time inspiratory maximum | [(60/Freq) - 0.6 sec] | [(60/Freq - 0.6 sec)] |
| Time espiratory minimum | na | 0-1.5 sec |
| Back-up Frequency | 1-60 bpm | 1-60 bpm |
| Frequency maximum | [60/(Tinsp + 0.6 sec)] | [60/(Tinsp + 0,6 sec) |
| Rise time | 0.1-1.0 sec | 0.1-1.0 sec |
| Trigger inspiratory (nasal cannula) | na | Auto |
| Trigger espiratory (nasal cannula) | na | Auto |
Table VI.
NPV parameters; we suggest start: Inspiratory pressure of -20, Expiratory pressure from 0 to 5, I:E Ratio from 1:1 to 1:2, back up frequency: set frequency at 2-4 breaths above patient’s own spontaneous rate.
| Negative pressure ventilation (Negavent) | |||
|---|---|---|---|
| Mode | T (timed) | ST (spontaneous/timed) | Syncro (syncrhronized) |
| Inspiratory pressure | Da -5 a -90 hPa | Da -5 a -90 hPa | Da -5 a -90 hPa |
| Expiratory pressure | Da +25 a -25 hPa | Da +25 a -25 hPa | Da +25 a -25 hPa |
| Back-up frequency | 5-60 bpm | 5-60 bpm | 5-60 bpm |
| I/E ratio | 1.0:9.9-9.9:1.0 | 1.0:9.9-9.9:1.0 | 1.0:9.9-9.9:1.0 |
| Trigger inspiratory (nasal cannula) | na | 1;9 | 1;9 |
| Trigger espiratory (nasal cannula) | na | 1;9 | na |
Conclusions
The use of MPV, IAPV and NPV is limited to a few centres, likely for the long time required to adapt and monitor the patients. The different possibilities of non-invasive mechanical ventilation to ensure the optimal interface for different patients should be known and applied.
Our goal must be to ensure the best possible quality of life for our patients. However, lack of local resources can also interfere with the diffusion of innovative technologies. MPV and IAPV are comfortable alternative to NIV, but more active participation than traditional masks is required when using MPV. For subjects with chronic disease who need to initiate NIV, both systems should be considered. In fact, they are useful for promoting a positive approach to NIV or for alternating the interface in patients who require 24-hour ventilatory support 24,31,37,43,45.
NPV, alternating with other techniques or in addition in case of patients with congenital or acquired facial deformities or not tolerating positive pressure, may have still a role in the treatment of patients with neuromuscular disorders 52-54.
Figures and tables
References
- 1.Drinker PA, McKhann CF, 3rd. Landmark perspective: the iron lung. First practical means of respiratory support. JAMA 1986;255:1476-1480. https://doi.org/10.1001/jama.255.11.1476 10.1001/jama.255.11.1476 [DOI] [PubMed] [Google Scholar]
- 2.Motley HL, Counrad A. Intermittent positive pressure breathing; a means of administering artificial respiration in man. J Am Med Assoc 1948;137:370-382. https://doi.org/10.1001/jama.1948.82890380005011a 10.1001/jama.1948.82890380005011a [DOI] [PubMed] [Google Scholar]
- 3.Motley HL, Lang LP, Gordon B. Use of intermittent positive pressure breathing combined with nebulization in pulmonary disease. Am J Med 1948;5:853-856. https://doi.org/10.1016/0002-9343(48)90165-x 10.1016/0002-9343(48)90165-x [DOI] [PubMed] [Google Scholar]
- 4.Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-865. https://doi.org/10.1016/s0140-6736(81)92140-1 10.1016/s0140-6736(81)92140-1 [DOI] [PubMed] [Google Scholar]
- 5.Kerby GR, Mayer LS, Pingleton SK. Nocturnal positive pressure ventilation via nasal mask. Am Rev Respir Dis 1987;135:738-740. https://doi.org/10.1164/arrd.1987.135.3.738 10.1164/arrd.1987.135.3.738 [DOI] [PubMed] [Google Scholar]
- 6.Pierson DJ. History and epidemiology of noninvasive ventilation in the acute-care setting. Respir Care 2009;54:40-52. PMID: 19111105 [PubMed] [Google Scholar]
- 7.Bersten AD, Holt AW, Vedig AE, et al. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med 1991;325:1825-1830. https://doi.org/10.1056/NEJM199112263252601 10.1056/NEJM199112263252601 [DOI] [PubMed] [Google Scholar]
- 8.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995;333:817-822. https://doi.org/10.1056/NEJM199509283331301 10.1056/NEJM199509283331301 [DOI] [PubMed] [Google Scholar]
- 9.Voulgaris A, Antoniadou M, Agrafiotis M, et al. Respiratory involvement in patients with neuromuscular diseases: a narrative review. Pulm Med 2019;2019:2734054. https://doi.org/10.1155/2019/2734054 10.1155/2019/2734054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo F, Annane D, Orlikowski D, et al. Invasive versus non-invasive ventilation for acute respiratory failure in neuromuscular disease and chest wall disorders. Cochrane Database Syst Rev 2017;12:CD008380. https://doi.org/10.1002/14651858.CD008380.pub2 10.1002/14651858.CD008380.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahni AS, Wolfe L. Respiratory care in neuromuscular diseases. Respir Care 2018;63:601-608. https://doi.org/10.4187/respcare.06210 10.4187/respcare.06210 [DOI] [PubMed] [Google Scholar]
- 12.Rabinstein AA. Non-invasive ventilation for neuromuscular respiratory failure: when to use and when to avoid. Curr Opin Crit Care 2016;22:94-9. https://doi.org/10.1097/MCC.0000000000000284 10.1097/MCC.0000000000000284 [DOI] [PubMed] [Google Scholar]
- 13.Pinto T, Chatwin M, Banfi P, et al. Mouthpiece ventilation and complementary techniques in patients with neuromuscular disease: a brief clinical review and update. Chron Respir Dis 2017;14:187-193. https://doi.org/10.1177/1479972316674411 10.1177/1479972316674411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach JR, Alba AS. Intermittent abdominal pressure ventilator in a regimen of non-invasive ventilatory support. Chest 1991;99:630-636. https://doi.org/10.1378/chest.99.3.630 10.1378/chest.99.3.630 [DOI] [PubMed] [Google Scholar]
- 15.Thomson A. The role of negative pressure ventilation. Arch Dis Child 1997;77:454-458. https://doi.org/10.1136/adc.77.5.454 10.1136/adc.77.5.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach JR, Mehta AD. Respiratory muscle aids to avert respiratory failure and tracheostomy: a new patient management paradigm. J Neurorestoratol 2014;2:25. https://doi.org/10.2147/JN.S49488 10.2147/JN.S49488 [DOI] [Google Scholar]
- 17.Bach JR, Gonçalves MR, Hon A, et al. Changing trends in the management of end-stage neuromuscular respiratory muscle failure: recommendations of an international consensus. Am J Phys Med Rehabil 2013;92:267-277. https://doi.org/10.1097/PHM.0b013e31826edcf1 10.1097/PHM.0b013e31826edcf1 [DOI] [PubMed] [Google Scholar]
- 18.Chatwin M, Gonçalves M, Gonzalez-Bermejo J, et al. ENMC Respiratory Therapy Consortium . 252nd ENMC international workshop: developing best practice guidelines for management of mouthpiece ventilation in neuromuscular disorders. March 6th to 8th 2020, Amsterdam, the Netherlands. Neuromuscul Disord 2020;30:772-781. https://doi.org/10.1016/j.nmd.2020.07.008 10.1016/j.nmd.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garuti G, Nicolini A, Grecchi B, et al. Open circuit mouthpiece ventilation: concise clinical review. Rev Port Pneumol 2014;20:211-218. https://doi.org/10.1016/j.rppneu.2014.03.004 10.1016/j.rppneu.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 20.Ogna A, Prigent H, Falaize L, et al. Bench evaluation of commercially available and newly developed interfaces for mouthpiece ventilation. Clin Respir J 2018;12:890-894. https://doi.org/10.1111/crj.12601 10.1111/crj.12601 [DOI] [PubMed] [Google Scholar]
- 21.Carlucci A, Mattei A, Rossi V, et al. Ventilator settings to avoid nuisance alarms during mouthpiece ventilation. Respir Care 2016;61:462-467. https://doi.org/10.4187/respcare.04217 10.4187/respcare.04217 [DOI] [PubMed] [Google Scholar]
- 22.Khirani S, Ramirez A, Delord V, et al. Evaluation of ventilators for mouthpiece ventilation in neuromuscular disease. Respir Care 2014;59:1329-1337. https://doi.org/10.4187/respcare.03031 10.4187/respcare.03031 [DOI] [PubMed] [Google Scholar]
- 23.Nardi J, Leroux K, Orlikowski D, et al. Home monitoring of daytime mouthpiece ventilation effectiveness in patients with neuromuscular disease. Chron Respir Dis 2016;13:67-74. https://doi.org/10.1177/1479972315619575 10.1177/1479972315619575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorentino G, Annunziata A, Cauteruccio R, et al. Mouthpiece ventilation in Duchenne muscular dystrophy: a rescue strategy for noncompliant patients. J Bras Pneumol 2016;42:453-456. https://doi.org/10.1590/S1806-37562016000000050 10.1590/S1806-37562016000000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorentino G, Esquinas AM. Tidal volume during mouthpiece non-invasive home ventilation: when the choice is the right answer. Chron Respir Dis 2016;13:383-384. https://doi.org/10.1177/1479972316661927 10.1177/1479972316661927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolini A, Barlascini C, Piroddi IM, et al. Effectiveness and safety of mouthpiece ventilation and nocturnal non-invasive ventilation in patients with kyphoscoliosis: short and long-term outcomes after an episode of acute respiratory failure. Rev Port Pneumol 2016;22:75-81. https://doi.org/10.1016/j.rppnen.2015.09.009 10.1016/j.rppnen.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 27.Niedermeyer S, Murn M, Choi PJ. Respiratory failure in amyotrophic lateral sclerosis. Chest 2019;155:401-408. https://doi.org/10.1016/j.chest.2018.06.035 10.1016/j.chest.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 28.Hobson EV, McDermott CJ. Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat Rev Neurol 2016;12:526-538. https://doi.org/10.1038/nrneurol.2016.111 10.1038/nrneurol.2016.111 [DOI] [PubMed] [Google Scholar]
- 29.Bédard ME, McKim DA. Daytime mouthpiece for continuous non-invasive ventilation in individuals with amyotrophic lateral sclerosis. Respir Care 2016;61:1341-1348. https://doi.org/10.4187/respcare.04309 10.4187/respcare.04309 [DOI] [PubMed] [Google Scholar]
- 30.Bach JR, Alba AS, Saporito LR. Intermittent positive pressure ventilation via the mouth as an alternative to tracheostomy for 257 ventilator users. Chest 1993;103:174-182. https://doi.org/10.1378/chest.103.1.174 10.1378/chest.103.1.174 [DOI] [PubMed] [Google Scholar]
- 31.Fiorentino G, Annunziata A, Gaeta AM, et al. Continuous non-invasive ventilation for respiratory failure in patients with amyotrophic lateral sclerosis: current perspectives. Degener Neurol Neuromuscul Dis 2018;8:55-61. https://doi.org/10.2147/DNND.S170771 10.2147/DNND.S170771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorentino G, Esquinas AM. Continuous mouthpiece daytime amyotrophic lateral sclerosis in noninvasive ventilation: definitive solid therapy? Respir Care 2017;62:387. https://doi.org/10.4187/respcare.05229 10.4187/respcare.05229 [DOI] [PubMed] [Google Scholar]
- 33.Nicolini A, Parrinello L, Grecchi B, et al. Diurnal mouthpiece ventilation and nocturnal non-invasive ventilation versus tracheostomy invasive ventilation in patients with amyotrophic lateral sclerosis. Panminerva Med 2020;62:19-25. https://doi.org/10.23736/S0031-0808.19.03644-9 10.23736/S0031-0808.19.03644-9 [DOI] [PubMed] [Google Scholar]
- 34.Sheehan DW, Birnkrant DJ, Benditt JO, et al. Respiratory management of the patient with Duchenne muscular dystrophy. Pediatrics 2018;142 (Suppl 2):S62-S71. https://doi.org/10.1542/peds.2018-0333H 10.1542/peds.2018-0333H [DOI] [PubMed] [Google Scholar]
- 35.McKim DA, Griller N, LeBlanc C, et al. Twenty-four hour non-invasive ventilation in Duchenne muscular dystrophy: a safe alternative to tracheostomy. Can Respir J 2013;20:e5-9. https://doi.org/10.1155/2013/406163 10.1155/2013/406163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutiérrez Gutiérrez G, Díaz-Manera J, Almendrote M, et al. Clinical guide for the diagnosis and follow-up of myotonic dystrophy type 1, MD1 or Steinert’s disease. Med Clin (Barc) 2019;153:82.e1-82.e17. https://doi.org/10.1016/j.medcli.2018.10.028 10.1016/j.medcli.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 37.Annunziata A, Fiorentino G, Esquinas A. Effect on lung function of mounthpiece ventilation in Steinert disease. A case report. Acta Myol 2017;36:33-35. PMID: 28690393; PMCID: PMC5479108. [PMC free article] [PubMed] [Google Scholar]
- 38.Koopman M, Vanfleteren LEGW, Steijns S, et al. Increased exercise tolerance using daytime mouthpiece ventilation for patients with diaphragm paralysis. Breathe (Sheff) 2017;13:225-229. https://doi.org/10.1183/20734735.005817 10.1183/20734735.005817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul RW. The Bragg-Paul pulsator: (section of therapeutics and pharmacology). Proc R Soc Med 1935;28:436-438. PMID: 19990165; PMCID: PMC2205276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McSweeney CJ. Bragg-Paul Pulsator for respiratory paralysis. Br Med J 1938;1:1206-1207. https://doi.org/10.1136/bmj.1.4039.1206 10.1136/bmj.1.4039.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weingarden SI, Belen JG. Alternative approach to the respiratory management of the high cervical spinal cord injury patient. Int Disabil Stud 1987;9:132-133. https://doi.org/10.3109/03790798709166342 10.3109/03790798709166342 [DOI] [PubMed] [Google Scholar]
- 42.Banfi P, Pierucci P, Volpato E, et al. Daytime non-invasive ventilatory support for patients with ventilatory pump failure: a narrative review. Multidiscip Respir Med 2019;14:38. https://doi.org/10.1186/s40248-019-0202-7 10.1186/s40248-019-0202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banfi PI, Volpato E, Bach JR. Efficacy of new intermittent abdominal pressure ventilator for post-ischemic cervical myelopathy ventilatory insufficiency. Multidiscip Respir Med 2019;14:4 https://doi.org/10.1186/s40248-019-0169-4 10.1186/s40248-019-0169-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Mattia E, Iatomasi M, Garabelli B, et al. Use of the intermittent abdominal pressure ventilation to guarantee speech in a tracheostomised amyotrophic lateral sclerosis patient. Rev Port Pneumol 2017;23:236-239. https://doi.org/10.1016/j.rppnen.2017.03.002 10.1016/j.rppnen.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 45.Pierucci P, Bach JR, Di Lecce Valentina V, et al. Daytime non-invasive ventilatory support via intermittent abdominal pressure for a patient with Pompe disease. Pulmonology 2020:S2531-0437(20)30184-7. https://doi.org/10.1016/j.pulmoe.2020.08.003 10.1016/j.pulmoe.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 46.Yang GF, Alba A, Lee M, et al. Pneumobelt for sleep in the ventilator user: clinical experience. Arch Phys Med Rehabil 1989;70:707-711 [PubMed] [Google Scholar]
- 47.Miller HJ, Thomas E, Wilmot CB. Pneumobelt use among high quadriplegic population. Arch Phys Med Rehabil 1988;69:369-372. PMID: 3163246 [PubMed] [Google Scholar]
- 48.Woollam CH. The development of apparatus for intermittent negative pressure respiration. (2) 1919-1976, with special reference to the development and uses of cuirass respirators. Anaesthesia 1976;31:666-685. https://doi.org/10.1111/j.1365-2044.1976.tb11849.x 10.1111/j.1365-2044.1976.tb11849.x [DOI] [PubMed] [Google Scholar]
- 49.Kacmarek RM. The mechanical ventilator: past, present, and future. Respir Care 2011;56:1170-1180. https://doi.org/10.4187/respcare.01420 10.4187/respcare.01420 [DOI] [PubMed] [Google Scholar]
- 50.Braun N. Negative Pressure Noninvasive Ventilation (NPNIV): history, rationale, and application. Basner R, Parthasarathy S, Eds. Nocturnal non-invasive ventilation. Boston MA. Springer; 2015. https://doi.org/10.1007/978-1-4899-7624-6_2 10.1007/978-1-4899-7624-6_2 [DOI] [Google Scholar]
- 51.Sonnesso G. Negative pressure ventilation: new uses for an old technique. AACN Clin Issues Crit Care Nurs 1990;1:313-317. https://doi.org/10.4037/15597768-1990-2009 10.4037/15597768-1990-2009 [DOI] [PubMed] [Google Scholar]
- 52.Hino H, Suzuki Y, Ishii E, et al. Biphasic cuirass ventilation for treatment of an air leak after pneumothorax in a patient with nemaline myopathy: a case report. J Anesth 2016;30:1087-1090. https://doi.org/10.1007/s00540-016-2250-x 10.1007/s00540-016-2250-x [DOI] [PubMed] [Google Scholar]
- 53.Grasso F, Engelberts D, Helm E, et al. Negative-pressure ventilation: better oxygenation and less lung injury. Am J Respir Crit Care Med 2008;177:412-418. https://doi.org/10.1164/rccm.200707-1004OC 10.1164/rccm.200707-1004OC [DOI] [PubMed] [Google Scholar]
- 54.Corrado A, Gorini M. Negative-pressure ventilation: is there still a role? Eur Respir J 2002;20:187-197. https://doi.org/10.1183/09031936.02.00302602 10.1183/09031936.02.00302602 [DOI] [PubMed] [Google Scholar]


