Abstract
Respiratory complications are common in the patient with muscular dystrophy. The periodic clinical and instrumental respiratory evaluation is extremely important. Despite the presence in the literature of updated guidelines, patient associations often report lack of knowledge of these pathologies, particularly in peripheral hospitals. The purpose of this work, inspired by the Italian Muscular Dystrophy Association (UILDM) is to improve management of respiratory problems necessary for the management of these patients complex. To this end, the main items that the specialist can meet in the follow-up of these pathologies have been analyzed and discussed, among which the respiratory basal evaluation, the criteria of adaptation to non-invasive ventilation, management of bronchial secretions, situations of respiratory emergency, indications for tracheostomy and the subject of advance directives of treatment (DAT).
Key words: respiratory failure, muscular dystrophy, cough efficacy, spirometry, polygraphy, non-invasive ventilation, arterial blood gases, cough machine, invasive ventilation, tracheostomy, mechanical ventilation
Introduction
Even though the lungs are not directly involved in the disease process, respiratory problems are common in neuromuscular disease (NMD) patients 1,2. Weakness of inspiratory and expiratory muscles causes decreased ability to expand the lungs and impairs alveolar ventilation leading to low oxygen and high carbon dioxide blood levels 3. Moreover, due to expiratory muscle weakness secretion management is also impaired because of ineffective expiratory flow during cough; saliva and mucus may accumulate in the upper airways and favour local infections, which may then propagate to lower respiratory tract and the lungs 4.
The extent to which respiratory involvement occurs and the pattern of the respiratory tests may change according to baseline disease and its progression. A number of diseases such as Duchenne Muscular Dystrophy (DMD) show a slowly progressive disease course and respiratory involvement occurs later on, in the advanced phases of the disease. In other diseases such as Facio-Scapolo-Humeral Dystrophy, acute respiratory insufficiency may be the presenting symptom 5. Moreover, other diseases, such as Myotonic Dystrophies (DM), predominantly show breathing disorders during sleep, which may disrupt diurnal gas exchange and aggravate centrally-driven symptoms such as excessive daytime sleepiness 6.
Standards of care and care recommendations for respiratory management are now available for DMD 7-9 and DM1 10 where death occurs primarily due to respiratory insufficiency and cardiac problems 11-14. This means that clinical centre neurologist and/or pulmonologist may have access to theoretical (pathophysiology) and practical (tests and parameters) information to manage NMD patients at best. However, since muscle disorders are rare, a doctor may happen to manage a very limited number of patients in his/her professional career. In addition, the quality of pulmonary function test and patients’ cooperation highly depend on expertise of the technician performing the examination. Finally, access to specialized respiratory centres may be difficult for NMD patients and their families, causing delayed screening and follow-up assessment.
Finally, as research progresses and new treatments for respiratory complications become available, patient and family expectations increase: for this reason, it is crucial that, all patients may be given the possibility to timely access novel respiratory therapies/devices in. Implementation and adherence to standards of care will slow down disease progression and will give the opportunity to include more patients in clinical trials.
The aim of our study was to describe standards of care for the management of respiratory complications in NMD patients and address some specific issues which are still a matter of controversy.
Materials and methods
Participants
Thirteen pulmonologists, 1 intensivist, 1 paediatrician, 1 psychologist and 2 respiratory physiotherapists with experience in respiratory care of paediatric and adult neuromuscular patients, from 16 different Italian sites, met in Milan to focus on the practical issues of respiratory management in muscular dystrophies in light of the existing standards of care for muscular dystrophies such as DMD and DM. One neuromuscular specialist was also included to integrate the respiratory clinical experience with disease-specific neuromuscular features and representatives from medical groups such as AIPO (Associazione Italiana Pneumologi Ospedalieri), SIP (Società Italiana Pneumologia), SIMRI (Società Italiana Medicina Respiratoria Infantile) as well as a patient representative were also present.
Methods
The method was inspired by the US NIH Consensus Program (http://consensus.nih.gov) and adapted from the Methodological Handbook of the Italian National Guideline System 15. This was the first Consensus Conference organized by the Italian muscular dystrophy association (UILDM). All activities were completed between September 2018 and April 2019. Planning and execution were carried out in 4 stages: (1) assignment, (2) scoping, (3) assessment, and (4) the consensus conference itself. The project included 4 workgroups (Box 1a).
DMD Standards of care implementation survey
In order to assess the level of implementation of respiratory SoC at each of the sites, a survey addressing each item described in the DMD SoC documents was used and given a score from 0 to 2, where 0 indicated that specific aspects were not carried out as described, 1 indicated that the recommendations were only partially addressed as described and 2 indicated that SoC recommendations for that specific item were fully covered. The results of the survey, described in Figures 1-3), showed that sleep studies and specifically nocturnal oximetry and/or capnography and polysomnography were only performed at some sites and, therefore, were not implemented as they should have. In addition, the assessment of maximal inspiratory and expiratory pressures (MIPs and MEPs) was not performed in the more advanced stages of the disease.
Figure 1.
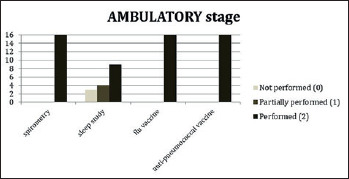
Survey results of participating centres for DMD ambulatory stage patients.
Figure 2.
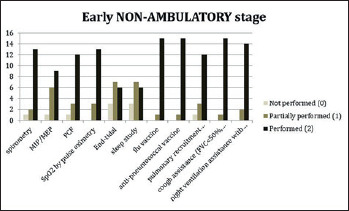
Survey results of participating centres for DMD early non-ambulatory stage patients.
Figure 3.
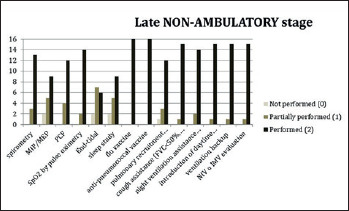
Survey results of participating centres for DMD late non-ambulatory stage patients.
Box 1a.
Consensus Conference methods Workgroups.
Scientific Committee (4 members): it planned and organized the whole project, nominated the Technical Committee and Workgroup members, chose the questions to be answered by the Workgroups, established the methods and rules of the Consensus Conference and chaired the Consensus Conference.
Technical Commettee (9 members): performed the systematic review with evidence mapping and assisted with defining questions: experts (16 members): synthesized and integrated information, provided shared answers to the proposed questions, and presented their findings during the Consensus Conference.
Consensus Development Panel (8 members): established the reviewing and presentation procedures and provided the final evaluation.
1 Candiani G, Colombo C, Daghini R, et al. Manuale metodologico: come organizzare una conferenza di consenso [online]. Rome: Istituto Superiore di Sanità, Sistema Nazionale Linee Guida SNLG; 2009 (http://www.psy.it/wp-content/uploads/2018/02/Manuale-Metodologico-Consensus.pdf).
Outcomes and endpoints
The overall aim of the workshop was to define baselines and follow-up respiratory assessments for children and adults affected by muscular dystrophy, to raise awareness among health professionals working in the acute settings that a specific approach is required for patients with muscular dystrophies having acute respiratory problems, while also providing caregivers with a practical guidelines for respiratory care. Specific aims of the project were: (i) define respiratory tests and procedures to be performed at baseline and at follow-up for all patients; (ii) determine criteria for starting non-invasive ventilation (NIV); (iii) provide indications for tracheostomy (IMV); (iv) define a protocol to manage acute respiratory insufficiency;(v) describe secretion management protocols; (vi) address end-of life protocols.
Unanimous consent was required to approve the care recommendations, protocol or pathway of care. In case of uncertainty, the panel agreed to declare that no consensus was reached and that further data were needed to define management for that specific aspect.
Respiratory management in patients with muscular dystrophies
Muscular dystrophies are characterized by progressive loss of skeletal muscle mass and progressive muscle weakness. In general, for most of them, respiratory decline becomes more obvious when patients lose ambulation. Weakness of the expiratory muscles causes an ineffective cough while weakness of the inspiratory muscles and scoliosis contribute to the restrictive ventilatory deficit, leading to hypoventilation, initially only at night-time and subsequently, even during the day 16.
a. Respiratory Core data set
Baseline assessments
Look for symptoms of respiratory involvement such as those suggestive of hypoventilation (tiredness, shortness of breath, morning headaches, fragmented sleep, excessive daytime sleepiness, concentration difficulties) and look for signs of pulmonary impairment (thoracic deformities, facial dysmorphism or paradoxical breathing, abdomen and thorax asynchronous movements suggestive of respiratory fatigue 17.
Test for respiratory function including sitting FVC both as an absolute value and as a percentage of the predicted value; maximal inspiratory and expiratory pressures (MIP, MEP), expiratory peak cough flow (PCF). Assessment of sleep-related breathing disorders (SRBD) such as Obstructive Sleep Apnea Syndrome (OSAS) with nocturnal oximetry or cardio-respiratory polygraphy. Additional tests that can be performed are end-tidal or transcutaneous partial pressure of carbon dioxide and arterial blood gas analysis in adults. These tests should be performed if SpO2 spot < 95% at RTP (Box 1).
Treat with air stacking exercises if FVC is < of 60% of predicted value using a self-inflating manual ventilation bag (AMBU bag) or mechanical insufflation-exsufflation device twice a day 18.
b. Follow-up assessments
The progression of respiratory involvement is variable within muscular dystrophies; in many of these such as Becker Muscular Dystrophy (BMD), Facio-scapulo-humeral Muscular Dystrophy (FSHD) and most of the Limb-Girdle Muscular Dystrophies (LGMD) it usually occurs over years and a follow-up is recommended every year. However, respiratory involvement may occur early in the disease and may be prominent in LGMD2I (FKRP mutation) or occur only later on in LGMD2C-F e LGMD1B 2. In these cases, follow-up should be more frequent and similar to the more aggressive approach in DMD (Box 2). Generally, the follow-up should include measurements FVC in sitting and supine and PCF, in addition to symptom and suggestive signs of nocturnal hypoventilation detection. In case of FVC below 50% of the predicted value, or of signs and symptoms of nocturnal hypoventilation, nocturnal pulse oximetry or polygraphy are necessary. The follow-up timeline will also have to be based on the patient’s conditions: if the patient is ambulatory, one assessment per year is sufficient, while non-ambulatory patients will have to be assessed every six months 19,20.
c. Focus on DMD
Spirometry should be first performed in DMD children from 6 years of age and it should be repeated every year. Sleep studies should be considered if there is weight gain subsequent to steroid treatment or if there are symptoms of sleep-related breathing disorders (decreased attention at school, irritability, excessive daytime sleepiness) 21. Regular vaccinations (flu and pneumococcal) should be highly recommended. Caregivers should be aware of initial signs of respiratory infections so that care can be started promptly.
Box 1.
Respiratory assessment: core data set.
Symptoms (fatigue, dyspnoea, morning headaches, frequent nocturnal awakenings, hypersomnolence, difficulty concentrating)
Objective signs (thoracic deformities, facial dysmorphisms, paradoxical breathing)
Spirometry with FVC, MIP, MEP, PCF
SpO2 spot measure
Nocturnal SpO2 or polygraphy
Additional test: end-tidal or transcutaneous partial pressure carbon dioxide
As disease progresses and adolescents lose the ability to walk at around a mean age of 13-14 years, respiratory monitoring (pulmonary examination, FVC, PCF, nocturnal SpO2) needs to be more frequent, and repeated every 6 months, specifically looking for symptoms of nocturnal hypoventilation. When FVC drops below 60% of the predicted value, air stacking techniques need to be introduced. Peak cough flow needs to be carefully determined when FVC drops below 50% of the predicted value or MEP is less than 60 cm H2O and if the PCF is less than 270 L/min, patient needs close monitoring. In the late non-ambulatory stages, the criteria for NIV initiation should be re-evaluated every 6 months 8.
d. Focus on myotonic dystrophies
Respiratory involvement is a typical feature of Myotonic Dystrophy type 1 (DM1), with pneumonia and arrhythmias being the main cause of death in these patients 12-21. Although reports on respiratory involvement and how this progresses over time are scanty in patients with Myotonic Dystrophy type 2 22, there is a general agreement that, although similar to DM1, respiratory involvement is less frequent.
Congenital Myotonic Dystrophy (CDM): respiratory insufficiency is the main cause of death in CDM and it is caused by weakness of the diaphragm and intercostals muscles as well as by the failure of cerebral respiratory control because of the severe cognitive impairment. Furthermore, the weak facial and oesophagus muscles may lead to swallowing inadequacy, and dysphagia mainly for liquids resulting in chronic lung inflammation, and/or aspiration pneumonia 23.
Box 2.
DMD and “DMD-like dystrophies” with respiratory involvement.
Duchenne Muscular Dystrophy (DMD)
Limb-Girdle Muscular Dystrophy 2I (LGMD2I)
Myofibrillar Myopathies (MFM)
Facioscapulohumeral Dystrophy (FSH) with:
small D4Z4 arrays (< 18 kb)
early onset
moderate/severe disease
Paediatric onset myotonic dystrophy: respiratory impairment is less frequent in this group of children and adolescents. However, weakness of the respiratory muscles may affect the ability to cough, resulting in atelectasis, chronic lung infections, chronic bronchitis and bronchiectasis. Furthermore, as in new-borns, dysphagia may be present, and children may not be aware of it, so that they may be at risk for aspiration pneumonia.
Adult onset myotonic dystrophy: weakness of the respiratory muscles affects the ability to cough, resulting in atelectasis, chronic lung infections, chronic bronchitis and bronchiectasis. Weakness of the diaphragm and possibly diaphragmatic and respiratory muscles myotonia 24 may lead to nocturnal hypoventilation. This condition is worsened by sleep apnoea, leading to disrupted sleep, excessive fatigue, and morning headaches potentially contributing to lethal cardiac arrhythmias. Excessive daytime sleepiness (EDS) is in fact one of the most frequent complaints reported in this patient population reaching a prevalence of up to 88% in some studies 25-27 and may be the presenting symptom of DM1, not infrequently, years preceding the diagnosis. Although mostly of central origin, EDS may coexist with sleep-related breathing disorders (SRBD) in some patients with DM1 28. Symptoms related to chronic respiratory insufficiency such as nocturnal hypoxemia and diurnal hypercapnia may be overlooked by the patients themselves probably because these gas abnormalities develop slowly, allowing brain/brainstem structures to adapt to these changes. It is not infrequent to find patients with unusually high levels of daytime hypercapnia not complaining of respiratory problems and who do not necessarily report EDS 29. Both peripheral and central components of EDS can be approached with existing treatment strategies. NIV is recommended to treat nocturnal hypoventilation related to chronic respiratory insufficiency but compliance is limited and despite NIV, EDS may persist. On the other hand, although off-label, modafinil may be used for the central component of EDS.
Late-onset myotonic dystrophy: respiratory impairment is not typically the most frequent complaint although the data on this specific subgroup of patients is scanty. The general impression is that disease progression may be more rapid than in the adult onset, so that respiratory monitoring is recommended despite the lack of symptoms or findings on initial assessments.
Criteria for starting non-invasive ventilation (NIV)
A reduction in vital capacity (VC), total lung capacity (TLC) and functional residual capacity (FRC) determine a respiratory deficiency which has a variable course between different disorders 30,31. Nocturnal Hypoventilation (NH) occurring especially during rapid eye movement sleep phase is the first manifestation of chronic respiratory insufficiency in neuromuscular disorders (NMD). It is unclear which definition of NH best relates to prognosis 32,33. A correlation between the reduction of VC and progression of sleep disordered breathing has been shown in patients with NMD 34. Daytime clinical assessments can be unreliable in early detection of respiratory failure because clinical symptoms of NH can be insidious and not always present 35. Early recognition of NH is very important because it can progress to daytime hypercapnia (partial carbon dioxide pressure [PaCO2] > 45 mmHg in arterial blood) or clinical symptoms related to hypoventilation if it is undiagnosed and therefore untreated with NIV 36 (Box 3). Well-timed use of NIV is effective to reduce NH and its progression towards daytime hypercapnia 37. NIV should be started in the presence of daytime hypercapnia and/or clinical symptoms as recommended in the current guidelines 38-40. NH diagnosis is not easy in NMD in which hypoventilation is defined as pCO2 > 50 mmHg for a period longer than 25% of sleep time 41,42 and this is because it is specifically studied in the paediatric population. Transcutaneous monitoring of pCO2 levels could detect NH, even in patients that don’t show symptoms and significant nocturnal hypoxaemia 43,44 with similar results reported although the study group included a much wider population of respiratory restrictive disorders other than NMD. Finally, Ogna et al. 45 demonstrated the usefulness of tcPCO2/SpO2 as a NH diagnostic tool and suggested that a better definition of the NH threshold is needed. However, it is still not clear if nocturnal monitoring can be used as an additional tool to decide when to start NIV in clinical setting. Nocturnal polysomnography (PSG) and/or pulse oximetry with carbon dioxide monitoring were recommended in the 2004 by the American Thoracic Society as an indication to NIV for DMD (39). However, PSG has some limits, because it is not universally available, it is expensive, time consuming and not available during routine evaluations 46. Besides, PSG attributes apneas and hypopneas only to obstructive and central events rather than to inspiratory muscle dysfunction. Assessment of symptoms related to inspiratory muscle dysfunction is often delayed in patients affected by DMD 47. Unfortunately, night-time ventilation may be insufficient, with development of daytime hypercapnia, even when appropriate NIV adjustments are made and a vigilant clinical follow-up is done 48.
Box 3.
Indications for NIV initiation in DMD muscular dystrophy (from Birnkrant DJ et al, Lancet Neurol 2018).
FVC less than 50% predicted value
MIP less than 60 cm H2O
PtcCO2 or petCO2 or paCO2 >/= 45 mm Hg
Baseline SpO2 less than 95% in room air (post airway clearance)
Critical issues with NIV
Age: patient age at initiation of NIV treatment is a prognostic factor, in fact, those patients that require NIV before the age of 17 have a worse prognosis than those starting NIV at an older age. Due to improvements in respiratory care death by cardiac causes has become more common, indicating the need for active cardiology support as this approach may improve outcome.
Facial interface: sometimes, young patients do not easily accept NIV treatment because there is a poor tolerance of the interface, and this can be induced by various factors, such as excessive oral air leakage, excessive pressure of the mask on the face, claustrophobia, anxiety (because sometimes the patient may not be able to call a family member), and patient-ventilator dyssynchrony 49. Hence, the interface plays an important role in tolerance and usefulness of NIV use. Interfaces that cover the nose alone or the nose and mouth (oronasal interface) are the most universally used; however, they can cause gastric distension, skin breakdown, conjunctivitis and claustrophobia 50. In addition, the application of an oronasal interface can worsen social life, since it makes it difficult to eat, drink and talk. Besides, this type of mask alters the patient’s perception of himself and may have negative psychological effects 51. Mouthpiece ventilation (MPV) via a 15-mm or 22-mm mouthpiece device is the preferable and more comfortable alternative; however, a more active participation of the patient is needed in this case. Patients requiring daytime NIV treatment (Box 4) better accepted the nasal mask treatment during the night hours, probably because the use of MPV during daytime hours made the patients feel safe, and gradually confident enough to be treated with NIV at night. The use of the nasal mask and MPV has enabled the treatment of patients who had formerly refused nasal, oral or oronasal interfaces. The possibility of using a mouthpiece as first choice interface for patients affected by DMD who need to start diurnal NIV treatment should always be kept in mind.
Box 4.
Daytime NIV options.
Multiple interfaces options (nasal, oro-nasal, mouthpiece)
Warning skin lesions prevention
IAPV (intermittent abdominal pressure ventilation)
Negative ventilation
NIV monitoring
Once home mechanical ventilation (HMV) is carried out, it is required a regular follow-up to assess both optimal tolerance and efficiency of the treatment is required. In addition, the measurement of both blood gases and HMV monitoring can be performed with more than one approach with an increasing level of complexity, starting from simple tools, such as oximetry, and moving to the most comprehensive sleep recording systems using in-hospital polysomnography 52. Recently a management strategy, with a simple initial screening based on nocturnal oxygen saturation monitoring (SpO2), followed by additional exams when there are pathological findings was suggested. Non-invasive transcutaneous measure of CO2 (TcCO2) has demonstrated to have acceptable accuracy in estimating PaCO2 over numerous hours in stable patients treated with HMV 53,54 showing a higher sensitivity than SpO2 in finding residual hypoventilation in NMD patients 55,56. Current recommendations regarding settings and monitoring of HMV are based on expert opinions 57. The European SOMNONIV Group suggests the use of an algorithm to monitor HMV, which includes oximetry as the first screening step to detect patients who require further nocturnal exams, and advises a mean nocturnal SpO2 over 90% for at least 90% of the total recording period as a therapeutic goal. The 2010, AASM clinical practice guidelines recommend adjusting the ventilator support if hypoventilation is present for ≥ 10 minutes. Recent data showed that TcCO2 can be an accurate estimation for PaCO2 in long-term mechanically ventilated patients, with the advantage of finding episodes of transient hypoventilation, not detected by punctual arterial blood gases analyses. The use of TcCO2 opens the possibility to evaluate the ventilation’s usefulness directly and several times at home, allowing a simplification in the management of HMV. However, although capnometry devices have registered technical improvements, TcCO2 accuracy is strongly dependent on appropriate handling and knowledge of the equipment and procedures. Risk assessment is an important part of discharge planning, and risk will vary according to the use of NIV or invasive ventilation, patient’s diagnosis, the degree of ventilator dependency, functional ability and comorbidities. In a UK study a total of 188 home visits in 6 months were to analyse home problems in 1,200 patients that used predominantly NIV. About one-quarter of these problems were caused by the ventilator, while 43 were caused by technical issues (noisy equipment and recurrent alarms). No patients died or experienced side effects as a result of equipment problems in these studies. More hospitalizations were seen in the “no fault” category, in which patients or caregivers reported a ventilator malfunction. However, when a home visit was performed, a ventilator malfunction was not found; a possible explanation of this is that the patient had become unwell (usually due to an infective exacerbation) and interpreted this event as a ventilator problem 58. These findings illustrate that patients, families and caregivers require different types of competencies, and shows that a clear problem-solving approach is needed in educating home care teams. In this area, the increasing competence to provide home telemonitoring and to observe data remotely from the ventilator has created great interest 59.
Focus on DMD: natural history studies in Duchenne muscular dystrophy (DMD), show that patients develop respiratory failure. This usually starts as nocturnal hypoventilation (NH) 60 and improves with the application of nocturnal non-invasive ventilation (NIV) 61,62. If not treated, almost 90% of DMD patients die from pulmonary complications associated with respiratory muscle weakness between 16 and 19 years of age 63,64. Nowadays, with the implementation of SoC, it is not infrequent to see that about half of this patient population reaches the age of 25.3-30.4 years as reported in four most favourable nocturnal NIV studies 65. In DMD patients the vital capacity (VC) peaks are registered between 9 and 16 years of age, and then the VC decreases by 5-10% per year until ventilatory support is needed for survival 66.
Focus on DM: adaptation to NIV is limited in these patients. Symptoms related to chronic respiratory insufficiency such as nocturnal hypoxemia and diurnal hypercapnia are overlooked by the patients themselves probably because these gas abnormalities develop slowly allowing brain/brainstem structures to adapt to these changes. When NIV is prescribed as a chronic treatment option, compliance is limited mainly because of the lack of symptoms immediately related to respiratory involvement and therefore the benefits of NIV use are not perceived in the short-term nor perceived as effective by the patients. Fatigue and EDS in fact usually persist despite NIV although SRBD improves with NIV constant use. The data on the effects of withdrawal and how this affects prognosis are still scanty 67-68.
Indications for tracheostomy
Use of home non-Invasive Ventilation (NIV) in neuromuscular disease (NMD) patients with chronic respiratory failure (CRF) may be expected to extend survival by many years, improve physiologic function and quality of life as well as decrease the frequency of episodes requiring acute care facilities 64. Based on these considerations and the fact that safety, comfort, satisfactory speech and swallowing have been reported by long-term users, NIV should be regarded as the therapy of choice in supporting breathing in DMD 69. Nevertheless, a significant proportion of DMD individuals are currently prescribed tracheostomy ventilation (TV) for home ventilatory care. Indeed, recent data published by one of the 14 reference centres for NMD in France, showed that 31 out of 150 DMD patients who had undergone long-term mechanical ventilation (LTMV) between 1997 and 2014 had initiated ventilatory assistance via a tracheostomy, although mechanical ventilation had increasingly started using a non-invasive interface over the course of the study period 70. In most cases, the decision to perform a tracheostomy is taken when NIV becomes ineffective: according to recent data collected by MD STARnet, the largest population-based surveillance system of individuals with DMD and Becker muscular dystrophy (BMD) in the United States, approximately 90% of patients had received tracheostomy following NIV treatment failure 71.
When to perform a tracheostomy?
Placement of a tracheostomy may be considered both in the event of a life-threatening acute illness that has required invasive management and when a slowly progressive ventilatory failure is present. Indeed, although the non-invasive approach, based on the combination of NIV and assisted coughing techniques, in particular Mechanical Insufflation–Exsufflation (MI-E), should be preferred as a first-line intervention for patients with DMD during an episode of Acute Respiratory Failure (ARF), moving 72 on to invasive ventilation with intubation becomes unavoidable in case of NIV failure, inability to clear secretions with cough assist and suctioning or the loss of ability to protect the airway with high risk of aspiration. Unfortunately 73, once intubated, a substantial proportion of NMD patients may encounter particular difficulties while being liberated from the endotracheal tube after recovery from the acute illness, due to weakness of the inspiratory muscles, inadequate cough and inability to handle oropharyngeal secretions, thereby having to switch to a tracheostomy 74. Of notice, a large uncontrolled study unexpectedly reported that the standardized use of NIV and cough assistance may lead to an effective extubation of the great majority of “unweanable” NMD patients who could not pass a spontaneous breathing trial 75.
In DMD patients with chronic, progressive ventilatory failure, indications for performing a tracheostomy have not been clearly defined 76. According to the consensus conference of the American College of Chest Physicians, and more recently the American Thoracic Society consensus for DMD respiratory care 39, severely impaired swallowing, leading to chronic aspiration and repeated pneumonia, and/or ineffective clearing of tracheobronchial secretions, despite the use of non-invasive manual or mechanical expiratory aids, have been considered to be indications for TV. In current practice, no level of pulmonary function or blood-gas abnormality absolutely mandates tracheostomy over NIV. However, a Vital Capacity (VC) value below 20% predicted, a PaCO2 level above or equal to 45 mmHg during assisted breathing, a need for increased ventilation time, and a severe clinical status at initiation of NIV, suggest an overall risk of NIV failure and the forthcoming need for a tracheostomy 77. Morphologic characteristics of the patient, as difficult intubation prediction, and environmental circumstances determining the ease or difficulty in using emergency service are to be taken into account as well.
In line with the recent German national guideline for treating CRF 78 indications for tracheostomy in NMD patients have been summarized in Box 5 and Box 6.
How and where to perform tracheostomy?
Performance of a tracheostomy as an elective procedure by skilled surgeons and follow-up care in specialized centres may reduce the risk of early and/or late postoperative complications 79.
In literature, there are no specific indications about tracheostomy implementation, percutaneous or surgical 80, but it has been agreed by the participants that, in case of long- term tracheostomy, the surgical technique is preferred. Such indications reflect the need for an easier and safer periodic tube change, a lower risk in case of stable surgical stoma, reduced accidental decannulations, always fearsome when dealing with totally ventilator dependent patients 81. Risk factors that can complicate the tracheostomy change include obesity, a short neck, anatomical abnormalities, excessive granulation tissue, lack of patient cooperation. In case of tracheostomy recently performed (in the previous 2 weeks) or in case of an anticipated difficult tracheal tube exchange, we suggest using the “railroad” technique with a guiding obturator 82.
Impact on patients and family
Once long-term TV is initiated, DMD patients require special considerations for care. Outcome and patient comfort are improved with the application of a well-conceived management plan including education for patients, families, and health-care providers, and by an active role by home-care agencies in providing care to these patients 83.
Box 5.
Indications for tracheostomy in neuromuscular disease patients (from Windisch W et al. Respiration 2018;96:171-203, mod.) 78.
Inability to fit an appropriate ventilation interface
NIV intolerance
NIV inefficiency
Severe bulbar symptoms with recurrent aspiration
Inefficiency of non-invasive secretion management
Failure to switch to NIV after intubation and invasive ventilation
Box 6.
Recommendations for patients who are expected to be on long-term IV (from Windisch W et al. Respiration 2018;96:171-203, mod.) 78.
Tracheostomy for long term ventilation should be performed surgically and not percutaneously
Patients on NIV ≥ 16 hours a day need to be equipped with 2 ventilators, one acting as a back-up, and need to have an external battery
Patients need to be equipped with an oximetry machine
Patients need to be provided with an extra tracheostomy tube of a smaller diameter than the one in place in case the tube gets removed accidentally and needs to be promptly replaced at home
In order to use a speaking valve, patients’ cuff must be deflated
The ventilator needs to be provided with active an humidifier so that the air inspired is sufficiently humified and warm
2 suction machines are required
Being unable to speak is a major cause of frustration for patients with a tracheostomy tube and their families: a tracheostomy, however, presents opportunities to promote articulated speech. Airflow through the upper airway and vocal cords is necessary for voice production: for this reason, partial cuff deflation may allow the patient to speak in a whisper during the inspiratory phase of the respiratory cycle. Adding a small amount of positive end-expiratory pressure produces a continued air leak and permits audible speech throughout the breathing cycle 84. Moreover, subjects with minimal ventilator requirements can be ventilated with cuffless tubes that allow a constant air leak and the ability to speak. Finally, the use of a one-way valve, such as a Passy-Muir valve, allows airflow through the tracheostomy tube during inspiration but does not permit air to exit the tracheostomy tube during exhalation. When the valve is employed with a cuffless or fenestrated tracheostomy tube, expiratory airflow is directed through the vocal cords and normal speech is facilitated 85.
A comparison of morbidity and causes of death in a number of DMD patients receiving full-time mechanical ventilation either by tracheostomy or by NIV, showed that the risk of complications was higher in tracheostomized compared with NIV patients, in particular mucus hypersecretion and tracheal injuries 86. Furthermore, data on mortality showed that the risk of death at 12 years does not significantly differ between DMD subjects undergoing long-term NIV or TV 87.
In conclusion, the decision to perform a tracheostomy in DMD ventilator-dependent individuals is complex and involves medical, ethical and financial considerations. Patients giving their consent to its application may live at home despite NIV failure.
Secretion management
Respiratory insufficiency and pneumonia are primary causes of mortality and comorbidity in many NMDs 2. Airway clearance techniques (ACT) are an essential component to the care of people with NMDs. During acute respiratory tract infections, patients with NMDs develop dyspnoea, hypercapnia and a reduction in both respiratory muscle strength and lung function 88.
What is important to control regularly?
Among, the various measurable parameters, the most useful, when referring to cough efficacy, are:
Vital Capacity (VC);
Maximal Insufflation Capacity (MIC);
Peak Cough Flow (PCF).
VC and MIC can be measured by a simple portable spirometer or flow meter. MIC, the maximum capacity of keeping air in the lungs, starting from vital capacity, through air-stacking manoeuvres, represents the best rib cage elasticity index: it should be measured when VC is below 2000 ml or at 50% of predicted in adults 89. In the evaluation of cough efficacy, PCF is the most reliable and simple to use assessment at the patient’s bedside 90,91, reference values are available for children 92 and adults: cut-off values for cough efficacy in normal adults range from 360 to 840 L/min 93. The PCF can be easily measured with a hand-held flow meter or a pneumotachograph/spirometer 94 using an oro-nasal mask or a mouthpiece 95. When the values are higher than 270-300 L/min, they are believed to be safe because it is expected that a PCF > 160 can be maintained during episodes of exacerbation 96. In clinical practice, an efficient cough requests a PCF higher than 160-200 L/min 97.
What to do when PCF < 270 L/min or VC < 50% or < 2000 ml?
It is important to regularly measure PFC and VC as, even in case of significative muscular weakness, the patient might not experience symptoms in everyday life. If PCF values are stably below 270 Litres/minute or VC < 50% of predicted or < 2000 ml in an adult patient, it is necessary to introduce cough assistance techniques, either manual or mechanical.
a. Manually assisted coughing
Manual cough assistance techniques can assist the inspiratory or expiratory phase, or both.
Assisted inspiration
In order to produce an efficient cough, deep inspiration preceding the expiratory phase is essential. The quantity of inhaled air can be increased by using an AMBU bag in the air-stacking manoeuvre, or by the use of mechanical ventilator in volumetric mode, with the inhalation of one or more consecutive breaths, without breathing out, in order to obtain a full deep breath. Some patients are able to learn glossopharyngeal breathing (GPB), that allows improved air stacking in the absence of any respiratory device.
Assisted expiration
Manual assistance manoeuvre in the cough expiratory phase consists in chest and abdomen compressions by the caregiver to improve the expiratory flow and promote secretions removal.
b. Mechanical in-exsufflation
Mechanical in-exsufflation (MI-E) is a very popular cough augmentation technique 98. MI-E devices produce inspiratory and expiratory assistance. MI-E is well tolerated 30 and may be delivered by non-invasive or invasive 99 interfaces. MI-E associated with manual assisted coughing, oximetry feedback and home use of non-invasive ventilation was shown to effectively decrease hospitalizations and respiratory complications and mortality in a program for patients with amyotrophic lateral sclerosis 100.
How to manage deep secretions?
Peripheral ACT incorporates the techniques that aim to improve ventilation and enhance mucus transport from the bronchi to the upper airways. Different techniques have the potential to loosen secretions and transport them from the peripheral to the proximal airways: these include High Frequency Chest Wall Oscillations (HFCWO), Intrapulmonary Percussive Ventilation (IPV), and Chest Wall Strapping (CWS) 101. Peripheral ACT does not require the patient’s co-operation. The use of these techniques is possible in infants, children and adults, even in the presence of a tracheostomy and/or bulbar failure or intellectual impairment. Carers must know that peripheral secretions cannot be mobilized in patients who have retained proximal airways secretions. Rather, it is recommended to use peripheral ACT after more central airways are cleaned of secretions by means of proximal ACT. In other words, sessions of airway clearance should first empty the proximal airways and then, if the patient is not too tired, mobilize secretions from the peripheral airways. If patients are exhausted, it is not recommended to approach the patient with peripheral ACT because these will not be tolerated and cough will be ineffective. This could put the patient at risk of having a respiratory arrest because of the excess of secretions without being able to get rid of them using a cough-machine. Recommendations to manage secretions are summarized in Box 7.
Management of acute respiratory failure
ARF most often occurs during otherwise benign upper respiratory tract infections favouring mucous encumbrance, and further weakening of respiratory muscles 88 or in cases of pneumonia, aspiration or atelectasis 73. Other causes of ARF in these patients are pneumothorax, fat embolism and abuse of sedative drugs 30. Several muscular dystrophies are associated with dilated cardiomyopathy 102,103, which may cause pulmonary edema and favour ARF 39.
A proactive clinical approach should be taken to prevent the onset of ARF and allow carers to recognize signs and symptoms potentially leading to ARF early, such as increased respiratory rate, tachycardia, tidal volume reduction in ventilated patients 9,64,104.
Admission to the hospital for ARF can be very disruptive for these patients 105, who could be successfully managed at home by experienced and well-trained family members and/or healthcare professionals 106. Bach and colleagues 96 described a protocol for managing these patients at home in case of respiratory tract infections, reporting a dramatic reduction in the need for hospitalization and a prolongation of life expectancy. More recently, Vianello et al. showed that active treatment provided by healthcare professionals is an effective alternative to hospital admission for selected NMD patients with respiratory infections 107. In particular, during respiratory infection, early use of antibiotics is mandatory if pulse oximetry is below 95% on room air 30. Moreover, according to Bach’s protocol 96, the patients should receive 24-h NIV during the exacerbation. Pulse oximetry should be monitored continuously and when oxygen saturation on room air falls below 95%, secretion removal should be aggressively induced using cough assistance until oxygen saturation returns to the 95% range. Oxygen should not be used to correct hypoxaemia at home, because it can worsen hypercapnia and it does not allow the recognition of a severe hypercapnia with the pulse oximetry. Finally, family members should be trained to use strict criteria leading to urgent hospital admission, and the home treatment protocol should be tailored according to local resources.
Box 7.
Secretion management: recommendations.
PCF and VC assessment are suggested at every follow-up visit
A spirometer or a hand-held flow meter can be used to measure PCF keeping the same type of interface, such as oro-nasal mask or mouthpiece, in the following evaluations
The use of MI-E is safe and effective through both invasive and non-invasive interface, in paediatric and adult patient
Ending the MI-E session during the inspiratory phase is recommended to avoid phenomena of atelectasis, especially in frail patients
To avoid secretion encumbrances in patients with ineffective cough, sessions of secretions removal from central airways must be performed before and after peripheral ACTs
If home respiratory management fails, patients must be hospitalized 73. Few prospective studies on the management of NMD with ARF 109 and some retrospective studies 64,109-112 reported the successful use of a non-invasive approach (i.e., NIV combined with assisted coughing) to improve gas exchange abnormalities and avoiding intubation. However, patient selection remains important for the success of this strategy. In particular, severe bulbar dysfunction increases the patient risk for aspiration, and hampers the elimination of airway secretions impeding successful use of non-invasive approach 108. Close monitoring of these patients is mandatory, and NIV should never delay endotracheal intubation for most severe cases 73. Monitoring must be tailored and personalized according to the clinical and respiratory severity of each case. In particular, PaCO2 measurement (i.e., capillary CO2 in mild disease and indwelling arterial line in most severe cases) must be included if supplemental oxygen is used to correct hypoxemia 113. It follows that these patients should be admitted in a unit where medical and nursing staff is adequately equipped to apply close monitoring and aggressive non-invasive respiratory assistance. Also in this setting the continuous presence of well-trained care-givers is important for the success of the treatment 73 Caregivers may provide continuous care, including repositioning of mask and administration of cough machine; otherwise, the presence of a skilled nurse is needed, with a nurse-patient ratio of 1:1 104.
If a non-invasive approach fails or is contraindicated, patients can be intubated as a short-term measure. In this case, assessment for a difficult intubation due to reduced mouth opening, macroglossia or limited mobility of the cervical spine is very important. If any of these conditions are present, intubation should be performed taking into account the guidelines for difficult airway management avoiding emergency intubation 114.
After recovery from the acute illness, patients with muscular dystrophies should be promptly extubated. Unfortunately, because of weakness of the inspiratory muscles, inadequate cough, and inability to handle oropharyngeal secretions, a substantial proportion of these patients fail the weaning process 115. Preventive application of NIV combined with assisted coughing after extubation provides a clinically important advantage to these patients by avoiding the need for reintubation or tracheostomy and shortening their stay in the ICU 116. Moreover, Bach and al. suggest using cough assistance devices before extubation to clear the airways. Once SpO2 is maintained > 95% on ambient air, patient should be extubated to full NIV support and aggressive cough machine to maintain or return to the SpO2 > 95%. The indication for a tracheostomy can be evaluated, but it should not be considered in the acute phase. In particular Bach and al. suggest to consider tracheostomy only in case of multiple failures with the application of the discontinuation protocol 75. Recommendations suggested for patients with muscular dystrophies in case of emergency management are summarized in Box 8.
Care choices and advanced directives
Although NMDs are uniformly fatal, each has a different life expectancy and disease trajectory that potentially influences health care decisions and raises unique ethical concerns. The burden of NMDs is high with consequences requiring repeated and extended hospitalizations, clinical management and frequent interactions with clinicians of many different specialties.
Some of the ethical challenges raised by NMDs include the choice and effectiveness of life-sustaining therapies and advance care planning: these issues involve informed consent and end-of-life care 117.
Palliative care (PC) is an “active and global care of patients suffering from diseases that cannot be cured, in order to control pain, dyspnoea and including psychological, social and spiritual aspects” 118. The uncertainties that arise in caring for NMDs, coupled with the increasing availability of therapies and technologies, create complex ethical quandaries for families, caregivers, society, school and clinicians. Such quandaries are exacerbated by the certainty from the time of diagnosis that these diseases are life limiting 117. The most frequent and stressful ethical challenges for NMDs occur in regards to ventilator support, ventilatory support (benefit vs harm), families wishes to receive long-term tracheostomy ventilation 119-121, palliative management, differences in opinion between family members and differences in physician opinions. For NMDs there are triggers for referral to palliative care services 122.
Box 8.
Emergency management: recommendations.
Clinicians must know that the development of respiratory tract infections in patients with muscular dystrophies, is a life-threatening event favouring the appearance of mucous encumbrance and further weakening of respiratory muscles that leads to ARF.
A proactive clinical approach should be taken to recognize pulmonary problems prior to the onset of respiratory compromise. Patients who have a FVC <50% of predicted value can be trained to use a protocol that provides indications for the use of NIV, cough assistance and pulse oximetry in case of respiratory infections.
NIV combined with mechanically assisted coughing has been established as standard practice in patients with muscular dystrophies affected by ARF either in the outpatient or in the inpatient (hospital). In particular, techniques to aid secretion removal must be applied aggressively if bronchial encumbrance is present.
During respiratory exacerbations they can be successfully managed at home if family members are well-trained to use NIV, cough assistance and pulse oximetry. Oxygen alone should not be used to correct hypoxemia. Early use of antibiotics is mandatory. Family members should be trained to use a protocol that defines also when patients need urgent hospitalization. This protocol should be tailored according to local resources.
If home respiratory management fails, patients with muscular dystrophies must be hospitalized and they should be placed in a unit where medical and nursing staff is adequately equipped for the aggressive management of these children and close monitoring. Monitoring must be tailored and personalized according to the clinical severity of each case.
The continuous presence of well-trained parents or other care-givers is important for the treatment success also in the critical care setting.
The use of NIV should not delay endotracheal intubation for most severe cases, avoiding emergency intubation.
After recovery from the acute illness, patients with muscular dystrophies should be promptly extubated and started immediately on NIV and cough assistance. Tracheostomy should not be considered in the acute phase and should be considered only in case of multiple failures of weaning protocol.
In summary, factors influencing patient/family decisions for ethical concerns are local tradition, level of home assistance, stress, patient’s age, where they lived, confusion about disease severity, internet information bias, variability in management across specialties and countries, cases reported in the media, paucity of quality-of-life data, lack of anticipatory care planning (ACP) resulting in critical decision making. NMDs patients frequently die in ICU and acute settings, have a low level of awareness about their disease prognosis 123. Italian respiratory units have, only in a minority of cases, a clear ACP and palliative/end of life plan 124. ACP is the process of communication between individuals and professional caregivers that includes, but is not limited to, options for end-of-life care and the completion of advanced directives. Typically, for NMDs and during an emergency, decisions may be made by clinicians who are unfamiliar with the child, and there is little time for confrontation 119. Like other types of preventive medicine, ACP are underutilized even though they are cheap, low-tech, and potentially highly effective 125. ACP facilitate the application of the proportionality care principle, pain/dyspnoea/anxiety treatment, informed consent, doctor/patient relationship, psychological assistance and trustee administrator presence. On the contrary, ACP could compromise the relationship between doctor and patient due to the mandatory respect of a pure contract; the possibility to refuse incongruous requests in the presence of new undefined therapies, the lack of clear patient informed competence, the risk of conflict between trustee administrator and family and the debate over artificial nutrition and hydration as care treatments, may remain unresolved problems. Recommendations for advanced directives are summarized in Box 9.
Conclusions
There is increasing evidence of a link between respiratory and mental health 126. In fact, literature suggests that in patients with chronic respiratory diseases, the evaluation of breathlessness perception, psychological disturbances and the recording of any stressful event should be considered as relevant as the physical and functional assessment of respiration 127.
In severe neurological conditions, ventilator users can present mainly two types of needs: respiratory related needs, including mode of ventilation prescription and selection, maintenance of lung recruitment and good airway clearance; non-respiratory related needs, including substantial nursing care, adequate nutrition, accessible communication and psychological support. It is relevant to pay attention to all of these needs with the aim to maintain patients’ quality of life (Qol) 128.
A UILDM - Telethon study provides evidence in favour of an integrated care model for muscular dystrophies that is suitable for: pharmacological treatment, rehabilitative interventions 129, psychological treatments, welfare and financial support 130. Medical care of a patient with DMD and his family is not complete without support for their psychosocial wellbeing 131. The families’ lives change significantly with the decision to place their child with NMD on HMV because of the experience of a recurrent sense of loss and uncertainty. It would be suitable to improve support by health care professionals, their extended family, and their community, to enable parents to fulfil their vital role 132.
Box 9.
Care choices and advanced directives.
1. A frank, early and individualized conversation is mandatory:
Listen and talk to your patients
Consider and imagine their preferences and future
2. Timing of conversation with patients/family:
ICU admission
Hospitalization for respiratory reasons
Continuous NIV for more than 16 hours/day
Persisting hypoxemia during NIV use
Severe comorbidities (congestive cardiac failure, gastro-intestinal pseudo-obstruction)
Severe malnutrition
Bulbar symptoms
Recurrent infection and severe malnutrition during tracheostomy ventilation
Cognitive deficit
Poor family network
3. Push for anticipatory care planning (ACP):
Decisions should be made in advance and not during an acute situation
Decisions must be individualized and based on the most objective criteria possible
Extend survival improving quality of life and facilitating the patient spending as much time as possible at home
ACP can be extended to all life support measures including the DNR and withdraw from MV
4. Doctor/team responsibility
The doctor has the legal and ethical responsibility to propose all options treatment including MV
The doctor should avoid personal perception
A multidisciplinary approach is recommended
An ethical committee involvement is welcomed also for moral distress and conflicts of conscience
Team training is needed
5. Taking care of end of life time
Check patient’s physical and psychosocial symptoms
Do not unduly prolong life and suffering
Patients who choose not to resort to MV should receive adequate end of life care
Facilitate the presence of family, friendly people and religious comfort
Consider hospice competencies and palliative care consultation services for your patients (“Home hospice” care could be preferred)
Interestingly, parents of paediatric neuromuscular patients requiring HMV did not refer significantly higher parental stress compared to parents of non-ventilated children, despite their children having a lower health-related Qol; this data suggests that parents living with a continuous care demand could undergo a progressive adjustment process allowing them to consider respiratory care as a part of “normal” life, thus without the perception of this being an additional source of stress 133.
A number of ethical challenges, or dilemmas, can arise alongside treatment progression: the decision-making process regarding whom HMV should be offered to, respect for patient and family wishes, Qol, dignity and equal access to dedicated assistance. Moreover there is uncertainty regarding the impacts of HMV on the patient, the family, the healthcare services and the allocation of resources. A better and broader understanding of these issues is crucial in order to improve the quality of care for both patient and family and to assist HMV professionals to improve the decision-making process and to keep the patient and his or her family highly involved 121.
Improvement and standardization of care pathways, with a better management of comorbidities related to neuromuscular diseases, has led to an increase in life expectancy and an increased number of patients reaching adulthood. Adolescence and adulthood are age groups in which new and challenging problems may develop. Care of children with chronic disorders is often complex, involving a high level of ongoing interaction between caregivers and the multidisciplinary health care team.
The transition from childhood to adulthood has therefore become an emerging problem that involves medical, psychological, social and economic aspects centred on the family. An unmanaged, non-standardized transition increases the risk of adverse outcomes. During this critical period, these patients are at increased risk for interrupted health care and related negative health consequences: They must cohabit with their progressive disability: decreased mobility, decreased independence for hygiene, increased needs of technological support. increased survival rate but at the same time increased morbidity. In general, the diagnosis is made in paediatric age and the co-morbidities develop starting from adolescence.
It is necessary to develop a standardized multidisciplinary transitional program focused on the needs of the patient around which the various professionals must gravitate. Health care providers and educators are among the best facilitators for discussions around health, education, sexuality, employment, social development and adult living. Therefore, the role of the care coordinator becomes fundamental in obtaining the goal of transition which is to optimize the quality of life and future potentiality of young patients with special health care needs.
Providing guidance on transfer of medical information and developing an individualized care plan for these children becomes essential to draw up a transition policy with planning tools (transition readiness assessment, portable medical summary and transition action plan).
Preparing young adults for the change in health care setting is crucial for a successful transition to adult care: there is no right time, but a timely and organized transfer must be discussed and planned before transitioning to adult health care providers.
The critical aspect of implementing the guidelines/recommendations, present in literature for each neuromuscular pathology, is usually determined by the difficulty in disseminating the scientific contents throughout the country, particularly at the local level of centres working with patients affected by neuromuscular diseases. This assumption was confirmed by the results of the survey, taken by all of the workshop participating specialist centres, from which it appears that not all guidelines on respiratory management of patients affected by DMD are applied in a homogeneous way by these centres.
At the end of the meeting, a flow-chart regarding rapid evaluation of dystrophic paediatric and adult patients, that can facilitate the respiratory classification of the patients, was developed (Fig. 4).
Figure 4.
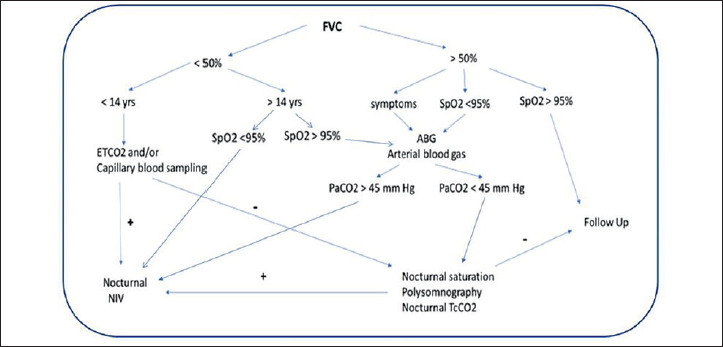
Flow-chart for rapid evaluation of respiratory function in muscular dystrophy.
The aim of this document is to facilitate the dissemination and application of essential respiratory care considerations for patients affected by muscular dystrophy, by hospitals and local clinical centres who do not routinely work with, but that could be involved in acute and chronic care of these patients. We are aware of the fact that the management of the respiratory involvement of paediatric and adult patients affected by muscular dystrophy should be as individualized as possible; nonetheless, we believe that patient educational training, and most important of the caregiver, has a significant impact in the course of treatment. For this reason, this paper has some, patient and caregiver cards attached that, describe the management of the most important respiratory issues that occur throughout the life of patients affected by muscular dystrophy such as:
air stacking exercises;
mechanical cough assistance;
non-invasive ventilation;
ventilation through tracheostomy critical aspects;
mouthpiece ventilation.
We hope the result of this work can encourage and facilitate the respiratory care for all the centres that will have to deal, even occasionally, with the respiratory management of patients affected by muscular dystrophy and their families.
Figures and tables
Acknowlegdement
“Special thanks to the Italian Muscular Dystrophy Association (UILDM) for their support
Appendix
Air-stacking and chest expansion exercises: why, how and when to do them
By Vilma Donizetti, Marino Iatomasi, Fabrizio Rao, Giancarlo Garuti
What does air-stacking mean?
In patients with neuromuscular disorders, that have inspiratory and expiratory respiratory muscle weakness, when vital capacity falls below a certain threshold, execution of chest wall expansion exercises is suggested. The objective is to reduce as much as possible acute episodes of secretion build up and maintain chest wall expansion.
When your respiratory therapist and your pneumologist suggest performing “air-stacking “or chest wall expansion exercises, it means that you will be trained to use devices that will permit your chest to expand as much as possible.
The type of expansion you will be trained to perform will help extend your rib cage to an extent allowing it to stretch and maintain all your chest muscles flexible, mobilize your joints as much as possible and allow air to enter all the alveoli present in your lungs.
How to perform it
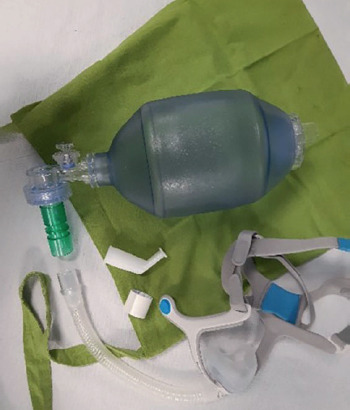
You can perform this type of stretching and trunk mobilization in two ways, by holding your breath or not (thanks to a big inhale or several consecutive inhales), and by using different devices such as an AMBU bag or a ventilator with a mouthpiece if you are already familiar with this type of ventilation and using it.
Which devices do I have to perform Air-stacking?
AMBU bag
The ambu bag is a self-inflating bag made of PVC/silicone that can hold 1200-1600 mL for adults and about 600 mL for paediatric patients
Air can be delivered to the patient via a mouthpiece or mask.
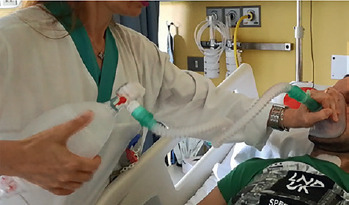
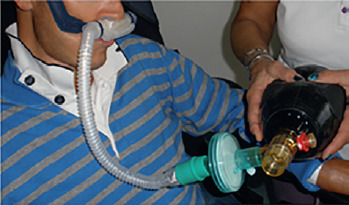
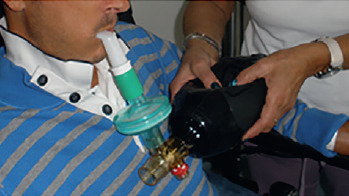
MPV = Mouth Piece Ventilation
MPV is a modality in which you can use the assistance of the ventilator only if required: when you need it you place your lips on the mouthpiece triggering the ventilator ready to assist you.
You can also use this modality to perform chest wall expansion exercises.
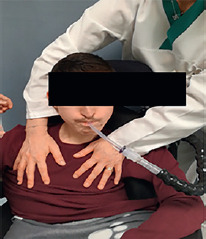
When I store air in my lungs do I necessarily need to hold my breath?
No, not everybody is able to store air inside the lungs. The respiratory therapist will try to adapt the best technique based on your lung function choosing between one of the following options:
re-expansion through single insufflation: in re-expansion thru single insufflation you will let as much air as possible into your lungs in one attempt and without necessarily holding it in;
Re-expansion through air-stacking. In re-expansion thru air-stacking you will be asked to progressively try to store air inside your lungs through one, two or more consecutive insufflations.
How often and how long do I have to perform this exercise?
Whichever method you are accustomed to using for chest wall expansion, the generally shared indication is to do it for at least around 15 minutes, twice a day.
During each session very frequent and close together expansions should be avoided as they may cause hyperventilation symptoms such as dizziness and tingling of hands and feet.
Try to perform this exercise daily and with due breaks between deep breaths!
When do I have to perform these exercises during the day?
You can perform chest wall expansion exercises when you prefer during the day but with the foresight to avoid the first two post-meal hours as insufflated air can go the wrong way and bloat the stomach and if that is full it may stimulate nausea or even retching.
Is it best to use a mask or mouthpiece when I perform exercise with the AMBU bag?
The respiratory therapist in charge of adapting and training you on expansion exercises will identify the appropriate technique and interface according to your motor function and skills, related to muscles in charge of air flow in the pharynx, larynx and mouth muscles.
For example if you have good motor function of the upper limbs, mouth, larynx, pharynx and mouth muscles the respiratory therapist will probably train you on the use of the AMBU bag using a mouthpiece to maintain a high level of independence in handling this technique.
If otherwise, when trying the mouthpiece air escapes around the lips, then training with a mask will be performed.
Please note: when using a mask you will need a caregiver to help you keep a tight seal of the mask on your face and to squeeze the resuscitation bag in synchrony with your breathing pattern.
How does air-stacking thru a ventilator and mouthpiece work?
Ventilators often have a dedicated program that can be stored for use with a mouthpiece. The pneumologist or respiratory therapist can set a dedicated program on your ventilator that will provide you with air volumes every time you ask for air from the mouthpiece.
If you are among those patients who already have a ventilator and mouthpiece ventilation then you could use this device to perform chest wall expansion and air-stacking exercises.
A dedicated program that will provide air volume on demand through the mouthpiece will be set on your ventilator.
The instruction you will be given will be to try to get as much air into your lungs through one or several inhales if you cannot hold air with your pharynx/larynx muscles.
This exercise can become ineffective and bothersome when air escape from the nose or mouth.
What if a ventilator does not have the way to store a ventilation through mouthpiece program?
If this is the case or when a patient cannot use this program the therapist will find an alternative solution such as suggesting use of night-time ventilation with a mask but setting the possibility to manually take a large breath on demand on the ventilator or storing a program with greater pressures or volumes to be used for short bouts and with this specific objective.
In conclusion
Chest expansion, using the AMBU bag or mouthpiece ventilator is a fundamental part of the physiotherapy program and should be carried out daily.
The respiratory therapist in charge of your program will try to adapt you to the most adequate modality in terms of efficacy and tolerability.
What is non-invasive ventilation and how can I manage it
By Vilma Donizetti, Marino Iatomasi, Fabrizio Rao, Giancarlo Garuti
What does NIV mean?
NIV is the abbreviation for Non-Invasive Ventilation.
Non-invasive ventilation is a way to help those muscles which make us breathe, and especially ventilate (exchange gases).
Sometimes in neuromuscular conditions, respiratory muscles are or become weak over time. Non-invasive ventilation supports the diaphragm and the other inspiratory muscles to allow the correct volume of air into the lungs and to exhale air from the lungs in a proper way, getting rid of carbon dioxide in the blood.
Carbon dioxide needs to be eliminated during ventilation because it is a gas that normally accumulates in the blood and that becomes noxious if it goes beyond normal levels.
When there is too much carbon dioxide in the blood you may feel excessively sleepy, you may have morning headaches, complain of difficulties concentrating while finding it hard to sleep well.
Non-invasive ventilation helps you sleep better and helps you feel wide awake during the day and to reduce the feeling of shortness of breath during the day if your respiratory muscles are weak.
What is NIV?
Having NIV means that you will be provided with a ventilator, a humidifier and a face mask, all connected by tubes between them.
The air is generated by the ventilator, then it passes through the humidifier so that it is warm enough when it reaches the face and finally the lungs.
The ventilator
There are different types of ventilators, with or without a built in battery, which can start different ventilatory programs.
Your referral centre will choose with you the ventilator best suited to fit your clinical needs.

The mask
The mask is a device that is placed on your face to allow air coming from the ventilator to enter your lungs through the mouth and/or nose. Generally the surface that comes in contact with the face is made of silicon.
There are different types available, all having the same objective that is the tightest fitting interface possible to avoid leaks and making it as comfortable as possible while ensuring the best ventilatory exchange.
On the top of the majority of commercially available masks there are holes from which air can exit: this air flow is very important because it allows carbon dioxide to be expelled thereby avoiding breathing toxic gas.
NEVER CLOSE THE HOLES ON THE MASK!!!
If the mask you are using does not have holes, carbon dioxide is expelled through a different circuit, for example a valve between your mask and the connecting tube. Make sure that blankets or other objects do not obstruct the passage of air outwards. Never add additional layers between mask and valve.
There are different types of masks that can be used depending on the objectives your physiotherapist has shared with you:
Nasal mask = mask that covers the nose only. This can be used to ventilate during the day or night, as needed.
Endonasal mask = mask having two small probes which partially enter your nostrils. This is the smallest possible mask, and it can be used during the day or at night. It allows concomitant use of glasses if needed and has small dimensions. However, it may move out of place more often than the other types of interfaces.
- Oro-nasal = this is the best solution for nocturnal non-invasive ventilation especially if you tend to open your mouth while sleeping with a nasal mask and you are not using a chin guard device because you do not tolerate it or it does provide additional help while breathing.
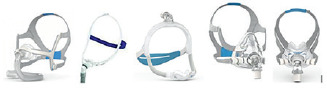
hybrid = oro-nasal mask which does not come into contact with your nasal bone. This may be convenient to avoid pressure lesions in the nasal area and to help you maintain a broader visual field.
MPV (= mouth piece ventilation) = ventilator with a mouth piece. This topic will be specifically addressed in a dedicated section.
We recommend washing your mask every day with water and neutral soap, drying it and placing it in a clean cloth when not in use. There is no need for you to use disinfectants because these may damage the mask itself.
Take care of your mask as best as you can because generally the National Health System provides you with only 2 masks per year.
The humidifier
To help you use NIV at its best you will most probably be given a humidifying system.
The purpose of the humidifier is to humidify air which would otherwise reach the ventilator as a cold and dry gas mixture: a plate warms distilled water to provide warm water droplets which evaporate into the tubes connecting the ventilator to your mask.
The distilled water is kept in a plastic gravity water chamber: never fill the chamber beyond the threshold indicated as the maximum level of water and never leave the water chamber empty as it may burn.
Once at home, you can modify and manage the level of humidification according to your needs and comfort, but avoid water accumulating in the tubes. To ensure this avoid abrupt changes in temperature between the tubes in the circuit and the room where the ventilator is used and kept.
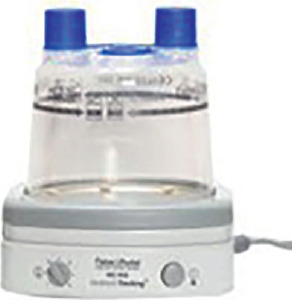
The circuit
This is made up of tubes connecting the humidifier to the mask. Their length is standard: they should not be shortened or lengthened.
Avoid water droplets accumulating in the tubes: this may cause the ventilator to break and facilitates bacterial growth.

FAQ
I only have one mask: am I allowed to have 2 types of masks each year?
Yes, it is actually recommended to have 2 masks of different types so that you can switch from one to the other to avoid pressure lesions on your face where the mask comes into contact.
I cannot remove the facial mask on my own and having an orofacial mask scares me because of the idea of not being able to ask for help or not being able to remove it quickly?
Talk about it with your physiotherapist or your pulmonologist, they will find an alternative solution, maybe using a chin support, a special alarm system or communication device which fits your needs.
I fear I will not be able to talk if I use the ventilator?
No, you will be able to speak, and actually the tone of your voice may sound stronger and of a higher pitch.
Is the air coming from the ventilator pure oxygen?
No, it is simply air taken from your surrounding (which does contain oxygen as well as other gases) which is directed through the circuit into your mask.
If there should be a need to add oxygen to your air supply, the pulmonologist and physiotherapist will provide you with an additional connector which will provide oxygen to the ventilator.
The air coming from the surroundings is full of dust, shouldn’t this air be filtered?
Yes, the ventilator has a spongy filter where air from the surroundings enters the ventilator. This needs to be washed weekly and has to be replaced when dry or when worn. Some ventilators also have a white filter against pollen dust which should not be washed but replaced monthly.
If the air that I breathe is the same as the air that the others inhale normally, why should I always use the humidifier with the ventilator?
Because the air which reaches the mask is pushed into the lungs and quickly passes through nose/mouth. The mouth alone cannot provide an adequate humidification and the nose can do this only if air passes slowly and one breathes normally.
May I use demineralized water, osmolarized water or mineral water instead of distilled water?
No, it is recommended to only use distilled water.
In case I travel by plane, how should I carry my ventilator?
The ventilator should always be carried in its original bag as a carry-on luggage and should never be checked in as regular luggage.
There needs to be a pre-printed travel form from the airlines and this needs to be filled out by your pulmonologist.
In case you need to use the ventilator during the flight you need to talk to the airline beforehand and organize your trip with an AMBU bag instead if needed.
Ask the airlines all the information you need before you travel.
May I use indifferently a mask with holes and one with a valve on the circuit?
No, the type of circuit prescribed to eliminate carbon dioxide is a medical prescription and is based on clinical grounds, and on the physiotherapeutic and mechanical features of the ventilator which was chosen. It is very dangerous for your health to change the parameters and settings that have been chosen specifically for you.
A friend, a technician, a provider, an internet video has prompted me to try a new mask/circuit/humidifier. They seem to be experts in the field. Can I follow their advices?
No, only the pulmonologist or physiotherapist who has experience in ventilation can help you. In case you have additional questions do not hesitate to call them.
I am using the ventilator for more than 16 hours a day. When I leave my house what should I bring with me?
When you leave your house you should definitely have:
a bag for emergency/accidents (cloth bag which should be hung on the wheelchair) containing the AMBU bag connected to your other mask which should be used in case your ventilator should fail to work and adhesive tape to close potential holes/leaks that may accidentally occur in the circuit;
saturimeter: if you think you will stay away from home for long (a whole day or longer) you should also carry your second ventilator and your cough assist machine.

MPV: Mouth Piece Ventilation
By Vilma Donizetti, Marino Iatomasi, Fabrizio Rao, Giancarlo Garuti
What is it?
Mouth piece ventilation is one of the oldest types of non-invasive ventilation born as an alternative to tracheostomy for patients affected by Polio in the Fifties.
For whom
Useful for patients affected by neuromuscular disorders, but also patients with diaphragmatic paralysis, spinal cord injuries, kyphoscoliosis, cystic fibrosis and COPD.
Conditions for use
Patient has to be awake, conscious and cooperative, able to access mouthpiece and with good control of upper airway muscles (no bulbar deficits).
When to use it
A Medical doctor or respiratory therapist will suggest this alternative ventilation technique when:
your carbon dioxide blood levels during the day increase beyond normal levels (> 45 mmHg) even though they are properly corrected by mechanical ventilation use at night;
you have during the day episodes of dyspnoea or shortness of breath;
you cannot talk for long periods of time or have such a low tone of voice that you cannot be heard or scream;
you use mechanical ventilation with a mask for more than 14/16 hours and are at risk of pressure sores on your face;
you have shortness of breath while eating and are progressively eating less therefore losing weight;
you have shortness of breath after a meal or while sitting on the toilet;
food goes the wrong way if you use the ventilator with a mask while eating or drinking;
you would like to cough on your own without always having to ask for help;
you would like to ventilate also during the day without this limiting your vision, speech or social life.
Why use it?
Using mouth piece ventilation improves quality of life and survival. Patients that use this type of ventilation appreciate the independence it provides by reducing fatigue in talking, breathing, eating, coughing sometimes even better than mask ventilation and also allows smelling.
No tube or masks hinder vision or interfere with social interactions. It is the patient that decides when and for how long he/she ventilates, breath by breath, according to his/her needs and is also able to alternate it with glossopharyngeal breathing or air-stacking manoeuvres (holding your breath after consecutive inhales).
How
Attach the ventilator to the wheelchair, turn it on and choose the MPV setting. After securing the circuit to your preferred support, and the mouth piece or straw close to the lips, come closer as if you were drinking a sip of air or just slightly touch the system (depending on the chosen ventilator): the tube will deliver enough air to fill your lungs to be able to breathe, scream, sing, cough, talk at length. When too much air is delivered, let it escape freely from your mouth without closing your lips too tight on the mouth piece; if too little air is delivered then hold two or three consecutive breaths (air-stacking) and then use the air to cough better or hold your breath longer while for example chewing food in your mouth or yelling.
Frequency, air quantity, regularity of ventilator use is decided by the patient. Normally no one tells us how much air is needed to speak louder or longer for example and everyone self regulates. The same thing happens when using MPV: it has an air reservoir the patient can tap into and he/she can decide when.
Maintenance
Wash and disinfect the mouthpiece daily with neutral detergent, the circuit weekly and the filter monthly.
Important
The mouthpiece has to be always fixed close to the mouth and easily accessed by the patient.
Pay attention to where the circuit support is anchored: if you usually tilt your wheelchair while ventilating, make sure that the mouth piece can follow your movements. There are commercially available supports that can be prescribed or you can adapt other systems such as the ones used to hold smartphones.
Never exhale into the circuit (if it does not have an exhale valve). After drinking from a straw would you then spit again in your glass?
In case of mouth dryness keep some water handy and drink regularly; on the contrary, with excessive salivation, ask for medical advice in regards to drugs that limit saliva production.
Always make sure the ventilator has enough battery charge before going far from an electrical outlet.
Based on your needs and/or for safety reasons, it is possible to program an alarm that is triggered after a set time of failed activation through the mouth piece (voluntary or involuntary).
Remember
Breathing is a vital necessity to survive not an addiction. MPV is needed to live better as it provides independency not addiction.

Cough-assist devices
By Vilma Donizetti, Marino Iatomasi, Fabrizio Rao, Giancarlo Garuti
Cough
Coughing is a physiological mechanism to protect our respiratory airways. We cough to manage secretions or to remove foreign matter in our airways or we may have in our throat.
Stages of cough
First there is a deep inspiration, then for a fraction of a second, the glottis closes and breath is held. Then air is suddenly and explosively expired and with the air any foreign particles of materials found in the airways
Cough efficacy
In order to understand whether cough is effective, the pulmonologist or the physiotherapist measures it with a specific instrument which quantifies the Peak Cough Flow. If this is greater than 270 l/min, cough is effective, if between 160 and 270 l/min cough is weak and if below 160 l/min, cough is definitely ineffective.
Mechanical cough-assisted devices
If air-stacking techniques and or manually-assisted manoeuvres prove to be inefficient (see document), either due to respiratory muscle weakness, fatigue or lack of compliance, the pulmonologist or the physiotherapist will prescribe a cough-assisted device.
There are different devices which can be prescribed and all are equally efficient. Each patient and caregiver will receive the device prescribed and with it, the specific instructions to use it properly.
What is it?
A cough-assist device is an instrument which mimics the act of coughing by mechanically inflating air within the airways and then by compressing air out of the lungs in the act of expiration. The machine introduces sufficient air within the lungs so as to inflate the lungs and the thorax and then the machine rapidly aspires the air in the lungs and with it the secretions in the mouth or in the cannula if there is a tracheostomy.

What it does not do?
A cough- assisted device is not an aspirator and it is not a ventilator. It is also not a cough stimulator or cough trainer. One should not expect to hear the typical sound of coughing when in use.
What is the purpose of a cough-assist device?
The purpose is to avoid secretions build-up and to mobilize them in the upper airways when the patient is unable to do so on his/her own. It also exerts action on the respiratory muscles by stretching them and by mobilizing the rib cage while maintaining its elastic properties.
Which techniques can be implemented with the cough-assist device?
To optimize the efficacy of this treatment it is recommended that the patient be asked to try and cough voluntarily when the cough-assist device is beginning to generate negative pressure within the airway system, eventually adding manual assistance (thoracic or abdominal thrust). To avoid tiring the patient in the first sequence, it is recommended to use the passive mode for the first cycles (to recruit and ventilate) and to add voluntary cough (and manual assistance) for the last cycle. Your therapist will teach you how to coordinate the different manoeuvres so that your participation will be as effective as possible.
Indications for prescription
A cough-assist device is recommended for patients with neuromuscular diseases having a PCF < 160 l/min, or when patients have a PCF < 270 l/min at rest, in a stable condition, but may get easily fatigued in case of acute respiratory disease.
Treatment protocol template
Preventive care: a cough-assisted device should be used even when secretions are within normal range as per quantity and colour. Recommendations are twice a day, five or six cycles for a total of 5 or 6 sequences away from meals . The patient can use the device in any position, while seated, supine or on his/her side. It is recommended to change position even during the same cycle so that air can reach different parts of the lungs. During this treatment the thorax should be monitored to make sure it expands and relaxes regularly as air passes through.
Acute care: in case of a respiratory infection, it is recommended to increase the frequency of the cycles, more than twice a day (even at night-time if needed) and every time saturation goes beneath 95%. Five or six sequences are usually needed for as many times to stabilize the patient. In these cases it is often useful to implement the treatment by asking the patient to try and cough on his/her own and to apply thoracic or abdominal thrusts too. If indicated by the physiotherapist or by your pulmonologist higher pressures may be needed.
If the patient is especially tired there may be the need to place the patient on his/her ventilator between sequences to allow for him/her to recover.
Parameters
Pressure: this is the strength with which air is forced into and out of the airway system. Sometimes the negative pressure is higher than the positive one, the opposite should not be done.
Time: this refers to the time required to insufflate and exsufflate the lungs. In general, the time to inflate is longer than the time needed to exsufflate. There are short intervals of time to recover between each sequence.
Air flow: this is the flow with which air can be delivered: it can be more or less intense or fast.
Trigger: if this is set by the technician, physiotherapist or pulmonologist, the patient can trigger air delivery with a minimum effort. This is of help for some patients because it may help them adapt and synchronize with the machine itself. The patient’s strength should be assessed prior to setting this trigger (this is especially true in case of an acute respiratory infection); if this occurs, then the health care professional should be informed and the program should be switched from trigger to automatic mode (if this is already set before hand).
Oscillations: these are vibrations that can be added during the insufflation and/or exsufflation phases.
Modality: a cough-assist device can be automatically shifted to the insufflation and the exsufflation modes or there may be the option to shift manually. At home the preferred option is the automatic modality (with or without trigger). In case the cough-assisted device is set to the manual mode, you will need to shift the lever to the inhale phase (trying to fill in the lungs as much as possible) and then towards the exhale phase (trying to empty your lungs as much and as quickly as possible).
Number of programs: there is the option to memorize 2 or more programs in the cough-assist device so that one is selected for the usual daily exercises and the other one (or more) can be selected during an acute phase, with higher pressures and different modalities. Precise indications for one or the other program will be provided.
Side effects and adverse events
These are usually few, rare and transitory. These are the most common ones:
ear pain;
chest pain (due to stretching of the thoracic musculo-skeletal structures);
abdominal tension with nausea and vomiting;
secretions with blood striations resulting from bronchial wall mobility;
desaturation in case of hemodynamic instability;
cardiac arrhythmias, bradycardia, tachycardia;
pneumothorax (lung collapse);
In case of problems, symptoms and signs of uncertain diagnosis please refer them as soon as possible to your physiotherapist or pulmonologist.
Controindications
Emphysema
Barotrauma predisposition
Parenchymal lesions
Hemodynamic instability
Reduced left ventricular function
Recent cardiogenic pulmonary edema
Tracheomalacia
Cleaning and maintenance
After each sequence the circuit and the face mask need to be cleaned with liquid detergent and need to be regularly disinfected with cold disinfectant. The catheter-mount needs to be replaced each time and needs to be washed and disinfected with cold disinfectant. Each part should then be dried.
The filter cannot be washed and needs to be replaced with the rest of the material when this is clearly worn out.
The external surface of the cough-assist device needs to be cleaned with a humid cloth.
The spongy machine filter needs to be washed weekly and has to be dried before it can be placed back on.
For any malfunctioning at home, please call the equipment provider.
What should not be done
DO NOT change the parameters without consulting the pulmonologist or your therapist
DO NOT let air leak out from the sides of the mask, but keep this tight on your face
DO NOT try to handle or try to fix the machine on your own
DO NOT prolong the time of treatment for more than 10 sequences in a row or for more than 30 minutes total
DO NOT interrupt the treatment after just one cycle thinking this is enough or that the machine is not doing anything. Stick to the instructions
Frequently asked questions
I have just eaten but I am already full of secretions. May I use my cough-assist device?
Yes, but with caution. It may cause you to vomit. Limit its use and avoid the abdominal thrust and stay seated for a couple of hours after your meal.
I choked myself with a small piece of food. Can I try and get rid of it, using the cough-assist device to remove this foreign material?
Yes, the machine can be used with the highest negative pressures and use it with the manual trusts until you have gotten rid of the foreign material
My chest hurts each time the air gets in and I have never experienced this type of feeling before
Stop using the machine immediately and contact your pulmonologist right away
I am going on holiday, can I leave my cough-assist device at home?
Your secretions do not go on holiday and your ability to cough does not improve on holiday. If you were given a cough-assist device it is because you need it and this means wherever you may be
I need to take a plane, can I bring it with me?
Yes, as hand-luggage and with a medical certificate saying that you need to use it and have it with you. It is best to contact the airline before you leave.
I have a gastrostomy, may I use it anyway?
Yes, with caution. In case of food or excess air in your stomach, let your physiotherapist or pulmonologist tell you what to do.
I was trained while lying, but now I am in my wheelchair, can I use it anyway?/I was trained while seated, but now I am in bed with a high temperature, can I use it anyway?
You can use the cough-assisted machine in any position, according to your needs and situations. Remember however, that it is best to change position to recruit and ventilate your lungs better.
I was trained some time ago and now I am not so sure I remember how it works, what should I do?
If you do not remember how to use it either because you do not remember or because things are still unclear to you, do not wait for an acute episode to occur but read this document over again and if, this is still unclear, contact your reference centre and ask to review the whole procedure again.
If I do not have trouble managing secretions should I use it anyway?
Yes, this device should not be used to manage secretions only. It also has the purpose of inspiring better and exercise your respiratory muscles. Ask your physiotherapist if you can perform different exercises on days in which you aren’t using it (i.e. air-stacking exercises with an AMBU bag for instance).
I have a tracheostomy. Is it enough for my caregivers to suction secretions from my cannula?
No, suction removes secretions if they are in the cannula or just below that. The cough-assist device mobilizes secretions from both lungs and brings them to the cannula and suction is then needed even more.
Invasive ventilation: critical issues
By Vilma Donizetti, Marino Iatomasi, Fabrizio Rao, Giancarlo Garuti
Tracheostomy is an artificial opening at neck level (between the Adam’s apple and the sternum) allowing air flow and direct communication between lungs-trachea and the outside, by-passing upper airways (nose-mouth-throat-vocal cords).
It is a respiratory pathway in alternative to the natural mouth/nose one, which is artificially created by a medical doctor (ENT or intensivist doctor) when breathing (even thru mechanical ventilation) and /or obstruction removal by natural means is not possible or no longer effective.
The tracheostomy is a shared decision between the patient and the multidisciplinary team, that can be planned in a stable stage or it can be a patient’s or caregiver/legal guardian’s decision in an emergency situation.
Tracheotomy or tracheostomy?
These terms indicate procedures performed to ensure better breathing. They are not synonyms although they are used interchangeably.
To be precise:
tracheotomy: simple incision and opening of the trachea which is usually temporary;
tracheostomy: surgical procedure that connects the trachea directly with the outside;
in both cases the result is an opening at throat level (tracheal stoma) with a tracheal cannula.
The cannula
The tracheostomy cannula is a curved plastic tube that crosses the stoma and directly connects the lungs with the outside, completely by-passing all structures above the vocal cords (nose-mouth-throat).
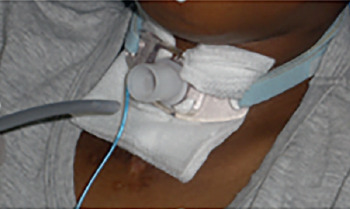
It can be equipped with:
cuff: a balloon that when inflated completely blocks air flow from the mouth and nose, preventing the patient from speaking;
inner cannula: cannula inside the outer cannula. It can be removed daily and temporarily by the patient to facilitate cleaning of the cannula itself;
fenestration: holes on the angled part of the cannula that in some specific cases can facilitate phonation.
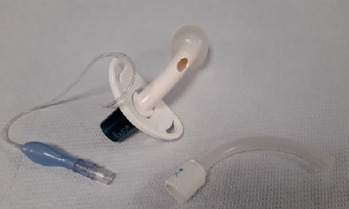
WHAT TO PAY ATTENTION TO DURING DAILY MANUEVERS?
There are some maneuvers to manage the tracheostomy that you might need to perform daily that require much attention.
ALWAYS REMEMBER to safely work with TWO HANDS: one hand supports the cannula and the flange to stabilize it, while the other hand connects or disconnects the catheter-mount, tracheal filter, etc.
It is a maneuver that has to be carried out with extreme attention to avoid harming the patient:

if the catheter-mount is pulled without keeping the cannula still with the fingers, there is a risk of moving or unthreading the cannula, especially if it is uncuffed;
if the catheter-mount is pushed against the cannula without keeping it still, this pushes directly on the patient’s trachea and can cause discomfort.
Always SUPPORT the ventilator circuit with a clip or similar device to avoid pulling the cannula with the tube’s weight provoking sores, cannula disconnection or even worse decannulation.
Secretion removal
Suction through tracheostomy cannula
To be performed:
when needed (for secretion highlighted by the patient or when these are audible inside the cannula);
only in cannula e not beyond.
The safest and most correct method is:
pre-measure the probe length (equal to the cannula length + ½ cm);
enter the cannula, with a sterile tube, only reaching the lower part of the cannula and not further;
enter without suctioning and start only when starting to retract the tube from the cannula;
suction duration should not last more than about 10 seconds;
do not use the same tube for more than two subsequent suctions unless sanitizing it before each use.
ATTENTION: deeper or prolonged maneuvers may lead to the risk of tracheal mucosa lacerations with possible bleeding and deep desaturations.
Mechanical cough assist
All secretions that did not reach the cannula can be mobilized and moved closer with mechanical cough assistance or AMBU bag. Follow the same directions for cough-assist devices by non-invasive administration simply substituting the mask with a catheter-mount and secretion removal by cough with suction. A cuffed cannula should be preferred when using cough-assist devices.
AMBU bag
The AMBU bag is a useful tool to mobilize secretions when the mechanical cough-assist devices are not available. For ways of using it please refer to instructions from your reference center.
Cleaning above the cuff
Cleaning above the cuff will be performed to avoid secretion or saliva build up above the balloon.
To clean above the cuff briefly deflate the balloon and suction any secretions from cannula and mouth.
This maneuver has to be performed according to the reference center’s recommendations. One way is for example to uncuff the cannula after a couple of cycles with a cuffed cannula, in order to use the cough assist machine. Uncuffing during cough assist machine use promotes secretion migration to the mouth, particularly if the patient during insufflation cooperates by scraping his throat.
ATTENTION: this maneuver must be performed with care and attention following all the indications from the physiotherapist in order to avoid patient desaturations and discomfort.
Urgency/emergency: what to do?
PROBLEM: ventilator malfunction
SOLUTION: disconnect patient from ventilator and uncuff cannula and let patient breath on his own (if he is usually able to and is not ventilated continuously) protecting the cannula with a tracheal filter, a phonatory valve or a plug. Call the home care provider’s toll-free number to report the malfunction manually ventilate patient with an AMBU bag while waiting for the back-up ventilator or medical assistance. Then call the toll-free number of the Home Care Provider to report the malfunction.
PROBLEM: although the patient is connected to his/her usual ventilator it appears he is not receiving enough air supply and the chest and abdomen do not inflate during insufflation.
This problem has different causes, and below are the ones that can be resolved at home.
Cannula is perfectly clean and free for air flow but there are many secretions below the cannula.
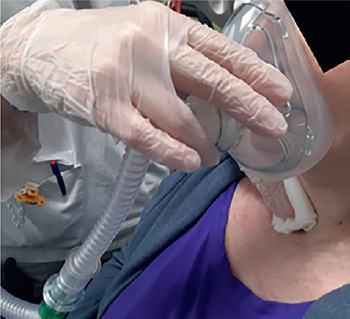
SOLUTION: use the cough assist machine and suction the cannula as needed to bring saturation levels above 94% and observe if the patient breathes better after obstruction removal.
Cannula is completely clogged by a plug of dry secretions that completely blocks the cannula and doesn’t allow air flow or the insertion of a suction tube.
SOLUTION: remove inner cannula, if present, substituting it with a clean one if an inner cannula is not used, completely uncuff the cannula and use the cough assist machine on the program with the highest pressure settings or the AMBU bag to try and mobilize the mucous plug.
Insist till removal keeping an eye on patient’s saturation and state of consciousness. Between uses of the cough assist/AMBU bag try to help patient breathe from natural airways using ventilator with mouthpiece or mask.
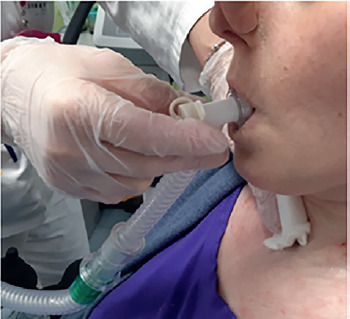
In the extreme case in which a secretion plug does not move and the patient, although uncuffed cannot breathe properly, IMMEDIATLY contact the emergency number and in the meantime, if the patient becomes cyanotic with saturation below 85%, remove the cannula, cover the stoma with a gloved finger and continue to ventilate the patient with a mask or mouthpiece (using mechanical ventilator or AMBU bag).
PROBLEM: if the patient severely desaturates and in a prolonged manner and seems to lose consciousness.
SOLUTION:
slightly uncuff the cannula;
start using the AMBU bag checking that the chest/abdomen lift at each AMBU bag squeeze;
call emergency number to request medical assistance (in case you are unable to resolve the problem in a short period of time).
PROBLEM: patient unplanned decannulation
SOLUTION: if patient usually:
is not continuously mechanically ventilated, place him in a position that favors spontaneous breathing (usually in sitting) and call the emergency number. Wait for medical assistance and monitor saturation levels;
is permanently ventilated or desaturates without ventilator assistance: close the stoma with a gloved finger and connect patient to ventilator with a mask, mouthpiece or the free end of the cannula itself used like a straw. Call emergency number as soon as possible.
If airflow is not resumed, the chest/abdomen do not lift and patient desaturates and becomes cyanotic, firmly position the AMBU bag mask on the stoma and try to ventilate the patient with the AMBU bag thru the tracheal stoma. Call the emergency number IMMEDIATELY
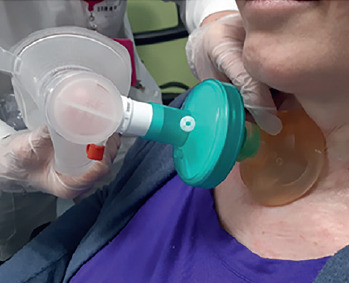
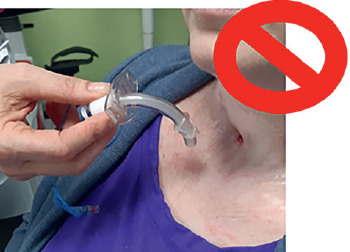
IMPORTANT: do not try to reposition the cannula if you haven’t received proper training from medical staff or if you are not in an extreme situation: if this maneuver is performed in a clumsy way it can lead to permanent damage. Should you need, if possible use a cannula without a cuff and with a smaller diameter compared to the usual one.
ADVICE: in the routine daily stoma cleaning maneuvers or change of neckplate avoid undoing or completely unthreading the neckplate. It is wiser to simply loosen the neckplate and keep it attached. If a collar change is needed insert the new collar in the flange and attach it before removing the old one.
Frequently asked questions
Can I use the same tube to first suction the mouth and after the cannula?
NO, the tube that enters the cannula has to be sterile so you can do the opposite and start suctioning in the cannula and then the mouth.
Which is the correct saturation value?
Normally blood oxygen saturation should be above 94%. If it falls below this limit start evaluating the situation: use the cough assist/suction device to remove any secretions, connect patient to ventilator (if not already on ventilation), check for temperature elevation, contact your primary care physician.
Saturation is low or lower than usual. Can I improve it by using a bit of oxygen?
Oxygen is considered a medication and as such should be administered only under medical prescription. Remember anyway that in your disorder pure oxygen consumption leads to carbon dioxide build up. In case of desaturation even after frequent use of the cough assist device while waiting for antibiotic therapy to become effective, increase hours of ventilation and if prescribed by a doctor, take oxygen preferably mixed with ventilator air flow. Reduce the time spent breathing pure oxygen thru nasal cannulas but prefer the use of the ventilator. This way you will tire less when breathing and you will avoid rapid carbon dioxide build up.
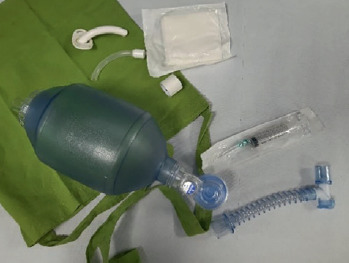
How many times a day do I have to use the cough assist machine?
At least twice a day, morning and evening and more if needed. Secretion production during the day is variable among different individuals and can be more or less.
Furthermore, most likely, you will need to use the cough assist machine just before or after postural changes such as bed to wheelchair transition, as this mobilizes secretions.
I use the ventilator more than 16 hours a day, when I go out what do I have to bring? When you go out of the house you should absolutely have with you:
emergency bag (readily available cloth bag attached to the wheelchair that contains an AMBU bag connected to a catheter-mount, adhesive tape to close any accidental holes in the circuit, a syringe to cuff and uncuff the cannula, a clean inner cannula wrapped in gauze, an uncuffed cannula of smaller diameter compared to the one used for emergencies);
suction machine (with charged battery, car charging cable) complete with tubes and bottle for water suction;
pulse oximeter.
If you are away from home for a long time (a whole day or more), it is best for you to also bring the cough assist machine and a back-up ventilator.
My secretions have been drier than usual for a couple of days. What can I do?
In case secretions are very dry it might be necessary to reduce the number of hours on ventilation with just a humidifying filter and/or uncuffed cannula, and increase the number of hours using an active humidifier preferably with a thermoregulated circuit.
It might be useful to use an aerosol dispenser or nebulizer with just physiological water: for practical guidance refer to your clinical center.
Contributor Information
UILDM Respiratory group:
Alessio Mattei, Grazia Crescimanno, Elisabetta Roma, Antonello Nicolini, Anna Mandelli, Alessia Fabiano, Mirco Lusuardi, Antonella Pini, Maurizio Grandi, Federico Sciarra, Emiliana Meleo, Claudia Profazio, Vilma Donizetti, and Marino Iatomasi
References
- 1.Shneerson JM, Simonds AK. Noninvasive ventilation for chest wall and neuromuscular disorders. Eur Respir J 2002;20:480-487. https://doi.org/10.1183/09031936.02.00404002 10.1183/09031936.02.00404002 [DOI] [PubMed] [Google Scholar]
- 2.Boentert M, Wenninger S, Sansone VA. Respiratory involvement in neuromuscular disorders. Curr Opin Neurol 2017;30:529-537. https://doi.org/10.1097/wco0000000000000470 10.1097/wco0000000000000470 [DOI] [PubMed] [Google Scholar]
- 3.Berger KI, Rapoport DM, Ayappa I, et al. Pathophysiology of hypoventilation during sleep. Sleep Med Clin 2014;9:289-300. [Google Scholar]
- 4.Auger C, Hernando V, Galmiche H. Use of mechanical insufflation-exsufflation devices for airway clearance in subjects with neuromuscular disease. Respir Care 2017;62:236-245. https://doi.org/10.4187/respcare.04877 10.4187/respcare.04877 [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer G, Povitz M, Gibson GJ, et al. Diagnosis of muscle diseases presenting with early respiratory failure. J Neurol 2015;262:1101-1114. https://doi.org/10.1007/s00415-014-7526-1 10.1007/s00415-014-7526-1 [DOI] [PubMed] [Google Scholar]
- 6.Bianchi ML, Losurdo A, Di Blasi C, et al. Prevalence and clinical correlates of sleep disordered breathing in myotonic dystrophy types 1 and 2. Sleep Breath 2014;18:579-589. https://doi.org/10.1007/s11325-013-0921-5 10.1007/s11325-013-0921-5 [DOI] [PubMed] [Google Scholar]
- 7.Birnkrant DJ, Bushby K, Bann CM, et al. DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 2018;17:251-267. https://doi.org/10.1016/S1474-4422(18)30024-3 10.1016/S1474-4422(18)30024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birnkrant DJ, Bushby K, Bann CM, et al. DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 2018;17:347-361. https://doi.org/10.1016/S1474-4422(18)30025-5 10.1016/S1474-4422(18)30025-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnkrant DJ, Bushby K, Bann CM, et al. DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol 2018;17:445-455. https://doi.org/10.1016/S1474-4422(18)30026-7 10.1016/S1474-4422(18)30026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashizawa T, Gagnon C, Groh WJ, et al. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurol Clin Pract 2018;8:507-520. https://doi.org/1212/CPJ.0000000000000531 10.1212/CPJ.0000000000000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Ruiten HJ, Marini Bettolo C, Cheetham T, et al. Why are some patients with Duchenne muscular dystrophy dying young: an analysis of causes of death in North East England. Eur J Paediatr Neurol 2016;20:904-909. https://doi.org/10.1016/j.ejpn.2016.07.020 10.1016/j.ejpn.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 12.de Die-Smulders CE, Howeler CJ, Thijs C, et al. Age and causes of death in adult-onset myotonic dystrophy. Brain 1998;121(Pt 8):1557-1563. https://doi.org/10.1093/brain/121.8.1557 10.1093/brain/121.8.1557 [DOI] [PubMed] [Google Scholar]
- 13.Mathieu J, Allard P, Potvin L, et al. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology 1999;52:1658-1662. https://doi.org/10.1212/wnl.52.8.1658 10.1212/wnl.52.8.1658 [DOI] [PubMed] [Google Scholar]
- 14.Camela F, Gallucci M, Ricci G. Cough and airway clearance in Duchenne muscular dystrophy. Paediatr Respir Rev Aug 2019;November 24. https://doi.org/10.1016/j.prrv.2018.11.001. [Epub ahead of print] 10.1016/j.prrv.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Candiani G, Colombo C, Daghini R, et al. Manuale metodologico: come organizzare una conferenza di consenso [online]. Rome: Istituto Superiore di Sanità, Sistema Nazionale Linee Guida SNLG, 2009. (http://www.psy.it/wp-content/uploads/2018/02/Manuale-Metodologico-Consensus.pdf). [Google Scholar]
- 16.Della Marca G, Frusciante R, Dittoni S, et al. Sleep disordered breathing in facioscapulohumeral muscular dystrophy. J Neurol Sci 2009;285:54-58. https://doi.org/10.1016/j.jns.2009.05.014 10.1016/j.jns.2009.05.014 [DOI] [PubMed] [Google Scholar]
- 17.McKim DA, McKim DA, Katz SL, et al. Lung volume recruitment slows pulmonary function decline in Duchenne muscular dystrophy. Arch Phys Med Rehabil 2012;93:1117-1122. https://doi.org/10.1016/j.apmr.2012.02.024 10.1016/j.apmr.2012.02.024 [DOI] [PubMed] [Google Scholar]
- 18.Moreira S, Wood L, Smith D, et al. Respiratory involvement in ambulant and non ambulant patients with facio-scapulo-homeral muscular dystrophy. J Neurol 2017;264:1271-1280. https://doi.org/10.1007/s00415-017-8525-9 10.1007/s00415-017-8525-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayanaswami P, Weiss M, Selcen D, et al. Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies. Neurology 2014;83:1453-1463. https://doi.org/10.1212/WNL.0000000000000892 10.1212/WNL.0000000000000892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawnani H, Thampratankul L, Szczesniak RD, et al. Sleep disordered breathing in young boys with Duchenne muscular dystrophy. J Pediatr 2015;166:640-5.e1. https://doi.org/10.1016/j.jpeds.2014.12.006 10.1016/j.jpeds.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 21.Groh WJ, Groh MR, Shen C, et al. Survival and CTG repeat expansion in adults with myotonic dystrophy type 1. Muscle Nerve 2011;43:648-651. https://doi.org/10.1002/mus.21934 10.1002/mus.21934 [DOI] [PubMed] [Google Scholar]
- 22.Schoser B, Montagnese F, Bassez G, et al. Consensus-based care recommendations for adults with myotonic dystrophy type 2. Neurol Clin Pract 2019;9:343-353. https://doi.org/10.1212/CPJ.0000000000000645 10.1212/CPJ.0000000000000645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell C, Sherlock R, Jacob P, et al. Congenital myotonic dystrophy: assisted ventilation duration and outcome. Pediatrics 2004;113:811-816. https://doi.org/10.1542/peds.113.4.811 10.1542/peds.113.4.811 [DOI] [PubMed] [Google Scholar]
- 24.Henke C, Spiesshoefer J, Kabitz HJ, et al. Characteristic of respiratory muscle involvement in myotonic dystrophy type 1. Neuromusc Disord 2020;30:17-27. https://doi.org/10.1016/j.nmd.2019.10.011 10.1016/j.nmd.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 25.Heatwole C, Bode R, Johnson N, et al. Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1). Neurology 2012;79:348-357. https://doi.org/10.1212/WNL.0b013e318260cbe6 10.1212/WNL.0b013e318260cbe6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laberge L, Begin P, Montplaisir J, et al. Sleep complaints in patients with myotonic dystrophy. J Sleep Res 2004;13:95-100. https://doi.org/10.1111/j.1365-2869.2004.00385.x 10.1111/j.1365-2869.2004.00385.x [DOI] [PubMed] [Google Scholar]
- 27.Laberge L, Gagnon C, Dauvilliers Y. Daytime sleepiness and myotonic dystrophy. Curr Neurol Neurosci Rep 2013;13:340-348. https://doi.org/10.1007/s11910-013-0340-9 10.1007/s11910-013-0340-9 [DOI] [PubMed] [Google Scholar]
- 28.Sansone VA, Gagnon C, participants of the 207th ENMC Workshop . 207th ENMC Workshop on chronic respiratory insufficiency in myotonic dystrophies: management and implications for research, 27-29 June 2014, Naarden, The Netherlands. Neuromuscul Disord 2015;25:432-442. https://doi.org/10.1016/j.nmd.2015.01.011 10.1016/j.nmd.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 29.Poussel M, Thil C, Kaminsky P, et al. Lack of correlation between the ventilatory response to CO2 and lung function impairment in myotonic dystrophy patients: evidence for a dysregulation at central level. Neuromusc Disord 2015;25:403-408. https://doi.org/10.1016/j.nmd.2015.02.006 10.1016/j.nmd.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 30.Hull J, Aniapravan R, Chan E, et al. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax 2012;67(Suppl 1):i1-i40. https://doi.org/10.1136/thoraxjnl-2012-201964 10.1136/thoraxjnl-2012-201964 [DOI] [PubMed] [Google Scholar]
- 31.Panitch HB. The pathophysiology of respiratory impairment in pediatric neuromuscular diseases. Pediatrics 2009;123(Suppl 4):S215-218. https://doi.org/10.1542/peds.2008-2952C 10.1542/peds.2008-2952C [DOI] [PubMed] [Google Scholar]
- 32.Katz SL. Assessment of sleep-disordered breathing in pediatric neuromuscular diseases. Pediatrics 2009;123(Suppl 4):S222-225. https://doi.org/10.1542/peds.2008-2952E 10.1542/peds.2008-2952E [DOI] [PubMed] [Google Scholar]
- 33.Ragette R, Mellies U, Schwake C, et al. Patterns and predictors of sleep disordered breathing in primary myopathies. Thorax 2002;57:724-728. https://doi.org/10.1136/thorax.57.8.724 10.1136/thorax.57.8.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz SL, Gaboury I, Keilty K, et al. Nocturnal hypoventilation: Predictors and outcomes in childhood progressive neuromuscular disease. Arch Dis Child 2010;95:998-1003. https://doi.org/10.1136/adc.2010.182709 10.1136/adc.2010.182709 [DOI] [PubMed] [Google Scholar]
- 35.Ward S, Chatwin M, Heather S, et al. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax 2005;12:1019-1024. https://doi.org/10.1136/thx.2004.037424 10.1136/thx.2004.037424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellies U, Ragette R, Dohna Schwake C, et al. Long-term noninvasive ventilation in children and adolescents with neuromuscular disorders. Eur Respir J 2003;22:631-636. https://doi.org/10.1183/09031936.03 10.1183/09031936.03 [DOI] [PubMed] [Google Scholar]
- 37.Young HK, Lowe A, Fitzgerald DA, et al. Outcome of noninvasive ventilation in children with neuromuscular disease. Neurology 2007;68:198-201. https://doi.org/10.1212/01.wnl.0000251299.54608.13 10.1212/01.wnl.0000251299.54608.13 [DOI] [PubMed] [Google Scholar]
- 38.Hahn A, Duisberg B, Neubauer BA, et al. Noninvasive determination of the tension-time index in Duchenne muscular dystrophy. Am J Phys Med Rehabil 2009;88:322-327. https://doi.org/10.1097/PHM.0b013e3181909dfa 10.1097/PHM.0b013e3181909dfa [DOI] [PubMed] [Google Scholar]
- 39.Finder JD, Birnkrant D, Carl J, et al. Respiratory care of the patient with duchenne muscular dystrophy: ATS consensus statement. In: Am J Respir Crit Care Med 2004;170:456-465. https://doi.org/10.1164/rccm.200307-885ST 10.1164/rccm.200307-885ST [DOI] [PubMed] [Google Scholar]
- 40.Wallgren-Pettersson C, Bushby K, Mellies U, et al. 117th ENMC workshop: Ventilatory support in congenital neuromuscular disorders – congenital myopathies, congenital muscular dystrophies, congenital myotonic dystrophy and SMA (II) 4-6 April 2003, Naarden, The Netherlands. Neuromuscular Disord 2004;14:56-69. https://doi.org/10.1016/j.nmd.2003.09.003 10.1016/j.nmd.2003.09.003 [DOI] [PubMed] [Google Scholar]
- 41.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 2012;8:597-619. https://doi.org/10.5664/jcsm.2172 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pautrat J, Khirani S, Boulé M, et al. Carbon dioxide levels during polygraphy in children with sleep-disordered breathing. Sleep Breath 2015;19:149-157. https://doi.org/10.1007/s11325-014-0980-2 10.1007/s11325-014-0980-2 [DOI] [PubMed] [Google Scholar]
- 43.Trucco F, Pedemonte M, Fiorillo C, et al. Detection of early nocturnal hypoventilation in neuromuscular disorders. Int Med Res 2018;46:1153-1161. https://doi.org/10.1177/0300060517728857 10.1177/0300060517728857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georges M, Nguyen-Baranoff D, Griffon L, et al. Usefulness of transcutaneous PCO2 to assess nocturnal hypoventilation in restrictive lung disorders. Respirology 2016;21:1300-1306. https://doi.org/10.1111/resp.12812 10.1111/resp.12812 [DOI] [PubMed] [Google Scholar]
- 45.Ogna A, Quera Salva MA, Prigent H, et al. Nocturnal hypoventilation in neuromuscular disease: prevalence according to different definitions issued from the literature. Sleep Breath 2016;20:575-581. https://doi.org/10.1007/s11325-015-1247-2 10.1007/s11325-015-1247-2 [DOI] [PubMed] [Google Scholar]
- 46.Toussaint M, Steens M, Soudon P. Lung function accurately predicts hypercapnia in patients with Duchenne muscular dystrophy. Chest 2007;131:368-375. https://doi.org/10.1378/chest.06-1265 10.1378/chest.06-1265 [DOI] [PubMed] [Google Scholar]
- 47.Hours S, Lejaille M, Pozzi D, et al. Perceived inspiratory difficulty in neuromuscular patients with primary muscle disorders. Neuromuscul Disord 2004;14:289-296. https://doi.org/10.1016/j.nmd.2004.01.008 10.1016/j.nmd.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 48.Toussaint M, Steens M, Wasteels G, et al. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J 2006;28:549-555. https://doi.org/10.1183/09031936.06.00004906 10.1183/09031936.06.00004906 [DOI] [PubMed] [Google Scholar]
- 49.Garuti G, Nicolini A, Grecchi B, et al. Open circuit mouthpiece ventilation: concise clinical review. Rev Port Pneumol 2014;20:211-218. https://doi.org/10.1016/j.rppneu.2014.03.004 10.1016/j.rppneu.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 50.Pisani L, Carlucci A, Nava S. Interfaces for noninvasive mechanical ventilation: technical aspects and efficiency. Minerva Anestesiol 2012;78:1154-1161. [PubMed] [Google Scholar]
- 51.Sferrazza Papa GF, Di Marco F, Akoumianaki E, et al. Recent advances in interfaces for non-invasive ventilation: from bench studies to practical issues. Minerva Anestesiol 2012;78:1146-1153. PMID: 23059519. [PubMed] [Google Scholar]
- 52.Janssens JP, Borel JC, Pépin JL. Nocturnal monitoring of home non-invasive ventilation: contribution of simple tools such as pulse oximetry, capnography, built-in ventilator software and autonomic markers of sleep fragmentation. Rev Mal Respir 2014;31:107-118. https://doi.org/10.1016/j.rmr.2013.08.003 10.1016/j.rmr.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 53.Aarrestad S, Tollefsen E, Kleiven AL, et al. Validity of transcutaneous PCO2 in monitoring chronic hypoventilation treated with non-invasive ventilation. Respir Med 2016;112:112-118. https://doi.org/10.1016/j.rmed.2016.01.017 10.1016/j.rmed.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 54.Orlikowski D, Prigent H, Ambrosi X, et al. Comparison of ventilator-integrated end-tidal CO2 and transcutaneous CO2 monitoring in home-ventilated neuromuscular patients. Respir Med 2016;117:7-13. https://doi.org/10.1016/j.rmed.2016.05.022 10.1016/j.rmed.2016.05.022 [DOI] [PubMed] [Google Scholar]
- 55.Nardi J, Prigent H, Adala A, et al. Nocturnal oximetry and transcutaneous carbon dioxide in home-ventilated neuromuscular patients. Respir Care 2012;57:1425-1430. https://doi.org/10.4187/respcare.01658 10.4187/respcare.01658 [DOI] [PubMed] [Google Scholar]
- 56.Paiva R, Krivec U, Aubertin G, et al. Carbon dioxide monitoring during long-term noninvasive respiratory support in children. Intensive Care Med 2009;35:1068-1074. https://doi.org/10.1007/s00134-009-1408-5 10.1007/s00134-009-1408-5 [DOI] [PubMed] [Google Scholar]
- 57.Berry RB, Chediak A, Brown LK, et al. NPPV Titration Task Force of the American Academy of Sleep Medicine. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med 2010;6:491-509. PMID: 20957853. [PMC free article] [PubMed] [Google Scholar]
- 58.Chatwin M, Heather S, Hanak A, et al. Analysis of home support and ventilator malfunction in 1,211 ventilator-dependent patients. Eur Respir J 2010;35:310-316. https://doi.org/10.1183/09031936.00073409 10.1183/09031936.00073409 [DOI] [PubMed] [Google Scholar]
- 59.Vitacca M, Bianchi L, Guerra A, et al. Tele-assistance in chronic respiratory failure patients: a randomised clinical trial. Eur Respir J 2009;33:411-418. https://doi.org/10.1183/09031936.00005608 10.1183/09031936.00005608 [DOI] [PubMed] [Google Scholar]
- 60.Inkley SR, Oldenburg FC, Vignos PJ. Pulmonary function in Duchenne muscular dystrophy related to stage of disease. Am J Med 1974;56:297-306. https://doi.org/10.1016/0002-9343(74)90611-1 10.1016/0002-9343(74)90611-1 [DOI] [PubMed] [Google Scholar]
- 61.Rideau Y, Gatin G, Bach J, et al. Prolongation of life in Duchenne’s muscular dystrophy. Acta Neurol (Napoli) 1983;5:118-124. Pubmed ID 6349272. [PubMed] [Google Scholar]
- 62.Vignos PJ. Respiratory function and pulmonary infection in Duchenne muscular dystrophy. Isr J Med Sci 1977;13:207-214 PMID: 863687. [PubMed] [Google Scholar]
- 63.Emery AE. Duchenne muscular dystrophy: genetic aspects, carrier detection and antenatal diagnosis. Br Med Bull 1980;36:117-122. https://doi.org/10.1093/oxfordjournals.bmb.a071624 10.1093/oxfordjournals.bmb.a071624 [DOI] [PubMed] [Google Scholar]
- 64.Gomez-Merino E, Bach JR. Duchenne muscular dystrophy: prolongation of life by noninvasive ventilation and mechanically assisted coughing. Am J Phys Med Rehabil 2002;81:411-415. https://doi.org/10.1097/00002060-200206000-00003 10.1097/00002060-200206000-00003 [DOI] [PubMed] [Google Scholar]
- 65.Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord 2002;12:926-929. https://doi.org/10.1016/s0960-8966(02)00140-2 10.1016/s0960-8966(02)00140-2 [DOI] [PubMed] [Google Scholar]
- 66.Bach J, Alba A, Pilkington LA, et al. Long-term rehabilitation in advanced stage of childhood onset, rapidly progressive muscular dystrophy. Arch Phys Med Rehabil 1981;62:328-331. PMID: 6941747. [PubMed] [Google Scholar]
- 67.Laberge L, Dauvillier Y. Myotonic dystrophy and sleepiness. Thorpy M, Billiard M, Eds. Sleepiness: causes, consequences, disorders and treatment. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- 68.O’Donoghue FJ, Borel JC, Dauvilliers Y, et al. Effects of 1-month withdrawal of ventilatory support in hypercapnic myotonic dystrophy type 1. Respirology 2017;22:1416-1422. https://doi.org/10.1111/resp.13068 10.1111/resp.13068 [DOI] [PubMed] [Google Scholar]
- 69.Bach JR, Bianchi C, Finder J, et al. Tracheostomy tubes are not needed for Duchenne muscular dystrophy. Eur Respir J 2007;30:179-180. https://doi.org/10.1183/09031936.00156806 10.1183/09031936.00156806 [DOI] [PubMed] [Google Scholar]
- 70.Boussaïd G, Lofaso F, Santos DB, et al. Impact of invasive ventilation on survival when non-invasive ventilation is ineffective in patients with Duchenne muscular dystrophy: a prospective cohort. Respir Med 2016;115:26-32. https://doi.org/10.1016/j.rmed.2016.04.009 10.1016/j.rmed.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 71.Andrews JG, Soim A, Pandya S, et al. Respiratory care received by individuals with Duchenne muscular dystrophy from 2000 to 2011. Respir Care 2016;61:1349-1359. https://doi.org/10.4187/respcare.04676 10.4187/respcare.04676 [DOI] [PubMed] [Google Scholar]
- 72.Vianello A, Bevilacqua M, Arcaro G, et al. Non-invasive ventilatory approach to treatment of acute respiratory failure in neuromuscular disorders. A comparison with endotracheal intubation. Int Care Med 2000;26:384-390. https://doi.org/10.1007/s001340051171 10.1007/s001340051171 [DOI] [PubMed] [Google Scholar]
- 73.Racca F, Del Sorbo L, Mongini T, et al. Respiratory management of acute respiratory failure in neuromuscular diseases. Minerva Anestesiol 2010;76:51-62. PMID: 20125073. [PubMed] [Google Scholar]
- 74.Cabrera Serrano M, Rabinstein AA. Causes and outcomes of acute neuromuscular respiratory failure. Arch Neurol 2010;67:1089-1094. https://doi.org/10.1001/archneurol.2010.207 10.1001/archneurol.2010.207 [DOI] [PubMed] [Google Scholar]
- 75.Bach JR, Gonçalves MR, Hamdani I, et al. Extubation of patients with neuromuscular weakness: a new management paradigm. Chest 2010;137:1033-1039. https://doi.org/10.1378/chest.09-2144 10.1378/chest.09-2144 [DOI] [PubMed] [Google Scholar]
- 76.Slutsky AS. Mechanical ventilation. American College of Chest Physicians Consensus Conference. Chest 1993;104:1833-1859. https://doi.org/10.1378/chest.104.6.1833 10.1378/chest.104.6.1833 [DOI] [PubMed] [Google Scholar]
- 77.Boussaïd G, Lofaso F, Santos DB, et al. Impact of invasive ventilation on survival when non-invasive ventilation is ineffective in patients with Duchenne muscular dystrophy: a prospective cohort. Respir Med 2016;115:26-32. https://doi.org/10.1016/j.rmed.2016.04.009 10.1016/j.rmed.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 78.Windisch W, Geiseler J, Simon K, et al. German National Guideline for treating chronic respiratory failure with invasive and non-invasive ventilation - Revised Ed. 2017: Part 2. Respiration. 2018;96:171-203. https://doi.org/10.1159/000488667 10.1159/000488667 [DOI] [PubMed] [Google Scholar]
- 79.Dhand R, Johnson JC. Care of the chronic tracheostomy. Respir Care 2006;51:984-1001. [PubMed] [Google Scholar]
- 80.Pappas S, Maragoudakis P, Vlastarakos P, et al. Versus percutaneous tracheostomy: an evidence-based approach, Eur Arch Otorhinolaryngol 2011;268:323-330. https://doi.org/10.1007/s00405-010-1398-5 10.1007/s00405-010-1398-5 [DOI] [PubMed] [Google Scholar]
- 81.White AC, Purcell E, Urquhart MB, et al. Accidental decannulation following placement of tracheostomy tube. Respir Care 2012;57:2019-2025. https://doi.org/10.4187/respcare.01627 10.4187/respcare.01627 [DOI] [PubMed] [Google Scholar]
- 82.Mirza S, Cameron DS. The tracheostomy tube change: a review of techniques. Hospital Medicine 2001;62:158-163. https://doi.org/10.12968/hosp.2001.62.3.1536 10.12968/hosp.2001.62.3.1536 [DOI] [PubMed] [Google Scholar]
- 83.Heffner JE. Management of the chronically ventilated patient with a tracheostomy. Chron Respir Dis 2005;2:151-161. https://doi.org/10.1191/1479972305cd084ra 10.1191/1479972305cd084ra [DOI] [PubMed] [Google Scholar]
- 84.Heffner JE, Martin-Harris B. Care of the mechanically ventilated patient with a tracheotomy. Tobin MJ, Ed. Principles and practice of mechanical ventilation 2nd Ed. New York: McGraw Hill; 2006, pp. 847-875. [Google Scholar]
- 85.Bach JR. A comparison of long-term ventilatory support alternatives from the perspective of the patient and care giver. Chest 1993;104:1702-1706. https://doi.org/10.1378/chest.104.6.1702 10.1378/chest.104.6.1702 [DOI] [PubMed] [Google Scholar]
- 86.Soudon P, Steens M, Toussaint M. A comparison of invasive versus noninvasive full-time mechanical ventilation in Duchenne muscular dystrophy. Chron Respir Dis 2008;5:87-93. https://doi.org/10.1177/1479972308088715 10.1177/1479972308088715 [DOI] [PubMed] [Google Scholar]
- 87.Boussaïd G, Lofaso F, Santos DB, et al. Impact of invasive ventilation on survival when non-invasive ventilation is ineffective in patients with Duchenne muscular dystrophy: a prospective cohort. Respir Med 2016;115:26-32. https://doi.org/10.1016/j.rmed.2016.04.009 10.1016/j.rmed.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 88.Poponick JM, Jacobs I, Supinski G, et al. Effect of upper respiratory tract infection in patients with neuromuscular disease. Am J Respir Crit Care Med 1997;156(2 Pt 1):659-664. https://doi.org/10.1164/ajrccm.156.2.9611029 10.1164/ajrccm.156.2.9611029 [DOI] [PubMed] [Google Scholar]
- 89.Kang SW, Bach JR. Maximum insulation capacity: vital capacity and cough flows in neuromuscular disease. Am J Phys Med Rehabil 2000;7:222-227. https://doi.org/10.1097/00002060-200005000-00002 10.1097/00002060-200005000-00002 [DOI] [PubMed] [Google Scholar]
- 90.Morrow B, Zampoli M, van Aswegen H, et al. Mechanical insufflation-exsufflation for people with neuromuscular disorders Cochraine Database Syst Rev 2013;30:CD010044. https://doi.org/10.1002/14651858.CD010044.pub2 10.1002/14651858.CD010044.pub2 [DOI] [PubMed] [Google Scholar]
- 91.Suárez AA, Pessolano FA, Monteiro SG, et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease Am J Phys Med Rihabil 2002;81:506-511. https://doi.org/10.1097/00002060-200207000-00007 10.1097/00002060-200207000-00007 [DOI] [PubMed] [Google Scholar]
- 92.Bianchi C, Baiardi P. Cough peak flows: standard values for children and adolescents. Am J Phys Med Rehabil 2008;87:461-467. https://doi.org/10.1097/PHM.0b013e318174e4c7 10.1097/PHM.0b013e318174e4c7 [DOI] [PubMed] [Google Scholar]
- 93.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J 2005;26:319-338. https://doi.org/10.1183/09031936.05.00034805 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 94.Kulnik ST, MacBean V, Birring SS, et al. Accuracy of portable devices in measuring peak cough flow. Physiol Meas 2015;36243-257. https://doi.org/10.1088/0967-3334/36/2/243 10.1088/0967-3334/36/2/243 [DOI] [PubMed] [Google Scholar]
- 95.Chatwin M, Toussaint M, Gonçalves MR, et al. Airway clearance techniques in neuromuscular disorders: a state of the art review. Respir Med 2018;136:98-110. https://doi.org/10.1016/j.rmed.2018.01.012 10.1016/j.rmed.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 96.Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest 1997;112:1024-1028. https://doi.org/10.1378/chest.112.4.1024 10.1378/chest.112.4.1024 [DOI] [PubMed] [Google Scholar]
- 97.Leger P, Paulus J. Recommendations of HAS: practical issues in home non-invasive ventilation in patients with neuromuscular disease. Rev Mal Respir 2006;23(Suppl 4):13s141-s143. PMID: 17057639. [PubMed] [Google Scholar]
- 98.American Thoracic Society/European Respiratory SocietyATS/ERS. Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518-624. https://doi.org/10.1164/rccm.166.4.518 10.1164/rccm.166.4.518 [DOI] [PubMed] [Google Scholar]
- 99.Toussaint M. The use of mechanical insufflation-exsufflation via artificial airways. Respir Care 2011;56:1217-1219. https://doi.org/10.4187/respcare.01448 10.4187/respcare.01448 [DOI] [PubMed] [Google Scholar]
- 100.Vitacca M, Paneroni M, Trainini D, et al. At home and on demand mechanical cough assistance program for patients with amyotrophic lateral sclerosis. Am J Phys Med Rehabil 2010;89:401-406. https://doi.org/10.1097/PHM.0b013e3181d89760 10.1097/PHM.0b013e3181d89760 [DOI] [PubMed] [Google Scholar]
- 101.Toussaint M, Chatwin M, Gonzales J, et al. ENMC Respiratory Therapy Consortium. 228th ENMC International Workshop: airway clearance techniques in neuromuscular disorders, Naarden, The Netherlands, 3-5 March, 2017. Neuromuscul Disord 2018;28:289-298. https://doi.org/10.1016/j.nmd.2017.10.008 10.1016/j.nmd.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 102.Goodwin FC, Muntoni F. Cardiac involvement in muscular dystrophies: molecular mechanisms. Muscle Nerve 2005;32:577-588. https://doi.org/10.1002/mus.20352 10.1002/mus.20352 [DOI] [PubMed] [Google Scholar]
- 103.Sveen ML, Thune JJ, Køber L, et al. Cardiac involvement in patients with limb-girdle muscular dystrophy type 2 and Becker muscular dystrophy. Arch Neurol 2008;65:1196-1201. https://doi.org/10.1001/archneur.65.9.1196 10.1001/archneur.65.9.1196 [DOI] [PubMed] [Google Scholar]
- 104.Vianello A, Corrado A, Arcaro G, et al. Mechanical insufflation-exsufflation improves outcomes for neuromuscular disease patients with respiratory tract infections. Am J Phys Med Rehabil 2005;84:83-88. https://doi.org/10.1097/01.phm.0000151941.97266.96 10.1097/01.phm.0000151941.97266.96 [DOI] [PubMed] [Google Scholar]
- 105.Bradley MD, Orrell RW, Clarke J, et al. Outcome of ventilatory support for acute respiratory failure in motor neurone disease. J Neurol Neurosurg Psychiatry 2002;72:752-756. https://doi.org/10.1136/jnnp.72.6.752 10.1136/jnnp.72.6.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sancho J, Servera E. Noninvasive ventilation for patients with neuromuscular disease and acute respiratory failure. Chest 2008;133;314-315. https://doi.org/10.1378/chest.07-2180 10.1378/chest.07-2180 [DOI] [PubMed] [Google Scholar]
- 107.Vianello A, Savoia F, Pipitone E, et al. “Hospital at home” for neuromuscular disease patients with respiratory tract infection: a pilot study. Respir Care 2013;58:2061-2068. https://doi.org/10.4187/respcare.02501 10.4187/respcare.02501 [DOI] [PubMed] [Google Scholar]
- 108.Servera E, Sancho J, Zafra MJ, et al. Alternatives to endotracheal intubation for patients with neuromuscular diseases. Am J Phys Med Rehabil 2005;84:851-857. https://doi.org/10.1097/01.phm.0000184097.17189.93 10.1097/01.phm.0000184097.17189.93 [DOI] [PubMed] [Google Scholar]
- 109.Bach JR, Bianchi C, Aufiero E. Oximetry and indications for tracheotomy for amyotrophic lateral sclerosis. Chest 2004;126:1502-1507. https://doi.org/10.1378/chest.126.5.1502 10.1378/chest.126.5.1502 [DOI] [PubMed] [Google Scholar]
- 110.Bach JR, Niranjan V, Weaver B. Spinal muscular atrophy type 1: a noninvasive respiratory management approach. Chest 2000;117:1100-1105. https://doi.org/10.1378/chest.117.4.1100 10.1378/chest.117.4.1100 [DOI] [PubMed] [Google Scholar]
- 111.Piastra M, Antonelli M, Caresta E, et al. Noninvasive ventilation in childhood acute neuromuscular respiratory failure: a pilot study. Respiration 2006;73:791-798. https://doi.org/10.1159/000090777 10.1159/000090777 [DOI] [PubMed] [Google Scholar]
- 112.Vianello A, Arcaro G, Guarnieri G, et al. Non-invasive ventilation for acute respiratory failure in Duchenne muscular dystrophy patients. Arch Bronconeumol 2021. (in press). https://doi.org/10.1016/j.arbres.2021.01.015 10.1016/j.arbres.2021.01.015 [DOI] [PubMed] [Google Scholar]
- 113.Kneyber MCJ, de Luca D, Calderini E, et al. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med 2017;43:1764-1780. https://doi.org/10.1007/s00134-017-4920-z 10.1007/s00134-017-4920-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Racca F, Mongini T, Wolfler A, et al. Recommendations for anesthesia and perioperative management of patients with neuromuscular disorders. Minerva Anestesiol 2013;79:419-433. PMID: 23419334. [PubMed] [Google Scholar]
- 115.Simonds AK. Streamlining weaning: protocols and weaning units. Thorax 2005;60:175-182. https://doi.org/10.1136/thx.2004.028688 10.1136/thx.2004.028688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vianello A, Arcaro G, Braccioni F, et al. Prevention of extubation failure in high-risk patients with neuromuscular disease. J Crit Care 2011;26:517-524. https://doi.org/10.1016/j.jcrc.2010.12.008 10.1016/j.jcrc.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 117.Geller G, Harrison KL, Rushton CH. Ethical challenges in the care of children and families affected by life-limiting neuromuscular diseases. J Dev Behav Pediatr 2012;33:548-561. https://doi.org/10.1097/DBP.0b013e318267c62d 10.1097/DBP.0b013e318267c62d [DOI] [PubMed] [Google Scholar]
- 118. www.who.int/cancer/palliative/definition.
- 119.Simonds AK. Respiratory support for the severely handicapped child with neuromuscular disease: ethics and practicality. Semin Respir Crit Care Med 2007;28:342-354. https://doi.org/10.1055/s-2007-981655 10.1055/s-2007-981655 [DOI] [PubMed] [Google Scholar]
- 120.Venkat A. The threshold moment: ethical tensions surrounding decision making on tracheostomy for patients in the intensive care unit. J Clin Ethics 2013;24:135-143. PMID: 23923812. [PubMed] [Google Scholar]
- 121.Dybwik K, Waage Nielsen E, Støre Brinchmann B. Ethical challenges in home mechanical ventilation: a secondary analysis. Nursing Ethics 2012;19:233-244. https://doi.org/10.1177/0969733011414967 10.1177/0969733011414967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang RS, Poon WS. “Triggers” for referral to neurology palliative care service. Ann Palliat Med 2018;7:289-295. https://doi.org/10.21037/apm.2017.08.02 10.21037/apm.2017.08.02 [DOI] [PubMed] [Google Scholar]
- 123.Vitacca M, Grassi M, Barbano L, et al. Last 3 months of life in home-ventilated patients: the family perception. Eur Respir J 2010;35:1064-1071. https://doi.org/10.1183/09031936.00061009 10.1183/09031936.00061009 [DOI] [PubMed] [Google Scholar]
- 124.Vitacca M, Vianello A. Outcomes of patients with als: an Italian Nationwide Survey. Respir Care 2013;58:1433-1441. https://doi.org/10.4187/respcare.02236 10.4187/respcare.02236 [DOI] [PubMed] [Google Scholar]
- 125.Gillick MR. Advance care planning. 2004;350:7-8. https://doi.org/10.1056/NEJMp038202 10.1056/NEJMp038202 [DOI] [PubMed] [Google Scholar]
- 126.Lunn S, Restrick L, Stern M. Managing respiratory disease: the role of a psychologist within the multidisciplinary team. Chronic Respir Dis 2017;14:45-53. https://doi.org/10.1177/1479972316688914 10.1177/1479972316688914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chetta A, Foresi A, Marangio E, et al. Psychological implications of respiratory health and disease. Respiration 2005;72:210-215. https://doi.org/10.1159/000084056 10.1159/000084056 [DOI] [PubMed] [Google Scholar]
- 128.Kwok TK. The palliative care needs for those advanced neurology patients on mechanical ventilator support. Annals Palliat Med 2018;7:332-334. https://doi.org/10.21037/apm.2017.08.18 10.21037/apm.2017.08.18 [DOI] [PubMed] [Google Scholar]
- 129.Politano L, Scutifero M, Patalano M, et al. Integrated care of muscular dystrophies in Italy. Part 1. Pharmacological treatment and rehabilitative interventions. Acta Myol 2017;36:19-24. PMID: 28690390. [PMC free article] [PubMed] [Google Scholar]
- 130.Magliano L, Scutifero M, Patalano M, et al. Integrated care of muscular dystrophies in Italy. Part 2. Psychological treatments, social and welfare support, and financial costs. Acta Myol 2017;36:41-45. PMID: 28781515. [PMC free article] [PubMed] [Google Scholar]
- 131.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. The Lancet Neurology 2010;9:77-93. https://doi.org/10.1016/S1474-4422(09)70271-6 10.1016/S1474-4422(09)70271-6 [DOI] [PubMed] [Google Scholar]
- 132.Mah JK, Thannhauser JE, McNeil DA, et al. Being the lifeline: the parent experience of caring for a child with neuromuscular disease on home mechanical ventilation. Neuromusc Disord 2008;18:983-988. https://doi.org/10.1016/j.nmd.2008.09.001 10.1016/j.nmd.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 133.Mah JK, Thannhauser JE, Kolski H, et al. Parental stress and quality of life in children with neuromuscular disease. Pediatr Neurol 2008;39:102-107. https://doi.org/10.1016/j.pediatrneurol.2008.04.011 10.1016/j.pediatrneurol.2008.04.011 [DOI] [PubMed] [Google Scholar]


