Key Points
Question
What trends for in-hospital mortality by age among hospitalized patients with SARS-CoV-2–positive tests are evident between March 1 and November 21, 2020?
Findings
This cohort study of 503 409 patients from 209 US acute care hospitals found a decrease in in-hospital mortality among patients with SARS-CoV-2–positive tests. Mortality rates were 10.6% in March, increased to 19.7% in April, and decreased to 9.3% in November.
Meaning
This large study supported findings of smaller, regional studies that reported in-hospital mortality decreased for all age groups during the pandemic period and suggests that this decrease cannot be solely attributed to hospital admission of increased proportions of younger patients.
This cohort study evaluates trends in in-hospital mortality among patients who tested positive for SARS-CoV-2.
Abstract
Importance
Mortality is an important measure of the severity of a pandemic. This study aimed to understand how mortality by age of hospitalized patients who were tested for SARS-CoV-2 has changed over time.
Objective
To evaluate trends in in-hospital mortality among patients who tested positive for SARS-CoV-2.
Design, Setting, and Participants
This retrospective cohort study included patients who were hospitalized for at least 1 day at 1 of 209 US acute care hospitals of variable size, in urban and rural areas, between March 1 and November 21, 2020. Eligible patients had a SARS-CoV-2 polymerase chain reaction (PCR) or antigen test within 7 days of admission or during hospitalization, and a record of discharge or in-hospital death.
Exposure
SARS-CoV-2 positivity.
Main Outcomes and Measures
SARS-CoV-2 infection was defined as a positive SARS-CoV-2 PCR or antigen test within 7 days before admission or during hospitalization. Mortality was extracted from electronically available data.
Results
Among 503 409 admitted patients, 42 604 (8.5%) had SARS-CoV-2–positive tests. Of those with SARS-CoV-2–positive tests, 21 592 (50.7%) were male patients. Hospital admissions among patients with SARS-CoV-2–positive tests were highest in the group aged 65 years or older (19 929 [46.8%]), followed by those aged 50 to 64 years (11 602 [27.2%]) and 18 to 49 years (10 619 [24.9%]). Hospital admissions among patients 18 to 49 years of age increased from 1099 of 5319 (20.7%) in April to 1266 of 4184 (30.3%) in June and 2156 of 7280 (29.6%) in July, briefly exceeding those in the group 50 to 64 years of age (June: 1194 of 4184 [28.5%]; 2039 of 7280 [28.0%]). Patients with SARS-CoV-2–positive tests had higher in-hospital mortality than patients with SARS-CoV-2–negative tests (4705 [11.0%] vs 11 707 of 460 805 [2.5%]; P < .001). In-hospital mortality rates increased with increasing age for both patients with SARS-CoV-2–negative tests and SARS-CoV-2–positive tests. In patients with SARS-CoV-2–negative tests, mortality increased from 45 of 11 255 (0.4%) in those younger than 18 years to 4812 of 107 394 (4.5%) in those older than 75 years. In patients with SARS-CoV-2–positive tests, mortality increased from 1 of 454 (0.2%) of those younger than 18 years to 2149 of 10 287 (20.9%) in those older than 75 years. In-hospital mortality rates among patients with SARS-CoV-2–negative tests were similar for male and female patients (6273 of 209 086 [3.0%] vs 5538 of 251 719 [2.2%]) but higher mortality was observed among male patients with SARS-CoV-2–positive tests (2700 of 21 592 [12.5%]) compared with female patients with SARS-CoV-2–positive tests (2016 of 21 012 [9.60%]). Overall, in-hospital mortality increased from March to April (63 of 597 [10.6%] to 1047 of 5319 [19.7%]), then decreased significantly to November (499 of 5350 [9.3%]; P = .04), with significant decreases in the oldest age groups (50-64 years: 197 of 1542 [12.8%] to 73 of 1341 [5.4%]; P = .02; 65-75 years: 269 of 1182 [22.8%] to 137 of 1332 [10.3%]; P = .006; >75 years: 535 of 1479 [36.2%] to 262 of 1505 [17.4%]; P = .03).
Conclusions and Relevance
This nationally representative study supported the findings of smaller, regional studies and found that in-hospital mortality declined across all age groups during the period evaluated. Reductions were unlikely because of a higher proportion of younger patients with lower in-hospital mortality in the later period.
Introduction
In-hospital mortality among patients with COVID-19 was high early in the pandemic and was reported to range from 12% to 28% in early case series.1,2,3 These studies also described a linear association between age and mortality.1,3,4 Since then, in-hospital mortality rates have decreased, likely reflecting improvements in survival.2,5,6 However, it has been proposed that at least some of the reduction in mortality rates in recent months can be attributed to the higher proportion of younger patients, who have lower mortality rates, among those hospitalized with COVID-19.7 In this analysis, in-hospital mortality rates for patients with SARS-CoV-2 infection were evaluated over time, and factors associated with observed trends were examined.
Methods
We conducted a multicenter, retrospective cohort analysis of data from 209 acute care facilities in the BD Insights Research Database (Becton, Dickinson and Company). The study data set was approved as a limited, deidentified data set for retrospective analysis and was exempted from patient consent by the New England institutional review board because it is a retrospective analysis of deidentified data. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.8
The hospitals included in the analysis have a range of bed sizes and are located in urban and rural areas throughout the United States. The database includes electronically captured laboratory results, US Census data, pharmacy orders, and admission, discharge, and transfer (ADT) data feeds.9 Hospitals in this analysis are a subset of facilities that are similar in bed size, census division, urban/rural designation, and teaching status to US hospitals included in the American Hospital Association survey.10
Eligible patients were hospitalized for at least 1 day, had results reported from a SARS-CoV-2 polymerase chain reaction (PCR) or antigen test 7 days prior to admission or during hospitalization, and a record of discharge or death in the hospital between March 1 and November 21, 2020. In this database, in-hospital mortality was defined as a designation of death, mortality, or presence in morgue in the ADT data feeds. We performed a validity check by randomly selecting 50 mortality cases and evaluating them for encounter-level clinical signs of mortality (eg, uncorrected severe metabolic acidosis determined by pH from arterial blood gases and uncorrected electrolyte changes incompatible with life). SARS-CoV-2 infection was defined as a positive SARS-CoV-2 PCR or antigen test 7 days prior to admission or during hospitalization.
Statistical Analysis
Frequencies were calculated for categorical variables. Means with SDs and medians with ranges were calculated for continuous variables. We used the χ2 and 2-sample t test to compare categorical and continuous variables, respectively. Linear regression was used to evaluate trends for mortality over time. All statistical analyses were performed using SAS software version 9.4 (SAS Institute). Statistical significance was set at P < .05, and tests were 2-tailed.
Results
We identified 503 409 unique patients hospitalized between March 1 and November 21, 2020, comprising 42 604 patients (8.5%) with laboratory-confirmed SARS-CoV-2–positive tests and 460 805 patients (91.5%) with SARS-CoV-2–negative tests. A minority of hospitalized patients were younger than 18 years (11 709 [2.3%]) (Table).
Table. Characteristics of Patients With SARS-CoV-2–Positive and SARS-CoV-2–Negative Tests, by Age Group.
| Characteristic | Patients, No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <18 y | 18-49 y | 50-64 y | 65-74 y | ≥75 y | Totala | |||||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| Patients tested for SARS-CoV-2 | 454 (3.9) | 11 255 (96.1) | 10 619 (7.1) | 138 311 (92.9) | 11 602 (9.7) | 108 071 (90.3) | 9642 (9.1) | 95 774 (90.9) | 10 287 (8.7) | 107 394 (91.3) | 42 604 (8.5) | 460 805 (91.5) |
| Malea | 210 (46.3) | 5788 (51.4) | 4973 (46.8) | 48 204 (34.9) | 6487 (55.9) | 59 534 (55.1) | 5112 (53.0) | 49 148 (51.3) | 4810 (46.8) | 46 412 (43.2) | 21 592 (50.7) | 209 086 (45.4) |
| Female | 244 (54.7) | 5467 (48.6) | 5646 (53.2) | 90 107 (65.1) | 5115 (44.1) | 48 537 (44.9) | 4530 (47.0) | 46 626 (48.7) | 5477 (53.2) | 60 982 (56.8) | 21 012 (49.3) | 251 719 (54.6) |
| ≥1 underlying severe illness or conditiona,b | 145 (31.9) | 2610 (23.2) | 6905 (65.0) | 58 767 (42.5) | 9474 (81.7) | 73 221 (67.8) | 8113 (84.1) | 67 738 (70.7) | 8611 (83.7) | 75 060 (69.9) | 33 248 (78.0) | 277 396 (60.2) |
| In-hospital deatha | 1 (0.2) | 45 (0.4) | 274 (2.6) | 1051 (0.8) | 922 (7.9) | 2576 (2.4) | 1359 (14.1) | 3223 (3.4) | 2149 (20.9) | 4812 (4.5) | 4705 (11.0) | 11 707 (2.5) |
| LOS for total populationa | ||||||||||||
| Mean (SD), d | 4.5 (11.2) | 5.3 (14.9) | 6.1 (8.7) | 4.1 (6.5) | 8.8 (12.0) | 5.5 (8.0) | 9.7 (11.3) | 5.8 (7.6) | 9.7 (19.7) | 5.7 (9.0) | 8.5 (13.6) | 5.2 (8.0) |
| Median (range), d | 2 (1-150) | 2 (1-317) | 4 (1-165) | 2 (1-620) | 5 (1-619) | 3 (1-457) | 6 (1-278) | 4 (1-482) | 6 (1-462) | 4 (1-514) | 5 (1-619) | 3 (1-620) |
| ICU admissiona | ||||||||||||
| Patients admitted to ICU | 92 (20.3) | 1736 (15.5) | 1810 (17.0) | 12 938 (9.4) | 2766 (23.8) | 18 162 (16.8) | 2630 (24.5) | 16 893 (17.5) | 2288 (22.2) | 15 988 (14.9) | 9586 (22.5) | 65 717 (14.3) |
| Total LOSa | ||||||||||||
| Mean (SD), d | 4.7 (5.0) | 6.6 (14.3) | 13.3 (14.9) | 8.5 (11.0) | 16.2 (14.8) | 9.8 (11.2) | 16.0 (13.3) | 9.7 (10.6) | 12.7 (11.0) | 8.7 (8.3) | 14.7 (13.7) | 9.2 (10.5) |
| Median (range), d | 3 (1-26) | 3 (1-249) | 9 (1-165) | 5 (1-303) | 12 (1-152) | 6 (1-218) | 12 (1-102) | 7 (1-370) | 10 (1-132) | 6 (1-241) | 11 (1-165) | 6 (1-370) |
| ICU LOSa | ||||||||||||
| Mean (SD), d | 3.3 (4.1) | 3.4 (8.6) | 7.6 (10.5) | 3.7 (5.5) | 9.6 (10.9) | 4.2 (5.9) | 9.2 (10.0) | 4.0 (5.6) | 6.4 (6.8) | 3.4 (4.3) | 8.3 (9.8) | 3.8(5.5) |
| Median (range), d | 2 (1-26) | 2 (1-191) | 4 (1-110) | 2 (1-95) | 6 (1-117) | 2 (1-114) | 6 (1-86) | 2 (1-253) | 4 (1-61) | 2 (1-81) | 5 (1-117) | 2 (1-253) |
Abbreviations: ICU, intensive care unit; LOS, length of stay.
P < .001 for comparison of patients with SARS-CoV-2–positive tests vs those with SARS-CoV-2–negative tests.
Includes the following baseline conditions occurring within the first 3 days of hospital admissions as determined by the maximum value of surrogate laboratory results: renal insufficiency (serum creatinine [SCr] >2.0 mg/dL [to convert to micromoles per liter, multiply by 88.4]); kidney failure (blood urea nitrogen >100 and SCr >3.0 mg/dL); suspected sepsis (lactic acid >2.0 or >4.0 mmol/L); suspected heart failure (brain-type natriuretic peptide [BNP] >400 pg/mL [to convert to nanograms per liter, multiply by 1.0] or N-terminal pro BNP >900 pg/mL); myocardial inflammation (doubling of troponin drawn within 6 hours final value: troponin ≥0.4 ng/L [to convert to micrograms per liter, multiply by 1.0], troponin T ≥15 ng/L [to convert to micrograms per liter, multiply by 1.0] in male patients, troponin T ≥10 ng/L in female patients, troponin I >0.04 ng/mL [to convert to micrograms per liter, multiply by 1.0]); liver dysfunction (any of the following: alanine aminotransferase >60 U/L [to convert to microkatals per liter, multiply by 0.0167], aspartate aminotransferase >80 U/L [to convert to microkatals per liter, multiply by 0.0167], serum albumin <3.0 g/dL [to convert to grams per liter, multiply by 10], international normalized ratio >2.0 [and not currently receiving warfarin, rivaroxaban, apixaban, edoxaban, or betrixaban]); cytokine stimulation (any of the following: fibrinogen <250 mg/dL [to convert to grams per liter, multiply by 0.01], C-reactive protein >7.0 mg/dL [to convert to milligrams per liter, multiply by 10], D-dimer [dimerized plasmin fragment D] >1000 ng/mL [to convert to nanomoles per liter, multiply by 5.476], erythrocyte sedimentation rate >30 mm/h, or triglycerides >265 mg/dL [to convert to millimoles per liter, multiply by 0.0113]).
Overall, the youngest age group (ie, <18 years) had the lowest proportion of patients with SARS-CoV-2–positive tests (454 of 11 709 [3.9%]), and patients 50 to 64 years of age had the highest proportion (11 602 of 119 673 [9.7%]) of patients with SARS-CoV-2–positive tests. Patients with SARS-CoV-2–positive tests, compared with those with SARS-CoV-2–negative tests, were more likely to be male (21 592 [50.7%] vs 209 086 [45.4%]; P < .001) and to have at least 1 underlying severe illness or condition (33 248 [78.0%] vs 277 396 [60.2%]; P < .001). Among patients with SARS-CoV-2–positive tests vs with SARS-CoV-2–negative tests, mean (SD) hospital length of stay was longer (8.5 [13.6] days vs 5.2 [8.0] days; P < .001), mean intensive care unit (ICU) length of stay was longer (8.3 [9.8] days vs 3.8 [5.5] days; P < .001), and ICU admission was more common (9586 [22.5%] vs 65 717 [14.3%]; P < .001) (Table).
Hospital Admission Trends by Age
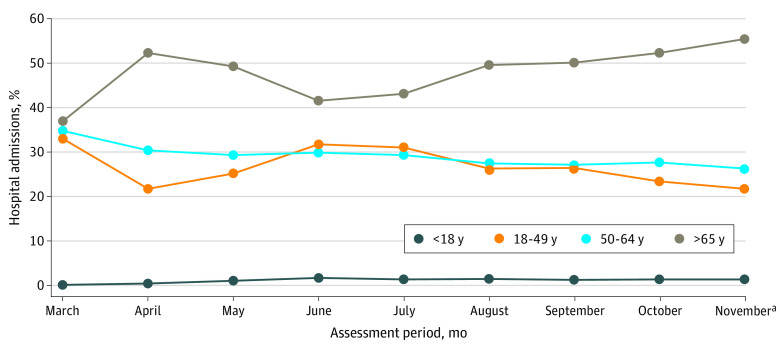
Hospital admissions among patients with SARS-CoV-2–positive tests were highest in the group aged 65 years or older (19 929 [46.8%]), followed by those aged 50 to 64 years (11 602 [27.2%]) and 18 to 49 years (10 619 [24.9%]) (Figure 1). Hospital admissions in patients aged 18 to 49 years increased from 1099 of 5319 (20.7%) in April to 1266 of 4184 (30.3%) in June and 2156 of 7280 (29.6%) in July and exceeded rates in patients aged 50 to 64 years (June: 1194 of 4184 [28.5%]; 2039 of 7280 [28.0%]). The proportion of patients admitted with SARS-CoV-2–positive tests who were younger than 18 years, while extremely low overall, also increased in June (Figure 1). These data indicate that hospital admissions among younger persons increased briefly in June and July but declined again thereafter.
Figure 1. Age Group Composition of All Hospitalized Patients With SARS-CoV-2–Positive Tests by Month of Admission.
The assessment period was from March 1 to November 21, 2020, and included data from 42 604 patients cared for in 209 hospitals.
aThe November time period extended from November 1 to November 21, 2020.
Mortality Trends
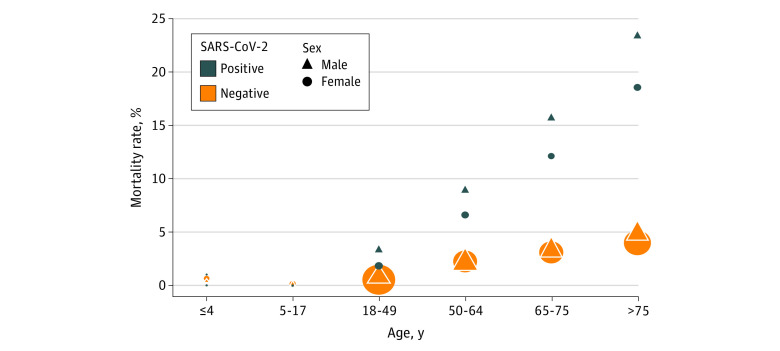
Patients with SARS-CoV-2–positive tests were significantly more likely to experience in-hospital mortality compared with those with SARS-CoV-2–negative tests (4705 [11.0%] vs 11 707 [2.5%]; P < .001) (Table; Figure 2). In-hospital mortality rates increased with increasing age in both patients with SARS-CoV-2–negative tests and those with SARS-CoV-2–positive tests. In patients with SARS-CoV-2–negative tests, mortality increased from 45 of 11 255 (0.4%) in those younger than 18 years to 4812 of 107 394 (4.5%) in those older than 75 years. In patients with SARS-CoV-2–positive tests, mortality increased from 1 of 454 (0.2%) of those younger than 18 years to 2149 of 10 287 (20.9%) in those older than 75 years (Table; Figure 2). While observed in-hospital mortality rates were similar in male and female patients with SARS-CoV-2–negative tests (6273 of 209 086 [3.0%] vs 5538 of 251 719 [2.2%]), higher in-hospital mortality was observed among male patients with SARS-CoV-2–positive tests compared with female patients with SARS-CoV-2–positive tests (2700 of 21 592 [12.5%] vs 2016 of 21 012 [9.6%]) (Figure 2). In the oldest age groups, in-hospital mortality rates were significantly higher among patients with SARS-CoV-2–positive tests compared with patients with SARS-CoV-2–negative tests (>75 years: 2149 of 10 287 [20.9%] vs 4812 of 107 394 [4.5%]; P < .001) (Figure 2).
Figure 2. In-Hospital Mortality Rates by Age and Sex for Patients With SARS-CoV-2–Positive and SARS-CoV-2–Negative Tests.
The assessment period was from March 1 to November 21, 2020, and included data from 209 hospitals. A larger symbol indicates a greater number of admissions.
Study Period Trends
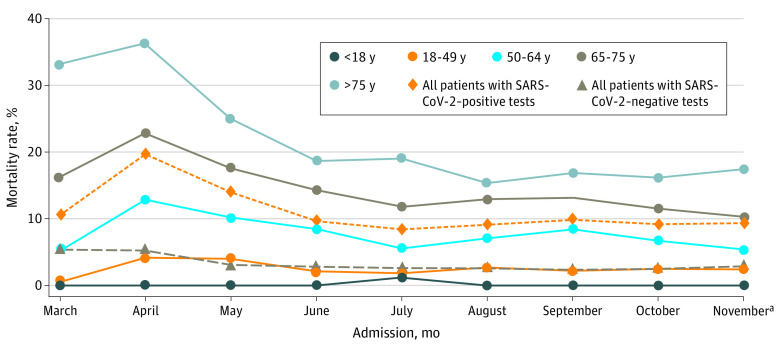
Figure 3 shows in-hospital mortality by month of admission for patients with SARS-CoV-2–negative tests and in-hospital mortality by month of admission overall and by age group for patients with SARS-CoV-2–positive tests. In-hospital mortality among patients with SARS-CoV-2–negative tests decreased slightly and then remained stable throughout the study period. In-hospital mortality among patients with SARS-CoV-2–positive tests increased from March to April (63 of 597 [10.6%] to 1047 of 5319 [19.7%]) and then decreased significantly during the study period (1047 of 5319 [19.7%] to 499 of 5350 [9.3%]; P = .04). Significant decreases in in-hospital mortality were also noted in the oldest age groups, including patients aged 50 to 64 years (197 of 1542 [12.8%] to 73 of 1341 [5.4%]; P = .02), 65 to 75 years (269 of 1182 [22.8%] to 137 of 1332 [10.3%]; P = .006), and older than 75 years (535 of 1479 [36.2%] to 262 of 1505 [17.4%]; P = .03) (Figure 3).
Figure 3. In-Hospital Mortality Rates by Month of Admission Among Patients With SARS-CoV-2–Positive and SARS-CoV-2–Negative Tests.
The assessment period was from March 1 to November 21, 2020, and included data from 42 604 patients cared for in 209 hospitals.
aThe November time period extended from November 1 to November 21, 2020.
Discussion
This large multicenter, geographically representative, retrospective cohort study found a significant decrease in in-hospital mortality among patients with SARS-CoV-2–positive tests from March to November. Mortality among patients with SARS-CoV-2–negative tests remained stable throughout the study period after the initial peak that occurred from March to May. In-hospital mortality decreases were not associated with a shift toward a higher proportion of younger (ie, 18 to 49 years of age) hospitalized patients with SARS-CoV-2–positive tests, who tend to have lower in-hospital mortality. While there was a slight shift in the proportion of hospitalized patients with SARS-CoV-2–positive tests to younger age groups in June and July, the proportion in this age group returned to levels seen earlier in the pandemic shortly afterward. In-hospital mortality decreased in all age groups, including the oldest age groups, who accounted for the highest proportion of all hospitalized patients with SARS-CoV-2–positive tests and had the highest in-hospital mortality rates. In-hospital mortality decreased overall, from a monthly peak of 19.7% in April to 9.3% in November, with every age group experiencing a decrease of approximately 50%. This large, national study is consistent with recently published smaller studies demonstrating decreases in in-hospital mortality and COVID-19 risk-adjusted mortality rates.2,11 Reasons for decreases in mortality since the start of the pandemic may include increased clinical experience in caring for and ventilating patients and use of prone positioning, systemic corticosteroids, and remdesivir.12,13,14
Strengths of this study include the extraordinarily large sample size, which included a diverse population across multiple geographic regions, including a large number of pediatric patients. Previously published studies have been conducted in single health systems in specific geographic areas with fewer patients.6,15 Longitudinal data for pediatric patients in our study showed that this age group had an increased proportion of all admissions with SARS-CoV-2–positive tests over time compared with other age groups—a finding that should be confirmed in other studies. In-hospital mortality among pediatric patients with SARS-CoV-2–positive tests was extremely rare (1 of 454 [0.2%]). Reasons for lower mortality among pediatric patients are likely multifactorial, ranging from lack of high-risk comorbid chronic conditions to differences in T cell–mediated humoral immune response in pediatric vs older populations.16,17,18 Most patients in our cohort were adults, and mortality in patients with SARS-CoV-2–positive tests was associated with male sex and having at least 1 underlying medical condition, consistent with other studies.1,15
Limitations
Limitations of this study include testing type and access. For the time interval assessed, the predominant diagnostic test for SARS-CoV-2 detection was PCR. Antigen testing was only readily available later in the pandemic and as a point-of-care test. These results may have been inconsistently captured in acute care emergency medical records. In addition, this study did not assess any discordance in test results among the 2 testing assays; for example, if an antigen test was positive and then a subsequent PCR test was negative for the same patient, the patient was considered as having a positive test. Furthermore, each hospital admission was counted as a single observation in the denominator. We did not link index hospital admissions and readmissions. We evaluated mortality trends by month and assumed that readmission rates were equal over time; therefore, the trends should not be affected. Additionally, the availability of diagnostic tests and public policy lockdowns increased during the pandemic and may have affected earlier mortality rates.19 We believe there was some ascertainment bias in the identification of mortality among patients with SARS-CoV-2–positive tests and those with SARS-CoV-2–negative tests in the month of March when the pandemic was beginning. Mortality among patients with SARS-CoV-2–negative tests declined from March to May and mortality among those with SARS-CoV-2–positive tests increased from March to April (Figure 3). It is likely that this was because of testing practices and a shortage of available SARS-CoV-2 diagnostics led to an initial inability to test all patients with suspected COVID-19 infections. Moreover, mortality was delineated in this database through ADT demarcations of expiration or death, and the completeness of such data was dependent on the governing institutional policies.
Conclusions
This nationally representative cohort study supported the findings of smaller, more geographically focused studies and found decreases in in-hospital mortality across all age groups throughout the pandemic period. Reductions in mortality rates did not appear to be associated with the age distribution of hospitalized patients with SARS-CoV-2–positive tests and were likely because of new therapies and improvements in the clinical management of patients with SARS-CoV-2 infection.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vahidy FS, Drews AL, Masud FN, et al. Characteristics and outcomes of COVID-19 patients during initial peak and resurgence in the Houston metropolitan area. JAMA. 2020;324(10):998-1000. doi: 10.1001/jama.2020.15301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wortham JM, Lee JT, Althomsons S, et al. Characteristics of persons who died with COVID-19—United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(28):923-929. doi: 10.15585/mmwr.mm6928e1 [DOI] [PubMed] [Google Scholar]
- 5.Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. doi: 10.1097/ccm.0000000000004747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16(2):90-92. doi: 10.12788/jhm.3552 [DOI] [PubMed] [Google Scholar]
- 7.Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess deaths associated with COVID-19, by age and race and ethnicity—United States, January 26-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1522-1527. doi: 10.15585/mmwr.mm6942e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 9.McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible gram-negative pathogens in ICU and non-ICU settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis. 2018;5(10):ofy241. doi: 10.1093/ofid/ofy241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed December 1, 2020. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- 11.Doidge JC, Mouncey PR, Thomas K, et al. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. Preprints. 2020;2020080267. doi: 10.20944/preprints202008.0267.v1 [DOI] [PMC free article] [PubMed]
- 12.Mittermaier M, Pickerodt P, Kurth F, et al. Evaluation of PEEP and prone positioning in early COVID-19 ARDS. EClinicalMedicine. 2020;28:100579. doi: 10.1016/j.eclinm.2020.100579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JAC, Murthy S, Diaz JV, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330-1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members . Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383(19):1813-1826. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox RJ, Brokstad KA. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat Rev Immunol. 2020;20(10):581-582. doi: 10.1038/s41577-020-00436-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westmeier J, Paniskaki K, Karaköse Z, et al. Impaired cytotoxic CD8+ T cell response in elderly COVID-19 patients. mBio. 2020;11(5):e02243-e20. doi: 10.1128/mBio.02243-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B, Fan CY, Wang AL, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81(1):e51-e60. doi: 10.1016/j.jinf.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhry R, Dranitsaris G, Mubashir T, Bartoszko J, Riazi S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine. 2020;25:100464. doi: 10.1016/j.eclinm.2020.100464 [DOI] [PMC free article] [PubMed] [Google Scholar]