Abstract
Characterizing the brain’s ability to adapt to changing environments has been at the forefront of neuroscience for decades. Newbold et al. build on this neuroplasticity work using precision neuroimaging and arm casting to unmask previously unknown pulses of spontaneous activity.
It has now been 60 years since David Hubel and Torsten Wiesel’s Nobel Prize winning work describing the formation of ocular dominance columns (ODC) in the cat visual cortex (Hubel and Wiesel, 1962). By using electrophysiologic recordings, they were able to show that information originating from either the left or the right eye is differentially represented by alternating columns of the primary visual cortex. Since these initial accounts, ocular representation in the primary visual cortex has been one of the most thoroughly studied neuroscientific phenomena and was an initial ‘seed’ into decades of work describing the mechanisms by which the brain rewires in response to changing functional demands.
For example, classic experiments on early-life sensory deprivation using chronic closure of one eyelid reveals a modification of the size and nature of ODC representation in the visual cortex (Antonini and Stryker, 1993). Representation of the sutured eye is significantly reduced, and representation of the unaffected eye is increased in size. This process is the result of a Hebbian-type mechanisms whereby connections are strengthened by correlated activity and weakened by uncorrelated activity (Hebb, 1949).
In another classic experiment, the laboratory of Michael Merzenich showed in the adult owl monkey that correlating the inputs of two adjacent fingers by surgically connecting the skin surfaces on the hand, leads to a significant change in the hand topography of the somatosensory cortex in the brain (Clark et al., 1988). The correlated inputs arising from the joined fingers abolish the normal discontinuities in digit representation in the motor system.
There is no question that our current thinking of the brain’s capacity to adapt to environmental changes, as well as its resilience to extreme perturbations, rests on this foundational work.
However, our understanding of such capacities is always limited by the methods used to study them. By necessity these early works on the mechanisms of brain plasticity have primarily been restricted to animal models and to a small number of focal, targeted areas in the brain. Since its inception, functional MRI has always been envisioned to expand our capacity for examining fundamental neural mechanisms in the human brain. fMRI’s ability to non-invasively measure neural activity across the entire brain has always provided hope that we would one day supplement early reductionist work with a broader view of brain function.
Nonetheless, this ‘merging’ of principles engendered by classical neuroscience work with new discoveries using functional MRI has been slow. This sluggish pace is likely the result of several factors. It has taken decades of work and experimentation to tease out unwanted artifacts in functional MRI. Only recently have human neuroimaging researchers begun to confront the challenges surrounding the sample sizes (Smith and Nichols, 2018) or the amount of data required within individuals to maximize reliability and reproducibility (Laumann et al., 2015). Perhaps most important is that the human systems neuroscience has generally shifted away from detailed experimentation and the intricate dissection of psychological processes supporting brain function. Rather it has now moved toward discovery science, where investigations are often simply describing observations in the data or characterizing the correspondence of complex behavioral phenotypes to networks and systems. Despite the merit of this shift, in many respects it may be slowing the contributions of non-invasive imaging to investigations of fundamental neuroscientific principles.
The research by Newbold et al. provides us with a turning point regarding the trend towards population-based discovery in human neuroscience. The publication elegantly lays down a blueprint for designing a well-controlled experimental study with non-invasive imaging. It complements well known foundational principles in neuroscience, builds on the growth of discovery science in the field, and provides the necessary parameters by which to reliably test interventions within individuals.
Specifically, the authors build on the early sensory deprivation work (Antonini and Stryker, 1993) and design a human version of the paradigm used by Clark and colleagues (Clark et al., 1988) to induce brain plasticity within individuals. To do this, they cast the dominant upper extremity for two weeks in 3 individuals. Using precision mapping (Laumann et al., 2015), they then tracked plasticity within each individual using 42–64 daily scans of resting state functional MRI. Consistent with what might be expected based on the existing lesion literature the authors saw a rapid (<48 hours) ‘disconnection’ of the circuits controlling the disused arm from the remainder of the brain’s somatomotor network. This observation supports a long-held hypothesis that functional connections are maintained in a Hebbian-like manner by co-activation of brain regions during behavior (Fair et al., 2007).
Even more important was an observation that connectivity within disused circuits became stronger during the cast period. Unlike the disconnection of the disused arm representation from contralateral somatomotor cortex, one would not predict such strengthening within the system based on experience-dependent Hebbian processes as outlined above. A possible reason for this unexpected observation relates to the author’s finding of spontaneous pulses of activity (Figure 1). These spontaneous activity pulses occurred frequently during the cast period and propagated throughout the disused circuits of the casted upper extremity.
Figure 1. Spontaneous activity pulses in disused somato-motor circuits.
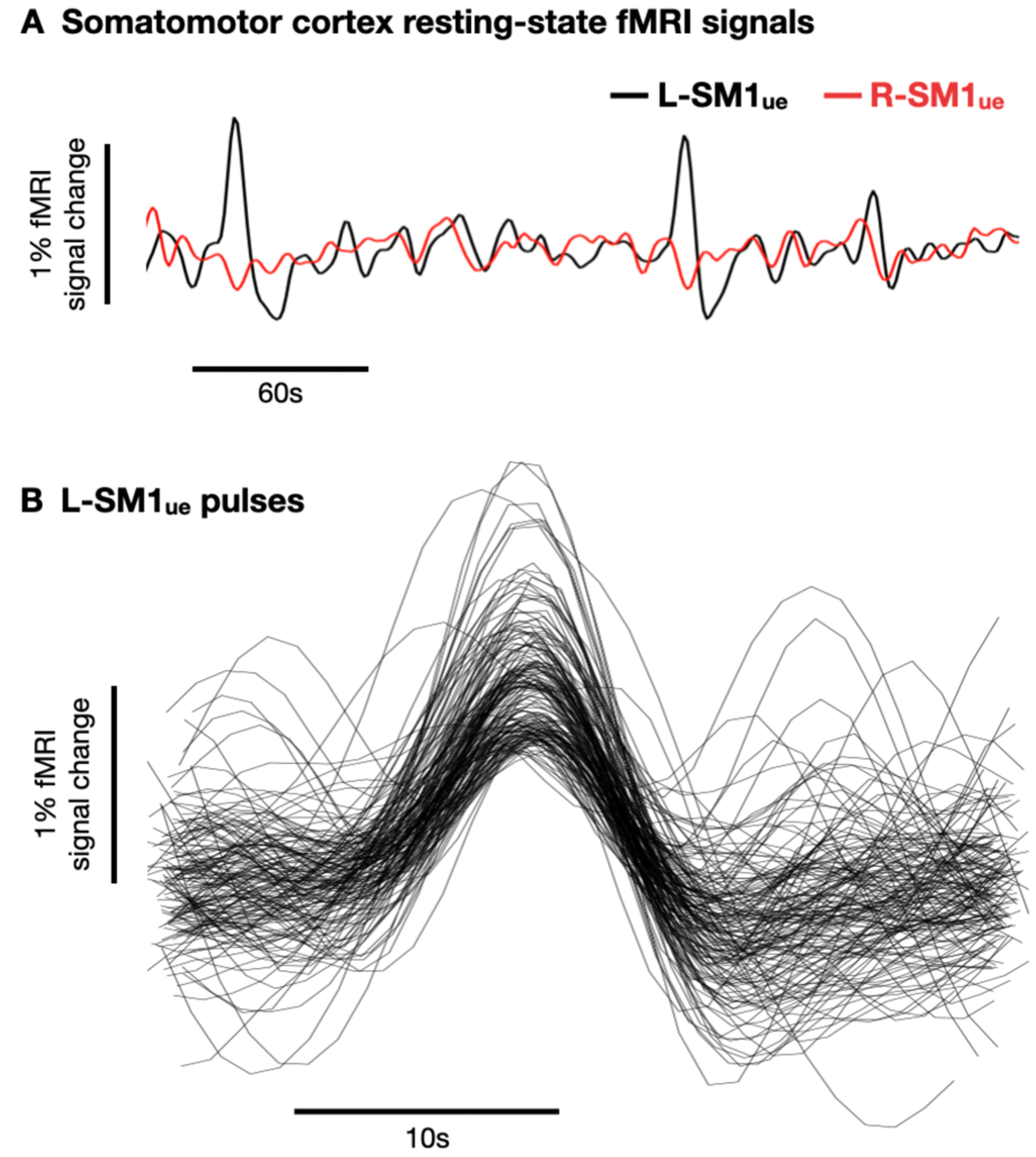
(A) Example resting-state functional MRI (fMRI) signals from left and right primary somatomotor cortex (L-SM1ue and R-SM1ue) during the cast period. (B) Recordings of 144 spontaneous activity pulses detected in an example participant.
What then is to account for such a phenomenon? Lessons learned in the aftermath of the work by Hubel and Wiesel on ODC plasticity are likely to provide some insights. Early developmental projections from the two eyes initially overlap in the visual cortex. It was originally determined that during the first postnatal months, visual experience promotes a preferential stabilization of some of the cortical connections and elimination of others resulting in a segregation of layer IV projections into well delineated columns (Sur and Leamey, 2001). However, it was later discovered that retinal inputs are not required for column formation. Spontaneous, correlated waves of activity from the retina through the thalamus can account for their formation (Sur and Leamey, 2001). It is possible that the pulses of activity observed in Newbold et al. represent similar spontaneous activity that strengthens and maintains the sub-circuit of the disused arm when sensory input and motor output are greatly reduced.
The implications of such a finding are sizeable. For those of us who study brain development the potential impact of such findings is clear. As noted above, chronic closure of one eyelid, early in life, reveals plastic mechanisms in which the size of ODC representation corresponding to the sutured eye is significantly reduced, whereas the representation of the unaffected eye is increased. In non-human primates, the sensitive period for this process begins at birth and slowly wanes until ~10 weeks of age (Note: A sensitive period is the time window during which an experimental or natural manipulation of a system will strongly affect the system’s development. A critical period is the point after which the effects of the manipulation are irreversible). Interestingly, sensitive and critical periods related to such phenomenon are intricately linked to inhibitory inputs. In ferrets, the maturation of inhibitory (GABAergic) circuits lags behind excitatory circuits. The timing of this maturation tightly correlates with the onset of critical periods (Sur and Leamey, 2001). Indeed, reducing GABA function in transgenic mice can keep the critical and sensitive periods open indefinitely. Increasing GABA-mediated inhibition can prematurely shorten the span of the sensitive period (Hensch, 2005). This decreased plasticity accompanying the emergence of inhibitory circuits in development is associated with the suppression of the spontaneous correlated bursts of activity. Following this line of thought to its logical conclusion provides a series of provocative questions arising from Newbold et al. Does sensory deprivation via casting reduce the influence of GABAergic circuits and ‘unmask’ these latent pulses of spontaneous activity? Does the plastic potential of the disused sub-circuit change by this simple manipulation?
Just like the ground-breaking work of Hubel and Wiesel, which set off decades of experiments and led to the characterization of fundamental principles surrounding brain plasticity, the work by Newbold et al. using whole brain fMRI clearly points to the next chapter in the story. There are a number of basic questions about the molecular and structural underpinnings of these functional signals that need to be answered. Insight into these findings would be vastly enhanced by translation and experimentation in animal models, where rich methodology for studying high-throughput genetic, histological, and physiological conditions in tightly controlled environment exists and can be accompanied by the same whole brain fMRI signals as a ‘bridge’ between the species (Stafford et al., 2014). The work by Newbold et al. provides the model by which interventional studies to monitor functional brain changes in individuals should be shaped. The work should be commended for its simplicity, originality, and advancement of human brain plasticity research.
Acknowledgements:
Funding for Dr. Fair and related work comes from the Gates Foundation, the National Institute of Mental Health (NIMH), and the National Institute on Drug Abuse (NIDA): R01MH115357, R01MH096773, R01MH105538, U01DA041148, U24DA041123.
Footnotes
Conflict Statement:
Damien A. Fair is a patent holder on the Framewise Integrated Real-Time Motion Monitoring (FIRMM) software. He is also a co-founder of Nous Imaging Inc. His university has put in place a plan to help ensure that this work is not affected by any financial interest.
Contributor Information
Damien Fair, Redleaf Endowed Director, Masonic Institute for the Developing Brain (MiDB), Professor, Institute of Child Development, College of Education and Human Development, Professor, Department of Pediatrics, Medical School, University of Minnesota
BT Thomas Yeo, Assistant Professor, Department of Electrical & Computer Engineering, Assistant Professor, Centre of Sleep & Cognition, Assistant Professor, N.1 Institute for Health, National University of Singapore
References:
- Antonini A, and Stryker MP (1993). Rapid remodeling of axonal arbors in the visual cortex. Science (80-.) 260, 1819–1821. [DOI] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, and Merzenich MM (1988). Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature 332, 444–445. [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, and Schlaggar BL (2007). Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. USA 104, 13507–13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO (1949). The organization of behavior (New York: John Wiley; ). [Google Scholar]
- Hensch TK (2005). Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Hubel DH, and Wiesel TN (1962). Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. London 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen MY, Gilmore AW, McDermott KB, Nelson SM, Dosenbach NUF, et al. (2015). Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron 87, 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, and Nichols TE (2018). Statistical Challenges in “Big Data” Human Neuroimaging. Neuron. [DOI] [PubMed] [Google Scholar]
- Stafford JM, Jarrett BR, Miranda-Dominguez O, Mills BD, Cain N, Mihalas S, Lahvis GP, Lattal KM, Mitchell SH, David SV, et al. (2014). Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. U. S. A 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, and Leamey CA (2001). Development and plasticity of cortical areas and networks. Nat. Rev. Neurosci 2, 251–262. [DOI] [PubMed] [Google Scholar]


