Abstract
Study Objectives
Cognitive behavioral therapy for insomnia (CBTI) for comorbid insomnia and obstructive sleep apnea (OSA) has had mixed results. We integrated CBTI with a positive airway pressure (PAP) adherence program and tested effects on sleep and PAP use.
Methods
125 veterans (mean age 63.2, 96% men, 39% non-Hispanic white, 26% black/African American, 18% Hispanic/Latino) with comorbid insomnia and newly-diagnosed OSA (apnea-hypopnea index ≥ 15) were randomized to 5-weekly sessions integrating CBTI with a PAP adherence program provided by a “sleep coach” (with behavioral sleep medicine supervision), or 5-weekly sleep education control sessions. Participants and assessment staff were blinded to group assignment. Outcomes (baseline, 3 and 6 months) included Pittsburgh Sleep Quality Index (PSQI), 7-day sleep diary (sleep onset latency [SOL-D], wake after sleep onset [WASO-D], sleep efficiency [SE-D]), 7-day actigraphy (SE-A), and objective PAP use (hours/night and nights ≥ 4 h). Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS), and Functional Outcomes of Sleep Questionnaire-10 (FOSQ-10) were also collected.
Results
Compared to controls, intervention participants showed greater improvement (baseline to 3 and 6 months, respectively) in PSQI (−3.2 and −1.7), SOL-D (−16.2 and −15.5 minutes), SE-D (10.5% and 8.5%), SE-A (4.4% and 2.6%) and more 90-day PAP use (1.3 and 0.9 more hours/night, 17.4 and 11.3 more nights PAP ≥ 4 h). 90-day PAP use at 3 months was 3.2 and 1.9 h/night in intervention versus controls. Intervention participants also had greater improvements in ISI, ESS, and FOSQ-10 (all p < 0.05).
Conclusions
An intervention integrating CBTI with a PAP adherence program delivered by a supervised sleep coach improved sleep and PAP use in adults with comorbid insomnia and OSA.
Trial Registration
ClinicalTrials.gov
Study name: Novel Treatment of Comorbid Insomnia and Sleep Apnea in Older Veterans
URL: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02027558&cntry=&state=&city=&dist=
Registration: NCT02027558
Keywords: insomnia, sleep apnea, cognitive behavioral therapy, randomized controlled trial
Statement of Significance.
Insomnia often coexists with newly diagnosed OSA and predicts lower PAP use, but recent CBTI trials in these patients have had mixed results. We integrated CBTI with a PAP adherence program provided by a BSM-supervised sleep coach (to facilitate future access and implementation), and tested effects of the intervention on sleep and PAP use. Compared to a placebo control condition, the intervention resulted in greater improvements in multiple sleep measures and PAP use for up to 6 months. These findings suggest that integrating CBTI with a PAP adherence program improves both sleep and PAP use in adults with comorbid insomnia and OSA. The use of sleep coaches (potentially drawing from a variety of providers) may increase access to this intervention.
Introduction
Insomnia often coexists with obstructive sleep apnea (OSA) [1–3], particularly among older adults [4] and US veterans [5], and predicts worse outcomes of OSA [6] such as more sleep disturbance and increased heart disease [7], greater absence from work [8], and worse health-related quality of life [9]. Untreated OSA limits response to treatment of insomnia [10], and untreated insomnia negatively impacts positive airway pressure (PAP) use [5]. This bidirectional problem is likely even more pressing in the veteran population, where both OSA and insomnia have a higher prevalence compared to the general population [11, 12], and prior work suggests nearly one-half of older outpatient veterans who meet diagnostic criteria for insomnia also have coexisting, previously unrecognized OSA [4, 13].
The relationship between insomnia and OSA is likely complex [14]. Insomnia associated with untreated OSA may be refractory to usual cognitive behavioral therapy for insomnia (CBTI), but there is some evidence that OSA treatment can improve symptoms of insomnia [15]. PAP may improve problems with sleep maintenance, but sleep onset insomnia and early morning awakening may persist regardless of PAP therapy and can have a negative effect on PAP use [16]. Targeted treatment for insomnia may be beneficial for patients with OSA comorbid with insomnia and has the potential to positively affect PAP use. The arousal threshold (i.e. the increase in inspiratory effort required to elicit arousal) is variable and arousability plays an important role in the clinical expression of OSA [17]. Of note, central impairment in the regulation of sleep depth has been suggested as a common pathogenic mechanism for insomnia and OSA [18]. There is also evidence for increased morbidity and greater illness severity when insomnia and OSA coexist. For example, decreased sleep quality in patients with OSA is associated with worse cardiometabolic abnormalities, which may reflect a negative additive effect with insomnia through activation of the hypothalamic−pituitary−adrenal axis and excessive sympathetic nervous activity [19].
Optimal management of the large number of patients with comorbid insomnia disorder and OSA is an area of growing interest. In the absence of OSA, clinical practice guidelines recommend CBTI as a first-line treatment for chronic insomnia [20–22]. CBTI is particularly recommended for insomnia in older adults, who are at increased risk for adverse effects of sedative-hypnotic medications [23], such as injurious falls [24] and cognitive decline [25]. PAP is the recommended first-line treatment for moderate and severe OSA [26, 27]. Poor adherence to PAP therapy is an important barrier to OSA management [28]. Numerous studies have examined predictors of greater PAP use (e.g. early PAP use predicts higher long-term use) [29], and behavioral and supportive interventions (e.g. educational programs, behavioral strategies, telemonitoring) have been tested to increase PAP use in OSA [30]. At least one recent guideline recommended offering PAP use interventions early in the course of treatment for patients with concurrent conditions such as insomnia [31]; these patients may be candidates for both CBTI and PAP adherence interventions. However, recent trials testing CBTI in patients presenting with comorbid insomnia and OSA have had mixed results in terms of increasing PAP use [32–34]. Challenges for treating patients when these conditions coexist include the delivery of insomnia and OSA treatment by different providers (e.g. psychologist and sleep medicine physician) and the lack of program materials that combine therapies for both insomnia and PAP adherence and address the bidirectional nature of these conditions.
Given prior evidence that behavioral and supportive interventions increase PAP use in OSA [30], we developed an integrated treatment that addresses both insomnia disorder and PAP use early in the course of OSA diagnosis and treatment to maximize treatment success when these conditions coexist. Further, we designed this structured, manual-based intervention to be delivered by a “sleep coach” (with telephone supervision by and consultation with a behavioral sleep medicine, BSM, specialist) to facilitate future implementation of the treatment in a variety of clinical settings. We hypothesized that sleep outcomes and PAP use would be better at a 3-month follow-up in the intervention group compared to controls, and these improvements would be maintained for up to 6 months.
Methods
Trial design
The intervention was tested in a randomized controlled trial among veterans (aged ≥ 50 years) in one VA healthcare system who were diagnosed with moderate or severe OSA (apnea-hypopnea index [AHI] ≥ 15) and prescribed PAP therapy, and who also met the International Classification of Sleep Disorders, 3rd edition (ICSD-3) [35] diagnostic criteria for chronic (≥3 months) insomnia disorder. The intervention integrated behavioral therapy for insomnia with a PAP adherence program, provided in 5 weekly sessions delivered in an individual (one-on-one) format, by non-clinician sleep coaches who had weekly supervision and access to BSM specialists. Participants were randomized to the intervention or an active control condition that involved general sleep education delivered one-on-one by a similar individual not trained in the intervention. Objective and subjective measures of sleep were collected at baseline, 3- and 6-month follow-up. Primary outcomes included subjective (sleep quality and sleep diary measures) and objective (wrist actigraphy) measures of sleep and objective PAP use (obtained from participants’ PAP machine equipment cloud data) at a 3-month follow-up. Repeat testing was performed at 6 months to assess whether treatment effects were maintained. Study procedures were approved by the medical center’s Institutional Review Board and written informed consent was obtained from all participants (ClinicalTrials.gov Identifier: NCT02027558).
Participants
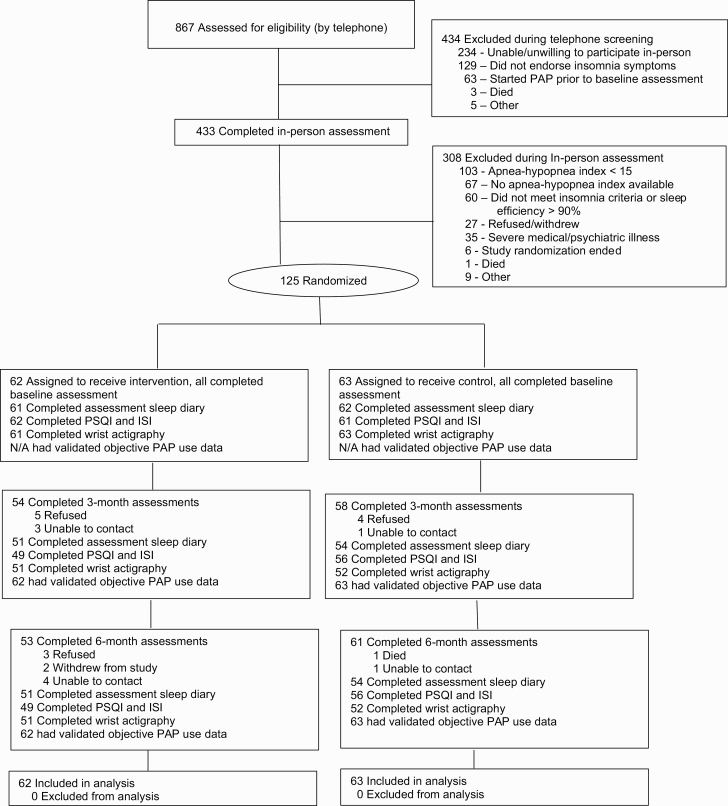
Eligible participants were community-dwelling veterans identified from consultation requests sent to the medical center’s sleep clinic. A two-step screening process was used to identify potential participants and determine study eligibility. During screening step 1, electronic records were reviewed to identify sleep clinic referrals requesting an evaluation to rule out OSA. Patients referred to sleep clinic with currently treated OSA were not contacted. If the veteran was 50 years of age or older and lived within 25 miles of our medical center, a brief letter of introduction was mailed, which included an opportunity to opt-out of being contacted by research staff (1,591 letters sent; 149 opted out). Of the 1,442 patients who did not opt out, we performed telephone screening for basic study eligibility. Telephone screening was completed in 867 patients (352 refused telephone screen, 127 unable to contact, 96 identified as ineligible prior to the telephone screen). Patients who self-reported a distant (but unverified) history of OSA that was currently untreated were not excluded. Overall, 433 were identified by telephone as potentially eligible for the study and agreed to come for an in-person visit where written informed consent was obtained and a baseline study assessment (screening step 2) to determine study eligibility was initiated (see Figure 1 for CONSORT 2010 [CONsolidated Standards Of Reporting Trials] [36] flow diagram). To assist with participant blinding, the informed consent process described the purpose of the study was to learn more about sleep problems and to test two sleep education programs. Insomnia diagnosis was determined by a consensus of a study physician certified in sleep medicine (CA, CF) and a study psychologist certified in behavioral sleep medicine (JM), using structured items (based on prior work) [13, 37, 38] from the baseline assessment that specifically addressed ICSD-3 diagnostic criteria for chronic insomnia disorder (excluding Criteria F [i.e. the sleep/wake difficulty is not better explained by another sleep disorder] since all participants had OSA) [35]. After completion of in-person baseline assessment, participants were excluded if: (1) their Mini-Mental State Examination (MMSE) [39] total score was <24 (indicative of cognitive impairment), (2) they were not diagnosed with moderate or severe OSA (i.e. AHI was <15) and/or were not prescribed PAP, (3) they did not meet diagnostic criteria for insomnia and/or had a sleep diary sleep efficiency >90%, or (4) medical record review identified a severe unstable medical disorder (e.g. <6 months life expectancy) or active severe mental disorder (e.g. current active substance abuse, psychiatric hospitalization within the past 90 days, documented bipolar disorder). Final eligibility was determined by consensus of a study physician and psychologist using information available from clinical electronic records and the screening and baseline assessment. One hundred and twenty-five participants met all eligibility criteria and were randomized. All data collection was performed by research staff at one Veterans Affairs healthcare system.
Figure 1.
Participant flow in the study.
Randomization
Randomization occurred after a baseline assessment was complete, and the sleep clinic had prescribed PAP and had scheduled the patient for a PAP pickup appointment. Participants meeting eligibility criteria were randomized 1:1 (using random allocation concealment) to the intervention or control condition. Prior to study commencement, a randomization sequence was created by a statistician using Stata 13.1 statistical software [40], stratified by AHI (15–29.9, ≥30). A separate senior research staff member (not involved in participant enrollment, assessment, or intervention) prepared opaque sequentially numbered envelopes and implemented the random allocation sequence. Participants and assessment research staff were blinded to group assignment. Sleep clinic providers and staff were also blinded to group assignment, and all intervention and control sessions occurred in a research building separate from sleep clinic locations.
Intervention and control conditions
All participants (intervention and control) received usual care from the sleep center during the trial, and all PAP prescriptions and PAP devices and supplies were provided by the sleep clinic. In our sleep center, patients typically undergo home sleep apnea testing (or in-laboratory polysomnography if central sleep apnea is suspected, and/or patients are suspected to have a comorbid sleep disorder such as a parasomnia). Patients with OSA are contacted by the sleep physician and provided information about their diagnosis. If the patient is agreeable a PAP prescription is written, and an in-person appointment is scheduled for the patient to pick up their PAP equipment from the sleep center. At that visit, a respiratory therapist orients patients to the PAP device and fits them with an interface. The first intervention or control session was scheduled to occur within one week before or after the PAP pickup appointment, with a preference for this session to occur before (or on the same day) as PAP pickup, if possible. Of the 125 randomized participants, 52 (41.9%) received session 1 during the week prior to PAP pickup (range 1–9 days), 40 (32.3%) received session 1 on the same day as PAP pickup, and 32 (25.8%) received session 1 during the week after PAP pickup (range 1–8 days). The number of days between session 1 and the PAP pickup appointment was not significantly different between groups.
Intervention condition
The intervention was delivered by one of two sleep coaches who had a master’s degree level of education (i.e. one in public health, the other in communication), but no clinical training or licensure. Prior to study onset, each sleep coach completed either a web-based or in-person CBTI training program (approximately 16 h of training). A psychologist investigator with BSM expertise (JLM or JMD) provided additional training on the study intervention materials and observed each sleep coach providing the intervention in a small number of pilot participants.
The intervention was structured and manual-based, delivered individually with hard-copy materials provided to participants (see Table 1 for a brief summary of intervention content). Five weekly 1-h sessions that integrated CBTI with a PAP adherence program were provided by the sleep coach. Intervention participants completed a 7-day intervention sleep diary between the intervention sessions. In addition to usual self-reported sleep measures, the intervention sleep diary also included self-reported items about the participant’s experience with nightly PAP use (e.g. specific challenges wearing PAP).
Table 1.
Intervention content
| Intervention session | Intervention session content |
|---|---|
| 1 |
PAP content: Understanding sleep apnea, health problems related to sleep apnea, PAP therapy, types of PAP, PAP comfort features, PAP mask features, getting started with PAP CBTI content: What causes insomnia, review of past week’s sleep diary, consistent bedtime and get-up time for the week (stimulus control) |
| 2 |
PAP content: Benefits of PAP use you’ve noticed so far, your challenges with PAP (patient, therapy and equipment-related challenges), your PAP use motivation, review of past week’s objective PAP use data, this week’s plan for PAP use CBTI content: Healthy sleep patterns, the “sleep bank,” pros/cons of napping (cognitive therapy), stimulus control techniques, relaxation techniques, review of past week’s sleep diary, adjust bedtime (sleep restriction) |
| 3 |
PAP content: Benefits of PAP use you’ve noticed so far, your challenges with PAP (patient, therapy and equipment-related challenges), review of past week’s objective PAP use data, this week’s plan for PAP use CBTI content: Behavioral and cognitive challenges to sleep plan (bedtime and get up time), additional relaxation techniques, review of past week’s sleep diary, adjust bedtime (sleep restriction) |
| 4 |
PAP content: Benefits of this treatment you’ve noticed so far, your challenges with PAP (patient, therapy and equipment-related challenges), review of past week’s objective PAP use data, this week’s plan for PAP use CBTI content: Sleep hygiene (e.g. caffeine, napping, alcohol, exercise), review of past week’s sleep diary, adjust bedtime (sleep restriction), finger trap behavioral experiment (cognitive therapy) |
| 5 |
PAP content: How has this treatment impacted your life, current status of your sleep apnea, sleep apnea self-management, review of past week’s objective PAP use data, my PAP planning contract CBTI content: Current status of your insomnia, adjusting your sleep schedule, insomnia self-management, expectations about future sleep (cognitive therapy), review of past week’s sleep diary, plans going forward |
Although listed separately in the table for clarity, the positive airway pressure (PAP) and cognitive behavioral therapy for insomnia (CBTI) content are integrated during the sessions.
CBTI components of the intervention included stimulus control, sleep restriction, sleep hygiene, relaxation techniques, and cognitive therapy techniques. Stimulus control began in session 1 by establishing a consistent bedtime “window,” with additional aspects added in subsequent sessions. Sleep hygiene and relaxation techniques were also briefly introduced in session 1 and expanded in subsequent sessions. Mean total sleep time and sleep efficiency were calculated by the sleep coach from the intervention sleep diary and reviewed at each intervention session with the participant, in addition to adherence with CBTI recommendations from the prior week’s session. Sleep restriction began in session 2 and was continued in the remaining sessions, with adjustment of time in bed based on sleep efficiency. Cognitive therapy strategies (which also began in session 2 and continued in subsequent sessions) included education on insomnia and sleep (to challenge misconceptions and dysfunctional beliefs), behavioral experiments (using the sleep diary to measure progress), pro/con discussion of napping (when indicated), addressing sleep effort with a behavioral experiment (finger trap), and developing adaptive thoughts around expectations for sleep in the future. PAP adherence components included education about OSA and how PAP treats OSA, personal motivations for using PAP, and a weekly review of the participant’s individual benefits (e.g. less daytime sleepiness) and challenges (patient, therapy, and equipment-related) experienced by the participant. Individualized recommendations and strategies to address challenges were identified with the sleep coach. Objective PAP use data from the prior week was also reviewed with the participant during the session.
During the intervention period, one weekly supervisory telephone call (lasting approximately 1 h) was held between the sleep coach and a supervising study psychologist (LF, CS, JMD) to discuss all currently active intervention participants (generally 4–6 participants were undergoing intervention each week). The purpose of these telephone calls was to review each participant’s progress in detail, and problem-solve issues of adherence with CBTI recommendations and PAP use. The sleep coach could contact the study psychologist briefly between the weekly supervising phone calls to receive specific additional guidance. The study psychologists had no direct contact with intervention participants. When appropriate, participants were encouraged by the sleep coach to contact their provider in the sleep clinic with symptoms that contributed to adherence challenges that might warrant specific intervention (e.g. nasal congestion) or when participant complaints suggested a change in the interface was needed.
Control condition
The control condition was a structured, manual-based, general sleep education program delivered at the same frequency and intervals as the intervention condition, to account for social attention and nonspecific treatment effects and to encourage participant retention. The control condition was delivered by a separate sleep coach (with a master’s degree in social work), who had no CBTI or PAP adherence training. Participants in the control condition did not complete weekly sleep diaries and weekly PAP use was not reviewed. The control session overall topics included sleep basics, sleep across the lifespan, sleep and the mind, evening activities and the sleep environment, and daytime activities and sleep, in sessions 1–5, respectively.
Fidelity of intervention and control
Fidelity of the intervention was monitored by session content checklists and participant recommendation forms (completed by the sleep coach during each intervention session). These forms were reviewed during the supervisory telephone call to verify that topics had been covered as outlined in the treatment manual and recommendations were appropriate.
Fidelity of the control condition was addressed by session content checklists (completed by the individual providing the control condition), which were reviewed to verify that control topics were covered. There was no overlap of content between the two groups, and key aspects of the intervention were not provided to control participants.
Measures
Participant characteristics
Descriptive characteristics (e.g. age, gender, race/ethnicity, educational level, marital status, employment status) were collected at baseline. Self-reported comorbidity was recorded as the number of health conditions endorsed (from a list of 36 common medical and psychiatric disorders) [41]. The Mini-Mental State Examination (MMSE) was performed as a measure of mental status [39]. The 7-item pain intensity subscale of the Geriatric Pain Measure (GPM), [42] the Patient Health Questionnaire-9 (PHQ-9, a 9-item scale, total score 0–27; higher score indicates more severe depressive symptoms) [43], and the Primary Care PTSD Screening Tool (PC-PTSD, a 4-item scale, total score 0–4; score >2 indicates more PTSD symptoms) [44] were also collected. All medications (prescription and over the counter) taken were recorded for one week during baseline assessment to calculate the total number of medications (excluding vitamins and other supplements). In addition, it was recorded whether the participant received a “medication commonly used for insomnia” (MCUFI), based on previously published definitions [45]. The Medical Outcomes Study 12-item Short-form Health Survey v2 (SF-12v2) [46] mental health composite (MCS) and physical health composite (PCS) scales (each scale total score 0–100; higher score indicates better functioning) were collected as measures of general health and function. The 16-item version of the Dysfunctional Beliefs and Attitudes about Sleep scale (DBAS-16) was used to assess participants’ beliefs and attitudes about sleep.
Outcome measures
Measures were collected at baseline and at 3 and 6 months from the date of randomization by assessment research staff blinded to group assignment, study research questions, and content of the intervention and control conditions.
Primary outcomes included subjective and objective measures of sleep and objective PAP use at a 3-month follow-up (the primary outcome endpoint). At each assessment timepoint (baseline, 3 and 6 months), participants completed the Pittsburgh Sleep Quality Index (PSQI, total score 0–21; higher score indicates worse sleep quality) and a 7-day assessment sleep diary including bedtime, nighttime awakenings, rise time, and other items to calculate sleep onset latency (SOL-D, i.e. the amount of time it takes to fall asleep), wake after sleep onset (WASO-D) and sleep efficiency (SE-D, time asleep over time in bed). Wrist actigraphy (Actiwatch Spectrum, Respironics) was performed at each timepoint during the same week (7 days) as the sleep diary (to use sleep diary bedtimes and rise times for actigraphy scoring) to estimate objective nighttime sleep efficiency (SE-A). The primary sleep measures included PSQI total score, SOL-D, WASO-D, SE-D, and SE-A at 3-month follow-up (i.e. five variables). Objective PAP use (downloaded from PAP equipment cloud data) was collected by research staff blinded to group assignment and verified by SD card download at study termination. Participants with no PAP use (from PAP equipment cloud data) were verified by blinded research staff who contacted participants to rule out equipment monitoring failure. The primary PAP measures included hours of PAP use per night and the number of nights with PAP used >4 h (both calculated over the past 30 and 90 days), at a 3-month follow-up (i.e. four variables). These sleep and PAP measures were repeated at a 6-month follow-up as secondary measures to estimate maintenance of treatment effects over time, but these findings should be considered exploratory.
Additional secondary sleep-related measures were collected at each timepoint and explored in analyses. The Insomnia Severity Index (ISI, total score 0–28; a higher score indicates worse insomnia severity) was included as a measure of insomnia symptoms and severity [47]. The Epworth Sleepiness Scale (ESS) is an 8-item questionnaire of daytime sleepiness that measures the chances that an individual would doze off or fall asleep under eight different circumstances [48]. The Functional Outcomes of Sleep Questionnaire 10-item version (FOSQ-10) was used as a measure of the effects of sleep disturbance and daytime sleepiness on the ability to perform daily activities [49]. The ISI, ESS, and FOS-Q were secondary measures, so the results of these analyses should also be considered exploratory.
At post-treatment, intervention and control participants rated the program they received on four credibility items (total score for each item 0–6, higher score indicates greater credibility) [50]. The items assessed how logical, successful, and acceptable they found the program and how confident they were that they would recommend the program to someone else.
Statistical analysis
Baseline differences between the treatment and control groups were assessed using two-sample t-tests for continuous variables and chi-square tests for categorical variables.
The a priori primary hypothesis was that sleep (by PSQI, sleep diary, and actigraphy) and PAP use would be better in the intervention group compared to controls at a 3-month follow-up. Secondary hypotheses were that better sleep and more PAP use would be maintained in the intervention group compared to controls at a 6-month follow-up. In the secondary analysis, we also tested for effects of the intervention on the additional sleep measures (i.e. ISI, ESS, FOSQ-10) at 3- and 6-month follow-up.
Multilevel models were used to test the hypotheses comparing the change in sleep outcomes for the intervention versus the control group. When applied to repeated measures designs, multilevel models accommodate incomplete data across timepoints (such as due to missing data when a follow-up visit is missed) and can permit the specification of a wide variety of residual covariance structures. Multi-level models were fit using previously described methods [51], with the Stata mixed command using the residuals() option to specify the residual covariance structure [52]. An unstructured residual covariance structure was used for all outcomes because it blends parsimony (with three timepoints, it estimates six parameters) with the assurance that the residual covariance structure will not be mis-specified.
The primary hypotheses for sleep variables were tested via an interaction contrast that compared the change in the outcome from baseline to 3 months for the treatment group versus the control group. The intervention effect was estimated as the change in the intervention group from baseline to 3 months minus the change in the control group from baseline to 3 months, and a 95% confidence interval for this effect was computed. Additionally, the intervention effect was estimated using a variation of Cohen’s d formulated for a pretest–posttest-control (PPC) design which forms a standardized measure of the change (from baseline to follow-up) for the intervention group versus the control group [53]. The secondary hypotheses regarding maintenance of treatment effects over time were tested in a similar manner, but instead focusing on the change from baseline to 6 months.
There was no PAP use at baseline (i.e. PAP was started after randomization), so our analytic strategy focused solely on post-baseline usage. Two-group t-tests were used for comparison of intervention and control group mean PAP use variables at 3- and 6-month follow-up. The outcome for these analyses was objective PAP use summarized over the past 30 days and over the past 90 days for each 3- and 6-month timepoint.
Sample size calculations performed prior to study commencement established that a total sample size of 120 randomized participants (using alpha = 0.05 and 80% power) would be adequate to test for differences between groups (intervention versus control) in both sleep and PAP use outcome variables (with an estimated 15.0% total loss to follow-up, resulting in at least 102 participants available for analyses at 6 months). All outcomes were assessed using intention-to-treat analyses. We tested for systematic attrition by using two-sample t-tests to compare the baseline characteristics for each outcome variable between those who did and did not have complete data at each follow-up timepoint. All such tests were not significant (p > 0.05), indicating no evidence of systematic attrition.
Data preparation and data analyses were performed using Stata version 15.1 [52]. All significance tests were two-tailed and tested using alpha = 0.05. No adjustments were made for multiple tests, thus the tests with regards to the secondary outcomes should be considered exploratory.
Results
Of the 433 participants who completed the in-person baseline assessment, 125 met all eligibility criteria and were randomized to the intervention (62 participants) or control (63 participants) group. Table 2 provides information on baseline demographics, sleep measures, and other characteristics of randomized participants. Participants were 96.0% male, 39.2% non-Hispanic white, 25.6% black/African American, 17.7% Hispanic/Latino, with a mean age of 63.2 years (range 50–87 years). OSA was diagnosed by Level 3 home sleep apnea testing in 109 (87.2%) participants, and by in-laboratory polysomnography in the remaining 16 (12.8%) participants. Mean AHI was 34.7 (AHI range 15.0–128.9) and mean ISI was 13.6. 87% of participants reported their sleep problems had been present for more than 12 months. There were no significant differences between groups on any of these baseline characteristics. Among participants who were not taking a medication commonly used for insomnia (MCUFI) at baseline, one participant in each group reported taking an MCUFI at 3- or 6-month follow-up.
Table 2:
Baseline demographic, sleep, and other characteristics of randomized participants
| Variable | Overall N = 125 |
Intervention group N = 62 |
Control group N = 63 |
t-value or chi-square (p-value) |
|---|---|---|---|---|
| Mean (SD) or % (n) |
Mean (SD) or % (n) | Mean (SD) or % (n) | ||
| Demographics | ||||
| Age, in years | 63.2 (7.1) | 62.8 (6.7) | 63.7 (7.6) | −0.72 (0.47) |
| Gender, male | 96.0% (119) | 98.4% (61) | 92.1% (58) | 2.73 (0.10) |
| Race/ethnicity | ||||
| % Non-Hispanic white | 39.2% (49) | 38.7% (24) | 39.7% (25) | |
| % Black/African American | 24.0% (30) | 22.6% (14) | 25.4% (16) | |
| % Hispanic/Latino white | 16.8% (21) | 19.4% (12) | 14.3% (9) | |
| % Asian | 5.6% (7) | 6.5% (4) | 4.8% (3) | |
| % American Indian | 2.4% (3) | 3.2% (2) | 1.6% (1) | 1.87 (0.93) |
| % More than one race | 8.8% (11) | 6.5% (4) | 11.1% (7) | |
| % Unknown | 3.2% (4) | 3.2% (2) | 3.2% (2) | |
| Education | ||||
| Less than high school | 2.4% (3) | 1.6% (1) | 3.2% (2) | 3.00 (0.56) |
| High school graduate | 21.6% (27) | 17.7% (11) | 25.4% (16) | |
| Some college | 43.2% (54) | 41.9% (26) | 44.4% (28) | |
| College graduate | 13.6% (17) | 17.7% (11) | 9.5% (6) | |
| Post baccalaureate | 19.2% (24) | 21.0% (13) | 17.5% (11) | |
| Marital status | ||||
| Married | 41.1% (51) | 40.3% (25) | 41.9% (26) | 4.90 (0.32) |
| Living as married | 6.5% (8) | 3.2% (2) | 9.7% (6) | |
| Divorced/separated | 31.5% (39) | 32.3% (20) | 30.6% (19) | |
| Widowed | 3.2% (4) | 1.6% (1) | 4.8% (3) | |
| Single/never married | 17.7% (22) | 22.6% (14) | 12.9% (8) | |
| Employment | ||||
| Working | 25.6% (32) | 24.2% (15) | 27.0% (17) | 0.13 (0.72) |
| Retired | 45.6% (57) | 46.8% (29) | 44.4% (28) | 0.07 (0.79) |
| Unemployed | 17.6% (22) | 17.7% (11) | 17.5% (11) | 0.00 (0.97) |
| Unable to work | 10.4% (13) | 11.3% (7) | 9.5% (6) | 0.10 (0.75) |
| Sleep measures | ||||
| Sleep onset latency (SOL-D) by sleep diary, min | 39.3 (34.7) | 40.9 (41.1) | 37.7 (27.3) | -0.51 (0.61) |
| Wake after sleep onset (WASO-D) by sleep diary, min | 53.4 (46.4) | 53.4 (49.6) | 53.5 (43.3) | 0.003 (0.99) |
| Sleep efficiency (SE-D) by sleep diary, % | 70.0 (15.9) | 70.1 (17.8) | 69.8 (14.0) | -0.07 (0.94) |
| Sleep efficiency, by wrist actigraphy (SE-A), % | 78.3 (9.2) | 77.7 (9.1) | 78.8 (9.4) | 0.71 (0.48) |
| Pittsburgh Sleep Quality Index (PSQI), total score | 11.3 (4.1) | 11.0 (3.9) | 11.5 (4.4) | 0.55 (0.59) |
| Insomnia Severity Index (ISI), total score | 13.6 (5.2) | 13.9 (4.5) | 13.3 (5.8) | 0.69 (0.49) |
| Epworth Sleepiness Scale (ESS), total score | 8.9 (5.6) | 9.7 (5.6) | 8.2 (5.5) | −1.50 (0.14) |
| Functional Outcomes of Sleep Questionnaire-10 item (FOSQ-10), total score | 28.6 (7.2) | 27.8 (7.3) | 29.5 (7.1) | 1.25 (0.21) |
| Dysfunctional Beliefs and Attitudes about Sleep (DBAS-16), total score | 85.6 (30.8) | 90.6 (29.0) | 80.7 (32.0) | −1.80 (0.07) |
| Self-reported duration of sleep problems > 12 months, % (number) of participants | 87.0 (107) | 90.2 (55) | 83.9 (52) | 1.08 (0.30) |
| Apnea−hypopnea index (AHI) | 34.7 (21.2) | 36.3 (23.4) | 32.7 (18.8) | -0.93 (0.36) |
| Other measures | ||||
| Comorbidity index, total score | 6.8 (3.7) | 7.0 (4.3) | 6.7 (2.9) | -0.41 (0.68) |
| Number of medications (excluding vitamins and supplements) | 6.6 (3.9) | 6.3 (4.1) | 6.8 (3.7) | 0.72 (0.47) |
| Medication commonly used for insomnia (MCUFI), percent (number) of participants | 18.4% (23) | 19.4% (12) | 17.5% (11) | 0.07 (0.79) |
| Mini-mental State Exam (MMSE), total score | 28.6 (1.4) | 28.6 (1.5) | 28.7 (1.3) | 0.41 (0.68) |
| Geriatric Pain Measure (GPM), total score | 17.1 (8.1) | 16.6 (8.2) | 17.6 (8.0) | -0.68 (0.50) |
| Depression (PHQ-9), total score | 7.9 (6.0) | 7.4 (5.4) | 8.5 (6.5) | 1.07 (0.29) |
| Primary Care Post-Traumatic Stress Disorder screening tool (PC-PTSD), % (number) high risk | 32.0% (40) | 27.4% (17) | 36.5% (23) | 1.19 (0.28) |
| Medical Outcomes Study 12-item Short-form Health Survey v2 (SF-12v2), mental health composite score (MCS) | 45.6 (11.6) | 46.6 (11.1) | 44.5 (12.0) | −1.03 (0.31) |
| SF-12v2 physical health composite score (PCS) | 37.0 (12.3) | 37.0 (12.8) | 36.9 (11.8) | −0.07 (0.94) |
The final column lists t-values or chi-square (with p-values in parentheses) for comparison of baseline characteristics between the intervention and control groups.
Program attendance, fidelity, and credibility
Attendance and participant-reported credibility were good for both the intervention and control conditions. All five intervention or control sessions were attended by 83.9% of intervention participants and 96.8% of control participants (p = 0.014). The mean number of minutes per session (as recorded by intervention and control staff for each session) ranged from 52 to 64 min in duration. The mean number of face-to-face intervention hours over the 5 weeks (totaled within each participant) was 4.4 h in both groups. At the conclusion of treatment, the range of mean scores for the credibility items among both intervention and control participants was 5.2–5.8 (on a 6-point scale, with higher scores indicating better credibility). Not surprisingly, compared to the control group, intervention participants reported higher (i.e. better) program credibility (p < 0.05). However, the largest difference in mean credibility between the intervention and control groups in an item was only 0.5 points (out of 6.0 points), suggesting there was adequate blinding of participants. No harms or unintended effects were identified in either the intervention or control groups. Treatment fidelity, as measured by completion of the session checklists, revealed that 97.0% of intervention topics and 99.4% of control topics were completed in the intervention and control sessions, respectively.
Outcomes
As indicated in Figure 1, at 3 months (the primary study endpoint), 54 (87.1%) intervention participants and 58 (92.1%) control participants completed an assessment. At 6 months, 53 (85.5%) intervention participants, and 61 (96.8%) control participants completed an assessment. Objective PAP use (obtained from participants’ PAP equipment cloud data) was available in all participants (including no use) at 3 and 6 months. Tables 3 and 4 show the mean values and 95% confidence intervals for sleep and PAP use measures at each timepoint (i.e. sleep measures at baseline, 3 and 6 months; PAP use at 3 and 6 months) for the intervention and control groups. There were no significant differences between the intervention and control groups in these measures at baseline (all p-values > 0.05).
Table 3.
Primary sleep measures at each timepoint by treatment group (N = 125; 62 intervention, 63 control)
| Outcome | Group | Baseline | 3 months | 6 months | Difference between groups in change from baseline (95% confidence intervals) p-value Effect size (d) |
|
|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Baseline vs 3 months | Baseline vs 6 months | ||
| Pittsburgh Sleep Quality Index (PSQI), total score |
Intervention Control |
11.0 (10.0, 12.1) 11.5 (10.4, 12.5) |
6.3 (5.2, 7.3) 9.9 (8.8, 10.9) |
7.2 (6.0, 8.4) 9.4 (8.2, 10.5) |
−3.2 (−4.6, −1.8) p < 0.001 d = −0.82 |
−1.7 (−3.3, −0.13) p = 0.039 |
| d = −0.45 | ||||||
| Sleep onset latency (SOL-D), by sleep diary, min |
Intervention Control |
40.9 (32.3, 49.6) 37.7 (29.2, 46.2) |
22.8 (15.2, 30.4) 35.8 (28.4, 43.0) |
22.5 (15.4, 29.5) 34.7 (28.2, 41.3) |
−16.2 (−29.0, −3.4) p = 0.013 d = −0.50 |
−15.5 (−27.6, −3.5) p = 0.011 |
| d = −0.46 | ||||||
| Wake after sleep onset (WASO-D), by sleep diary, min |
Intervention Control |
53.2 (41.7, 64.8) 53.0 (41.5, 64.6) |
20.4 (12.0, 28.9) 40.7 (32.5, 48.8) |
19.3 (12.3, 26.4) 34.2 (27.7, 40.8) |
−20.5 (−37.6, −3.3) p = 0.019 d = −0.39 |
−15.2 (−30.9, 0.62) p = 0.06 |
| d = −0.32 | ||||||
| Sleep efficiency (SE-D) by sleep diary, % |
Intervention Control |
70.1 (66.1, 74.0) 70.0 (66.1, 73.9) |
86.2 (82.9, 89.6) 75.7 (72.5, 78.9) |
85.4 (82.2, 88.5) 76.8 (73.9, 79.7) |
10.5 (4.5, 16.4) p = 0.001 d = 0.64 |
8.5 (2.5, 14.4) p = 0.005 |
| d = 0.58 | ||||||
| Sleep efficiency (SE-A), by actigraphy, % |
Intervention Control |
77.6 (75.3, 79.8) 78.7 (76.5, 81.0) |
81.7 (79.6, 83.8) 78.5 (76.4, 80.6) |
80.4 (78.0, 82.8) 78.9 (76.6, 81.3) |
4.4 (1.9, 6.8) p = 0.001 d = 0.52 |
2.6 (0.15, 5.1) p = 0.038 |
| d = 0.34 | ||||||
Mean values (and 95% confidence intervals) at each timepoint are provided. Mixed models with random intercepts were used to compare change from baseline for the intervention versus control group. p-values represent treatment versus control comparison using repeated measures analysis. Intervention effect was estimated as the change in the intervention group from baseline (at 3 and 6 months) minus the change in the control group from baseline (at 3 and 6 months). Effect size (d) was estimated using a variation of Cohen’s d for a pretest−posttest-control (PPC) design. The a priori primary outcome timepoint was 3 months follow-up. Assessments were repeated at 6 months as secondary estimates of maintenance of treatment effects, so these results should be considered exploratory.
Table 4.
Primary PAP use measures at each timepoint by treatment group (N = 125; 62 intervention, 63 control)
| Outcome | Group | Baseline | 3 months | 6 months | Difference between groups at each follow-up timepoint (95% Confidence Intervals) p-value effect size (d) |
|
|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | 3 months | 6 months | ||
| PAP use per night (in hours, over the past 30 days) | Intervention | NA | 3.0 (2.3, 3.7) | 2.4 (1.7, 3.1) | 1.1 (2.0, 0.16) p = 0.021 d = 0.42 |
0.9 (1.8, 0.05) p = 0.038 d = .37 |
| Control | NA | 1.9 (1.3, 2.5) | 1.5 (.85, 2.0) | |||
| PAP use per night (in hours, over the past 90 days) | Intervention | NA | 3.2 (2.5, 3.8) | 2.5 (1.9, 3.2) | 1.3 (2.1, 0.51) p = 0.001 d = 0.59 |
0.9 (1.7, 0.02) p = 0.045 d = 0.38 |
| Control | NA | 1.9 (1.4, 2.4) | 1.7 (1.1, 2.2) | |||
| Number of nights with PAP used ≥ 4 h (over the past 30 days) | Intervention | NA | 12.1 (9.2, 15.0) | 9.9 (7.1, 12.6) | 4.7 (8.4, 0.96) p = 0.014 d = 0.44 |
4.3 (7.9, 0.63) p = 0.022 d = 0.42 |
| Control | NA | 7.4 (5.0, 9.8) | 5.6 (3.1, 8.1) | |||
| Number of nights with PAP used > 4 h (over the past 90 days) | Intervention | NA | 38.6 (30.8, 46.3) | 30.7 (22.8, 38.6) | 17.4 (27.3, 7.6) p < 0.001 d = 0.63 |
11.3 (21.8, 0.82) p = 0.035 d = 0.38 |
| Control | NA | 21.2 (14.9, 27.4) | 19.4 (12.2, 26.5) | |||
NA = not applicable (no PAP use at baseline).
Mean values (and 95% confidence intervals) at each timepoint are provided. p-values represent treatment versus control comparison using two group t-test. Intervention effect was estimated as the difference between the intervention and control groups at each timepoint (at 3 and 6 months). Effect size (d) was estimated using a variation of Cohen’s d for a pretest–posttest-control (PPC) design. The a priori primary outcome timepoint was 3 months follow-up. Assessments were repeated at 6 months as secondary estimates of maintenance of treatment effects, so these results should be considered exploratory.
Tables 3 and 4 also present the results of the repeated measures analysis comparing the change in outcomes for the intervention group between baseline and 3 months minus the change in the control group between baseline and 3 months. These differences are also shown for the comparison between baseline and 6 months. The intervention group had greater improvements in all primary sleep (PSQI, SOL-D, WASO-D, SE-D, and SE-A) and PAP use measures at 3 months (i.e. the primary outcome timepoint) compared to controls (all p-values < 0.05). At 6 months, improvements in sleep and PAP use measures remained significantly better in the intervention group compared to controls (all p-values < 0.05) except for WASO-D (p = 0.06). For example, at 3 months, compared to their baseline values, the intervention group had a mean of 3.2 points greater improvement in PSQI (compared to baseline) than controls (p < 0.001). Likewise, at 3 months (compared to their baseline values), the intervention group took a mean of 16.2 fewer minutes to fall asleep (SOL-D, p = 0.013) than controls (compared to their baseline values), they were awake 20.5 min less once they fell asleep (WASO-D, p = 0.019) than controls, and they had a greater improvement in sleep efficiency compared to baseline of 10.5% (SE-D, p = 0.001) compared to controls. The absolute values of the effect sizes for improvement in PSQI were 0.82 and 0.45 at 3 and 6 months, respectively. The absolute values of the effect sizes in sleep diary and actigraphy outcomes ranged from 0.32 to 0.64. Of note, a negative effect size indicates a decrease in mean value and a positive effect size indicates an increase in mean value; all effects sizes were in the direction of a better outcome with intervention.
The intervention group also had greater PAP use than controls at 3 and 6 months (see Table 4), regardless of whether the data was summarized over the prior 30 days or the prior 90 days. Compared to controls, over the prior 30 days the intervention group used PAP on average 1.1 more hours per night and 0.9 more hours per night at 3 and 6 months, respectively. Similarly, when PAP use was averaged over the prior 90 days, compared to controls the intervention group used PAP on average 1.3 and 0.9 more hours per night at 3 and 6 months, respectively. In addition, compared to controls, over the prior 30 days the intervention group used PAP for ≥4 h per night on 4.7 and 4.3 more nights at 3 and 6 months, respectively; and 17.4 and 11.3 more nights over the prior 90 days, at 3 and 6 months, respectively. Effect sizes for more PAP use with intervention ranged from 0.42 to 0.63 at 3 months, and from 0.36 to 0.42 at 6 months.
In additional secondary analyses, we summarized individual-level PAP data to further explore the impact of the intervention on PAP use (not shown in tables). We identified participants who had “any” PAP use (i.e. defined as a cumulative total of at least 60 min of use over the prior 90 days) at 3 and 6 months. At 3 months, the percent of participants who had any PAP use over the prior 90 days was 87.1% and 82.5% among the intervention and control groups, respectively (p = 0.62). Similarly, at 6 months, the percent of participants who had any PAP use (at least 60 min) over the prior 90 days was 79.0% and 71.4% in the intervention and control groups, respectively (p = 0.41). We believe these findings suggest the impact of the intervention was not attributable to PAP acceptance alone. In additional exploratory analysis, we found no significant differences between the intervention and control groups in total sleep time (estimated by sleep diary and wrist actigraphy) at baseline, 3- and 6-month follow-up (data not shown), which suggests that increased total sleep time alone was unlikely to be a major determinant of increased PAP use with the intervention.
Results of the other secondary measures are shown in Table 5. There was a greater improvement compared to baseline in intervention participants compared to controls at 3 and 6 months for ISI, ESS, and FOSQ-10, with effect sizes (absolute values) that ranged from 0.31 to 0.81. For example, compared to controls, intervention participants had greater decreases (i.e. more improvement) in ISI total score (compared to baseline) of −4.5 and −3.1 at 3 and 6 months, respectively (all p-values < 0.05), with effect sizes that ranged from 0.81 to 0.58. DBAS-16 scores also showed greater improvement from baseline among intervention participants compared to controls at 3 and 6 months.
Table 5.
Other secondary measures at each timepoint by treatment group (N = 125; 62 intervention, 63 control)
| Outcome | Group | Baseline | 3 months | 6 months | Difference between groups in change from baseline (95% confidence intervals) p-value effect size (d) |
|
|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Baseline vs 3 months | Baseline vs 6 months | ||
| Insomnia Severity Index (ISI), total score |
Intervention Control |
13.9 (12.6, 15.2) 13.2 (11.9, 14.5) |
6.4 (4.7, 8.0) 10.2 (8.6, 11.8) |
7.7 (6.1, 9.3) 10.1 (8.6, 11.6) |
−4.5 (−6.6, −2.4) p < 0.001 d = −0.81 |
−3.1 (−5.3, −0.90) p = 0.006 |
| d = −0.58 | ||||||
| Epworth Sleepiness Scale (ESS) | Intervention | 9.7 (8.3, 11.1) | 5.9 (4.5, 7.4) | 5.9 (4.6, 7.2) | −2.4 (−3.8, −0.9) p = 0.001 d = −0.31 |
−2.1 (−3.5, −0.7) p = 0.003 d = −0.34 |
| Control | 8.2 (6.9, 9.6) | 6.8 (5.4, 8.2) | 6.5 (5.3, 7.8) | |||
| Functional Outcomes of Sleep Questionnaire-10 (FOSQ-10) |
Intervention Control |
28.0 (26.2, 29.8) 29.4 (27.6, 31.2) |
33.2 (31.3, 35.0) 30.8 (29.0, 32.6) |
32.4 (30.6, 34.2) 31.6 (29.9, 33.4) |
3.7 (1.4, 6.1) p = 0.002 d = 0.49 |
2.1 (−0.3, 4.5) p = 0.081 |
| d = 0.33 | ||||||
| Dysfunctional Beliefs and Attitudes-16 (DBAS-16) |
Intervention Control |
90.6 (83.1, 98.2) 80.7 (73.2, 88.2) |
64.0 (55.7, 72.2) 73.1 (65.1, 81.2) |
71.9 (64.5, 79.4) 75.0 (67.8, 82.1) |
−19.1 (−27.7, −10.4) p = 0.000 d = −0.54 |
−13.0 (−21.0, −4.9) p = 0.002 d = −0.42 |
Mean values (and 95% confidence intervals) at each timepoint are provided. Mixed models with random intercepts were used to compare change from baseline for the intervention versus control group. p-values represent treatment versus control comparison using repeated measures analysis. Intervention effect was estimated as the change in the intervention group from baseline (at 3 and 6 months) minus the change in the control group from baseline (at 3 and 6 months). Effect size (d) was estimated using a variation of Cohen’s d for a pretest–posttest-control (PPC) design. Results of these secondary outcomes should be considered exploratory.
Discussion
In this randomized controlled trial, we found that a structured, manual-based, 5-session intervention that integrates CBTI with a PAP adherence program, delivered by a sleep coach with weekly telephone supervision by a BSM psychologist, improves both sleep, and PAP use among middle-aged and older veterans with comorbid insomnia and newly diagnosed moderate or severe OSA. The overall effectiveness of the intervention on sleep outcomes was similar to findings from other CBTI studies [54], with demonstrated improvements in sleep diary, questionnaire and actigraphy measures of sleep, and most sleep-related improvements were maintained at 6 months. PAP use was also significantly greater with the intervention, with 1.3 and 0.9 more hours of PAP use per night over the prior 90 days at 3 and 6 months, respectively.
Recent controlled trials testing CBTI in adults with comorbid insomnia and OSA have had mixed results. Bjorvatn et al. performed a randomized controlled trial testing CBTI provided in a self-help workbook (which was demonstrated to improve insomnia in participants without OSA in prior work) [55] versus sleep hygiene advice as the control condition [32]. Insomnia symptoms (on the Bergen Insomnia Scale and ISI) improved with both the CBTI self-help workbook and with the sleep hygiene control condition at 3 months follow-up, but there were no significant differences between these two groups. Likewise, there were no significant differences between the two groups in PAP use at 3 months (mean PAP use was 2.9 h per night in each group at 3 months) [32]. In another study that examined both the timing and addition of CBTI to PAP initiation, Ong et al. compared: (a) CBTI (provided in 4 weekly sessions by a trained therapist) followed by PAP initiation, (b) CBTI concurrent with PAP initiation, or (c) PAP initiation only (without CBTI) [33]. All three groups had improvement in PSQI total score at 90 days follow-up, but there were no significant differences between groups. Both groups receiving CBTI had greater improvement in ISI compared to the group that received PAP initiation alone. There were no significant differences in PAP use between the three groups over 90 days of follow-up in either hours of PAP use per night (2.5 mean hours of PAP use per night in each group) or percent of nights with use ≥4 h (33.0%, 34.8%, and 39.6% of nights, respectively). Finally, Sweetman et al. studied CBTI (4 weekly sessions provided by a psychologist prior to PAP initiation) versus PAP initiation alone (i.e. treatment as usual, TAU, without CBTI) [34]. At 6 months follow-up, the CBTI group had greater improvement in ISI, but not in sleep diary or polysomnography measures, compared to TAU. Compared to TAU, the CBTI group had significantly more PAP use, with a mean of 57.3 min (i.e. 1.0 h) and 63.9 min (1.1 h) more of PAP use per night at 3 and 6 months, respectively.
Like the studies mentioned above, the current study found low PAP use among patients with comorbid insomnia and OSA. The low PAP use in controls (particularly by 6 months) suggests that in the absence of evidence-based treatment for insomnia and support in the initial phase of treatment, veterans with comorbid insomnia and OSA are unlikely to successfully use PAP and are likely to continue to experience insomnia as well. Few participants in either group sustained nightly PAP use that reached the ≥4 h per night Centers for Medicare and Medicaid Services (CMS) threshold [56] (although PAP use was significantly greater with the intervention). Increasing evidence suggests there may not be a PAP threshold that must be reached to reverse the adverse effects of OSA [57]. Instead, there is evidence for a linear dose-response where more PAP use is associated with greater improvements in daytime sleepiness, functional status, blood pressure, and other outcomes [58, 59]. For example, 1 h more of PAP use per night is associated with less sleepiness [57] and decreased mortality [60]. In fact, recent guidelines recommend continued use of PAP even if the 4-h threshold is not met as some benefits are seen with even lower levels of use, such as reduced sleepiness [31]. Of note, we found improvements in daytime sleepiness and functional outcomes of sleep with intervention, suggesting improvements in sleep and PAP use were associated with improvement in these other measures of well-being.
For the primary and secondary measures of sleep and related outcomes, in addition to statistically significant improvements, we believe our findings also suggest clinically meaningful improvements with the intervention. For example, at 3 months follow-up the difference between groups in change from baseline exceeded (was better than) previously reported expert opinion on minimally clinically important differences (MCID) for the PSQI (MCID 2.5 points) [61] and ESS (MCID variably reported as two to three points) [62]. Our results did not meet the previously reported minimally important difference of 6.0 points for the ISI (which was defined by improvement in other health-related outcomes) [63]. We were unable to identify a reported MCID for the FOSQ-10. In addition, using a simple mathematical estimate of minimally important difference (calculated as 0.3–0.5 times the baseline standard deviation, SD) [64, 65], at 3 months follow-up the difference between groups in change from baseline for all sleep diary, actigraphy and questionnaire variables exceeded (was better than) 0.3 times SD. At 3 months, the difference between groups in change from baseline exceeded (was better than) 0.5 times SD for sleep onset latency and sleep efficiency (by sleep diary) and all questionnaires (i.e. PSQI, ISI, ESS, FOSQ).
We believe our approach provides a promising option for adults with comorbid insomnia and OSA that integrates CBTI with a PAP adherence program at the initiation of PAP. This integrated approach represents an alternative to sequential treatments and may facilitate long-term PAP use. Furthermore, the approach of addressing insomnia at the initiation of PAP treatment is consistent with at least one recent guideline that recommends offering PAP adherence interventions early in treatment for individuals with comorbid insomnia and OSA to address this important barrier to PAP use [31]. In the current study, the CBTI and PAP adherence components of the intervention were fully integrated, so we are unable to estimate the potential impact of each component separately. Future research would be needed to estimate the relative impact of each component of the intervention.
We designed our intervention to facilitate implementation into clinical practice in a variety of settings. The intervention is similar in length to some formats of CBTI alone (i.e. five weekly sessions), and the use of supervised sleep coaches may expand the reach of the intervention to settings with limited availability of BSM providers, such as practices in rural areas. Both the highly structured format of the intervention (designed for use by a variety of individuals as sleep coaches) and the weekly supervision by BSM specialists (by telephone) are essential elements of our intervention, which is meant to demonstrate the effectiveness of a model of care designed to increase access to treatment. The coordination of the intervention with routine clinical care for OSA (i.e. prescription of PAP therapy and provision of PAP equipment and supplies were performed by sleep clinic providers) also increases the likelihood of adoption.
There were also several key strengths of the study design, particularly the rigorous randomized controlled trial methodology with 6 months follow-up and the objective PAP use data as a main outcome measure. Another strength was the use of a clinical population with limited exclusionary criteria, which enhances generalizability. In addition, the high rates of attendance with intervention sessions suggest good feasibility of providing the intervention within clinical care pathways. Finally, the active control condition delivered by an individual with a comparable educational level using similarly formatted program materials provided a strong comparison group to address potential non-specific placebo effects; a key factor to consider in the design of trials involving behavioral treatment [66]. The use of standard clinical measures as our key outcomes further enhances generalizability. For CBTI, the key outcome measures were derived from patient sleep diaries and questionnaires. For PAP use, we used objective monitoring data, which is available through remote monitoring in most clinical settings.
A potential limitation of the study was the predominantly male veteran population; findings may not be generalizable to non-veteran patients. However, 20 million Americans are veterans, and the Veterans Administration provides a unique opportunity to develop and test interventions that may have wider applicability to the larger US population. In addition, since nearly all participants were men, the results may not be generalizability to women. Also, these findings may not be generalizable to patients with comorbid insomnia and mild OSA, since all participants had OSA that was moderate or severe. In addition, we did not formally measure each participant’s adherence to the specific components of CBTI. Also, the higher credibility reported by the intervention group versus controls could be interpreted as a limitation in our ability to adjust for placebo effect. Finally, this work does not address sleep and PAP use outcomes beyond six months; future research is needed to evaluate how to encourage sustained use after initial intervention for comorbid insomnia and OSA. It is possible that additional clinical support (if PAP use levels decline over time) could encourage the sustained use of PAP therapy and increase even longer-term benefits of this treatment.
In summary, we developed and tested a structured, manual-based treatment integrating CBTI with a PAP adherence program provided by a “sleep coach” with weekly telephone supervision by BSM specialists, which significantly improved both sleep and PAP use among middle-aged and older veterans with comorbid insomnia and moderate or severe OSA, and these improvements were maintained for up to 6 months. Given evidence that insomnia predicts less PAP use [5], and early PAP use predicts better long-term PAP use [29], we believe this intervention provides a promising approach to assist patients in the common clinical situation where OSA and insomnia disorder coexist.
Acknowledgments
We thank Sergio Martinez, Diane Lee, Lisa Partch, Simone Vukelich and our other outstanding research staff who made this work possible. We also thank the providers and staff of the Sleep Disorders Center in the VA Greater Los Angeles Healthcare System.
The views expressed in this paper are those of the authors only and do not reflect the official policy or position of the institutions with which the authors are affiliated, the Department of Veteran’s Affairs, nor the United States Government.
This work was performed at the VA Greater Los Angeles Healthcare System.
Funding
This work was funded by Veterans Administration Health Services Research and Development (IIR 12–353, PI: Alessi and RCSA 20–191, PI: Martin), National Institutes of Health/National Heart, Lung and Blood Institute (K24HL143055, PI: Martin), National Institutes of Health/National Institute on Aging (K23AG049955, PI: Dzierzewski) and the Veterans Administration Greater Los Angeles Geriatric Research, Education and Clinical Center.
Conflicts of interest statement. The authors report no other conflicts.
Disclosure Statement
Financial Disclosure: None.
Non-financial Disclosure: None.
References
- 1. Luyster FS, et al. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6(2):196–204. [PMC free article] [PubMed] [Google Scholar]
- 2. Sweetman AM, et al. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med Rev. 2017;33:28–38. [DOI] [PubMed] [Google Scholar]
- 3. Krakow B, et al. Prevalence of sleep breathing complaints reported by treatment-seeking chronic insomnia disorder patients on presentation to a sleep medical center: a preliminary report. Sleep Breath. 2013;17(1):317–322. [DOI] [PubMed] [Google Scholar]
- 4. Fung CH, et al. Prevalence and symptoms of occult sleep disordered breathing among older veterans with insomnia. J Clin Sleep Med. 2013;9(11):1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallace DM, et al. Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath. 2018;22(1):5–15. [DOI] [PubMed] [Google Scholar]
- 6. Wickwire EM, et al. Insomnia and sleep-related breathing disorders. Chest. 2010;137(6):1449–1463. [DOI] [PubMed] [Google Scholar]
- 7. Cho YW, et al. Comorbid insomnia with obstructive sleep apnea: clinical characteristics and risk factors. J Clin Sleep Med. 2018;14(3):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sivertsen B, et al. The joint contribution of insomnia and obstructive sleep apnoea on sickness absence. J Sleep Res. 2013;22(2):223–230. [DOI] [PubMed] [Google Scholar]
- 9. Tasbakan MS, et al. Quality of life in obstructive sleep apnea is related to female gender and comorbid insomnia. Sleep Breath. 2018;22(4):1013–1020. [DOI] [PubMed] [Google Scholar]
- 10. Benetó A, et al. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13(4):287–293. [DOI] [PubMed] [Google Scholar]
- 11. Sharafkhaneh A, et al. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5(4):345–350. [DOI] [PubMed] [Google Scholar]
- 12. Baldwin CM, et al. Obstructive sleep apnea and ischemic heart disease in southwestern US veterans: implications for clinical practice. Sleep Breath. 2005;9(3):111–118. [DOI] [PubMed] [Google Scholar]
- 13. Alessi C, et al. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J Am Geriatr Soc. 2016;64(9):1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bahr K, et al. Current treatment of comorbid insomnia and obstructive sleep apnea with CBTI and PAP-Therapy: a systematic review. Front Neurol. 2018;9:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyên XL, et al. Insomnia symptoms and CPAP compliance in OSAS patients: a descriptive study using Data Mining methods. Sleep Med. 2010;11(8):777–784. [DOI] [PubMed] [Google Scholar]
- 16. Björnsdóttir E, et al. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep. 2013;36(12):1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malhotra A, et al. The importance of arousal in obstructive sleep apnea-updates from the American Thoracic Society 2016. J Thorac Dis. 2016;8(Suppl 7):S542–S544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eckert DJ, et al. Impaired central control of sleep depth propensity as a common mechanism for excessive overnight wake time: implications for sleep apnea, insomnia and beyond. J Clin Sleep Med. 2020;16(3):341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. André S, et al. Cardiometabolic comorbidities in obstructive sleep apnea patients are related to disease severity, nocturnal hypoxemia, and decreased sleep quality. Respir Res. 2020;21(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morgenthaler T, et al. ; American Academy of Sleep Medicine. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- 21. Riemann D, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. [DOI] [PubMed] [Google Scholar]
- 22. Qaseem A, et al. ; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 23. Hanlon JT, et al. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu NW, et al. Association of benzodiazepine and Z-drug use with the risk of hospitalisation for fall-related injuries among older people: a nationwide nested case-control study in Taiwan. BMC Geriatr. 2017;17(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J, et al. Use of sedative-hypnotics and the risk of Alzheimer’s dementia: A retrospective cohort study [published correction appears in PLoS One]. 2018 Oct 16;13(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patil SP, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2019;15(2):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patil SP, et al. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15(2):301–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehrtash M, et al. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung. 2019;197(2):115–121. [DOI] [PubMed] [Google Scholar]
- 29. Aloia MS, et al. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med. 2007;5(3):229–240. [DOI] [PubMed] [Google Scholar]
- 30. Askland K, et al. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Systematic Rev. 2020. (4). doi: 10.1002/14651858.CD007736.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mysliwiec V, et al. The Management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guidelines. Ann Intern Med. 2020;172(5):325–336. [DOI] [PubMed] [Google Scholar]
- 32. Bjorvatn B, et al. No effect of a self-help book for insomnia in patients with obstructive sleep apnea and comorbid chronic insomnia – a randomized controlled trial. Front Psychol. 2018;9:2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ong JC, et al. A randomized controlled trial of CBT-I and PAP for obstructive sleep apnea and comorbid insomnia: main outcomes from the MATRICS study. Sleep. 2020;43(9). doi: 10.1093/sleep/zsaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sweetman A, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12). doi: 10.1093/sleep/zsz178 [DOI] [PubMed] [Google Scholar]
- 35. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014:21–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CONSORT 2010. Flow Diagram. http://www.consort-statement.org/consort-2010
- 37. Martin JL, et al. Estimated prevalence of insomnia among women veterans: results of a postal survey. Womens Health Issues. 2017;27(3):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryden AM, et al. Insomnia disorder among older veterans: results of a postal survey. J Clin Sleep Med. 2019;15(4):543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Folstein MF, et al. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 40. StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP. [Google Scholar]
- 41. Selim AJ, et al. Comorbidity assessments based on patient report: results from the Veterans Health Study. J Ambul Care Manage. 2004;27(3):281–295. [DOI] [PubMed] [Google Scholar]
- 42. Ferrell BA, et al. The geriatric pain measure: validity, reliability and factor analysis. J Am Geriatr Soc. 2000;48(12):1669–1673. [DOI] [PubMed] [Google Scholar]
- 43. Kroenke K, et al. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prins A, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Prim Care Psych. 2004;9:9–14. [Google Scholar]
- 45. Bertisch SM, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ware JE, Jr, et al. User’s Manual for the SF-12v2 Health Survey With a Supplement Documenting SF-12 Health Survey. Lincoln, RI: Quality Metric, Inc; 2002. [Google Scholar]
- 47. Bastien CH, et al. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 48. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:450–545. [DOI] [PubMed] [Google Scholar]
- 49. Chasens ER, et al. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32(7):915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borkovec T, et al. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3;257–260. [Google Scholar]
- 51. Singer JD, et al. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press, Inc.; 2003. [Google Scholar]
- 52. StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- 53. Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11:364–386. [Google Scholar]
- 54. Brasure M, et al. Management of Insomnia Disorder. Comparative Effectiveness Review No. 159. AHRQ Publication No. 15(16)-EHC027-EF. Rockville, MD: Agency for Healthcare Research and Quality; December 2015. [PubMed] [Google Scholar]
- 55. Bjorvatn B, et al. A self-help book is better than sleep hygiene advice for insomnia: a randomized controlled comparative study. Scand J Psychol. 2011;52(6):580–585. [DOI] [PubMed] [Google Scholar]
- 56. Naik S, et al. Centers for medicare and medicaid services positive airway pressure adherence criteria may limit treatment to many medicare beneficiaries. J Clin Sleep Med. 2019;15(2):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weaver TE, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bakker JP, et al. Adherence to CPAP: what should we be aiming for, and how can we get there? Chest. 2019;155(6):1272–1287. [DOI] [PubMed] [Google Scholar]
- 59. Krakow B, et al. Adherence and subthreshold adherence in sleep apnea subjects receiving positive airway pressure therapy: a retrospective study evaluating differences in adherence versus use. Respir Care. 2016;61(8):1023–1032. [DOI] [PubMed] [Google Scholar]
- 60. Campos-Rodriguez F, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128(2):624–633. [DOI] [PubMed] [Google Scholar]
- 61. Sandahl H, et al. Treatment of sleep disturbances in trauma-affected refugees: study protocol for a randomised controlled trial. Trials. 2017;18(1):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Patel S, et al. The Epworth sleepiness scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(7):961–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang M, et al. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487–2494. [DOI] [PubMed] [Google Scholar]
- 64. Farivar SS, et al. Half standard deviation estimate of the minimally important difference in HRQOL scores? Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):515–523. [DOI] [PubMed] [Google Scholar]
- 65. Norman GR, et al. The truly remarkable universality of half a standard deviation: confirmation through another look. Exp Rev Pharmacoeconomics Outcomes Res. 2004;4:581–585. [DOI] [PubMed] [Google Scholar]
- 66. Enck P, et al. Placebo effects in psychotherapy: a framework. Front Psychiatry. 2019;10:456. [DOI] [PMC free article] [PubMed] [Google Scholar]