Abstract
Increasing evidence has indicated that long noncoding RNAs (lncRNAs) are involved in the progression of laryngeal squamous cell carcinoma (LSCC). Here, we aimed to disclose the role of MNX1-AS1 in LSCC progression, and explore whether MNX1-AS1 participates in LSCC progression via targeting miR-744-5p to active BCL9/β-catenin signaling. Sixty-five human LSCC tissues and the paracancerous normal tissues were recruited to determine the levels of MNX1-AS1, miR-744-5p and BCL9 using qRT-PCR. The interaction of miR-744-5p and MNX1-AS1/BCL9 was determined by using the RNA immunoprecipitation (RIP) assay and/or luciferase gene reporter assay. Cell viability, in vivo tumor formation, invasion and migration abilities were detected by MTT, Xenograft models and Transwell assays. MNX1-AS1 level was increased significantly in human LSCC tissues as compared with the normal tissues, which showed a positive correlation with BCL9 level while a negative correlation with miR-744-5p level. High level of MNX1-AS1 predicted a poor prognosis and an advanced clinical process in LSCC patients. miR-744-5p targeted upregulation weakened the luciferase activity of MNX1-AS1 and /BCL9, and downregulated their expression levels-wt, while showed no effect when the binding sites were mutated. Knockdown of MNX1-AS1 markedly weakened cell viability, migration, and invasion abilities, while BCL9 overexpression abolished these tendencies. In addition, MNX1-AS1 downregulation induced decreases in tumor volumes and weights in vivo, accompanied by reductions in BCL9, Ki-67 and β-catenin expression and an increase in miR-744-5p expression. Collectively, this study reveals that MNX1-AS1 contributes to cell growth and migration by regulating miR-744-5p/BCL9/β-catenin axis in LSCC.
Keywords: MNX1-AS1, miR-744-5p, BCL9, laryngeal squamous cell carcinoma, cell growth
Introduction
Laryngeal squamous cell carcinoma (LSCC), as one of the most common malignances in head and neck, has a high mortality rate of 50%1. It is reported that LSCC causes >20,000 deaths in the United States annually2 and is thought to be one of the main causes of cancer‐associated fatalities in the globe, especially in China3. Thus, it is important to further disclose the mechanisms of the occurrence and development of LSCC, such as the molecular mechanisms by which some crucial genes and signaling pathways change.
Long noncoding RNAs (lncRNAs) are >200 nucleotides in length and characterized by limited protein coding capacity4. Dysregulation of lncRNAs has been demonstrated to be a frequent event and closely linked to carcinogenesis, with important values in the treatment and diagnosis of cancer5–7. For instance, lncRNA MNX1-AS1 (MNX1 antisense RNA1) is overexpressed in several kinds of cancers and serves as an oncogene, including gastric cancer8, ovarian cancer 9,10, lung cancer11,12, cervical cancer13, breast cancer,14 and prostate cancer15. However, the role and mechanisms underlying MNX1-AS1 in LSCC still remain to be illustrated.
MicroRNA (miRNA) is another kind of non-coding RNA molecules, which contains ∼22 nucleotides in length and suppresses gene expression through combination with the 3’-UTR of target mRNAs in an imperfectly complementary manner16. In addition to regulation of gene expression at transcriptional, post-transcriptional and epigenetic levels, evidence has shown that lncRNA can work as an endogenous sponge of miRNA to abrogate their role in inhibiting the expression of target genes17. MNX1-AS1 sponges miR-218-5p and upregulates RAB1A expression, leading to increases in tumor growth and metastasis of bladder cancer18. MNX1-AS1 sponges miR-527 to increase BRF2 expression, thereby promoting lung cancer progression 11. With the help of miRDB online software, miR-744-5p is demonstrated to be a predicted target of MNX1-AS1. However, whether MNX1-AS1 involves in LSCC progression via sponging miR-744-5p remains unclear.
The present paper was designed to reveal the role of MNX1-AS1 in cell growth and migration of LSCC, and to determine whether MNX1-AS1 could sponge miR-744-5p to increase BCL9 (B-cell lymphoma 9) expression and subsequently activates Wnt/β-catenin signaling.
Materials and Methods
Tissue Samples
Sixty-five paired human LSCC tissues and the adjacent normal tissues were obtained from patients with LSCC who diagnosed with LSCC. Informed consents were obtained from all patients. None of them received chemotherapy, radiation therapy, or immunotherapy before surgery. Experiments including human samples obtained the approval of the Ethic Committee of the First Affiliated Hospital, Huzhou University, the First People’s Hospital of Huzhou (China). Written informed content was obtained.
Cell Lines and Culture Methods
Two LSCC cell lines, AMC-HN-8 and TU686 cells and the kidney epithelial cell line HEK-293 T cells were sourced from the Cell Bank, China Academy of Sciences (Shanghai, China). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS; Gibco, USA) in a cell incubator (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 5% CO2 at a constant temperature of 37°C.
Modification of Gene Expression
The short hairpin RNAs (shRNAs) used to silence MNX1-AS1 (called sh-MNX1-AS1), the plasmid used to upregulate MNX1-AS1/BCL9 (called MNX1-AS1/BCL9) and the mimic applied to overexpress miR-744-5p (called miR-744-5p mimic), together with the negative control vectors (shRNA, vector, NC mimic) were produced by GenePharma Co., Ltd (Shanghai, China). MNX1-AS1, BCL9, Vector, miR-744-5p mimic and NC mimic were introduced into cells with the help of cell transfection using Lipofectamine 3000 reagent (Invitrogen, Waltham, MA, USA). Sh#1MNX1-AS1, sh#2MNX1-AS1 and shRNA were introduced into cells by cell infection with the help of polybrene (Hanbio Co., Ltd, Shanghai, China).
Quantitative Real Time PCR (qRT-PCR) Assay
Total RNA was extracted with Trizol regent (Thermo Fisher Scientific, MA, USA) in accordance with the manufactory’s instructions. After quantification with Nanodrop (Thermo Fisher Scientific, MA, USA), 1 µg RNA sample was reversely transcripted into cDNA by using a First Strand cDNA Synthesis Kit purchased from CWBIO (Jiangsu, China). Subsequently, the qRT-PCR assay was performed based on the descriptions of a SYBR Premix Ex Taq II kit (Takara Biotechnology, Dalian, China) in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). U6 and GAPDH were used as the internal references for miR-744-5p, and MNX1-AS1 and BCL9. The calculation of mRNA levels was executed with the 2-ΔΔCt method 19. Table 1 displayed the primers used in this experiment.
Table 1.
Primer Sequences.
| Gene | Sense (5’-3’) | Antisense (5’-3’) |
|---|---|---|
| MNX1-AS1 | CCCGCATTTTCAGATTCAC | GCTCTCAGCCTCGCCATA |
| BCL9 | ATACCAGGAGGCCAGGGATT | GCCACCCAATCAGATGGGAA |
| GAPDH | ACCTGACCTGCCGTCTAGAA | TCCACCACCTGTTGCTGTA |
| miR-744-5p | TGCGGGGCTAGGGCTA | GGCCCAGTGTTCAGACTAC |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
RNA Immunoprecipitation (RIP) Assay
RIP assay used to evaluate the relationship of MNX1-AS1 and miR-744-5p was performed as previously reported 20. In brief, cells were lysed with 25 mM Tris-HCl buffer (pH 7.5) made up of 1 mM EDTA, 150 mM NaCl, 5% (v/v) glycerol, 1% (v/v) Nonidet P-40, and 100 U/ml RNase inhibitor (Thermo Fisher Scientific, Shanghai, China). Then, the whole-cell extracts were incubated with protein-A Sepharose beads which are precoated with anti-Ago2 antibody (3 μg; No. ab32381, Abcam, Cambridge, MA, USA) or control anti-IgG antibody (No. ab109489, Abcam, USA) for 30 min at room temperature. The immune complex was then resuspended with 900 µL of RIP wash buffer and probed with another 100 mL of cell lysate overnight at 4°C, followed by collection with protease K and analysis by qRT-PCR.
Western Blotting Assay
Total protein was collected by using radioimmunoprecipitation assay lysis buffer (Beyotime, Jiangsu, China), supplemented with 1% (v/v) protease inhibitors (Beyotime). Then, 30 µg protein sample was obtained from every group and separated by using 8% sodium dodecyl ulfate-polyacrylamide gel electrophoresis, followed by transformation into the polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were then successively incubated with 5% fat-free milk for 1 hour at room temperature and proved with the primary antibodies, including BCL9 (1:2,000 dilution; No. PA5-93229, Thermo Fisher Scientific, USA), β-catenin antibody (1:2,000 dilution; No. PA5-16762, Thermo Fisher Scientific, USA) and β-actin (1:5,000 dilution, No. AM4302, Thermo Fisher Scientific) at 4°C for overnight. After that, the membranes were probed with secondary antibodies (Abcam, USA) for 1 hour. Protein signals were detected using iBright CL750 (Thermo Fisher Scientific) after incubation with ECL (Thermo Fisher Scientific).
MTT Assay
Cell growth was detected by using the MTT (Methyl Thiazolyl Tetrazolium) assay (No. ab211091, Abcam, USA). In brief, LSCC cells were placed in 96-well plates with 3,000 cells each well. At 24, 48, 72, and 96 hours post culture, 10 µL of MTT solution was added into the culture medium. Following incubation at 37°C for another 4 hours, 100 µL DMSO (D5879-100ML, Sigma-Aldrich, St. Louis, MO, USA) was added into each well. The optical density (OD) values at 490 nm for every well was measured once the formazan crystals were fully dissolved by a microplate reader (Shanghai Puyuan Instrument Co., Ltd, Shanghai, China).
Transwell Chambers Assay
To assess cell invasions, LSCC cells suspended in FBS-free DMEM was seeded in the Matrigel pre-coated membrane in the top chamber (BD Bioscience, USA), with 1×105 cells for each well, and the lower chamber was added 600 µL of 10% FBS-DMEM. At 24 hours post incubation, the non-invaded cells were removed with cotton swabs, and the invaded cells in the lower surface were fixed with 10% methanol and stained with 0.1% crystal violet (Solarbio Co., Ltd, Beijing, China) for 8 min. A similar procedure was carried out to test cell migration ability with no pre-coated transwell chambers. Pictures were taken under a microscope with a magnification of ×200, and the invaded cells were counted. Five randomly selected fields were recorded for each well.
Luciferase Gene Reporter Assay
With the help of miRDB online software (http://mirdb.org/), MNX1-AS1 is shown to be a predicted target of miR-744-5p. BCL9 is predicted as a target of miR-744-5p using the starBase online software (http://starbase.sysu.edu.cn/). The wild-type (wt) and mutant (mut) sequences of MNX1-AS1/BCL9 DNA were inserted into the pGL3-control vector (Promega, Madison, Wisconsin, USA). After that, HEK-293 T cells were co-transfected with miR-744-5p mimics or NC mimic and MNX1-AS1/BCL9-wt or MNX1-AS1/BCL9-mut using Lipofectamine 3000 reagent (Invitrogen, USA). Following 48 hours of transfection, cells were harvested and submitted to detection of the luciferase activity using the Dual-Luciferase Reporter Assay System (Promega, USA).
Xenograft Models
Male BALB/c nude mice (six-week-old) obtained from the Guangdong Medical Laboratory Animal Center (Guangdong, China) were used in this study. A total of 12 mice were randomly divided into shRNA and shMNX1-AS1 groups, with 6 mice in each group. A total of 3×106 TU686 cells with shRNA or shMNX1-AS1 stable expression suspended with 100 µL PBS were injected into the armpits of mice. After 5 weeks of cell injection, mice were euthanized and the tumors were collected. In addition, 1×106 TU686 cells with MNX1-AS1 stable overexpression or without were also injected into the armpits of mice to assess MNX1-AS1 upregulation on cell tumorigenesis. The protocols involving mice were in accordance with the Ethic Committee of the First Affiliated Hospital, Huzhou University, the First People’s Hospital of Huzhou (China).
Immunohistochemistry
Following taking pictures and weighing, tumors from one side of the mice were submitted for qRT-PCR and tumors from the other side was fixed by using 4% formalin and embedded in paraffin. Sections were then cut into 4 µm slides and submitted to immunohistochemistry experiment. After removing the endogenous peroxides, the slides were incubated overnight at 4°C with diluted specific antibodies, including anti-BCL9 antibody (No. PA5-93229), anti-Ki-67 antibody (No. PA5-19462) and anti-β-catenin antibody (No. PA5-16762), all purchased from Thermo Fisher Scientific (USA). After that, the slides were immersed in HRP-conjugated secondary antibodies at 37°C for 1 hour, following by counterstaining with a 3,3-diaminobenzidine solution (Solarbio, China) for 3 min. Then, the nuclear was stained with hematoxylin.
Statistical Analysis
Data are expressed as mean±standard deviation (SD) from ≥3 times experiments. Statistical analyses were performed using SPSS software (version 21.0, SPSS Inc., Chicago, IL, USA). Qualitative data were analyzed by the chi-square test. Correlations between the expression levels of miR-744-5p and BCL9/MNX1-AS1 were determined by Pearson correlation analysis. Two-side t tests and one-way ANOVA with Bonferroni post-hoc tests were used to compare differences between two and ≥ 3 groups. The value of P < 0.05 was considered as statistically significant.
Results
MNX1-AS1 is Upregulated in Human LSCC Tissues and Links to Advanced Clinical Process and Poor Prognosis
First, we determined the expression patterns of MNX1-AS1 in human LSCC tissues and the adjacent normal tissues. As detected by the qRT-PCR, MNX1-AS1 level was significantly elevated in LSCC tissues as compared with the normal tissues (Fig. 1A), with higher levels in advanced stages (Fig. 1B). In addition, MNX1-AS1 levels in tissues from lymphatic metastasis patients were significantly higher than those without lymphatic metastasis (Fig. 1C). Compared with MNX1-AS1 low expression group, the overall survival rate in MNX1-AS1 high expression group was decreased (Fig. 1D). Moreover, we analyzed the relationship between MNX1-AS1 expression levels and the clinicopathologic features of patients with LSCC. As shown in Table 2, MNX1-AS1 level was positively associated with the T stage (P = 0.009), lymphatic metastasis (P = 0.000) and clinical stage (P = 0.000). Overall, these above results showed that MNX1-AS1 was upregulated in human LSCC tissues, which was closely related to the advanced clinical process and poor prognosis of LSCC.
Figure 1.
High level of MNX1-AS1 associated with poor prognosis in LSCC. (A) QRT-PCR was used to detect MNX1-AS1 levels in human LSCC tissues and the paired normal tissues (n = 65, **P < 0.01, compared with normal group). (B) QRT-PCR was used to detect MNX1-AS1 levels in human lscc tissues with different stages, (n = 22, I group; n = 22, II group; n = 12, III group; n = 9, IV group; **P < 0.01, compared with stage I group). (C) MNX1-AS1 levels in human LSCC tissues with or without lymphatic metastasis (n = 48, no group, n = 17, yes group; **P < 0.01, compared with no group). (D) Kaplan-Meier Survival Curves with log rank tests were used to determine the effect of MNX1-AS1 expression levels on the overall survival of LSCC patients. LSCC: laryngeal squamous cell carcinoma; qRT-PCR: Quantitative Real Time PCR.
Table 2.
The Jaspar Website Predicted the Presence of SP1 Binding Sites on the Mnx1-AS1 Promoter.
| Matrix ID | Name | Score | Relative score | Sequence ID | Start | End | Strand | Predicted sequence |
|---|---|---|---|---|---|---|---|---|
| MA0079.3 | SP1 | 17.3958 | 0.999999996047 | NC_000007.14:157008857-157011057 | 1941 | 1951 | + | GCCCCGCCCCC |
| MA0079.3 | SP1 | 14.6752 | 0.965770927897 | NC_000007.14:157008857-157011057 | 1684 | 1694 | + | GCCCCGCCCCG |
| MA0079.3 | SP1 | 11.5405 | 0.926332815414 | NC_000007.14:157008857-157011057 | 87 | 97 | - | TCCCCACCCCA |
| MA0079.1 | SP1 | 9.58261 | 0.921119537438 | NC_000007.14:157008857-157011057 | 899 | 908 | + | TCGGCGGGGT |
| MA0079.3 | SP1 | 11.1204 | 0.921047809752 | NC_000007.14:157008857-157011057 | 2053 | 2063 | + | CGCCCTCCCCT |
| MA0079.3 | SP1 | 10.7022 | 0.915786524757 | NC_000007.14:157008857-157011057 | 2049 | 2059 | + | TGCCCGCCCTC |
| MA0079.3 | SP1 | 10.7019 | 0.915783429192 | NC_000007.14:157008857-157011057 | 1876 | 1886 | - | GGCCCTCCCCG |
| MA0079.1 | SP1 | 9.30469 | 0.911425278761 | NC_000007.14:157008857-157011057 | 1585 | 1594 | + | GGGGCTCGGT |
| MA0079.1 | SP1 | 9.07176 | 0.903300263217 | NC_000007.14:157008857-157011057 | 1996 | 2005 | + | CGGGCGGGGA |
| MA0079.3 | SP1 | 9.45802 | 0.900133367416 | NC_000007.14:157008857-157011057 | 256 | 266 | - | TGCCCTCCTCT |
MNX1-AS1 Promotes the Viability, Migration and Invasion of LSCC Cells
Next, we explored the effects of MNX1-AS1 on cell growth, migration and invasion in LSCC with loss/gain-of-function assays. MNX1-AS1 expression was significantly increased following cell transfection with MNX1-AS1, while decreased after cell infection with sh#1MNX1-AS1 and sh#2MNX1-AS1 in AMC-HN-8 (Fig. 2A) and TU686 cells (Fig. 2B). Sh#1MNX1-AS1 was used in the following assays as it showed the highest knockdown efficiency. Upregulation of MNX1-AS1 significantly enhanced cell viability while downregulation of MNX1-AS1 inhibited cell viability (Fig. 2C, D). In addition, upregulation of MNX1-AS1 induced significantly increases in cell migration and invasion abilities, while knockdown of MNX1-AS1 induced an opposite result in both AMC-HN-8 and TU686 cells (Fig. 2C, D). These results demonstrated that MNX1-AS1 positively regulated cell viability, migration and invasion in LSCC cells.
Figure 2.
MNX1-AS1 promoted LSCC cell viability, migration, and invasion. (A) MNX1-AS1 expression levels following cell transfections with control, MNX1-AS1, shRNA, sh#1MNX1-AS1, and sh#2MNX1-AS1 were determined by qRT-PCR. (B) MTT assay was used to detect cell viability. (C, D) Transwell chambers were applied to test cell migration and invasion abilities. (n = 3, ** P < 0.01, Compared with control group; ## P < 0.01, compared with shRNA group). qRT-PCR: Quantitative Real Time PCR.
MNX1-AS1 Targets miR-744-5p in LSCC Cells
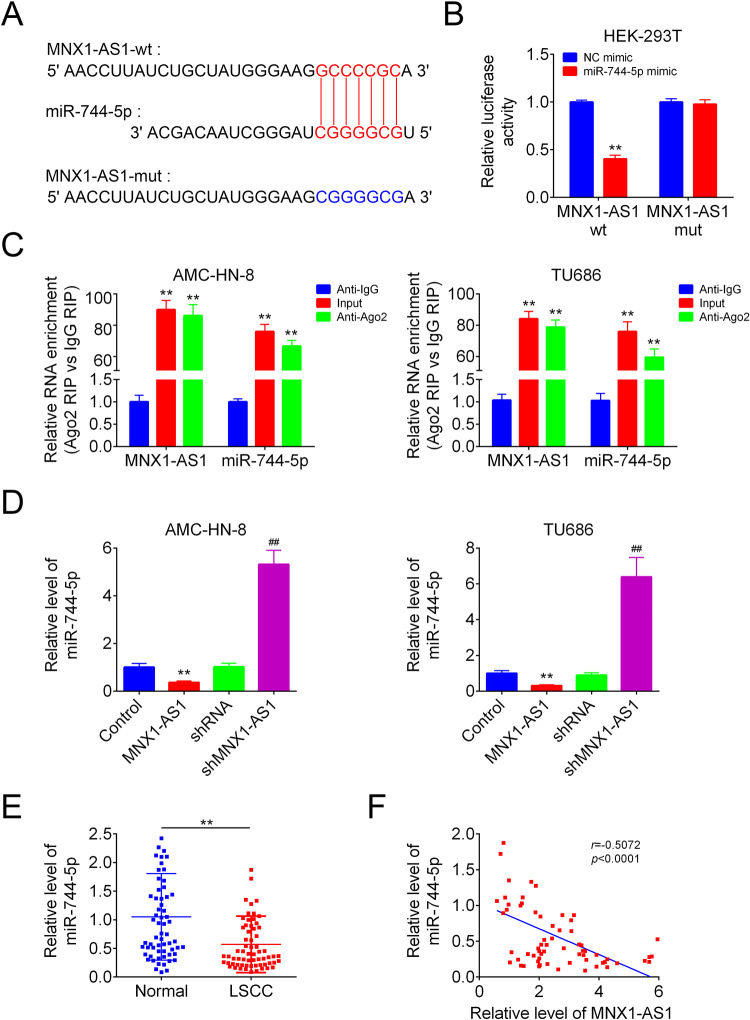
Then, we determined whether miR-744-5p was a target of MNX1-AS1. Fig. 3A displayed the putative binding sites among miR-744-5p and MNX1-AS1 predicted by miRDB online software, as well as the mutative type within the binding sites. Upregulation of miR-744-5p with mimic transfection impaired the luciferase activity of MNX1-AS1-wt vector significantly, while this tendency was abolished following cell transfection with the MNX1-AS1-mut vector in HEK-293 T cells, as detected by the luciferase gene reporter assay (Fig. 3B). Similarly, the RIP assay also revealed that MNX1-AS1 could bind to miR-744-5p in both AMC-HN-8 and TU686 cells (Fig. 3C). In addition, overexpression of MNX1-AS1 induced an apparent decrease in miR-744-5p level, while downregulation of MNX1-AS1 enhanced miR-744-5p level in AMC-HN-8 and TU686 cells (Fig. 3D). Furthermore, we observed a reduction in miR-744-5p level in human LSCC tissues as compared with the non-tumor tissues (Fig. 3E), which showed a negative correlation with MNX1-AS1 level in LSCC tissues (Fig. 3F). These findings uncovered that miR-744-5p was a direct target of MNX1-AS1 in LSCC cells.
Figure 3.
MNX1-AS1 targeted miR-744-5p. (A) The putative binding sites between miR-744-5p and MNX1-AS1. (B) The luciferase activity of MNX1-AS-wt/mut was detected by luciferase gene reporter assay (n = 3, **P < 0.01, compared with NC mimic group). (C) RIP assay was used to detect the link of MNX1-AS1 and miR-744-5p (n = 3, ** P < 0.01, compared with anti-IgG group). (D) QRT-PCR was applied to analysis the expression of miR-744-5p in different groups (n = 3, ** P < 0.01, compared with control group; ## P < 0.01, compared with shRNA group). (E) QRT-PCR was used to detect miR-744-5p levels in human LSCC tissues and the paired normal tissues (n = 65, ** P < 0.01, compared with normal group). (F) correlations between the expression levels of miR-744-5p and MNX1-AS1 in 65 cases of human LSCC tissues were determined by Pearson Correlation Analysis. LSCC: laryngeal squamous cell carcinoma; qRT-PCR: Quantitative Real Time PCR.
MNX1-AS1 Positively Regulates BCL9 Expression Via Targeting miR-744-5p in LSCC Cells
Next, we explored the relationship between miR-744-5p and BCL9. The putative binding sites between miR-744-5p and BCL9 were shown in Fig. 4A. Overexpression of miR-744-5p significantly decreased the luciferase activity of BCL9-wt, while mutation of the binding sites abrogated this effect, as detected by using the luciferase gene reporter assay in HEK-293 T cells (Fig. 4B). The western blotting result showed that miR-744-5p overexpression decreased the protein level of BCL9 in AMC-HN-8 and TU686 cells significantly (Fig. 4C), and rescued MNX1-AS1-mediated increase in BCL9 expression level (Fig. 4D). In addition, we detected BCL9 expression levels in human LSCC tissues and the adjacent normal tissues. As shown in Fig. 4E, BCL9 expression was significantly increased in human LSCC tissues as compared with the normal group. And, BCL9 level was positively correlated with MNX1-AS1 level in LSCC tissues, while negatively correlation with miR-744-5p level (Fig. 4F). These results indicated that MNX1-AS1 positively regulated BCL9 expression via targeting miR-744-5p in LSCC cells.
Figure 4.
MNX1-AS1 increased BCL9 expression via targeting miR-744-5p. (A) The putative binding sites between miR-744-5p and BCL9. (B) The luciferase activity of BCL9-wt/mut was detected by luciferase gene reporter Assay (n = 3, **P < 0.01, compared with NC mimic group). (C) BCL9 protein levels in NC mimic or miR-744-5p mimic transfected cells were detected by using Western blotting (n = 3, **P < 0.01, compared with NC mimic group). (D) BCL9 protein levels in AMC-HN-8 and TU686 cells in control+NC mimic, MNX1-AS1+NC mimic and MNX1-AS1+miR-744-5p mimic groups were detected by Western blotting (n = 3, ** P < 0.01, compared with control+NC mimic group; ## P < 0.01, compared with mnx1-as1+nc mimic group). (E) QRT-PCR was used to detect the mRNA level of BCL9 in human LSCC tissues and the paired normal tissues (n = 65, ** P < 0.01, compared with normal group). (F) Correlations between the expression levels of miR-744-5p and BCL9/MNX1-AS1 in 65 cases of human LSCC tissues were determined by Pearson Correlation Analysis.
Knockdown of MNX1-AS1 Inhibits Cell Viability, Migration and Invasion Via Decreasing BCL9 Expression in LSCC Cells
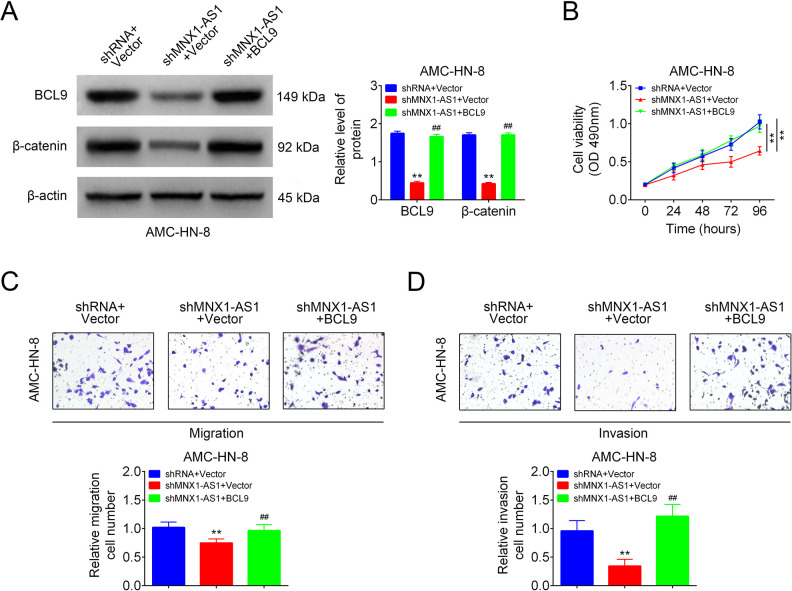
We then explored the roles of MNX1-AS1/BCL9 in LSCC cell viability, migration and invasion in AMC-HN-8. BCL9 and β-catenin expressions were decreased following cell infection with shMNX1-AS1, while increased after cell transfection with BCL9 vector (Fig. 5A). BCL9 overexpression neutralized MNX1-AS1 downregulation-mediated repressions in cell viability (Fig. 5B), migration (Fig. 5C) and invasion (Fig. 5D) in AMC-HN-8 cells. These findings suggested that MNX1-AS1 downregulation inhibited cell viability, migration and invasion via decreasing BCL9 expression in LSCC cells
Figure 5.
MNX1-AS1 inhibited LSCC cell viability, migration and invasion via inhibiting BCL9/β-catenin signaling. AMC-HN-8 cells in shRNA+vector group, shMNX1-AS1+vector group and shMNX1-AS1+BCL9 groups were submitted to the following assays. (A) Western blotting technology used for the detection of BCL9 and β-catenin expression levels (n = 3, **P < 0.01, compared with shRNA+vector group; ##P < 0.01, compared with shMNX1-AS1+vector group). (B) MTT assay for cell viability detection (n = 3, ** P < 0.01, compared with shRNA+vector group). (C, D) Transwell chambers used for cell migration and invasion detection (n = 3, ** P < 0.01, compared with shRNA+vector group; ## P < 0.01, compared with shMNX1-AS1+vector group). LSCC: laryngeal squamous cell carcinoma.
Knockdown of MNX1-AS1 Impairs Cell Tumorigenesis in LSCC Cells
Moreover, we also explored MNX1-AS1 role in LSCC progression in vivo. In comparison with the shRNA group, tumor volume and weight were significantly decreased in shMNX1-AS1 group (Fig. 6A, B). In addition, the levels of MNX1-AS1, BCL9, Ki-67 and β-catenin were decreased and miR-744-5p level was increased in shMNX1-AS1 group as compared with the shRNA group (Fig. 6C, D). However, overexpression of MNX1-AS1 enhanced cell tumorigenesis with increases in tumor volume and weight (Supplemental Fig. S1). These results confirmed that knockdown of MNX1-AS1 inhibited cell tumorigenesis in LSCC cells via targeting miR-744-5p/BCL9 axis.
Figure 6.
Knockdown of MNX1-AS1 inhibited cell tumorigenesis in LSCC cells. (A) Tumor shapes in shRNA and shMNX1-AS1 groups. (B) Tumor volumes and weights in shRNA and shMNX1-AS1 groups. (C) The levels of miR-744-5p, MNX1-AS1 and BCL9 mRNA were detected by using qRT-PCR assay in tumor tissues from the shRNA and shMNX1-AS1 groups. (D) Immunohistochemistry technology was used to detect the protein levels of BCL9, Ki-67 and β-catenin in tumor tissues from the shRNA and shMNX1-AS1 groups. (** P < 0.01, compared with shRNA group.). LSCC: laryngeal squamous cell carcinoma.
Discussion
Many lncRNAs have been identified to be involved in the progression of LSCC, such as UCA121, SNHG2022, XIST (X inactivate-specific transcript)23 and PTCSC324. In the current paper, we explored the role of MNX1-AS1 in the progression of LSCC. The results demonstrated that MNX1-AS1 was overexpressed in human LSCC tissues as compared to the normal tissues, and served as an oncogene in LSCC through activating the BCL9/β-catenin signaling by targeting miR-744-5p.
Up to now, MNX1-AS1 roles in several kinds of cancers were disclosed. For instance, Lv et al 9 reported that higher level of MNX1-AS1 was closely associated with International Federation of Gynecology and Obstetrics stage and lymphatic metastasis of ovarian cancer, and knockdown of MNX1-AS1 caused significant inhibitions in cell proliferation and migration capacities. Liu et al13 demonstrated that MNX1-AS1 facilitated cervical cancer cell proliferation and inhibited apoptosis through activating MAPK pathway. MNX1-AS1 also serves as an oncogene in gastric cancer25, non-small cell lung cancer (NSCLC)12, prostate cancer15, breast cancer14, and glioblastoma26. Similar to the expression pattern and role of MNX1-AS1 in other cancers, a significant increase in MNX1-AS1 expression was also observed in human LSCC tissues as compared with the normal tissues, which was linked to higher clinic stage and lymphatic metastasis. In addition, upregulation of MNX1-AS1 led to apparent increases in cell viability, migration and invasion abilities, while knockdown of MNX1-AS1 repressed cell viability, migration, invasion and tumorigenesis in LSCC, indicating MNX1-AS1 plays an oncogenic role in LSCC.
Similar to lncRNAs, miRNAs are also demonstrated to be closely involved in carcinogenesis27. Researchers have reported that miR-744-5p is downregulated in multiple types of cancers, such as ovarian cancer28,29, NSCLC30, cervical cancer31, colorectal cancer32, and glioblastoma33, and serves as a tumor suppressor. Here, we first demonstrated that miR-744-5p was also lowly expressed in human LSCC tissues in comparison with the normal tissues, and showed a negative correlation with MNX1-AS1 level. In addition, MNX1-AS1 could direct target miR-744-5p and reduced its expression levels in both LSCC AMC-HN-8 and TU686 cell lines.
Afterwards, we first confirmed that miR-744 directly targeted BCL9 in LSCC. The human BCL9 gene was first found in lymphoblastic leukemia cells34. As the study went on, BCL9 is identified as a co-activator of β-catenin transcription that plays important roles in carcinogenesis of multiple kind of human cancers, including LSCC and is considered as a therapeutic target for cancer35,36. Overexpression of BCL9 has been found in many cancers, resulting in the aberrant activation of Wnt/β-catenin signaling and the subsequently malignant process of tumors, like hepatocellular carcinoma37 and adrenocortical carcinoma38. The current study revealed that BCL9 expression level was increased in human LSCC tissues, and was positively correlated with MNX1-AS1 level and negatively correlated with miR-744-5p level in LSCC. Using the luciferase gene reporter and western blotting assays, we demonstrated that MNX1-AS1 increased BCL9 and β-catenin expression via sponging miR-744-5p. Moreover, overexpression of BCL9 rescued the repressions in cell viability, migration and invasion abilities induced by MNX1-AS1 downregulation in LSCC.
We also assessed MNX1-AS1 effect on the viability of human oral epithelial cells (HOEC), and the results showed that MNX1-AS1 overexpression increased HOEC viability and downregulation of MNX1-AS1 inhibited cell viability (Supplemental Fig. S2A). The results from the current study provides a reasonable theoretical basis and possible molecular markers for the diagnosis and treatment of LSCC. However, a lot of experiments should to be done for the application of MNX1-AS1 in clinical treatment of LSCC. Another main limitation of this study is that we don’t explore the mechanism underlying MNX1-AS1 dysregulation in LSCC. It has been reported that E2F1 and TEAD4 can bind to the promoter region of MNX1-AS1 and promote its transcription39,40. In the present study, we predicted that SP1 can also bind to the promoter region of MNX1-AS1 with high score using the JASPAR prediction tool (http://jaspar.genereg.net/). In addition, the qRT-PCR result showed that MNX1-AS1 level was increased following E2F1, TEAD4 and SP1 overexpression LSCC cells AMC-HN-8 and TU686 (Supplemental Fig. S2B-C), suggesting that the upregulation of MNX1-AS1 in LSCC may be caused by E2F1, TEAD4, and SP1. However, the relationship between E2F1, TEAD4, SP1, and MNX1-AS1 should be further verified in our further study, as well as their role in LSCC progression.
In conclusion, this study reveals that MNX1-AS1 is overexpressed in LSCC, which contributes to LSCC progression by regulating miR-744-5p/BCL9/β-catenin axis, as shown in Fig. 7. Our study demonstrates that MNX1-AS1 might be a potential biomarker for the diagnosis and prognosis prediction, and a therapeutic target of LSCC.
Figure 7.
The schematic diagram of this study. MNX1-AS1 promotes LSCC cell growth and migration through targeting miR-744-5p to activate the BCL9/β-catenin signaling. LSCC: laryngeal squamous cell carcinoma.
Supplemental Material
Supplemental Material, sj-jpg-1-cll-10.1177_09636897211005682 for LncRNA MNX1-AS1 Contributes to Laryngeal Squamous Cell Carcinoma Growth and Migration by Regulating mir-744-5p/bcl9/β-Catenin Axis by Bingliang Ma, Gang Ren, Jue Xu, Chenyi Yin and Yuye Shi in Cell Transplantation
Supplemental Material, sj-jpg-2-cll-10.1177_09636897211005682 for LncRNA MNX1-AS1 Contributes to Laryngeal Squamous Cell Carcinoma Growth and Migration by Regulating mir-744-5p/bcl9/β-Catenin Axis by Bingliang Ma, Gang Ren, Jue Xu, Chenyi Yin and Yuye Shi in Cell Transplantation
Footnotes
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Authors’ contributions: Bingliang Ma and Ren Gang designed the study, supervised the data collection, analyzed the data, Jue Xu interpreted the data and prepare the manuscript for publication, Chenyi Yin and Yuye Shi supervised the data collection, analyzed the data and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Ethics approval: Ethical approval was obtained from the Ethics Committee of the First Affiliated Hospital, Huzhou University, the First People’s Hospital of Huzhou.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Animal Ethics Committee of the First Affiliated Hospital, Huzhou University, the First People’s Hospital of Huzhou approved protocols.
Statement of Informed Consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Foundation of Huzhou Bureau of Science and Technology (Grant No. 2018GYB78).
ORCID iD: Gang Ren  https://orcid.org/0000-0002-0107-0524
https://orcid.org/0000-0002-0107-0524
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer. 2005;5(2):127–135. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3. Wei Q, Yu D, Liu M, Wang M, Zhao M, Jia W, Ma H, Fang J, Xu W, Chen K, Xu Z, et al. Genome-wide association study identifies three susceptibility loci for laryngeal squamous cell carcinoma in the Chinese population. Nat Genet. 2014;46(10):1110–1114. [DOI] [PubMed] [Google Scholar]
- 4. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. [DOI] [PubMed] [Google Scholar]
- 5. Kong X, Liu W, Kong Y. Roles and expression profiles of long non-coding RNAs in triple-negative breast cancers. J Cell Mol Med. 2018;22(1):390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang XZ, Liu H, Chen SR. Mechanisms of long non-coding rnas in cancers and their dynamic regulations. Cancers (Basel). 2020;12(5):1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malissovas N, Ninou E, Michail A, Politis PK. Targeting long non-coding RNAS in nervous system cancers: new insights in prognosis, diagnosis and therapy. Curr Med Chem. 2019;26(30):5649–5663. [DOI] [PubMed] [Google Scholar]
- 8. Zhang W, Huang L, Lu X, Wang K, Ning X, Liu Z. Upregulated expression of MNX1-AS1 long noncoding RNA predicts poor prognosis in gastric cancer. Bosn J Basic Med Sci. 2019;19(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lv Y, Li H, Li F, Liu P, Zhao X. Long noncoding RNA MNX1-AS1 knockdown inhibits cell proliferation and migration in ovarian cancer. Cancer Biother Radiopharm. 2017;32(3):91–99. [DOI] [PubMed] [Google Scholar]
- 10. Li AH, Zhang HH. Overexpression of lncRNA MNX1-AS1 is associated with poor clinical outcome in epithelial ovarian cancer. Eur Rev Med Pharmacol Sci. 2017;21(24):5618–5623. [DOI] [PubMed] [Google Scholar]
- 11. Liu H, Han L, Liu Z, Gao N. Long noncoding RNA MNX1-AS1 contributes to lung cancer progression through the miR-527/BRF2 pathway. J Cell Physiol. 2019;234(8):13843–13850. [DOI] [PubMed] [Google Scholar]
- 12. Liu G, Guo X, Zhang Y, Liu Y, Li D, Tang G, Cui S. Expression and significance of LncRNA MNX1-AS1 in non-small cell lung cancer. Onco Targets Ther. 2019;12:3129–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu X, Yang Q, Yan J, Zhang X, Zheng M. LncRNA MNX1-AS1 promotes the progression of cervical cancer through activating MAPK pathway. J Cell Biochem. 2019;120(3):4268–4277. [DOI] [PubMed] [Google Scholar]
- 14. Cheng Y, Pan Y, Wang O. MNX1-AS1 is a functional oncogene that induces EMT and activates the AKT/mTOR pathway and MNX1 in breast cancer. Cancer Manag Res. 2019;11:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Z, Wang F, Zhang S. Knockdown of lncRNA MNX1-AS1 suppresses cell proliferation, migration, and invasion in prostate cancer. FEBS Open Bio. 2019;9(5):851–858. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, Francis SE, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126(7):2495–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li C, Wan L, Liu Z, Xu G, Wang S, Su Z, Zhang Y, Zhang C, Liu X, Lei Z, Zhang HT. Long non-coding RNA XIST promotes TGF-beta-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018;418:185–195. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Xing H, Nikzad AA, Liu B, Zhang Y, Li S, Zhang E, Jia Z. Long noncoding RNA MNX1 antisense RNA 1 exerts oncogenic functions in bladder cancer by regulating miR-218-5p/RAB1A Axis. J Pharmacol Exp Ther. 2020;372(3):237–247. [DOI] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 20. Kuwano Y, Nishida K, Kajita K, Satake Y, Akaike Y, Fujita K, Kano S, Masuda K, Rokutan K. Transformer 2beta and miR-204 regulate apoptosis through competitive binding to 3’ UTR of BCL2 mRNA. Cell Death Differ. 2015;22(5):815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun S, Gong C, Yuan K. LncRNA UCA1 promotes cell proliferation, invasion and migration of laryngeal squamous cell carcinoma cells by activating Wnt/beta-catenin signaling pathway. Exp Ther Med. 2019;17(2):1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Xu J, Guo YN, Yang BB. LncRNA SNHG20 promotes the development of laryngeal squamous cell carcinoma by regulating miR-140. Eur Rev Med Pharmacol Sci. 2019;23(8):3401–3409. [DOI] [PubMed] [Google Scholar]
- 23. Liu C, Lu Z, Liu H, Zhuang S, Guo P. LncRNA XIST promotes the progression of laryngeal squamous cell carcinoma via sponging miR-125b-5p to modulate TRIB2. Biosci Rep. 2020;40(4):BSR20193172. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Xiao D, Cui X, Wang X. LncRNA PTCSC3 inhibits cell proliferation in laryngeal squamous cell carcinoma by down-regulating lncRNA HOTAIR. Biosci Rep. 2019;39(6):BSR20182362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma JX, Yang YL, He XY, Pan XM, Wang Z, Qian YW. Long noncoding RNA MNX1-AS1 overexpression promotes the invasion and metastasis of gastric cancer through repressing CDKN1A. Eur Rev Med Pharmacol Sci. 2019;23(11):4756–4762. [DOI] [PubMed] [Google Scholar]
- 26. Gao Y, Xu Y, Wang J, Yang X, Wen L, Feng J. lncRNA MNX1-AS1 promotes glioblastoma progression through inhibition of miR-4443. Oncol Res. 2019;27(3):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tam C, Wong JH, Tsui SKW, Zuo T, Chan TF, Ng TB. LncRNAs with miRNAs in regulation of gastric, liver, and colorectal cancers: updates in recent years. Appl Microbiol Biotechnol. 2019;103(12):4649–4677. [DOI] [PubMed] [Google Scholar]
- 28. Kleemann M, Schneider H, Unger K, Sander P, Schneider EM, Fischer-Posovszky P, Handrick R, Otte K. MiR-744-5p inducing cell death by directly targeting HNRNPC and NFIX in ovarian cancer cells. Sci Rep. 2018;8(1):9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao LG, Wang J, Li J, Li QF. miR-744-5p inhibits cellular proliferation and invasion via targeting ARF1 in epithelial ovarian cancer. Kaohsiung J Med Sci. 2020;36(10):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen S, Shi F, Zhang W, Zhou Y, Huang J. miR-744-5p inhibits non-small cell lung cancer proliferation and invasion by directly targeting PAX2. Technol Cancer Res Treat. 2019;18:1533033819876913. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Chen XF, Liu Y. MicroRNA-744 inhibited cervical cancer growth and progression through apoptosis induction by regulating Bcl-2. Biomed Pharmacother. 2016;81:379–387. [DOI] [PubMed] [Google Scholar]
- 32. Shen J, Li M. MicroRNA-744 inhibits cellular proliferation and invasion of colorectal cancer by directly targeting oncogene notch1. Oncol Res. 2018;26(9):1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng Y, Li Y, Fang Q, Luo H, Zhu G. microRNA-744 is downregulated in glioblastoma and inhibits the aggressive behaviors by directly targeting NOB1. Am J Cancer Res. 2018;8(11):2238–2253. [PMC free article] [PubMed] [Google Scholar]
- 34. Willis TG, Zalcberg IR, Coignet LJ, Wlodarska I, Stul M, Jadayel DM, Bastard C, Treleaven JG, Catovsky D, Silva ML, Dyer MJ. Molecular cloning of translocation t(1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21. Blood. 1998;91(6):1873–1881. [PubMed] [Google Scholar]
- 35. Yang N, Wang Y, Hui L, Li X, Jiang X. SOX 1, contrary to SOX 2, suppresses proliferation, migration, and invasion in human laryngeal squamous cell carcinoma by inhibiting the Wnt/beta-catenin pathway. Tumour Biol. 2015;36(11):8625–8635. [DOI] [PubMed] [Google Scholar]
- 36. Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109(1):47–60. [DOI] [PubMed] [Google Scholar]
- 37. Yang C, Xu Y, Cheng F, Hu Y, Yang S, Rao J, Wang X. miR-1301 inhibits hepatocellular carcinoma cell migration, invasion, and angiogenesis by decreasing Wnt/beta-catenin signaling through targeting BCL9. Cell Death Dis. 2017;8(8):e2999. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Brown TC, Nicolson NG, Korah R, Carling T. BCL9 Upregulation in adrenocortical carcinoma: a novel wnt/beta-catenin activating event driving adrenocortical malignancy. J Am Coll Surg. 2018;226(6):988–995. [DOI] [PubMed] [Google Scholar]
- 39. Ye Y, Gu B, Wang Y, Shen S, Huang W. E2F1-mediated MNX1-AS1-miR-218-5p-SEC61A1 feedback loop contributes to the progression of colon adenocarcinoma. J Cell Biochem. 2019;120(4):6145–6153. [DOI] [PubMed] [Google Scholar]
- 40. Shuai Y, Ma Z, Liu W, Yu T, Yan C, Jiang H, Tian S, Xu T, Shu Y. TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Mol Cancer. 2020;19(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-jpg-1-cll-10.1177_09636897211005682 for LncRNA MNX1-AS1 Contributes to Laryngeal Squamous Cell Carcinoma Growth and Migration by Regulating mir-744-5p/bcl9/β-Catenin Axis by Bingliang Ma, Gang Ren, Jue Xu, Chenyi Yin and Yuye Shi in Cell Transplantation
Supplemental Material, sj-jpg-2-cll-10.1177_09636897211005682 for LncRNA MNX1-AS1 Contributes to Laryngeal Squamous Cell Carcinoma Growth and Migration by Regulating mir-744-5p/bcl9/β-Catenin Axis by Bingliang Ma, Gang Ren, Jue Xu, Chenyi Yin and Yuye Shi in Cell Transplantation