Abstract
POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome is rare, with polyneuropathy and monoclonal plasma cell disorder generally considered as essential diagnostic symptoms. We report two cases of POEMS syndrome without monoclonal protein expression. The first case was a 72-year-old man who had experienced recurrent edema of the lower limbs for 2 years and abdominal distention for 2 months. The other case was a 62-year-old man with a 5-year history of recurrent numbness of the extremities and muscle weakness, which had become serious over the preceding 3 months. Both patients had various symptoms that matched those of POEMS syndrome, but neither had monoclonal protein expression. However, a diagnosis of POEMS syndrome was made in each case. Both patients were treated with lenalidomide and dexamethasone, after which their symptoms improved and laboratory test results normalized. The findings in these two cases suggest the possibility that POEMS syndrome may occur without monoclonal protein expression. The diagnostic criteria of POEMS syndrome may thus need further investigation.
Keywords: POEMS syndrome, atypical, monoclonal protein, case report, diagnostic criteria, symptom
Introduction
POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome is a rare syndrome characterized by the presence of monoclonal plasma cell disorder, multiple peripheral neuropathy, and one or more of the following features: organomegaly, endocrinopathy, typical skin changes, edema, papilledema, osteosclerotic myeloma, Castleman disease, and increased serum level of vascular endothelial growth factor (VEGF).1 Polyneuropathy and monoclonal plasma cell disorder are generally considered essential for the diagnosis of POEMS syndrome.2 In the current study however, we report on two cases of POEMS syndrome without monoclonal protein (M protein) expression.
Case reports
A 72-year-old man had experienced abdominal distention and edema of the lower limbs for 2 years and was subsequently hospitalized in 2016. Skin pigmentation was noted on the ankles, and later on the dorsum of the hands. The patient developed massive ascites 2 months prior to the current hospital admission. The ascites appeared to be exudative and the symptoms recurred several times, despite intermittent diuretic therapy. The cause of the ascites could not be determined, even after laparoscopic exploration. The patient also experienced paresthesia and numbness in both hands and loss of physical strength before admission to our hospital.
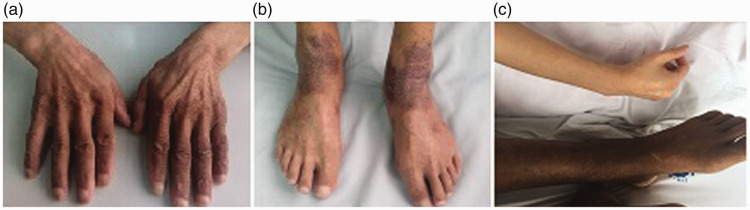
Examinations performed at hospital admission revealed bean-sized lymph nodes in both sides of the neck, clavicle, armpit, and groin, a distended abdomen, obvious pitting edema in the lower limbs, and the dorsa of the hands and ankles were dark (Figure 1).
Figure 1.
Pigment deposition. (a, b) Pigment deposition in the dorsum of hands and ankles in case 1. (c) Pigment deposition in the lower limbs in case 2 (upper, healthy female; lower, patient limbs)
The patient showed hypothyroidism, with thyroid-stimulating hormone, free triiodothyronine, and free thyroxine levels of 8.52 (normal range: 0.35–4.94 mIU/L), 1.55 (normal range: 2.63–5.70 pg/mL), and 0.63 (normal range: 0.7–1.48 ng/dL), respectively. His testosterone level was 0.42 (normal range: 1.75–7.81 µg/L) and pituitary prolactin level was 15.58 (normal range: 2.64–13.13 µg/L). The patient was therefore considered to have hypogonadism. His cortisone levels at midnight, 8 am, and 4 pm were 5.55 (normal range: 0.00–5.00 µg/dL), 7.92 (normal range: 6.70–22.60 µg/dL), and 7.06 (normal range: 0.00–10.00 µg/dL), respectively, and his adrenocorticotropic hormone (ACTH) levels at 8 am and 4 pm were 55 (normal range: 1–80 ng/L) and 34 (normal range: 5–40 ng/L), respectively. He was therefore diagnosed with mild adrenal insufficiency. His serum VEGF level was 557.2 (normal range: 0–142 pg/mL).
B-scan ultrasonography indicated a small amount of bilateral pleural effusion, pericardial effusion, and massive effusion in the abdomen and pelvic cavity. Puncture drainage showed that the ascites was exudative. Malignancy and tuberculosis were ruled out. Radiography showed no osteolytic lesions in the skull or pelvic cavity. Echocardiography showed moderate pulmonary hypertension. Biopsy of the left supraclavicular lymph node demonstrated reactive hyperplasia. Electromyography revealed peripheral nerve damage in the upper and lower limbs (axonal degeneration and demyelination) and a significantly prolonged F-M interval, indicating decreased nerve conduction and prolonged distal latency, with a distal, symmetric, and progressive distribution and gradual proximal spread.
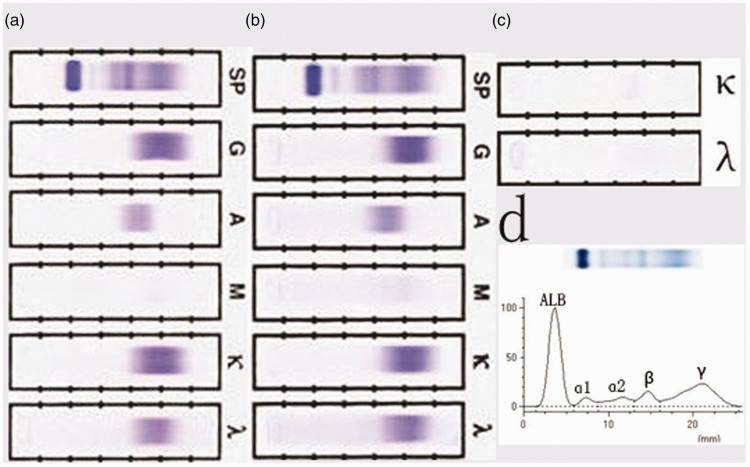
The patient’s serum immunoglobulin (Ig) G, kappa light chain, and lambda light chain levels were 2400 (normal range: 751.0–1560.0 mg/dL), 1970 (normal range: 629–1350 mg/dL), and 962 (normal range: 313–723 mg/dL), respectively, urinary Ig kappa and lambda light chain levels were 18.7 (normal: <1.85 mg/dL) and <5.00 (normal: <5.00 mg/dL), respectively, and his 24-hour urinary protein level was 250 mg. Serum and urine immunofixation and urine electrophoresis revealed no M protein expression (Figure 2). No free Ig monoclonal light chain was detected. Bone marrow aspiration and biopsy revealed hyperproliferation without plasmacytoma, and immunohistochemistry demonstrated no monoclonal hyperplasia. Amyloidosis was also ruled out based on skin and rectum biopsy findings.
Figure 2.
Lack of monoclonal protein detection. No monoclonal protein was detected by (a) serum immunofixation, (b) ascites immunofixation, (c) urine immunofixation, or (d) serum electrophoresis. SP, serum protein; G, immunoglobulin G; A, immunoglobulin A; M, immunoglobulin M; κ, kappa light chain; λ, lambda light chain; ALB, albumin
The patient had typical multiple peripheral neuropathy, as an essential diagnostic criterion of POEMS syndrome. He also demonstrated the major criterion of high serum VEGF, as well as many minor features, including extravascular volume overload, organomegaly, endocrinopathy, and typical skin changes. However, the patient lacked M protein. Other diagnoses, including Castleman disease, were ruled out because the pathology was not supported. After a multidisciplinary discussion, the patient was diagnosed with POEMS syndrome. He received levothyroxine 12.5 µg/day for hypothyroidism, hydrocortisone 20 mg every morning for adrenal insufficiency, diuretics for ascites, and mecobalamin for peripheral neuropathy. He also received six 4-week cycles of lenalidomide 10 mg for 21 days and dexamethasone 20 mg for 5 days (LD regimen) over the following 6 months. The patient’s ascites and edema improved after one cycle of the LD regimen, and both his hypothyroidism and testosterone level normalized after four cycles, even without levothyroxine sodium tablets and cortisone replacement therapy. The patient completed six cycles of the LD regimen, after which all his symptoms improved and the laboratory test results, including VEGF levels, normalized. The patient is currently undergoing follow-up.
In another case, a 62-year-old man was admitted to our hospital in February 2017 with a 5-year history of recurrent numbness in the extremities and muscle weakness. He experienced consistent tingling and paresthesia in the fingers and feet and darkening of the skin. The patient received gamma globulin for 5 days at a local hospital in 2013, followed by prednisone 55 mg/day for 1 month. His symptoms seemed to improve and the dosage of prednisone was gradually decreased to 5 mg. However, the numbness in the extremities and muscle weakness worsened again, preventing him from walking or grasping for 3 months before presenting to our hospital. He had also been diagnosed with hypothyroidism 1 year previously and took levothyroxine every day.
On admission, the patient had several bean-like lymph nodes in his left axilla, obvious pigmentation in the lower limbs (Figure 1), and mild edema in both extremities. Muscle power in his upper limbs was normal, his lower extremity proximal muscle power was level 5− and his distal muscle power was level 2. His symptoms were distal, symmetric, and progressive, with a gradual proximal spread. The patient showed a weak tendon reflex and no tibial nerve reflex. The distal limbs showed sock-like hypoalgesia. Both the finger-to-nose test and heel-knee-tibia test indicated instability. Roberg sign was positive. Electromyography revealed peripheral nerve injury in the upper and lower limbs, involving both motor and sensory sensations. The F wave was absent in both the upper and lower limbs, and the tibial nerve reflex was also lacking. The patient revealed proximal, symmetrical polyneuropathy involvement with motor and sensory sensations, consistent with demyelinating polyradiculoneuropathy.
Biochemical examination revealed renal dysfunction (creatinine, 151 µmol/L). His thyroid function was within the normal range due to levothyroxine treatment, his testosterone level was 1.31 µg/L (normal range: 1.75–7.81 µg/L), and his pituitary prolactin level was 27.03 µg/L (normal range: 2.64–13.13 µg/L). The patient was therefore considered to show hypogonadism. His cortisone and ACTH levels were slightly decreased (morning 47 ng/L [normal range: 10–80 ng/L], and afternoon 3 ng/L [normal range: 5–40 ng/L]), and an ACTH stimulation test indicated adrenal insufficiency. Unfortunately, the patient’s VEGF level was not available.
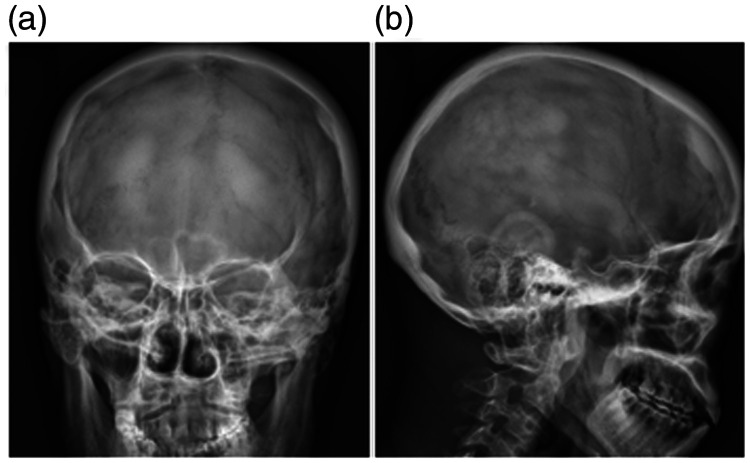
Radiography showed obvious osteosclerotic lesions in the skull (Figure 3). Other examinations revealed splenomegaly (spleen thickness 4.7 cm), and ultrasound showed minor pericardial effusion, pleural effusion, and abdominal pelvic effusion.
Figure 3.
Obvious osteosclerotic lesions in the skull in case 2
The patient underwent several serum and urine electrophoresis and immunofixation assessments, but no M protein was observed. Bone marrow aspiration and biopsy showed no plasmacytosis or monoclonal light chain. Similar findings were obtained with lymph node biopsy. Amyloidosis was also ruled out based on skin and rectum biopsy findings.
The patient had obvious polyneuropathy, as a mandatory criterion of POEMS syndrome, typical osteosclerotic lesions, endocrine disorders, skin changes, and splenomegaly. He was therefore finally diagnosed with POEMS syndrome based on one mandatory, one major, and several minor criteria. He was administered LD treatment. His nerve symptoms improved after five cycles of treatment, and his kidney, adrenal, and gonad functions all became normal. The patient’s condition remained stable during follow-up.
Both patients provided written informed consent for publication of this report.
Discussion
The acronym POEMS was suggested in the 1980s to define the disorder characterized by the following constellation of findings: polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes.3 Many additional features have been described for this syndrome, including sclerotic bone lesions, Castleman disease, elevated VEGF levels, papilledema, pleural effusion, edema, ascites, erythrocytosis, and thrombocytosis.1 However, not all the features are required to make the diagnosis. The International Myeloma Working Group criteria considered both polyneuropathy and monoclonal plasma cell proliferative disorder to be mandatory, while osteosclerotic or mixed sclerotic/lytic lesions, Castleman disease, and elevated serum or plasma VEGF levels were major criteria, and the other features were minor criteria. A diagnosis of POEMS syndrome is confirmed when patients demonstrate both mandatory major criteria, one of the three other major criteria, and one of the six minor criteria.2
Most patients in previous studies showed M protein in the serum and/or urine, and others showed clonal lambda plasma cell proliferation by immunohistochemical staining of biopsy specimens.1,4 M protein was detected in the serum in 85% of patients, with a median serum M-protein spike of 1.1 g/dL, with only 7% of patients having an M spike > 2 g/dL. In addition, 40% of the patients had M protein in the urine, with a median value of 100 mg per 24 hour, according to the Mayo Clinic series.1 The M protein detected was normally the lambda light chain type, and the associated heavy chain was mostly IgA or IgG.5
Atypical cases of POEMS syndrome have been described in the literature.6–8 Morizane et al. described a case of atypical POEMS syndrome without polyneuropathy.6 A 43-year-old woman showed M protein, elevated VEGF, organomegaly, endocrinopathy, and skin lesions, as well as typical renal and sclerotic bone lesions. However neurological examinations and peripheral nerve conduction tests were both normal, even 5 years after the onset of skin eruptions. A nationwide survey in Japan reported that 25% of 102 patients with POEMS syndrome lacked M protein4. Suichi et al. investigated 392 cases of POEMS syndrome and found that all patients showed polyneuropathy, but only 89% had monoclonal plasma cell proliferation.9 He et al. analyzed the medical documents of all patients referred to Peking Union Medical College Hospital from August 2012 to July 2017, and reviewed the clinical and laboratory features of 13 patients with atypical POEMS syndrome with undetectable monoclonal gammopathy.10 There are several possible explanations for this presentation. First, there may be non-secretory plasma cells present in POEMS syndrome, as occasionally seen in multiple myeloma. Second, levels of M protein may be too low to be detected by currently available assays, especially in the past, when sensitive assessments such as immunofixation electrophoresis were unavailable. If M protein is found to be negative by immunofixation, bone marrow biopsy, positron emission tomography, and free light chain should be tested to detect monoclonal plasma cell proliferation. Suichi et al.11 considered that the diagnosis of POEMS syndrome should be judged based on the patient’s abnormalities as a whole, especially in these unusual cases.11
Both the current cases had one mandatory, one or two major, and several minor criteria, but neither had M protein. However, based on the overwhelming number of other characteristic signs and symptoms, we made a provisional diagnosis of special POEMS syndrome without M protein in both these cases. Although the first patient’s serum VEGF levels were not high enough to diagnose POEMS syndrome, they became normal after treatment. Plasma VEGF may be useful for monitoring disease activity after treatment, and may correlate with clinical improvements better than hematologic response.12 The efficacy of the treatment also supported the diagnosis.
These two cases were considered unlikely to show Castleman disease because the lymph node pathology supported neither hyaline vascular-type nor plasma-type Castleman disease. Detailed electrophysiological analysis of the duration of distal compound muscle action potentials may help to distinguish between POEMS syndrome and Castleman disease.13 Amyloidosis was also ruled out based on skin and rectum biopsy findings.
There have been no randomized controlled clinical trials and there is currently no standard treatment for POEMS syndrome.14 Targeted radiation therapy is generally the preferred treatment option for patients with isolated bone lesions,15 while patients with widespread osteosclerotic lesions or evidence of bone marrow involvement or obvious symptoms are treated with chemotherapy followed by autologous hematopoietic cell transplantation, similar to the treatment for multiple myeloma. Suitable chemotherapy regimens include melphalan plus dexamethasone,1 and lenalidomide-,16–18 thalidomide-,15 and bortezomib-based therapies.19
Regarding the prognosis of POEMS syndrome, most patients have chronic courses, with patient survival being about three times longer than that for multiple myeloma. Overall survival in the Mayo Clinic series was about 13.7 years,1 but the median overall survival times of patients with extravascular volume overload or clubbing were only 6.6 and 2.6 years, respectively.20
Conclusion
These two case reports suggest that patients may present with special POEMS syndrome without monoclonal protein expression. However, further studies are required to determine if these patients subsequently develop monoclonal protein expression, and also to reappraise the current nomenclature and diagnostic criteria for POEMS syndrome.
Acknowledgement
We thank Dr. Xiufeng Yin for work on the graphics involved in this article.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Zhejiang Provincial Science and Technology Department of China [grant number LGF20H160024].
ORCID iD: Hua Ping Du https://orcid.org/0000-0001-5922-4111
References
- 1.Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood 2003; 101: 2496–2506. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A. POEMS syndrome. Blood Rev 2007; 21: 285–299. [DOI] [PubMed] [Google Scholar]
- 3.Bardwick PA, Zvaifler NJ, Gill GN, et al. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine (Baltimore) 1980; 59: 311–322. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi T, Sobue I, Toyokura Y, et al. The Crow-Fukase syndrome: a study of 102 cases in Japan. Neurology 1984; 34: 712–720. [DOI] [PubMed] [Google Scholar]
- 5.Abe D, Nakaseko C, Takeuchi M, et al. Restrictive usage of monoclonal immunoglobulin lambda light chain germline in POEMS syndrome. Blood 2008; 112: 836–839. [DOI] [PubMed] [Google Scholar]
- 6.Morizane R, Sasamura H, Minakuchi H, et al. A case of atypical POEMS syndrome without polyneuropathy. Eur J Haematol 2008; 80: 452–455. [DOI] [PubMed] [Google Scholar]
- 7.Ofran Y, Yishay O, Elinav E, et al. POEMS syndrome: failure of newly suggested diagnostic criteria to anticipate the development of the syndrome. Am J Hematol 2005; 79: 316–318. [DOI] [PubMed] [Google Scholar]
- 8.Charli-Joseph Y, Fernández-Sánchez M, Saeb-Lima M, et al. POEMS syndrome: are current diagnostic criteria too exclusive. J Am Acad Dermatol 2011; 65: 415–417. [DOI] [PubMed] [Google Scholar]
- 9.Suichi T, Misawa S, Beppu M, et al. Prevalence, clinical profiles, and prognosis of POEMS syndrome in Japanese nationwide survey. Neurology 2019; 93: e975–e983. doi: 10.1212/WNL.0000000000008062 [DOI] [PubMed] [Google Scholar]
- 10.He T, Zhao A, Zhao H, et al. Clinical characteristics and the long-term outcome of patients with atypical POEMS syndrome variant with undetectable monoclonal gammopathy. Ann Hematol 2019; 98: 735–743. [DOI] [PubMed] [Google Scholar]
- 11.Suichi T, Misawa S, Sato Y, et al. Proposal of new clinical diagnostic criteria for POEMS syndrome. J Neurol Neurosurg Psychiatry 2019; 90: 133–137. doi: 10.1136/jnnp-2018-318514 [DOI] [PubMed] [Google Scholar]
- 12.D'Souza A, Hayman SR, Buadi F, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood 2011; 118: 4663–4665. doi: 10.1182 [DOI] [PubMed] [Google Scholar]
- 13.Lau YH, Mohd Unit H, Lee LP, et al. Temporal dispersion in demyelination of POEMS syndrome and Castleman disease. Clin Neurophysiol Pract 2020; 5: 112–117. Published 2020 May 31. doi: 10.1016/j.cnp.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwabara S, Dispenzieri A, Arimura K, et al. Treatment for POEMS (polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes) syndrome. Cochrane Database Syst Rev 2012; 2012: CD006828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dispenzieri A. How I treat POEMS syndrome. Blood 2012; 119: 5650–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kourelis TV, Buadi FK, Kumar SK, et al. Long-term outcome of patients with POEMS syndrome: An update of the Mayo Clinic experience. Am J Hematol 2016; 91: 585–589. [DOI] [PubMed] [Google Scholar]
- 17.Vannata B, Laurenti L, Chiusolo P, et al. Efficacy of lenalidomide plus dexamethasone for POEMS syndrome relapsed after autologous peripheral stem-cell transplantation. Am J Hematol 2012; 87: 641–642. [DOI] [PubMed] [Google Scholar]
- 18.Chu BF, Shana’ah A, Hofmeister CC, et al . Long-term therapy with lenalidomide in a patient with POEMS syndrome. Eur J Case Rep Intern Med 2014; 1: 2014_000093. doi: 10.12890/2014_000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dispenzieri A. POEMS syndrome: update on diagnosis, risk-stratification, and management. Am J Hematol 2015; 90: 951–962. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood 2012; 120: 56–62. [DOI] [PubMed] [Google Scholar]