Abstract
Objective
To explore the clinical importance of the distribution of pulmonary artery embolism in acute pulmonary embolism (APE).
Methods
Sixty-four patients with APE were classified into mixed-type and distal-type pulmonary embolism groups. Their right ventricular systolic pressure (RVSP) and disease duration were recorded, and the diameter of their right ventricles was measured by ultrasound. The computed tomography angiographic clot load was determined as a Mastora score.
Results
Patients with distal-type pulmonary embolisms had significantly lower RVSPs (44.92 ± 17.04 vs 55.69 ± 17.66 mmHg), and significantly smaller right ventricular diameters (21.08 ± 3.06 vs 23.37 ± 3.48 mm) than those with mixed-type pulmonary embolisms. Additionally, disease duration was significantly longer in patients with distal-type pulmonary embolisms (14.33 ± 11.57 vs 8.10 ± 7.10 days), and they had significantly lower Mastora scores (20.91% ± 18.92% vs 43.96% ± 18.30%) than patients with mixed-type pulmonary embolisms. After treatment, RVSPs decreased significantly in patients with both distal-type and mixed-type pulmonary embolisms. Right ventricle diameters also decreased significantly in patients with mixed-type pulmonary embolisms after treatment.
Conclusion
Patients with mixed-type pulmonary embolisms are significantly more susceptible to pulmonary hypertension, enlarged right ventricular diameters, and shorter durations of disease than those with distal-type pulmonary embolisms. The distribution of pulmonary artery embolism in APE can provide a clinical reference.
Keywords: Pulmonary artery, pulmonary embolism, pulmonary hypertension, ventricular systolic pressure, Mastora score, ventricular diameter
Introduction
Acute pulmonary embolism (APE) is a common and potentially fatal disease with a mortality of 8% to 15% despite effective anticoagulation treatment.1 It occurs following intrapulmonary changes, which lead to a series of cardiopulmonary consequences that can even cause sudden death in severe cases. Additionally, acute pulmonary arterial hypertension (APAH) greatly influences its prognosis. Clinical symptoms play a pivotal role in the diagnosis of pulmonary embolism, and diagnosis may be delayed because of atypical symptoms that are easily ignored; however, a delayed diagnosis and treatment can result in poor prognosis. The diagnostic imaging of computed tomography pulmonary angiography (CTPA) can identify different locations of pulmonary embolisms, and determine if they are accompanied by pulmonary infarction. The present study aimed to investigate the distribution of pulmonary artery embolisms, time to diagnosis, right ventricular systolic pressure (RVSP), right ventricular diameters, and other indicators to provide clinical references for the diagnosis and treatment of APE.
Materials and Methods
Ethics statement
This retrospective study was approved by the Ethics Committee of Linyi People’s Hospital (Linyi, China; approval no. YX30056). It was conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived by the Ethics Committee because of the retrospective nature of this study. Nevertheless, patient data were protected for confidentiality.
Study subjects
Sixty-four patients (25 women and 39 men) with APE who were admitted to Linyi People’s Hospital between June 2018 and December 2019 were enrolled in the study. All patients were diagnosed by CTPA before admission. Patient hemodynamics were stable upon admission, and blood gas analysis and cardiac ultrasound were performed. Patients were given low-molecular-weight heparin and calcium combined with warfarin, and their international normalized ratios were maintained at 2 to 3. All patients were followed-up with CTPA, cardiac ultrasound, routine blood analysis, and coagulation function testing after 1 month of treatment. According to the 2014 European Society of Cardiology Guidelines,2 patients with a disease duration exceeding 30 days were excluded from the study. Patients with cardiopulmonary diseases, such as heart valve disease, chronic obstructive pulmonary disease, and pulmonary heart disease, were also excluded.
CTPA imaging characteristics
Thromboembolism was determined by the location of the thrombus. The following three locations were identified: (I) proximal thrombus, which only involved the main pulmonary artery trunk, right and left pulmonary arteries, and lobar artery; (II) distal thrombus, which only involved the segmental and/or subsegmental artery; and (III) mixed-type thrombus, which was defined as a thromboembolism involving both proximal and peripheral pulmonary arteries.
No proximal-type embolisms were identified, so patients were assigned to two groups depending on whether they had mixed-type or distal-type pulmonary embolisms. All patients were examined on a 64-channel dual-source CT system (Somatom Definition, Siemens Medical Solutions, Forchheim, Germany) using acquisition parameters: 120 kV, 125 effective mAs, collimation of 0.6 mm, pitch of 1.4, rotation time of 0.5 s, and a reconstructed slice thickness of 2 mm. Contrast enhancement was achieved through antecubital venous access with a contrast volume of 100 mL Iomeprol-400 (Iomeron, Bracco Imaging S.p.A., Milan, Italy), followed by a saline flush of 30 mL with an injection rate of 4 mL/s. In all examinations, the entire chest was examined in the caudocranial direction during deep inspiration breath hold. From each dataset, the following images were constructed: (a) diagnostic images, consisting of continuous 1-mm-thick lung (a medium sharp kernel (B50f)) and mediastinal (a soft kernel (B20f)) images; and (b) perfusion images (2-mm thickness; 1-mm intervals; D23 kernel) using dual-energy post-processing software (Syngo MultiModality; Siemens Healthcare, Erlagen, Germany).
The CT images were read by two radiologists in a randomized order and the CT angiographic clot load score was obtained using Mastora scoring.3 The scoring system included five mediastinal arteries (main pulmonary artery, right and left pulmonary arteries, and right and left interlobar pulmonary arteries), six lobar pulmonary arteries, and 20 segmental arteries (three in the upper lobes, two in the middle lobe or lingual area, and five in the lower lobes on each side). The severity score was adopted based on the percentage of luminal obstruction in the arteries by emboli, and each artery was scored from 0 to 5 (where 0 = 0%, 1 = 1%–24%, 2 = 25%–49%, 3 = 50%–74%, 4 = 75%–99%, and 5 = 100%). Summing the scores of mediastinal, lobar, and segmental arteries gave a global score with a maximum of 155, corresponding to a 100% obstruction index (Table 1).
Table 1.
Location of the thrombus and corresponding Mastora score.
| Thrombus location | Arteries scored | Range of Mastora score |
|---|---|---|
| Distal thrombus | 20 segmental | 0–100 |
| Proximal thrombus | Five mediastinal and six lobar | 0–55 |
| Mixed-type thrombus | Five mediastinal, six lobar, and 20 segmental | 0–155 |
Transthoracic echocardiography
All patients underwent standard echocardiographic assessment using GE Vivid S5 ultrasound (GE Healthcare, Chicago, IL, USA) including two-dimensional, pulsed-wave Doppler, color Doppler, and M-mode echocardiography with 2.5- to 4.0-MHz transducers. The following measurements were retrospectively measured in all patients: the diameter of the main pulmonary artery, diameters of right and left pulmonary arteries, the anteroposterior diameter of right and left ventricles, the right ventricular volume in the systolic and diastolic phase, the thickness of the right ventricular anterior wall, and the interventricular septum thickness. Echocardiographic examinations were undertaken at diagnosis and at the 1-month follow-up. Additionally, videotapes of the echocardiograms were read by a highly experienced echocardiographer, who was blinded to clinical data. The anteroposterior diameter of the right ventricle in end-diastolic period was measured at the four-chamber view. Right ventricular dilatation was defined as a right ventricular diastolic anterior–posterior diameter >26 mm. Pulmonary hypertension was defined as a RVSP >40 mmHg. The right ventricular–right atrium (RV–RA) gradient was estimated by the peak velocity of the tricuspid regurgitant flow signal using the simplified Bernoulli equation (continuous-wave Doppler in apical 4-chamber view), and RA pressure was estimated from the end-expiratory diameter and respiratory changes in the inferior vena cava in subcostal view.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software (IBM Corp., Armonk, NY, USA). Continuous variables were compared by the Student’s t-test, and categorical variables were analyzed by the chi-squared test. P < 0.05 was considered statistically significant.
Results
Of the 64 patients with pulmonary embolism, 25 were female and 39 were male. The median age of patients with distal-type pulmonary embolisms was 60.83 ± 11.02 years, and the median age of patients with mixed-type pulmonary embolisms was 59.35 ± 14.45 years.
No patients had a central pulmonary embolism. Distal-type pulmonary embolisms occurred in 12 patients (18.75%), mixed-type pulmonary embolisms in 52 patients (81.25%), and 54 patients had APAH (84.36%). In the distal-type pulmonary embolism group, seven patients had pulmonary hypertension (58.33%), compared with 47 patients in the mixed-type pulmonary embolism group (90.38%; P < 0.05). Eleven patients had an enlarged right ventricle diameter (17.19%), and significantly more patients with mixed-type pulmonary embolisms had an enlarged right ventricular diameter compared with those with distal-type pulmonary embolisms (P < 0.05).
The average time to diagnosis in the distal-type pulmonary embolism group was significantly longer than in the mixed-type pulmonary embolism group (14.33 ± 11.57 days vs 8.10 ± 7.10 days, respectively; P < 0.05). Moreover, Mastora’s index was significantly lower in the distal-type pulmonary embolism group than in the mixed-type pulmonary embolism group (20.91% ± 18.92% vs 43.96% ± 18.30%, respectively; P < 0.05) (Table 2).
Table 2.
Effects of pulmonary embolism distribution.
| Parameter | Distal-type thrombus (n = 12) | Mixed-type thrombus (n = 52) | P-value |
|---|---|---|---|
| Male | 8 (20.51%) | 31 (79.49%) | 0.902 |
| Female | 4 (16.00%) | 21 (84.00%) | |
| Age (years) | 60.83 ± 11.02 | 59.35 ± 14.45 | 0.740 |
| Mastora score (%) | 20.91 ± 18.92 | 43.96 ± 18.30 | <0.001 |
| Duration of disease (days) | 14.33 ± 11.57 | 8.10 ± 7.10 | 0.019 |
| RVSP before treatment (mmHg) | 44.92 ± 17.04a | 55.69 ± 17.66b | 0.042 |
| RVSP after treatment (mmHg) | 39.08 ± 16.85a | 39.21 ± 20.42b | 0.750 |
| RVD before treatment (mm) | 21.08 ± 3.06NS | 23.37 ± 3.48c | 0.048 |
| RVD after treatment (mm) | 21.50 ± 1.78NS | 21.79 ± 2.67c | 0.972 |
Lowercase superscript letters represent significant differences in comparisons: a, P = 0.018; b, P < 0.001; c, P = 0.021; NS, not significant.
RVSP: right ventricular systolic pressure, RVD: right ventricular diameter.
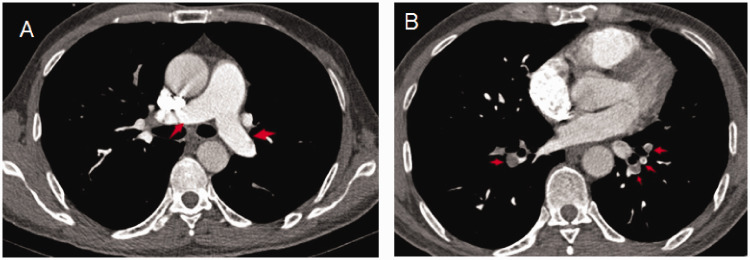
Figure 1 shows the CT characteristics of a 58-year-old male patient in the distal-type pulmonary embolism group who was admitted to hospital because of chest tightness after activity for 20 days. CTPA found a pulmonary artery embolus in the pulmonary segment arteries but no thrombus in the left and right pulmonary arteries or lobar arteries. Color Doppler ultrasound revealed a right ventricle diameter of 22 mm and an RVSP of 30 mmHg.
Figure 1.
CT characteristics of a patient with a distal-type pulmonary embolism. No thrombus was seen in the left and right pulmonary arteries or lobar arteries (a). A pulmonary artery embolus was distributed mainly in the pulmonary segment arteries (b). Red arrows represent embolisms.
CT, computed tomography.
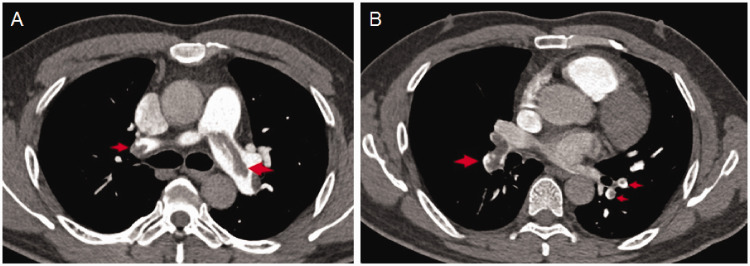
Figure 2 shows the CT characteristics of a 69-year-old male patient in the mixed-type pulmonary embolism group who was admitted to hospital because of dyspnea for 7 days. CTPA showed that the embolism was distributed in left and right pulmonary arteries, left and right pulmonary lobe arteries, and pulmonary segment arteries. Color Doppler ultrasound revealed a right ventricular diameter of 32 mm and an RVSP of 56 mmHg.
Figure 2.
CT characteristics of a patient with a mixed-type pulmonary embolism. The embolism was shown to be distributed in left and right pulmonary arteries (a), as well as left and right pulmonary lobe arteries, and pulmonary segment arteries (b). Red arrows represent embolisms.
CT, computed tomography.
Changes in the RVSP and right ventricle diameter after anticoagulant treatment
One month after anticoagulant treatment, color Doppler ultrasound was performed to evaluate changes in the RVSP and right ventricle diameter. The RVSP after treatment was significantly lower than that before treatment in both patient groups (P < 0.05). Similarly, the right ventricle diameter after treatment was significantly smaller than before treatment in the mixed-type pulmonary embolism group (P<0.05). However, this difference was not significant in the distal-type pulmonary embolism group. Comparing the two groups after treatment, no significant difference was seen in the RVSP or in the diameter of the right ventricle (Table 2).
Discussion
CTPA is used to assess the distribution of pulmonary embolism, and can readily distinguish distal-type from mixed-type pulmonary embolisms. Cardiopulmonary changes are the main manifestations of pulmonary embolism in the acute phase and the distribution of pulmonary arterial embolisms is related to the severity of clinical symptoms, short-term and long-term treatment effects, and disease complications.
In the present study, patients with APE were classified as having mixed-type pulmonary embolisms (81.5%) or distal-type pulmonary embolisms (18.75%). Although no patients had central pulmonary embolisms, previous work has reported that such patients are particularly susceptible to pulmonary hypertension.4–8 The likelihood of chronic thromboembolic pulmonary hypertension (CTEPH) was also shown to significantly increase when the acute RVSP exceeds 50 mmHg.9–11 The acute phase of pulmonary hypertension is often temporary, and most patients improve after anticoagulation treatment.12 However, the RVSP can be persistently increased in a number of patients. The optimal timing and diagnostic test for CTEPH after acute pulmonary embolism are unknown.
Takayuki et al.13 reported that CTEPH was mainly associated with vascular remodeling and thrombus organization, and continuous increases in CTEPH were separately linked with proximal pulmonary artery remodeling.14–16 Additionally, Klok et al.17 observed associations between CTEPH and the embolization site and degree of embolism, as well as with diagnostic delay. Several studies reported a correlation between a delay in treatment and pulmonary embolism and pulmonary arterial hypertension.16–18 Although the location of thromboembolisms in the acute phase can be associated with sudden death,19,20 Gonzalez et al.21 conversely found no significant correlation between the thrombus location and disease severity.
Mastora scoring is extensively used in the assessment of obstruction severity, although it is relatively tedious to perform and cannot predict the incidence of chronic thromboembolic pulmonary hypertension.10 In the present study, the Mastora score differed significantly between the two patient groups, indicating that the scope and extent of mixed-type pulmonary embolism was significantly larger than those of distal-type pulmonary embolism, as seen in previous studies.3,22–24 Additionally, the clinical symptoms of mixed pulmonary embolism presented significantly earlier than those of peripheral pulmonary embolism (P < 0.05), indicating that mixed-type pulmonary embolism may cause more obvious clinical symptoms.25 The distribution of pulmonary embolism is a continuous process. As the embolization range expands, an increase in the RVSP gradually increases the diameter of the right ventricle. Recently, Jeong et al.26 reported that patients with extensive pulmonary embolism are prone to early elevation of the RVSP and right ventricular damage, whereas right ventricular enlargement is a manifestation of disease progression.27–29 Indeed, this has been associated with short-term mortality,30,31 and plays a key role in diagnosis and treatment.
In the present study, we showed that the distribution of pulmonary embolism in the acute phase significantly correlates with the RVSP, duration of disease, and right ventricle enlargement, but is not associated with patient sex or age. Moreover, it directly reflects the severity of acute pulmonary embolism. Because the therapeutic effect is most notable in the acute phase, the clinical evaluation of acute pulmonary embolism should be conducted intuitively and rapidly.
Our study has some limitations. First, the influence of etiology on the distribution of the lesions was not assessed when assessing the distribution of pulmonary embolism in the acute phase. Second, primary lesions were not targeted during anticoagulation treatment. Thus, further studies are required to clarify the effects of primary disease on pulmonary embolism.
Conclusions
The present study shows that mixed-type pulmonary embolism is more susceptible to changes in both pulmonary artery systolic pressure and right ventricular diameter than distal-type pulmonary embolism. Additionally, the clinical symptoms of mixed-type pulmonary embolism are more obvious, leading to a shorter duration of disease and an earlier diagnosis. Thus, the distribution of APE indicates the severity of disease. CTPA readily determines the type of pulmonary thrombosis and therefore provides a reliable basis for clinical evaluation.
Footnotes
Data availability: The data used to support the findings of this study are included within the article.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Innovation Plan in Linyi City (Grant No. 201919001).
ORCID iD: Miao Guo https://orcid.org/0000-0002-8302-562X
References
- 1.Doğan H, De Roos A, Geleijins J, et al. The role of computed tomography in the diagnosis of acute and chronic pulmonary embolism. Diagn Interv Radiol 2015; 21: 307–316. DOI:10.5152/dir.2015.14403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstantinides SV. 2014 ESC guidelines on the diagnosis and management of acute pulmonaryembolism. Eur Heart J 2014; 35: 3145–3146. [DOI] [PubMed] [Google Scholar]
- 3.Mastora I, Remy-Jardin M, Masson P, et al. Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol 2003; 13: 29–35. [DOI] [PubMed] [Google Scholar]
- 4.Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Haemost 2014; 112: 598–605. [DOI] [PubMed] [Google Scholar]
- 5.Pengo V, Lensing AWA, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350: 2257–2264. [DOI] [PubMed] [Google Scholar]
- 6.Bach AG, Nansalmaa B, Kranz J, et al. CT pulmonary angiography findings that predict 30-day mortality in patients with acute pulmonary embolism. Eur J Radiol 2015; 84: 332–337. [DOI] [PubMed] [Google Scholar]
- 7.Bellofiore A, Roldán-Alzate A, Besse M, et al. Impact of acute pulmonary embolization on arterial stiffening and right ventricular function in dogs. Ann Biomed Eng 2013; 41: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auer RC, Schulman AR, Tuorto S, et al. Use of helical CT is associated with an increased incidence of postoperative pulmonary emboli in cancer patients with no change in the number of fatal pulmonary emboli. J Am Coll Surg 2009; 208: 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok FA, Dzikowska-Diduch O, Kostrubiec M, et al. Derivation of a clinical prediction score for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. J Thromb Haemost 2016; 14: 121–128. DOI:10.1111/jth.13175 [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Ma Z, Guo X, et al. Computed tomographic pulmonary angiography in the assessment of severity of chronic thromboembolic pulmonary hypertension and right ventricular dysfunction. Eur J Radiol 2011; 80: e462–e469. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro A, Lindmarker P, Johnsson H, et al. Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation 1999; 99: 1325–1330. [DOI] [PubMed] [Google Scholar]
- 12.Mehta D, Barnett M, Zhou L, et al. Management and outcomes of single subsegmental pulmonary embolus: a retrospective audit at North Shore Hospital, New Zealand. Intern Med J 2014; 44: 872–876. DOI:10.1111/imj.12507 [DOI] [PubMed] [Google Scholar]
- 13.Jujo T, Sakao S, Ishibashi-Ueda H, et al. Correction: evaluation of the microcirculation in chronic thromboembolic pulmonary hypertension patients: the impact of pulmonary arterial remodeling on postoperative and follow-up pulmonary arterial pressure and vascular resistance. PLoS One 2015; 10: e0138040. DOI:10.1371/journal.pone.0138040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quarck R, Wynants M, Ronisz A, et al. characterization of proximal pulmonary arterial cells from chronic thromboembolic pulmonary hypertension patients. Respir Res 2012; 13: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Gan HL, Liu Y, et al . The distinguishing cellular features of pulmonary artery smooth muscle cells from chronic thromboembolic pulmonary hypertension patients. Exp Lung Res 2013; 39: 349–358. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Yang L, Zhang Y, et al. Incidence and risk factors of chronic thromboembolic pulmonary hypertension in patients with diagnosis of pulmonary embolism for the first time in real world. Clin Respir J 2018; 12: 2551–2558 [DOI] [PubMed] [Google Scholar]
- 17.Klok FA, Barco S, Konstantinides SV, et al. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: results from the European CTEPH Registry. Eur Respir J 2018; 52: 1801687. DOI:10.1183/13993003.01687-2018 [DOI] [PubMed] [Google Scholar]
- 18.Kumamaru KK, Hunsaker AR, Kumamaru H, et al. Correlation between early direct communication of positive ct pulmonary angiography findings and improved clinical outcomes. Chest 2013; 144: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 19.Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest 2012; 142: 1417–1424. DOI:10.1378/chest.11-2739. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesh SK, Wan SC. Central clot score at computed tomography as a predictor of 30-day mortality after acute pulmonary embolism. Ann Acad Med Singap 2010; 39: 442–447. [PubMed] [Google Scholar]
- 21.Gonzalez Della Valle A, Blanes Perez A, Lee YY, et al. The clinical severity of patients diagnosed with an in-hospital pulmonary embolism following modern, elective joint arthroplasty is unrelated to the location of emboli in the pulmonary vasculature. J Arthroplasty 2017; 32: 1304–1309. DOI:10.1016/j.arth.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 22.Zhao DJ, Ma DQ, He W, et al. Cardiovascular parameters to assess the severity of acute pulmonary embolism with computed tomography. Acta Radiol 2010; 51: 413–419. [DOI] [PubMed] [Google Scholar]
- 23.Varol K, Gumus C, Yucel H, et al . Correlation of right ventricular dysfunction on acute pulmonary embolism with pulmonary artery computed tomography obstruction index ratio (PACTOIR) and comparison with echocardiography. Jpn J Radiol 2015; 33: 311–316. [DOI] [PubMed] [Google Scholar]
- 24.Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 2001; 176: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 25.Heyer CM, Lemburg SP, Knoop H, et al. Multidetector-CT angiography in pulmonary. embolism—can image parameters predict clinical outcome? Eur Radiol 2011; 21: 1928–1937. [DOI] [PubMed] [Google Scholar]
- 26.Park JR, Chang SA, Jang SY, et al. Evaluation of right ventricular dysfunction and prediction of clinical outcomes in acute pulmonary embolism by chest computed tomography: comparisons with echocardiography. Int J Cardiovasc Imaging 2012; 28: 979–987. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Shi H, Wang Y, et al. Assessment of correlation between CT angiographic clot load score, pulmonary perfusion defect score and global right ventricular function with dual-source CT for acute pulmonary embolism. Br J Radiol 2012; 85: 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi KJ, Cha SI, Shin KM, et al. Prognostic implications of computed tomographic right ventricular dilation in patients with acute pulmonary embolism. Thromb Res 2014; 133: 182–186. [DOI] [PubMed] [Google Scholar]
- 29.Casazza F, Bongarzoni A, Forgione C, et al. Echocardiographic evolution of pulmonary artery pressure after acute pulmonary embolism. Results from IPER registry. Thromb Res 2014; 134: 1224–1228. DOI:10.1016/j.thromres.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Barco S, Mahmoudpour SH, Planquette B, et al. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 2019; 40: 902–910. DOI:10.1093/eurheartj/ehy873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furlan A, Aghayev A, Chang CC, et al. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology 2012; 265: 283–293. DOI:10.1148/radiol.12110802. [DOI] [PMC free article] [PubMed] [Google Scholar]