Abstract
Hormonal contraceptives have been on the market for over fifty years and, while their formulations have changed, the basic mechanism of action has remained the same. During this time, numerous studies have been performed documenting side effects, some of which appear over time, some within weeks or months, but all can have a serious impact on health and quality of life. An effort was made to perform a series of comprehensive literature surveys to better understand immediate and long-term side effects of these agents. The results of this literature review uncovered a number of potential side effects, some of which are acknowledged and many of which are not noted in the prescribing information for these agents. Among the unacknowledged side effects are: an increased risk of HIV transmission for depot medroxyprogesterone acetate (DMPA), and for combination contraceptives breast cancer, cervical cancer, Crohn’s disease, ulcerative colitis, systemic lupus erythematosus, depression, mood disorders and suicides (especially among women twenty-five years of age and younger, in the first six months of use), multiple sclerosis, interstitial cystitis, female sexual dysfunction, osteoporotic bone fractures (especially for progesterone-only contraceptives), and fatty weight gain. Misleading prescribing information regarding cardiovascular and thrombotic risks are also noted. Women seeking birth control have a right to be informed and educated about risk avoidance through the use of effective nonhormonal methods like fertility awareness methods. In one case—that of DMPA—the increased risk of HIV acquisition has been conclusively demonstrated to be both real and unique to this drug. Considering the availability of numerous alternatives, there is no justification for the continued marketing of DMPA to the public.
Summary:
We reviewed the effect of hormonal contraceptives on women’s health. A number of potential side effects were noted including increased risks of breast cancer, cervical cancer, inflammatory bowel disease, lupus, multiple sclerosis, cystitis, bone fractures, depression, mood disorders and suicides, fatty weight gain, and female sexual dysfunction. With the long-acting injectable contraceptives there is an increased risk of getting HIV. Misleading prescribing information regarding the risks of heart attacks, strokes and blood clotting problems were also noted. Women seeking birth control have a right to know about how to avoid these risks by using effective hormone-free Fertility Awareness Methods.
Keywords: Autoimmune disease, Cancer, Contraception, Fractures, Human immunodeficiency virus, Osteoporosis, Sexual dysfunction, Suicide, Thromboembolic disease, Weight gain
In 1965 the Supreme Court created a right to privacy in the Constitution and used it to overturn Connecticut’s laws against the marketing and distribution of contraception. This decision in effect legalized contraception for married couples in 1965.1 Twenty-nine other states began bringing their laws in line with the Supreme Court’s decision. Within two years, contraception began to be legalized for unmarried individuals.2
A seismic shift took place in our culture. Beginning with married couples and then spreading to the unmarried, the procreative meaning of sex had been split off from the bonding and pleasurable aspects of sex. At first, the change seemed small. After all, it was thought all the pill and other forms of contraception did was prevent conception. The fact that it fundamentally changed human sexuality and, by extension, marriage, family, and the value of human life was invisible.
The pill was not available before 1960. Usage diffused through the population gradually from 0 percent users up until 1959 to 1 percent users in 1960 to still only about 18 percent users in 1965 when the US Supreme Court legalized contraception for married people, to about 55 percent users in 1968 when Humanae Vitae came out (Paul VI 1968), to “96 percent users by 1973, a mere eight years—less than a decade—after the Court’s decision.”3 Use of modern contraception saturated the population. People no longer saw sexual behavior as necessarily procreative. Sex changed from necessarily being about family to being about whatever consenting adults—or teens—wanted it to be.
Today, hormonal contraceptives have been widely used for over fifty years and, while their formulations have changed, the basic mechanism of action has remained the same. During this time, numerous studies have been performed documenting side effects, some of which appear over time, some within weeks or months, but all can have a serious impact on health. An effort was made to perform a series of comprehensive literature surveys to better understand immediate and long-term side effects of these agents. Some of these have been previously published (Peck and Norris 2012; Williams 2017; Keenan et al. 2018; Williams et al. 2018), while others are included here for the first time. Comparison of the scientific literature with the current prescribing information revealed that many of these risks, although well documented in the peer-reviewed literature, are not included in the prescribing information.
Hormonally active contraceptives are in some sense a unique class of drugs: they use potent synthetic steroids to nullify fertility. There is no pathology or abnormal condition being treated, but instead a normal functioning body system (the female reproductive system) is incapacitated. Under the Hippocratic principle to do no harm, the safety and tolerability of these agents would have to be exemplary to justify their ethical use.
However, many common side effects are acknowledged in the prescribing information for these steroidal contraceptives. Among the most widely prescribed combination contraceptives are YAZ® (drospirenone/ethinyl estradiol),4 ORTHO TRI-CYCLEN® (norgestimate/ethinyl estradiol),5 ALESSE® (Levonorgestrel/ethinyl estradiol),6 Lo LOESTRIN Fe® (norethindrone acetate and ethinyl estradiol tablets, ethinyl estradiol tablets and ferrous fumarate tablets),7 and MIRCETTE® (desogestrel/ethinyl estradiol and ethinyl estradiol),8 while for progesterone-only contraceptives, the most common include NEXPLANON® (etonogestrel implant)9 and DEPO-PROVERA CI® (medroxyprogesterone acetate).10 Serious side effects that are acknowledged include venous and arterial thrombotic disease (including serious cardiovascular disease and strokes), high blood pressure, depression, and migraines. More common side effects are also noted, such as breast tenderness, nausea, vomiting, and fluid retention, but these are inconsistently noted across the products. Many more are poorly stated, and many more are not acknowledged. The purpose of this article is to review the clinical and scientific literature on the side effects focusing on those which are not fully acknowledged in the prescribing information. These observations served as the basis for a citizen’s petition to the Food and Drug Administration (FDA) to update the prescribing information of hormonally active contraceptives and to remove depot medroxyprogesterone acetate (DMPA) from the market. The specific recommendations are noted here with the supporting information following. Supplemental Tables with the full literature search results and other supporting documentation are available in the online version of this manuscript (see Supplemental Materials).
Medical Risks
Risk of HIV Transmission
One of the most common forms of steroidal contraception is the injectable contraceptive: DMPA. DMPA is highly effective and requires only quarterly injections, as opposed to daily oral ingestion. As a long-acting type of effective contraceptive, it is not unique, as there are other injectable or implantable contraceptives in wide use, for example, norethisterone enanthate (NET), as well as other delivery systems such as vaginal rings and patches.
However, evidence began emerging in the 1990s, which has become compelling in recent years, that DMPA is unique among contraceptives in its property of facilitating the transmission of HIV. This dangerous characteristic has been abundantly and unequivocally documented through several lines of evidence which are summarized below:
Epidemiological Evidence
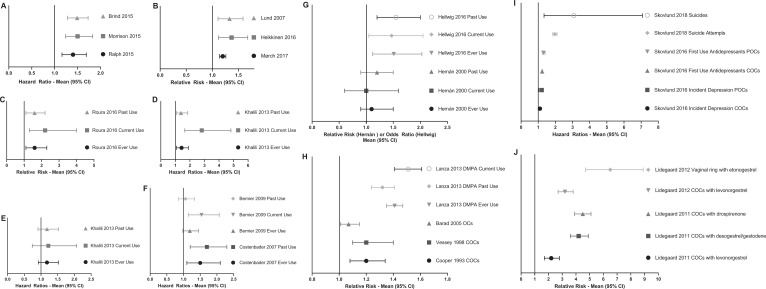
Four meta-analyses (three reports) were published in 2015. Each used different inclusion criteria and compiled the data on different numbers of studies, yet all four came up with essentially the same result of significantly increased risk of male-to-female HIV transmission in women using DMPA (Figure 1A and Supplementary Table 1).
Figure 1.
Increases in risks of diseases with hormonal contraceptives. Depending on the study, the data shown are either hazard ratios (HR), relative risk (RR), or odds ratio (OR). The 95% confidence interval (95% CI) is also shown. The full data are in Supplementary Tables 1-15. (A) Increase in the risk of HIV infection with depot medroxyprogesterone acetate (DMPA) from 3 meta-analyses. (B) Increase in the risk of breast cancer with combination oral contraceptives (COCs) from the three best cohort studies. (C) Increase in the risk of invasive cervical cancer with COCs from the best cohort study with any use (ever use), current use, and past use as noted. (D) Increase in the risk of Crohn’s disease with COCs from the best cohort study with ever use, current use, and past use as noted. (E) Increase in the risk of ulcerative colitis with COCs from the best cohort study with ever use, current use, and past use as noted. (F) Increase in the risk of systemic lupus erythematosus with COCs from the best cohort studies with ever use, current use, and past use as noted. (G) Increase in the risk of multiple sclerosis with COCs from the best cohort study and the best case–control study with ever use, current use, and past use as noted. (H) Increase in the risk of osteoporotic fractures (all fractures) with COCs and progestogen-only contraceptives (POCs; especially DMPA) from the best cohort studies with ever use, current use, and past use as noted. (I) Increase in the risk of depression, first antidepressant use, suicide attempts and suicides with COCs and POCs from the best cohort study with Ever Use, Current Use and Past Use as noted. (J) Increase in the risk of venous thromboembolism with various COCs from the best cohort studies.
Ten primary studies (all longitudinal, published between 2003 and 2014, listed in Supplementary Table 2) were methodologically robust enough to meet the inclusion criteria of all 3 published reviews. Importantly, no consistent association has emerged with regard to oral contraceptives (OCs) or other injectable or implantable contraceptives and the facilitation of HIV transmission.
Mechanistic Evidence
Heffron et al. (2012) reported the increased presence of HIV-1 RNA in genital fluids of women using DMPA. Maritz et al. (2018) reported experimental evidence of increased replication of HIV in human blood monocytes in vitro with medroxyprogesterone acetate (MPA). There is also experimental evidence of agonistic binding to the glucocorticoid receptor (GR) as the mechanism for DMPA’s immunosuppression. Over the last fifteen years, abundant experimental evidence of cytotoxic and immunosuppressive action of DMPA via its agonistic binding to the GR of human leukocytes has been reported (Schindler et al. 2003; Hapgood and Tomasicchio 2010; Hapgood et al. 2014). Thus, Huijbregts, Michel, and Hel (2014) reported experimental evidence of immunosuppression of human T cells in vitro by MPA. Tomasicchio et al. (2013) reported experimental evidence of increased human T cell destruction in vitro via the GR with MPA. Hapgood et al. (2014) reported:
that MPA, unlike NET and progesterone, represses inflammatory genes in human PBMCs (peripheral blood mononuclear cells) in a dose-dependent manner, via the glucocorticoid receptor (GR), at concentrations within the physiologically relevant range. These and published results collectively suggest that the differential GR activity of MPA versus NET may be a mechanism whereby MPA, unlike NET or progesterone, differentially modulates HIV-1 acquisition and pathogenesis in target cells where the GR is the predominant steroid receptor expressed.
Experimental evidence of MPA-mediated suppression of inflammatory genes via GR in cultured human cells (Govender et al. 2014) demonstrated the suppression of inflammatory genes in cultured human endocervical cells.
One of the systematic reviews noted above (Morrison et al. 2015) concluded that their analysis “adds to the evidence that DMPA may increase HIV risk.” They further suggested that “A randomized control trial would provide more definitive evidence about the effects of hormonal contraception, particularly DMPA, on HIV risk.”
The results of a randomized controlled trial were published in the Lancet by the ECHO Trial Consortium, the trial having been designed by a group of ten that includes three of the authors of the 2015 Morrison review (Morrison, Baeten and Rees in Morrison et al. 2015). However, in stark contrast to their 2015 conclusion, the ECHO group, which studied DMPA in comparison to the copper intrauterine device (CuIUD) and the levonorgestrel (LNG) implant, concluded: “We did not find a substantial difference in HIV risk among the methods evaluated, and all methods were safe and highly effective.”
However, a careful analysis of the design and results of the ECHO Trial reveals that in fact, the ECHO Trial results of 2019 provide a near perfect confirmation of the results of the 2015 Morrison systematic review and meta-analysis (SRMA), and that the authors misrepresent them as the opposite; as the exculpation of DMPA as “safe.” The performance of an appropriate randomized control trial presented the ECHO Trial Consortium with serious ethical and scientific challenges. First, the very idea of the need to conduct such a trial of a medical drug or device for an elective condition (contraception) which had already been shown to present significant risk elevation for the contraction of a potentially life-threatening infection (HIV) by three independent SRMAs, reviewing data that had been accumulated in dozens of studies dating back over a quarter century, is ethically problematic, to say the least. This is especially true in the case of DMPA, a contraceptive progestin that stands alone among many such available, in its property of being a glucocorticoid agonist, the likely mechanism by which it increases HIV risk. Indeed, the Morrison SRMA was the most mildly worded of the three 2015 SRMAs, with that of Brind et al. (2015), concluding that the evidence that DMPA increases the risk of HIV transmission was now “compelling.”
Second, assuming that conducting such a study at all would be ethically valid, the World Health Organization (WHO 2017) changed the guidance for the use of DMPA from category 1 (“no restriction”) to category 2 (“a condition where the advantages of using the method generally outweigh the proven or theoretical risks”), relating to the use of DMPA as a contraceptive in women at high risk of HIV acquisition. This guidance was based on the 2015 SRMAs. At the very least, this new WHO guidance would need to be disclosed to all the ECHO study subjects, and the ECHO study notes that all participants in the study “were provided with this updated information.” But the providing of such information presents a clear ethical challenge itself, in the context of a trial wherein subjects are randomly assigned to one of three groups, only one of which was already thought to facilitate HIV transmission. What of women who were assigned to the DMPA group? On the one hand, advising them that they were now in the highest risk group regarding HIV infection would unblind them and likely encourage them to opt out of the study, thus rendering the study scientifically invalid. On the other hand, not so advising these women would constitute withholding information on the risks of the proposed medication, a clear violation of the need to obtain informed consent. Determined to conduct a scientifically valid study, it would seem, the ECHO Consortium opted to make “concerted efforts to not provide additional or differential information or counseling to women in the DMPA-IM group.”
Third, assuming (arguendo) that the ethical challenges have been adequately met by the study design, there is the challenge of scientific validity. In such a trial, a critical aspect of study design is the statistical power of the study; to ensure that the statistical power is adequate to either confirm or reject the earlier findings. As noted in this article, the three 2015 SRMAs arrived at virtually the same results, in the comparison of women using DMPA to those using no form of hormonal contraception. The results obtained by Morrison et al. included an overall HR = 1.50 (95% CI 1.24–1.83). In contrast, there was no significant increase in HIV infection risk with either the long-acting injectable contraceptive norethisterone enanthate (NET-EN; HR = 1.24; 95% CI 0.84–1.82) or combined oral contraceptive (COC) use (HR = 1.03; 95% CI 0.88–1.20). Importantly, they also compared the use of each of the three methods to each other, and they reported that, compared to NET-EN, DMPA use was still associated with significantly elevated HIV infection risk: HR = 1.32 (95% CI 1.08–1.61). In the new ECHO study, three contraceptive methods (DMPA, CuIUD, and LNG implants) were compared only to each other. Therefore, the key comparison (in regard to DMPA) was clearly between the effects of DMPA versus the effects of LNG, another long-acting, progestin-only contraceptive steroid, and one which, like NET-EN and unlike DMPA, has neither been found to significantly elevate HIV infection risk nor to interact with the GR. Since the appropriate comparison in the 2015 Morrison SRMA was between DMPA and NET-EN use, a perfect replication of the result they obtained thereby would be a statistically significant elevation in the neighborhood of HR = 1.3. Hence, it is puzzling that the ECHO study was statistically designed thus: “The trial was designed with 80 percent power to detect a 50 percent increase in the hazard of HIV for each contraceptive method compared with each of the others (i.e., DMPA-IM vs. CuIUD, DMPA-IM vs LNG implant, and CuIUD vs LNG implant). We chose a 50% increase in HIV risk on the basis of formative work with stakeholders to determine a meaningful difference that would inform policy change.”
In other words, the ECHO consortium was not concerned with the scientific imperative of repeating or refuting their earlier results. Rather, they had made the decision that anything less than a 50 percent increase in HIV infection risk was not “a meaningful difference,” thus to be ignored. Not surprisingly then, the result obtained in the ECHO study, comparing DMPA use to LNG use, was (in their “continuous use” data set, Table 2 in ECHO 2019) HR = 1.29 (95% CI 0.98–1.71). Therefore, although this result is virtually identical to the result Morrison et al. (2015) obtained in their 2015 SRMA, it just failed to achieve statistical significance at the 0.05 level (p = .06), due to the study’s having been underpowered to detect this difference. Yet the ECHO Consortium seized upon this result to call DMPA “safe and effective” (along with the other two methods tested), and their literal bottom line conclusion was: “These results support continued and increased access to these three contraceptive methods.”
From both a scientific and an ethical point of view, this conclusion is clearly erroneous. Six women died in the DMPA group out of the 2609 enrolled, compared to only one death in the LNG group (There were 143 vs. 116 cases of HIV infection in the DMPA vs. LNG, respectively.) Thus, DMPA appeared responsible for five excess deaths (and twenty-seven excess cases of HIV infection); about 0.19% of its users, in a maximum follow-up period of only twelve to eighteen months (average 1.33 years follow-up for the 7,829 women participating with a total of 10,409 woman-years of follow-up time). If a woman’s reproductive life is estimated to be about twenty-five times the follow-up period, from age thirteen until about age forty-five, that would come out to a death rate of about 5 percent of women using DMPA compared to other injectable or implantable progestins over the course of their fertile years. The excess rate of HIV acquisition would likely cause far more excess deaths. This increased risk of death is clearly not acceptable.
Yet, even more troubling than the flouting of both appropriate scientific and ethical considerations by the ECHO Consortium was the cover given the Consortium by the WHO itself. Specifically, the WHO issued a puzzling public statement, claiming there was “no evidence of a causal association between DMPA use and women’s risk of HIV acquisition” in October, 2015, shortly before recruitment began for the ECHO trial. This outrageously false statement was immediately challenged by an open letter of protest from a group comprised of over twenty AIDS advocacy organizations and several individuals, the letter demanding that the WHO’s “demonstrably false” statement of “no evidence” be removed from the WHO website. The statement was subsequently removed, but not before the ECHO trial was registered with the National Institutes of Health clinical trial database (Sathyamala 2020). More recently, the highly questionable guidance issued by the WHO was the subject of an editorial in the British Medical Journal (BMJ; Sathyamala 2019). It is also noteworthy that this editorial still stands unchallenged by the WHO or anyone supporting the agency, on the pages of the BMJ. We can see no justification whatsoever for the continued availability of DMPA in the US market. This detailed analysis of the ECHO Consortium study only underscores our earlier conclusion.
Summary and Conclusions
DMPA, in contrast to all other steroidal contraceptives, has now conclusively been demonstrated to significantly increase the risk of HIV transmission from infected men to women. The robust epidemiological association has been supported by in vivo evidence of increased HIV RNA in the female genital tracts of women using DMPA. Moreover, abundant experimental evidence has shown that MPA, due to its agonistic binding of the GR, specifically represses the innate immune responses of both circulating human leukocytes and endocervical cells and allows for increasing HIV replication. The demonstration in the literature of the chain of causation is therefore compelling.
In the United States, where the availability of a wide range of contraceptive drugs and devices is virtually universal, and where, among these contraceptive choices, one and only one particular method—DMPA—is now known to increase the transmission of an often-fatal viral infection (HIV/AIDS), there can be no justification for such a drug’s continued availability in the marketplace. It should be removed from the marketplace by the FDA without further delay.
Cancer
The bulk of this information has been published (Williams et al. 2018). Significant findings are noted here.
Many of the COC prescribing information surveyed has the following assertion regarding cancer risk:
There is substantial evidence that COCs do not increase the incidence of breast cancer. Although some past studies have suggested that COCs might increase the incidence of breast cancer, more recent studies have not confirmed such findings.
Some studies suggest that COCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which these findings may be due to differences in sexual behavior and other factors.
Noting that the World Health Organization International Agency for Research on Cancer (IARC) has classified COCs as Group I carcinogens (World Health Organization International Agency for Research on Cancer 2012), a literature search as performed evaluating the published literature on this association. Papers were accessed from a PubMed literature review as noted (Williams et al. 2018). Each paper was rated based on the parameters noted in the STROBE statement (von Elm et al. 2007). As noted previously,
In agreement with the IARC, the recent literature confirms an increased risk of breast cancer and cervical cancer with use of OCs. The recent literature also confirms the IARC conclusion that OCs decrease the risk of ovarian and endometrial cancers. However, there is little support from recent studies for the IARC conclusion that OCs decrease the risk of colorectal cancer or increase the risk of liver cancer. For liver cancer, this may be due to the recent studies having been performed in areas where hepatitis is endemic. In one large observational study, POCs also appear to increase the overall risk for developing cancer.
The relevant data on breast cancer and cervical cancer are summarized here.
Breast Cancer
Breast cancer remains the most commonly diagnosed cancer (excluding nonmelanoma skin cancers) in women in developed nations with over 2 million cases diagnosed worldwide in 2018 (https://www.wcrf.org/dietandcancer/cancer-trends/breast-cancer-statistics). According to the Surveillance, Epidemiology and End Results (SEER) statistics (https://seer.cancer.gov/statfacts/html/breast.html), in 2017, there were an estimated 3,577,264 women living with female breast cancer in the United States. In the United States, it is estimated there will be 276,480 new cases of breast cancer in 2020, accounting for 15.3 percent of all new cancer cases, with about 42,170 deaths, accounting for 7.0 percent of all cancer deaths. Any risk factors that are controllable should be minimized. The data for breast cancer are shown split into cohort studies (Figure 1B and Supplementary Table 3), case control studies (Supplementary Table 4), and meta-analyses (Supplementary Table 5).
The World Health Organization International Agency for Research on Cancer (IARC) working groups has repeatedly classified combined estrogen–progestogen contraceptives as Group I carcinogens (WHO 1999, 2007, 2012). The National Toxicology Program classifies steroidal estrogen as a known human carcinogen (Report on Carcinogens, Fourteenth Edition available at https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html).
In agreement with IARC, recent data confirm an increased risk of breast cancer with use of COCs (Figure 1B). Key articles confirming this association include Yager and Davidson (2006) and Kahlenborn et al. (2006). The most dramatic increases appear to be seen for recent use (within one to five years) or current use of COCs in premenopausal women, odds ratios (OR) of 1.5 for recent use (Beaber et al. 2014). Use for a year or more is associated with a 2.5-fold increased risk of triple-negative breast cancer, a very aggressive and difficult to treat subtype (Dolle et al. 2009). In postmenopausal women on hormone replacement therapy (HRT) prior use of COCs further increase the risk of breast cancer (Lund et al. 2007). There also appears to be an increased risk for African American women on COCs within the past five years (Bethea et al. 2015).
Use of the LNG-releasing intrauterine contraceptive (IUC) increases the risk of developing breast cancer in postmenopausal (Heikkinen et al. 2016). A large prospective cohort study included 1.8 million Danish women the risk of breast cancer among current or recent users for both COCs and OCs, and the risk increased with length of use (Mørch et al. 2017). The increased risk persisted after discontinuing use if COCs were used for five years or more. The risk extends to women with the BRCA1 gene mutation if COCs are if begun under the age of twenty (Mehrgou and Akouchekian 2016; Kotsopoulos et al. 2014) with the risk increasing with length of use.
Data from recent, large cohort studies (see Figure 1B and Mørch et al. 2017; Heikkinen et al. 2016; Poosari et al. 2014) show RRs ranging from 1.2 to 1.37, while a large registry study of progestogen-only contraceptives (POCs; Soini et al. 2014) showed an increased RR for breast cancer of 1.19. Again, duration of use increases the risk of breast cancer for COCs as does use early in life (Mørch et al. 2017).
Cervical Cancer
According to the SEER statistics (https://seer.cancer.gov/statfacts/html/cervix.html), it is estimated that there are 291,704 women in the United States with cervical cancer in 2017. There will be about 13,800 new cases of cervical cancer in 2020, with 4,290 deaths. The five-year survival for cervical cancer is 66 percent. The IARC evaluation of an increased risk of cervical cancer with COCs is also supported especially by a large, high-quality cohort study (Roura et al. 2016), especially for invasive cervical cancer (Figure 1C and Supplementary Table 6). The risk increases with duration of use with current use conferring a higher risk than past use. This is supported by a meta-analysis of case–control studies (Moreno et al. 2002). Increased risk is also likely for POCs (McFarlane-Anderson et al. 2008; International Collaboration et al. 2007).
Autoimmune Diseases
For evaluation of autoimmune diseases which may be related to the use of COCs and POCs, papers were accessed from a PubMed literature review as previously described (Williams 2017). Each paper was rated based on the parameters noted in the STROBE statement (von Elm et al. 2007). As noted previously (Williams 2017, 275), “…substantial evidence exists linking the use of COCs to a lower incidence of hyperthyroidism, an increase in multiple sclerosis (MS), ulcerative colitis, Crohn’s disease, systemic lupus erythematosus (SLE) and interstitial cystitis. Progesterone only contraceptives are linked to progesterone dermatitis and, in one large developing world concurrent cohort study, are associated with increases in arthropathies and related disorders, eczema and contact dermatitis, pruritis and related conditions, alopecia, acne and urticaria.” The evidence with regard to COCs and MS, ulcerative colitis, Crohn’s disease, SLE, and interstitial cystitis are summarized here.
Crohn’s Disease
Overall, 17 primary studies and two meta-analyses were identified which evaluated the effect of COCs on the later development of Crohn’s disease (Figure 1D and Supplementary Table 7). Most studies indicate a significantly increased risk for ever use, current use or past use (Ng et al. 2012; Sicilia et al. 2001; Katschinski 1993; Khalili et al. 2013; Godet, May, and Sutherland 1995). Some analyses suggest that the risk is highest for current use (Cornish et al. 2008). Overall, these studies indicate that the use of COCs conveys an increased risk of Crohn’s disease, especially current use.
Ulcerative Colitis
Overall fourteen primary studies and one meta-analysis were identified which evaluated the effect of COCs on the later development of ulcerative colitis (Figure 1E and Supplementary Table 8). While most of the studies do not show a significantly increase risk with ever use of COCs, there are some that do (Boyko et al. 1994; Parrello et al. 1997) with none showing a decreased risk. One meta-analysis showed a significantly increased risk for current use (Cornish et al. 2008). Overall, these studies suggest that the use of COCs conveys an increased risk of ulcerative colitis, especially current use.
SLE
Seven studies evaluating the effect of COCs on susceptibility to SLE (Figure 1F and Supplementary Table 9) indicate a significantly increased risk for development of SLE with ever use of COCs (Costenbader et al. 2007; Sanchez-Guerrero et al. 1997), current use (Bernier et al. 2009), and for past use (Costenbader et al. 2007). None of the studies showed a decreased risk. COCs are an important risk factor for the subsequent development of SLE.
MS
A total of six studies were identified which evaluated the impact of COCs on the subsequent development of MS (Figure 1G and Supplementary Table 11). Significantly increased risk has been shown for ever use of COCs (Hellwig et al. 2016; Kotzamani et al. 2012) and for current use or past use (Hellwig et al. 2016). Overall, these studies suggest that the use of COCs may convey an increased risk for the subsequent development of MS.
Interstitial Cystitis
Significantly higher use of birth control pills in cases of interstitial cystitis versus controls has been noted (Konkle et al. 2012). COCs appeared to markedly increase the risk of the disease whether past or current use (Gardella et al. 2011). One meta-analysis (Champaneria, D’Andrea, and Latthe 2016) showed that ever use of COCs significantly increased the risk of interstitial cystitis. Overall, use of COCs appears to be associated with an increased risk for the development of interstitial cystitis.
Other Diseases and Disorders
Osteoporotic Bone Fractures
Prescribing information for POCs typically includes a warning regarding the development of osteoporosis. However, the more relevant outcome is fracture risk. Therefore, articles were sought that looked at the effect of COCs and POCs on fracture risk. Data were initially derived from a systematic review of the evidence from observational studies of hormonal contraceptive use for contraception and the risk of fracture in women by Lopez et al. (2015). They noted that in 2004, the US FDA added a warning to DMPA labeling about the potential loss of bone mineral density (BMD), which might limit long-term use. A systematic review of progestin-only methods found an association between DMPA use and loss of BMD (Curtis and Martins 2006). Lopez et al. (2015) identified 559 articles, 524 of which did not meet their inclusion criteria. Thirty-five full-text reports remained, 11 of which were excluded. Of the remaining 24, 10 were secondary articles. That left 14 articles: the 14 studies examined OCs (N = 12), DMPA (N = 4), and the hormonal IUD (N = 1). Similar search terms to Lopez were used for papers published since 2015, and two additional papers were retrieved. The resulting studies are shown in Supplementary Table 12 with selected studies shown in Figure 1H.
COCs
Three early studies (Cooper et al. 1993; Tuppurainen et al. 1993; Vessey, Mant, and Painter 1998) showed an increase risk of fracture with use of COCs. These studies predominately evaluated premenopausal fracture risk. Others that evaluated wrist fracture linked to falling had few cases but showed a trend to decreased risk (O’Neill et al. 1996). One study that evaluated postmenopausal fracture risk based on prior OC use (Barad et al. 2005) also found an increased fracture risk. Another study looking at hip fracture risk in elderly women (Michaëlsson et al. 1999) showed a decreased risk but is compromised in that “The exposure time for oral contraceptives may thus maximally have spanned 5 years…” Two studies by Vestergaard, Rejnmark, and Mosekilde (2006, 2008b) looked at any fracture with OC use and did not show a significant effect when multivariate analyses were performed. However, these studies only looked at use within the past five years and did not take into account remote use or cumulative lifetime use. A small cross-sectional study in southern Tasmania (Wei et al. 2011) was stratified by duration of use and showed a reduction in vertebral deformities for five to ten years of use, but no effect for shorter or longer duration of use and no effect on number of vertebral deformities. A large case–control study which evaluated incident fracture risk with varying numbers of COC prescriptions showed an increased risk for more than ten prescriptions with current use (Meier et al. 2010). A similar study failed to confirm this for most prescription numbers (Kyvernitakis et al. 2017), but this study had fewer subjects reducing its power. A case–control study (Memon, Iversen, and Hannaford 2011) nested in an earlier cohort study (Cooper et al. 1993) failed to show an effect.
Overall, the weight of evidence for use of COCs suggests an increased risk of bone fracture with protracted use. The cohort study by Barad et al. (2005) appears to have the largest number of subjects and was the only study that evaluated postmenopausal fracture risk with prior use of COCs.
POCs
Virtually all the studies evaluating POCs show an elevated risk (Lanza et al. 2013; Vestergaard, Rejnmark, and Mosekilde 2008a; Meier et al. 2010; Kyvernitakis et al. 2017). The relative risk ranges from 1.32 for past use to 1.51 or current use in the one cohort study (Lanza et al. 2013) with the case–control studies showing an elevated risk for patients who have had at least three prescriptions of POCs with ORs ranging from 1.36 to 2.41 (Vestergaard, Rejnmark, and Mosekilde 2008a; Meier et al. 2010; Kyvernitakis et al. 2017). This risk appears to increase with duration of use as measured by the number of prescriptions.
Impact of Contraceptives on Body Mass
Weight gain is a common complaint among contraceptive users but whether use of contraceptives is causally related remains undefined. Progestin-only contraceptives are most commonly associated with weight gain complaints and discontinuation. A recent Cochrane review (Gallo et al. 2014) examined the effect of COCs on weight gain and concluded existing data do not support a causal relationship. A second review of progestin-only contraceptives on weight gain (Lopez et al. 2016) found most studies of low to moderate quality but did conclude weight gain of up to two kilograms (4.4 lbs) within the first year of use with continued increases thereafter. The authors advised appropriate counselling on expected weight changes to minimize discontinuation due to perceived weight gain.
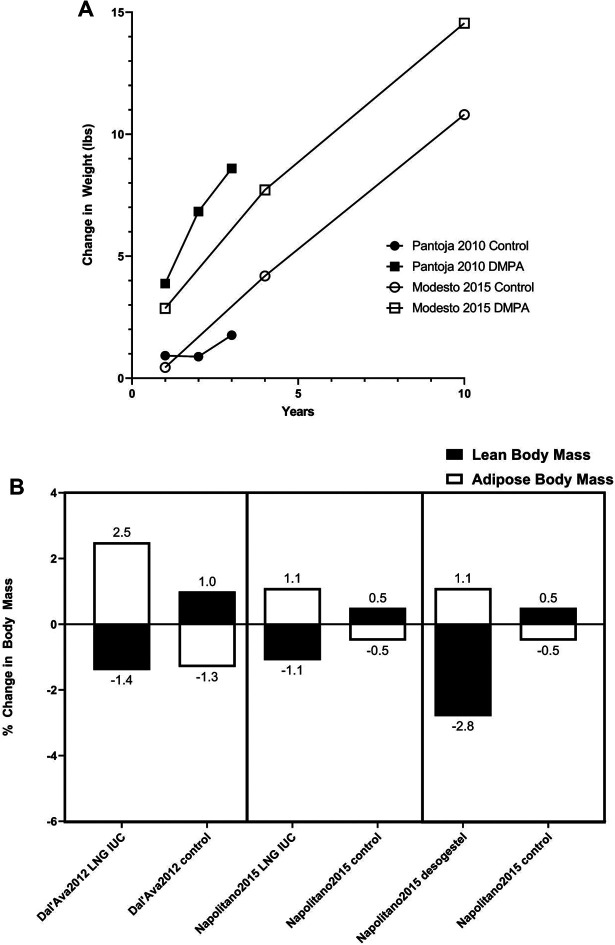
Supplementary Table 13 summarizes studies of one year or longer that examined weight and body mass changes in contraceptive users in comparison to nonhormonal contraceptives or no method. Selected studies are also depicted graphically in Figure 2, with Figure 2A showing data from two studies which followed women over three to ten years following starting DMPA, and Figure 2B showing weight and body composition changes over one year for women on DMPA, those using LNG-releasing IUC, and those using desogestrel. The studies show similar results with weight gain (albeit not reaching statistical significance) compared with controls that continues through 10 years of observation, an increase in fat mass and a decrease or lesser increase in lean mass compared to controls.
Figure 2.
Changes in weight and body composition with depot medroxyprogesterone acetate (DMPA) use from the best available prospective studies. The full data are in Supplementary Table 13. (A) The increase in weight (in pounds) over time in women using DMPA compared with use of copper intrauterine contraceptive (CuIUC; control) is shown from two large retrospective studies. (B) The change in body composition (change in %fat weight and %lean weight) with one year of use is compared for DMPA and CuIUC (Dal’Ava et al. 2012) in the left panel; for a levonorgestrel (LNG)-releasing IUC and CuIUC (Napolitano et al. 2015) in the middle panel; and for desogestrel and no hormonal contraception (Napolitano et al. 2015) in the right panel. Note that all the changes for body composition were statistically significant. Dal’Ava et al. (2012) difference between groups for fat mass p = .0009 and for lean body mass = .027. Fat mass changes were also significant in Napolitano et al.’s (2015) study with LNG versus control p = .02 and Desogetrel versus control p = .0001.
The strongest data appear to be the deleterious effects of LNG-releasing IUDs on percent lean and fat body mass. Total body weight change does not appear different between groups, and several large studies have shown no significant differences. However, a significant increase in % fat mass with a corresponding decrease in % lean body mass was observed in both studies where these were measured. A similar effect was seen from oral desogestrel in a single study.
Thus, while limited to date, data suggest that the use of progestin-only contraceptives may have deleterious effects on % fat and % lean body mass with no statistically significant overall effect on total body weight. A review of current Mirena labeling makes no mention of changes in lean or fat body mass composition.
Retrospective, but not more recent, prospective studies also show DMPA use is associated with significant gains in weight. The data appear too mixed to draw firm conclusions.
Urogenital Effects of Contraceptives
In addition to cervical cancer and interstitial cystitis, noted above, there are other adverse urogenital effects of COCs that should be communicated to patients. These include bacteriuria (Zahran et al. 1976; calculated OR = 3.57), urinary tract infection (Engel 1979: 27 percent to 50 percent incidence), bladder trabeculation (Zahran et al. 1976; calculated OR = 11.7), recurrent vulvovaginal candidiasis (Spinillo et al. 1995; Yusuf et al. 2007; OR = 2.08), vaginal dryness (Lee, Low, and Ang 2017), vulvar vestibulitis (Champaneria, D’Andrea, and Latthe 2016: OR = 2.1 95% CI 1.26–3.49; also noted in Lee, Low, and Ang 2017), and female sexual dysfunction (FSD; Lee, Low, and Ang 2017). FSD appears related to OC-induced dyspareunia, reduced sexual desire, and reduced libido (Lee, Low, and Ang 2017). This risk is increased if COCs are used in adolescents and the duration of OC use is at least two years (Lee, Low, and Ang 2017) although some newer COCs containing drospirenone 3 mg plus EE 30 mg and gestodene 75 mg plus EE 20 mg appear to have a reduction in these risks (Lee, Low, and Ang 2017). These urogenital risks, especially FSD where there is substantial literature, should be referenced in prescribing information and patient pamphlets.
Cardiovascular Effects
Venous Thromboembolism (VTE) and Contraceptives
The current language on the black box warning of certain contraceptives regarding risk of cardiovascular events clearly misleads women about the real risks of these drugs. It says: WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS. A study (Gomer 2009) conducted among 300 women concluded “that most of them believe that certain risks are only associated with being over 35 years of age and/or smoking.” Instead, the label should clearly state that anyone taking the medications without good knowledge of the risk factors could experience a potentially life-threatening cardiovascular event and should discuss the risks with a medical provider.
The incidence of VTE for healthy women can significantly increase with the use of hormonal contraceptives, even women under thirty-five and not-smoking. In a 2012 article about birth control side effects, Dr. Rebecca Peck (Peck and Norris 2012) report that “Oral contraceptives are associated with a three to five times higher risk of VTE” (43). (Van Hylckama et al. 2009). Third and fourth generation combined hormonal contraceptives (COC) have been found to put women at an even much higher risk, leading to major lawsuits against some manufacturers and changes in regulations in several countries. In his opinion published in Drug Safety, Dr. Lidegaard, the author of several studies on the subject, states: “Of 14 studies specifically assessing the risk in users of COC with desogestrel or gestodene, 13 found a higher risk with use of these products when compared to the use of COC with levonorgestrel” (Lidegaard 2014, 1354). Drospirenone, the progestin contained in Yaz and Jasmine, also increases the risk of VTE over LNG by a factor of 1.5–2.8. “The rate ratio of VTE between users of CHC with drospirenone vs levonorgestrel was 1.5 - 2.8, and the relative risk was 6.3 as compared with nonusers in both the large Dutch (Van Hylckama et al. 2009) and Danish (Lidegaard et al. 2011) study” (Lidegaard 2014, 1354). The author comments that “the studies demonstrating risk differences between COC with different progestins are generally methodologically more transparent and more robust than those demonstrating no difference, especially concerning exclusion of women with predispositions for VTE” (1354). Another large study published in 2015 (Vinogradova, Coupland, and Hippisley-Cox 2015) reviewed 10,552 cases of VTE reported between 2001 and 2013 in the UK and found similar elevated risks of VTE with these COC: “Corresponding risks associated with current exposure to desogestrel (4.28, 3.66 to 5.01), gestodene (3.64, 3.00 to 4.43), drospirenone (4.12, 3.43 to 4.96), and cyproterone (4.27, 3.57 to 5.11) were significantly higher than those for second generation contraceptives LNG (2.38, 2.18 to 2.59).” Note that the ORs were “adjusted for smoking status, alcohol consumption, ethnic group, body mass index, comorbidities, and other contraceptive drugs.”
Most importantly, the risk levels are multiplied if women have other risk factors. For instance, women who have the genetic blood condition known as Factor V Leiden could have a risk as high as 18 per 10,000 woman-years. If these women stay on the product for 10 years, their risks could be 250 per 10,000 woman-years, or 2.5 percent as risks increase with aging (Lidegaard 2014).
Dr. Lidegaard (2014) concludes:
Therefore, women with known risk factors of VTE are advised to be reluctant to use COC. The relative risk of VTE with different dispositions is as follows: previous thrombosis: > 50 (Le Moigne et al. 2013), genetic abnormalities such as factor V Leiden mutation (heterozygous): 6, deficiency of protein C: 10, of protein S: 10, of antithrombin: 25, and of prothrombin 20210A: 3 (Phillippe et al. 2014). Pregnancy with delivery on average: 8, adiposity: 2–3 and immobilization 2–5 depending on how long [a] time you are immobilized. Family disposition (first-degree relatives with VTE before their 50th year) doubles the risk of VTE. Women with such dispositions are generally recommended to use progestin-only contraception, which does not increase the risk of VTE except perhaps for medroxyprogesterone depots. A genetic screening should until further also be restricted to women with a family disposition. (1356)
In a 2018 systematic review (Keenan et al. 2018) of the most evidenced-based articles from the 1960s to 2018 comparing users of COCs to nonusers, with a confirmed diagnosis of VTE, and including more than 17 million woman-years of observation, women on hormonal contraceptives (HC) increase their risk by three- to ninefold. However, the first year of use has the highest risk for clot formation, and if a woman is younger than thirty, her risk is increased thirteenfold in the first year. Obesity can increase the risk of being on hormonal contraception, about doubling the risk compared to a woman of normal weight on the pill. It is not considered cost-effective to check for thrombophilia, a genetic disposition to form blood clots, but for those with thrombophilia, the risk can be as high as sixty-two-fold in the first year.
This systematic review of the literature concludes that 136–260 women die from VTE a year in the United States from hormonal contraception. Combined with the added risk of stroke and heart attack from the COCs, 300–400 women die each year in the United States simply due to their choice of using HC for family planning (Keenan et al. 2018). To give some perspective, meningitis killed forty-five people (of all ages) in 2017: most US states mandate meningitis vaccination for college and university students. A summary of studies is shown in Supplementary Table 14 with data from the best cohort studies shown graphically in Figure 1J.
Atherosclerosis and Cardiovascular Events
Noting that previous studies had demonstrated women on OCs faced a fourfold increased risk of heart attack (Hennekens and MacMahon 1977; Vessey et al. 1976; Beral 1976), researchers in 1982 set out to understand the pathogenesis of vascular disease related to COCs. They found that COCs caused “greater cell proliferation and incorporation…in both human arterial smooth muscle cells and dermal fibroblasts.” Smooth muscle cell proliferation is an integral feature of all atherosclerotic lesions (Bagdade and Subbaiah 1982).
In 2007, a presentation at the American Heart Association meeting described a study of 1,301 Belgian women, which showed that women had a 20 percent to 30 percent increase of plaque for every decade on COCs (Rietzschel et al. 2007). They performed a multivariate adjustment for age; smoking; blood pressure (BP); lipids; obesity; diabetes; physical activity; fruit, vegetable, and alcohol intake; educational level; and drug therapy (lipid-lowering, antihypertensive, aspirin). Use of OC was associated with a significant increase in carotid or femoral unilateral plaque (OR per ten years of OC exposure were: carotid plaque 1.17 (1.00–1.33) and femoral plaque 1.28 (1.10–1.47). When evaluating the prevalence of bilateral disease (involvement of right and left carotid/femoral artery) as a more stringent phenotype of atherosclerosis, the OR per ten years of OC exposure were 1.42 (1.03–1.84) for carotid plaque and 1.34 (1.05–1.63) for femoral plaque.
They later noted that active OC users had elevated C-reactive protein (CRP) levels, three times higher than nonusers. CRP is a biomarker for many inflammation-related arterial (and autoimmune) diseases, which was recently the subject of another presentation (Rietzschel et al. 2018). After a similar multivariate analysis, they found the high sensitivity CRP levels were (adjusted geometric means [95% CI]): nonusers 1.0 [0.9 –1.1]; HRT users 1.2 [1.1–1.5] and OC users 3.3 [3.0 –3.6] (OC vs. nonusers: p < .001; HRT vs. NoH: p < .05).
This group also evaluated the carotid and femoral pulse wave velocity (PWV), a measure of arterial stiffness (Rietzschel et al. 2008). They found the average PWV among nonusers was 6.6 m/sec, while the average among current OC users was 6.75 m/sec. The BP of current OC users was also significantly higher (systolic BP [+4.4 ± 0.9 mmHg; p < .001], diastolic BP [+2.3 ± 0.6mmHg; p < .001]). They noted that duration of OC use is a significant determinant of PWV, even after adjustment for age, BP, lipid levels, body size, heart rate, drug therapy (lipid-lowering, antihypertensive), glycemic status, and smoking: F = 6.1; p = .013. Per ten years of OC exposure, PWV increased by 0.1 m/s (0.02–0.18; p = .013). They concluded that current OC use is associated with increased PWV because OCs increase BP, while long-term use is an independent determinant of PWV, increasing PWV by 0.10 m/s per ten years exposure (probably through structural remodeling of the vessels). These findings were supported by an evaluation of large artery stiffness in the ENIGMA study (Hickson et al. 2011) although other smaller studies have shown conflicting data (Yu et al. 2014; Priest, Shenouda, and MacDonald 2018).
A study of homocysteine and nitric oxide (NO) levels compared fifty healthy women with normal menstrual cycles as a control group and fifty healthy women receiving OC pills for at least three menstrual cycles (Fallah et al. 2012). They noted that after three months of treatment, homocysteine levels were significantly increased (p = .027), and there was a significant and considerable decrease (p = .048) in NO concentration of COC consumers. Another study evaluated the effect of COCs on homocysteine and CRP levels in women (Norouzi et al. 2011). This observational cross-sectional analysis included ninety healthy, nonobese women (mean age twenty-five years). Forty-five healthy women on OCP and forty-five healthy controls were studied. COC users had a minimum of three cycles on COCs. The results showed that the homocysteine (13.268 ± 3.475 vs. 7.288 ± 2.621 μmol/L) and CRP (5863.0 ± 1349.5 vs. 1138.3 ± 691.12 ng/ml) levels were significantly higher in women receiving OCP in comparison with the control group (p = 0.027 and p < 0.001). Similarly, a cross-sectional study, in 2011–2012, evaluated sixty healthy premenopausal women (thirty cases of COC consumers and thirty controls as nonconsumers), aged between twenty-five and forty-five years who were current users for at least a three-year period. They evaluated brachial artery endothelial function (using flow-mediated dilatation [FMD]) and common carotid artery intima–media thickness (Heidarzadeh et al. 2014). They noted that there was a significant FMD% difference between two groups of cases and controls: 11 ± 3.53 versus 15.80 ± 9.22 (p = .01). In addition, a significant mean carotid artery intima–media thickness difference was detected: 0.53 ± 0.07 versus 0.44 ± 0.08 (p = .00). Although these results were not significant after multiple regression analysis, the authors noted that their results were in favor of early atherosclerotic changes in prolonged users of COCs.
The Danish Heart Association released the results of a fifteen-year historic cohort study looking at thrombotic stroke and myocardial infarction, which observed over 1.6 million women. The results demonstrated that women taking COCs with ethinyl estradiol at a dose of 20 µg had a risk of arterial thrombosis that was 0.9 to 1.7 times higher than nonusers, while those taking a dose of 30 to 40 µg had a 1.3 to 2.3 higher risk (Lidegaard et al. 2012). The risk of thrombotic stroke appeared to be independent of duration of use, while the risk for myocardial infarction increased with duration of use (Supplementary Table 15).
Together, these studies suggest that protracted use of COCs can induce atherosclerotic changes independent of any pro-thrombotic effect. These changes may contribute to the increase in thrombotic stroke and myocardial infarction seen in COC users.
Psychological Effects
Risk of Depression, Mood Disorders, and Suicide
The effects of contraceptive steroid hormones on depression, mood disorders, and suicide have been investigated (Supplementary Table 10 with Figure 1 depicting data from the best cohort studies). The largest study of incident depression and use of antidepressant medication (Skovlund et al. 2016) indicates significantly increased risks for both COCs and POCs for both outcomes. The same group studied suicide attempts and suicides (Skovlund et al. 2018). Elevated risks were seen, and this was the case for both COCs and POCs. The recent National College Health Assessment study (Gregory et al. 2018) showed a similar trend. One study (Keyes et al. 2013) showed a lower risk of depression but was not measuring clinically diagnosed depression but rather the presence of depressive symptoms within seven days prior to the survey. They also found a lower rate of suicide attempts among COC users. Similar findings were seen in two studies that also used a questionnaire looking at current COC or POC use (Toffol et al. 2011, 2012). An analysis of the development of mood disorders found a higher incidence with POCs but a lower incidence with COCs (Svendal et al. 2012). A study of postpartum depression as a reported adverse drug reaction showed higher rates for levonogestrel, etonogestrel, sertraline, and drospirenone (Horibe et al. 2018). A study of postpartum DMPA versus CuIUD use showed significant increases in depression scores and major depressive episodes with DMPA (Singata-Madliki et al. 2016). A retrospective cohort study showed an increased risk for antidepressant use in patients who used ethinyl estradiol/etonogestrel (ring) and a decreased risk of depression diagnosis with norethindrone-only pills or the LNG intrauterine system (Young et al. 2007). A small retrospective chart review of the effect of immediate postpartum DMPA did not show significant effects on postpartum depression (Tsai and Schaffir 2010). All the papers, which have broken out the age groups of users, show maximum increased risk for depression, suicide risk, and suicide within three months of beginning to use the drugs and tapering off after six months, partly due to attenuation of symptoms, partly due to discontinuation due to adverse effects. These risks need to be adequately conveyed in prescribing information and patient-related materials.
However, little attention has been paid to the effects of blocking the important actions of estradiol and progesterone with progestins during the time of active brain remodeling. Estradiol and progesterone in normal sequence are essential for brain remodeling from ages fifteen to nineteen years particularly for myelination, dendritic pruning, and establishment of new synaptic connections (Del Rio et al. 2018). Suppressing these with synthetic progestins can have far-reaching, untoward effects. See Griksiene below in Supplementary Table 10 as well as Del Rio et al. (2018).
Conclusion
Hormonal agents have a variety of effects on various organs and organ systems which may result in a deleterious impact on women’s health. The data reviewed above reflect a vast body of information which has come to light since the introduction of these agents as contraceptives over fifty years ago. While the information for patients and prescribers currently reflects many of the known side effects, others have come to light which are not adequately represented in the current prescribing information. These should be added and made obvious to patients. In one instance, that of VTE, while the warning information is present, it is phrased in a misleading manner which misinforms the patients into drawing incorrect conclusions regarding the risks. In addition, one agent (DMPA) appears to convey a specific risk for HIV transmission which is not shared by other agents. DMPA should be considered for revoking of marketing authorization and removed from the market. The risks of depression, mood disorders, and suicide have not been adequately emphasized.
The manufacturers of these agents are morally obligated and should be legally required to widely publicize these risks. Many millions of women are currently receiving COCs and POCs. Many millions more have been exposed to these agents at some point in their lives. They should receive updated information regarding risks which, in the past, have been conveyed inadequately or, not at all. All women who have been exposed to COCs or POCs should be informed so that they can take this information into account, as they may encounter some of these adverse effects many years after ceasing use. Women seeking birth control have a right to be informed and educated about risk avoidance through the use of effective nonhormonal methods like fertility awareness methods. Based on these observations, these specific recommendations are noted.
Specific Recommendations
Removal from the Market:
-
DMPA
Remove from the market the injectable contraceptive DMPA (Depo Provera) based on conclusive evidence that it facilitates the transmission of HIV from men to women. Numerous alternatives are available.
Addition of Black Box Warnings to Prescribing Information
-
Breast Cancer
Combined estrogen–progestogen contraceptives (COCs, including oral, intravaginal and transdermal formulations) are acknowledged by World Health Organization International Agency for Research on Cancer (IARC) as Group I carcinogens. Substantial data support an increased risk of breast cancer with the use of COCs. A black box warning should be added to the labeling of all COCs that they have been shown to increase the risk of breast cancer.
POCs have not been extensively studied, but one large registry study did show a significantly increased risk of breast cancer with use of POCs. Lacking evidence to the contrary, a similar warning should be added to all POCs.
-
Cervical Cancer
COCs have been linked to a significantly increased risk of cervical cancer. Similar data have been shown for POCs. A black box warning should be added to the labeling of all COCs and POCs that they have been shown to increase the risk of cervical cancer.
-
Inflammatory Bowel Disease
Significantly higher risk for the development of inflammatory bowel disease, especially Crohn’s disease, but also ulcerative colitis, has been shown for COCs. A black box warning should be added to the labeling of all COCs that their use is linked to a significantly increased risk for the development of inflammatory bowel disease.
-
SLE
Significantly higher risk for the development of SLE has been shown for COCs in several studies, especially the best-designed, largest cohort studies. A black box warning should be added to the labeling of all COCs that their use is linked to a significantly increased risk of the development of SLE.
-
Depression and Suicide
Substantive evidence indicates there is a 25% risk of depression for women under twenty-five years of age especially within six months of starting COCs. A black box warning should be added to the labeling of all COCs that their use is linked to a significantly increased risk of the development of depression.
The relative risk for suicide attempts ranges from 1.91 for COCs to 2.29 for oral progestins, 2.58 for vaginal ring and 3.28 for patch among adolescents and young women—mean age 21 years—peaking within two months of onset of medication. A black box warning should be added to the labeling of all COCs that their use is linked to a significantly increased risk of suicide. Close monitoring is essential especially in the first year of use.
-
Venous Thrombosis and Cardiovascular Events
The current black box warning regarding thrombotic events on some formulations, notes “WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS.” This is misleading and has shown to be misinterpreted by many women who infer that the increased risk only occurs with cigarette smoking and/or with being over thirty-five years of age. The warnings should be amended to state, “WARNING: INCREASED RISK OF SERIOUS CARDIOVASCULAR EVENTS INCLUDING BLOOD CLOTS.”
This warning should be required for hormonal birth control products including oral, intravaginal and transdermal formulations. The patient-related materials should clearly explain the genetic risk factors, other risk factors, and the signs and symptoms. This warning should be included in ALL direct-to-consumer advertising (television, print, radio, etc.).
Additional Safety Information Which Should Be Added to Prescribing Information
-
MS
Significantly higher risk for the development of MS has been shown for COCs in several studies, especially the best-designed, largest case–control studies. A warning should be added to the labeling of all COCs that their use appears to be linked to a significantly increased risk of the development of MS.
-
Bone Fractures
Use of POCs is clearly associated with a higher risk of bone fractures. A warning should be added to the labeling of all POCs that their use is linked to a significantly increased risk of the development of bone fractures.
Protracted use of COCs has been associated with an increased risk of bone fractures. A warning should be added to the labeling of all COCs that their prolonged use may be linked to a significantly increased risk of the development of bone fractures.
-
Body Mass Effects
-
For ANY progestin-releasing IUD, the following should be added to the prescribing information in side effects/precautions:
Progestin-releasing IUDs (IUCs) have demonstrated in clinical trials to significantly increase % fat body mass with a corresponding decrease in % lean body mass over one year of use.
Although the current evidence is less for progestin-only contraceptives, it tends in the same direction. Similar labeling should be considered.
-
-
Urogenital Problems
Interstitial Cystitis: Significantly higher risk for the development of interstitial cystitis has been shown for COCs in two studies. A warning should be added to the labeling of all COCs that their use appears to be linked to a significantly increased risk of the development of interstitial cystitis.
COCs have also been linked to an increased risk of bacteriuria, urinary tract infections, bladder trabeculation, vulvovaginal candidiasis, vaginal dryness, vulvar vestibulitis, and FSD caused by COC-induced dyspareunia and reduced sexual desire and libido. These risks should be adequately conveyed in the prescribing information, especially FSD where there is substantial literature evidence.
Supplemental Material
Supplemental Material, sj-pdf-1-lqr-10.1177_0024363920982709 for Hormonally Active Contraceptives Part I: Risks Acknowledged and Unacknowledged by William V. Williams, Joel Brind, Laura Haynes, Michael D. Manhart, Hanna Klaus, Angela Lanfranchi, Gerard Migeon, Mike Gaskins, Elvis I. Seman, Lester Ruppersberger and Kathleen M. Raviele in The Linacre Quarterly
Biographical Notes
William V. Williams, MD, FACP, is President and CEO of BriaCell Therapeutics Corporation and adjunct professor of medicine of the University of Pennsylvania. He has participated in drug development for over twenty years. He is also a permanent deacon in the Archdiocese of Philadelphia. He and his wife Lorraine have three children and four grandchildren.
Joel Brind earned his PhD from New York University in Basic Medical Sciences, just retired after thirty-four years as a professor of biology and endocrinology at Baruch College, City University of New York. He specializes in research into sex steroid hormone synthesis and metabolism and their connections to human disease. He co-founded the Breast Cancer Prevention Institute in 1999, and from 2003 to 2006, he served on the federal CDC Advisory Committee on the early detection and control of breast and cervical cancer. He has published extensively on the connections between abortion and breast cancer and between contraceptive steroids and HIV transmission.
Laura Haynes earned her PhD in counseling psychology from Biola University, La Mirada, CA; MA in experimental-general psychology from Southern Methodist University. She reviews research, writes, and speaks internationally on sexuality and gender. She has testified before legislative hearing committees in several states in the United States, trained therapists from twenty-six nations, and presented to U.N. diplomats and high-level government officials. She retired from clinical psychology practice in 2018 after more than forty years’ experience.
Michael D. Manhart, PhD, currently serves as a senior scientific consultant for the Couple to Couple League (CCL) and is a member of the FACTS Advisory Council. After receiving his doctorate in Microbiology from the University of Cincinnati College of Medicine, he embarked on a career in Research & Development with Procter& Gamble. While there, he held numerous management roles of increasing responsibility in various global health care and pharmaceutical organizations; he retired in 2008 as a director of R&D. He also served on the CCL Board of Directors from 2007 to 2009. In July 2009, he was appointed CCL Executive Director and led the organization until 2016.
Hanna Klaus, MD, FACOG, is a Medical Mission Sister who has served in Pakistan and Bangladesh and on the faculties of Washington, St. Louis, and George Washington University medical schools. She is co-founder of the international Teen STAR (Sexuality Teaching in the context of Adult Responsibility) program.
Angela Lanfranchi, MD, FACS, is a clinical assistant professor of surgery at Rutgers-Robert Wood Johnson Medical School and president of the Breast Cancer Prevention Institute. She practiced breast surgery in New Jersey for thirty-three years.
Gerard Migeon, ESCP, is the co-founder and CEO of Natural Womanhood and naturalwomanhood.org, an organization dedicated to transforming reproductive health and relationships by empowering women and couples to embrace fertility awareness, natural family planning, and comprehensive women’s health.
Mike Gaskins, BA, a journalist and author of the book, In the Name of The Pill, has researched and written extensively about the history of birth control and the risks associated with various contraceptives. His email address is mgaskins@gmail.com.
Elvis I. Seman, MBBS, FRANZCOG, EUCOGE, FRCOG, NFPMC, PhD, trained in obstetrics and gynecology in Australia and the United Kingdom. He is currently medical lead in urogynecology at Flinders Medical Centre and senior lecturer at Flinders University, South Australia. He is actively involved in MaterCare and the Catholic Medical Association of South Australia. He also has an interest in restorative reproductive medicine and gained a PhD in laparoscopic pelvic floor repair. He is married to Marija. They have two sons and a delightful nineteen-month-old granddaughter.
Lester Ruppersberger, DO, FACOOG, is a retired board-certified NFP-only Ob/Gyn with thirty-seven years of clinical experience. He is a past president of the Catholic Medical Association and current president of the Philadelphia Guild of the CMA. He was a host with his wife of fifty years, Betty, on Catholic radio for fifteen years discussing NFP. He is the Medical Director for two crisis pregnancy centers in the Philadelphia area. He serves on the Board of the Couple to Couple League. He has authored several articles and a self-published book for parents called Wonderfully Made Babies. He is a frequent speaker for CMA-sponsored programs. He has two grown children and ten grandchildren.
Kathleen M. Raviele, MD, is a fellow in the American College of Obstetricians and Gynecologists and is a past president of the Catholic Medical Association. She practiced gynecology in the Atlanta area for thirty-nine years. Her email address is ravielek@gmail.com.
Notes
PBS (1999–2002a). Timeline: The pill, Genesis-1950. American Experience, http://www.pbs.org/wgbh/amex/pill/timeline/index.html.
PBS (1999–2002b). Timeline: The pill, 1951–1990. American Experience, http://www.pbs.org/wgbh/amex/pill/timeline/timeline2.html.
Michael, R. (1988): Table A-1, pp. 394–95, where DF represents “the diffusion of new contraceptive technological possibilities embodied in the pill and IUD” (p. 376) and DRATE represents “divorce rate—the number of divorces per 1000 married women” age greater than or equal to 15 (p. 374). DF reached 97 percent in 1974, a mere nine years after the Supreme Court legalized contraception.
Prescribing information available at http://labeling.bayerhealthcare.com/html/products/pi/fhc/YAZ_PI.pdf
Prescribing information available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021241s027lbl.pdf
Product monograph available at https://www.pfizer.ca/sites/default/files/201812/Alesse_PM_E_219900_03_December_2018.pdf
Prescribing information available at https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/NDA-22501-Lo-Loestrin-Fe-PI-CLEAN-08-2017_ver1.pdf
Prescribing information available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020713s010lbl.pdf
Prescribing information available at https://www.merck.com/product/usa/pi_circulars/n/nexplanon/nexplanon_pi.pdf
Prescribing information available at http://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: William V. Williams, MD, FACP  https://orcid.org/0000-0003-3611-9284
https://orcid.org/0000-0003-3611-9284
Michael D. Manhart, PhD  https://orcid.org/0000-0003-1727-1846
https://orcid.org/0000-0003-1727-1846
Supplemental Material: The supplemental material for this article is available online.
References
- Bagdade J. D., Subbaiah P. V.. 1982. “Serum from Oral Contraceptive Users Stimulates Growth of Arterial Smooth Muscle Cells.” AHA Journals, Arteriosclerosis 2, no. 2: 170–76. [DOI] [PubMed] [Google Scholar]
- Barad D., Kooperberg C., Wactawski-Wende J., Liu J., Hendrix S. L., Watts N. B.. 2005. “Prior Oral Contraception and Postmenopausal Fracture: A Womens’ Health Initiative Observational Cohort Study.” Fertility and Sterility 84:374–83. [DOI] [PubMed] [Google Scholar]
- Beaber E. F., Malone K. E., Tang M. T., Barlow W. E., Porter P. L., Daling J. R., Li C. I.. 2014. “Oral Contraceptives and Breast Cancer Risk Overall and by Molecular Subtype among Young Women.” Cancer Epidemiology, Biomarkers & Prevention 23:755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V. 1976. “Cardiovascular Disease Mortality Trends and Oral Contraceptive Use in Young Women.” Lancet 2:1047–52. [DOI] [PubMed] [Google Scholar]
- Bernier M. O., Mikaeloff Y., Hudson M., Suissa S.. 2009. “Combined Oral Contraceptive Use and the Risk of Systemic Lupus Erythematosus.” Arthritis and Rheumatism 61:476–81. [DOI] [PubMed] [Google Scholar]
- Bethea T. N., Rosenberg L., Hong C. C., Troester M. A., Lunetta K. L., Bandera E. V., Schedin P., et al. 2015. “A Case-control Analysis of Oral Contraceptive Use and Breast Cancer Subtypes in the African American Breast Cancer Epidemiology and Risk Consortium.” Breast Cancer Research 17:22:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko E. J., Theis M. K., Vaughan T. L., Nicol-Blades B.. 1994. “Increased Risk of Inflammatory Bowel Disease Associated with Oral Contraceptive Use.” American Journal of Epidemiology 140:268–78. [DOI] [PubMed] [Google Scholar]
- Brind J., Condly S. J., Mosher S. W., Morse A. R., Kimball J.. 2015. “Risk of HIV Infection in Depot Medroxyprogesterone Acetate (DMPA) Users: A Systematic Review and Meta-analysis.” Issues in Law & Medicine 30:129–39. [PubMed] [Google Scholar]
- Champaneria R., D’Andrea R. M., Latthe P. M.. 2016. “Hormonal Contraception and Pelvic Floor Dysfunction: A Systematic Review.” International Urogynecology Journal 27:709–22. [DOI] [PubMed] [Google Scholar]
- Cooper C., Hannaford P., Croft P., Kay C. R.. 1993. “Oral Contraceptive Pill Use and Fractures in Women: A Prospective Study.” Bone 14, no. 1: 41–45. [DOI] [PubMed] [Google Scholar]
- Cornish J. A., Tan E., Simillis C., Clark S. K., Teare J., Tekkis P. P.. 2008. “The Risk of Oral Contraceptives in the Etiology of Inflammatory Bowel Disease: A Meta-analysis.” American Journal of Gastroenterology 103:2394–2400. [DOI] [PubMed] [Google Scholar]
- Costenbader K. H., Feskanich D., Stampfer M. J., Karlson E. W.. 2007. “Reproductive and Menopausal Factors and Risk of Systemic Lupus Erythematosus in Women.” Arthritis and Rheumatism 56:1251–62. [DOI] [PubMed] [Google Scholar]
- Curtis K. M., Martins S. L.. 2006. “Progestin-only Contraception and Bone Mineral Density: A Systematic Review.” Contraception 73:470–87. [DOI] [PubMed] [Google Scholar]
- Del Rio J. P., Allende M. I., Molina N., Serrano F. G., Molina S., Vigil P.. 2018. “Steroid Hormones and Their Action in Women’s Brains: The Importance of Hormonal Balance.” Frontiers in Public Health 6:141:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal’Ava N., Bahamondes L., Bahamondes M. V., de Oliveira Santos A., Monteiro I.. 2012. “Body Weight and Composition in Users of Levonorgestrel-releasing Intrauterine System.” Contraception 86, no. 4:350–53. [DOI] [PubMed] [Google Scholar]
- Dolle J. M., Daling J. R., White E., Brinton L. A., Doody D. R., Porter P. L., Malone K. E.. 2009. “Risk Factors for Triple-negative Breast Cancer in Women under the Age of 45 Years.” Cancer Epidemiology, Biomarkers & Prevention 18:1157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel H. J. 1979. “Adverse Effects of Oral Contraceptives.” Medizinische Monatsschrift für Pharmazeuten 2:199–204. [PubMed] [Google Scholar]
- Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. 2019. “HIV Incidence among Women Using Intramuscular Depot Medroxyprogesterone Acetate, A Copper Intrauterine Device, or a Levonorgestrel Implant for Contraception: A Randomised, Multicentre, Open-label Trial.” Lancet 394, no. 10195: 303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah S., Nouroozi V., Seifi M., Samadikuchaksaraei A., Aghdashi E. M.. 2012. “Influence of Oral Contraceptive Pills on Homocysteine and Nitric Oxide Levels: As Risk Factors for Cardiovascular Disease.” Journal of Clinical Laboratory Analysis 26:120–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Talk Paper. 2004. “U.S. Food and Drug Administration. Black box Warning Added Concerning Long-term Use of Depo-Provera Contraceptive Injection.” http://web.archive.org/web/20070809090332/http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01325.html.
- Gallo M. F., Lopez L. M., Grimes D. A., Carayon F., Schulz K. F., Helmerhorst F. M.. 2014. “Combination Contraceptives: Effects on Weight.” Cochrane Database of Systematic Reviews 9:Art. No.CD003987. [DOI] [PubMed] [Google Scholar]
- Gardella B., Porru D., Nappi R. E., Daccò M. D., Chiesa A., Spinillo A.. 2011. “Interstitial Cystitis Is Associated with Vulvodynia and Sexual Dysfunction—A Case-control Study.” The Journal of Sexual Medicine 8:1726–34. [DOI] [PubMed] [Google Scholar]
- Godet P. G., May G. R., Sutherland L. R.. 1995. “Meta-analysis of the Role of Oral Contraceptive Agents in Inflammatory Bowel Disease.” Gut 37:668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer K. 2009. Women, Birth Control Pills, and Thrombophilia: An Analysis of Risk Communication Kerry Gomer. Clemson University. https://tigerprints.clemson.edu/cgi/viewcontent.cgi?referer=&httpsredir=1&article=1573&context=all_theses. [Google Scholar]
- Govender Y., Avenant C., Verhoog N. J., Ray R. M., Grantham N. J., Africander D., Hapgood J. P.. 2014. “The Injectable-only Contraceptive Medroxyprogesterone Acetate, Unlike Norethisterone Acetate and Progesterone, Regulates Inflammatory Genes in Endocervical Cells via the Glucocorticoid Receptor.” PLoS One 9:e96497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S. T., Hall K., Quast T., Gatto A., Bleck J., Storch E. A., DeBate R.. 2018. “Hormonal Contraception, Depression and Academic Performance among Females Attending College in the United States.” Psychiatry Research 270:111–16. [DOI] [PubMed] [Google Scholar]
- Hapgood J. P., Ray R. M., Govender Y., Avenant C., Tomasicchio M.. 2014. “Differential Glucocorticoid Receptor-mediated Effects on Immunomodulatory Gene Expression by Progestin Contraceptives: Implications for HIV-1 Pathogenesis.” American Journal of Reproductive Immunology 71, no. 6: 505–12. [DOI] [PubMed] [Google Scholar]
- Hapgood J. P., Tomasicchio M.. 2010. “Modulation of HIV-1 Virulence via the Host Glucocorticoid Receptor: Towards Further Understanding the Molecular Mechanisms of HIV-1 Pathogenesis.” Archives of Virology 155:1009–19. [DOI] [PubMed] [Google Scholar]
- Heffron R., Donnell D., Rees H., Celum C., Mugo N., Were E., de Bruyn G., et al. 2012. “Use of Hormonal Contraceptives and Risk of HIV-1 Transmission: A Prospective Cohort Study.” Lancet Infectious Diseases 12:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidarzadeh Z., Asadi B., Saadatnia M., Ghorbani A., Fatehi F.. 2014. “The Effect of Low-dose Combined Oral Contraceptive Pills on Brachial Artery Endothelial Function and Common Carotid Artery Intima–Media Thickness.” Journal of Stroke and Cerebrovascular Diseases 23:675–80. [DOI] [PubMed] [Google Scholar]
- Heikkinen S., Koskenvuo M., Malila N., Sarkeala T., Pukkala E., Pitkäniemi J. 2016. “Use of Exogenous Hormones and the Risk of Breast Cancer: Results from Self-reported Survey Data with Validity Assessment.” Cancer Causes & Control 27:249–58. [DOI] [PubMed] [Google Scholar]
- Hellwig K., Chen L. H., Stancyzk F. Z., Langer-Gould A. M. 2016. “Oral Contraceptives and Multiple Sclerosis/Clinically Isolated Syndrome Susceptibility.” PLoS One 11:e0149094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekens C. H., MacMahon B.. 1977. “Oral Contraceptive and Myocardlal Infarction.” New England Journal of Medicine 296:1166–67. [DOI] [PubMed] [Google Scholar]
- Hickson S. S., Miles K. L., McDonnell B. J., Yasmin K. M., Cockcroft J. R., Wilkinson I. B., McEniery C. M., Study Investigators ENIGMA. 2011. “Use of the Oral Contraceptive Pill Is associated with Increased Large Artery Stiffness in Young Women: The ENIGMA Study.” Journal of Hypertension 29, no. 6: 1155–59. [DOI] [PubMed] [Google Scholar]
- Horibe M., Hane Y., Abe J., Matsui T., Kato Y., Ueda N., Sasaoka S., et al. 2018. “Contraceptives as Possible Risk Factors for Postpartum Depression: A Retrospective Study of the Food and Drug Administration Adverse Event Reporting System, 2004-2015.” Nursing Open 5, no.2: 131–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts R. P., Michel K. G., Hel Z.. 2014. “Effect of Progestins on Immunity: Medroxyprogesterone but not Norethisterone or Levonorgestrel Suppresses the Function of T Cells and pDCs.” Contraception 90, no. 2: 123–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Collaboration of Epidemiological Studies of Cervical Cancer, Appleby P., Beral V, Berrington de González A, Colin D, Franceschi S, Goodhill A, et al. 2007. “Cervical Cancer and Hormonal Contraceptives: Collaborative Reanalysis of Individual Data for 16,573 Women with Cervical Cancer and 35,509 Women without Cervical Cancer from 24 Epidemiological Studies.” Lancet 370:1609–21. [DOI] [PubMed] [Google Scholar]
- Kahlenborn C., Modugno F., Potter D. M., Severs W. B.. 2006. “Oral Contraceptive Use as a Risk Factor for Premenopausal Breast Cancer: A Meta-analysis.” Mayo Clinic Proceedings 81:1290–1302. [DOI] [PubMed] [Google Scholar]
- Katschinski B. 1993. “Smoking and Ovulation Inhibitor in Inflammatory Bowel Diseases.” Medizinische Klinik (Munich, Germany) 88 (Suppl 1): 5–8. [PubMed] [Google Scholar]
- Keenan L., Kerr T., Duane M., Van Gundy K.. 2018. “Systematic Review of Hormonal Contraception and Risk of Venous Thrombosis.” The Linacre Quarterly 85, no. 4: 470–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes Katherine T., Cheslack-Postava K., Westhoff C., Heim C. M., Haloossim M., Walsh K., Koenen K.. 2013. “Association of Hormonal Contraception Use with Reduced levels of Depressive Symptoms: A National Study of Sexually Active Women in the United States.” American Journal of Epidemiology 178, no. 9: 1378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili H., Higuchi L. M., Ananthakrishnan A. N., Richter J. M., Feskanich D., Fuchs C. S., Chan A. T.. 2013. “Oral Contraceptives, Reproductive Factors and Risk of Inflammatory Bowel Disease.” Gut 62:1153–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle K., Berry S. H., Elliott M. N., Hilton L., Suttorp M. J., Clauw D. J., Clemens J. Q.. 2012. “Comparison of an Interstitial Cystitis/Bladder Pain Syndrome Clinical Cohort with Symptomatic Community Women from the RAND Interstitial Cystitis Epidemiology Study.” Journal of Urology 187:508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsopoulos J., Lubinski J., Moller P., Lynch H. T., Singer C. F., Eng C., Neuhausen S. L., et al. 2014. “Timing of Oral Contraceptive Use and the Risk of Breast Cancer in BRCA1 Mutation Carriers.” Breast Cancer Research and Treatment 143:579–86. [DOI] [PubMed] [Google Scholar]
- Kotzamani D., Panou T., Mastorodemos V., Tzagournissakis M., Nikolakaki H., Spanaki C., Plaitakis A.. 2012. “Rising Incidence of Multiple Sclerosis in Females associated with Urbanization.” Neurology 78:1728–35. [DOI] [PubMed] [Google Scholar]
- Kyvernitakis I., Kostev K., Nassour T., Thomasius F., Hadji P.. 2017. “The Impact of Depot Medroxyprogesterone Acetate on Fracture Risk: A Case-control Study from the UK.” Osteoporosis International 28, no. 1: 291–97. [DOI] [PubMed] [Google Scholar]
- Lanza L. L., McQuay L. J., Rothman K. J., Bone H. G., Kaunitz A. M., Harel Z., Ataher Q., Ross D., Arena P. L., Wolter K. D.. 2013. “Use of Depot Medroxyprogesterone Acetate Contraception and Incidence of Bone Fracture.” Obstetrics and Gynecology 121, no. 3: 593–600. [DOI] [PubMed] [Google Scholar]
- Lee J. J. M. L., Low L. L., Ang S. B.. 2017. “Oral Contraception and Female Sexual Dysfunction in Reproductive Women.” Sexual Medicine Reviews 5:31–44. [Google Scholar]
- Le Moigne E., Delluc A., Tromeur C., Nowak E., Mottier D., Lacut K., Le Gal G.. 2013. “Risk of Recurrent Venous Thromboembolism among Young Women after a First Event While Exposed to Combined Oral Contraception versus Not Exposed To: A Cohort Study.” Thrombosis Research 132:51–55. [DOI] [PubMed] [Google Scholar]
- Lidegaard Ø. 2014. “Hormonal Contraception, Thrombosis and Age.” Expert Opinion on Drug Safety 13:1353–60. [DOI] [PubMed] [Google Scholar]
- Lidegaard Ø., Nielsen L. H., Skovlund C. W., Løkkegaard E.. 2012. “Venous Thrombosis in Users of Non-oral Hormonal Contraception: Follow-up Study, Denmark 2001–10.” British Medical Journal 344:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidegaard Ø., Nielsen L. H., Skovlund C. W., Skjeldestad F. E., Løkkegaard E.. 2011. “Risk of Venous Thromboembolism from Use of Oral Contraceptives Containing Different Progestogens and Estrogen Doses: Danish Cohort Study 2001–2009.” British Medical Journal 343:d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L. M., Chen M., Mullins Long S., Curtis K. M., Helmerhorst F. M.. 2015. “Steroidal Contraceptives and Bone Fractures in Women: Evidence from Observational Studies (Review).” Cochrane Database of Systematic Reviews 7: Art. No. CD009849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L. M., Ramesh S., Chen M., Edelman A., Otterness C., Trussell J., Helmerhorst F. M.. 2016. “Progestin-only Contraceptives: Effects on Weight (Review).” Cochrane Database of Systematic Reviews 8: Art. No. CD008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Bakken K., Dumeaux V., Andersen V., Kumle M.. 2007. “Hormone Replacement Therapy and Breast Cancer in Former Users of Oral Contraceptives—The Norwegian Women and Cancer Study.” International Journal of Cancer 121:645–48. [DOI] [PubMed] [Google Scholar]
- Maritz M. F., Ray R. M., Bick A. J., Tomasicchio M., Woodland J. G., Govender Y., Avenant C., Hapgood J. P.. 2018. “Medroxyprogesterone Acetate, Unlike Norethisterone, Increases HIV-1 Replication in Human Peripheral Blood Mononuclear Cells and an Indicator Cell Line, via Mechanisms Involving the Glucocorticoid Receptor, Increased CD4/CD8 Ratios and CCR5 Levels.” PLoS One 13, no. 4: e0196043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane-Anderson N., Bazuaye P. E., Jackson M. D., Smikle M., Fletcher H. M.. 2008. “Cervical Dysplasia and Cancer and the Use of Hormonal Contraceptives in Jamaican Women.” BMC Womens Health 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrgou A., Akouchekian M.. 2016. “The Importance of BRCA1 and BRCA2 Genes Mutations in Breast Cancer Development.” Medical Journal of the Islamic Republic of Iran 30:369. [PMC free article] [PubMed] [Google Scholar]
- Meier C., Brauchli Y. B., Jick S. S., Kraenzlin M. E., Meier C. R.. 2010. “Use of Depot Medroxyprogesterone Acetate and Fracture Risk.” Journal of Clinical Endocrinology and Metabolism 95:4909–5016. [DOI] [PubMed] [Google Scholar]
- Memon S., Iversen L., Hannaford P. C.. 2011. “Is the Oral Contraceptive Pill associated with Fracture in Later Life? New Evidence from the Royal College of General Practitioners Oral Contraception Study.” Contraception 84:40–47. [DOI] [PubMed] [Google Scholar]
- Michael R. 1988. “Why Did the U.S. Divorce Rate Double within a Decade?” Research in Population Economics 6:367–99. ISBN: 0-89232-626-3. Obtained from the author by request. Contact: Robert T. (Bob) Michael, rmichael@uchicago.edu. [Google Scholar]
- Michaëlsson K., Baron J. A., Farahmand B. Y., Persson I., Ljunghall S.. 1999. “Oral-contraceptive Use and Risk of Hip Fracture: A Case-control Study.” Lancet 353:1481–84. [DOI] [PubMed] [Google Scholar]
- Mørch L. S., Skovlund C. W., Hannaford P. C., Iversen L., Fielding S., Lidegaard Ø. 2017. “Contemporary Hormonal Contraception and the Risk of Breast Cancer.” New England Journal of Medicine 377:2228–39. [DOI] [PubMed] [Google Scholar]
- Moreno V., Bosch F. X., Muñoz N., Meijer C. J., Shah K. V., Walboomers J. M., Herrero R., Franceschi S.. 2002. “International Agency for Research on Cancer. Multicentric Cervical Cancer Study Group. Effect of Oral Contraceptives on Risk of Cervical Cancer in Women with Human Papillomavirus Infection: The IARC Multicentric Case-control Study.” Lancet 359:1085–92. [DOI] [PubMed] [Google Scholar]
- Morrison C. S., Chen P.-L., Kwok C., Baeten J. M., Brown J., Crook A. M., Van Damme L., et al. 2015. “Hormonal Contraception and the Risk of HIV Acquisition: An Individual Participant Data Meta-analysis.” PLOS Medicine 12:e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano A., Zanin R., Palma F., Romani C., Grandi G., Di Carlo C., Cagnacci A.. 2015. “Body Composition and Resting Metabolic Rate of Perimenopausal Women Using Continuous Progestogen Contraception.” European Journal of Contraception and Reproductive Health Care. 10.3109/13625187.2015.1079610. [DOI] [PubMed] [Google Scholar]
- Ng S. C., Woodrow S., Patel N., Subhani J., Harbord M.. 2012. “Role of Genetic and Environmental Factors in British Twins with Inflammatory Bowel Disease.” Inflammatory Bowel Disease 18:725–36. [DOI] [PubMed] [Google Scholar]
- Norouzi V., Seifi M., Fallah S., Korani M., Samadikuchaksaraei A.. 2011. “Effect of Oral Contraceptive Therapy on Homocysteine and C-reactive Protein Levels in Women: An Observational Study.” Anadolu Kardiyol Derg 11:698–702. [DOI] [PubMed] [Google Scholar]
- O’Neill T. W., Marsden D., Adams J. E., Silman A. J.. 1996. “Risk Factors, Falls, and Fracture of the Distal Forearm in Manchester, UK.” Journal of Epidemiology and Community Health 50:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrello T., Pavia M., Angelillo I. F., Monteleone G., Riegler G., Papi G., D’Incà R., Annese V., Tonelli F., Caprilli R., Pallone F.. 1997. “Appendectomy Is an Independent Protective Factor for Ulcerative Colitis: Results of a Multicentre Case Control Study. The Italian Group for the Study of the Colon and Rectum (GISC).” Italian Journal of Gastroenterology and Hepatology 29:208–11. [PubMed] [Google Scholar]
- Paul VI, Pope. 1968. Humanae Vitae. http://w2.vatican.va/content/paul-vi/en/encyclicals/documents/hf_p-vi_enc_25071968_humanae-vitae.html.
- Peck R., Norris C. W.. 2012. “Significant Risks of Oral Contraceptives (OCPs).” Linacre Quarterly 79:41–56. doi: 10.1179/002436312803571447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillippe H. M., Hornsby L. B., Treadway S., Armstrong E. M., Bellone J. M.. 2014. “Inherited Thrombophilia.” Journal of Pharmacy Practice 27:227–33. [DOI] [PubMed] [Google Scholar]
- Poosari A., Promthet S., Kamsa-ard S., Suwanrungruang K., Longkul J., Wiangnon S.. 2014. “Hormonal Contraceptive Use and Breast Cancer in Thai Women.” Journal of Epidemiology 24:216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest S. E., Shenouda N., MacDonald M. J.. 2018. “Effect of Sex, Menstrual Cycle Phase, and Monophasic Oral Contraceptive Pill Use on Local and Central Arterial Stiffness in Young Adults.” American Journal of Physiology: Heart and Circulatory Physiology 315:H357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietzschel E., De Buyzere M., De Baquer D., Bekaert S., Segers P., Cassiman P., Verdonck P., De Backer G., Gillebert T., Investigators ASKLEPIOS. 2007. “Anticonceptive Drug Use and Increased Carotid and Femoral Plaque Prevalence: Population Data from Asklepios.” Circulation 116:820. [Google Scholar]
- Rietzschel E., De Buyzere M., De Baquer D., Langlois M., Bekaert S., Segers P., Van Damme P., Verdonck P., De Backer G., Gillebert T., Investigators ASKLEPIOS. 2018. “Oral Contraceptives Cause Major C-reactive Protein Rises in the Female General Population.” Circulation 116:800–801. [Google Scholar]
- Rietzschel E., De Buyzere M., Segers P., Bekaert S., De Baquer D., De Backer G., Gillebert T.. 2008. “Long Term Oral Contraceptive Use is an Independent Risk Factor for Arterial Stiffening.” Circulation 118:S803–4. [Google Scholar]
- Roura E., Travier N., Waterboer T., de Sanjosé S., Bosch F. X., Pawlita M., Pala V., et al. 2016. “The Influence of Hormonal Factors on the Risk of Developing Cervical Cancer and Pre-cancer: Results from the EPIC Cohort.” PLoS One 11:e0147029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guerrero J., Karlson E. W., Liang M. H., Hunter D. J., Speizer F. E., Colditz G. A.. 1997. “Past Use of Oral Contraceptives and the Risk of Developing Systemic Lupus Erythematosus.” Arthritis and Rheumatism 40:804–8. [DOI] [PubMed] [Google Scholar]
- Sathyamala C. 2019. “Depot Contraception and HIV: An Exercise in Obfuscation.” British Medical Journal 367:l5768. doi: 10.1136/bmj.l5768. [DOI] [PubMed] [Google Scholar]
- Sathyamala C. 2020. “Depo-Provera and HIV Transmission: WHO to Trust?” Different Takes 95:1–6. https://sites.hampshire.edu/popdev/files/2020/01/DT-95.pdf. [Google Scholar]
- Schindler A. E., Campagnoli C., Druckmann R., Huber J., Pasqualini J. R., Schweppe K. W., Thijssen J. H.. 2003. “Classification and Pharmacology of Progestins.” Maturitas 46 (Suppl 1): S7–16. [DOI] [PubMed] [Google Scholar]
- Sicilia B., López Miguel C., Arribas F., López Zaborras J., Sierra E., Gomollón F.. 2001. “Environmental Risk Factors and Crohn’s Disease: A Population-based, Case-control Study in Spain.” Digestive and Liver Disease 33:762–67. [DOI] [PubMed] [Google Scholar]
- Singata-Madliki M., Hofmeyr G. J., Lawrie T. A.. 2016. “The Effect of Depot Medroxyprogesterone Acetate on Postnatal Depression: A Randomised Controlled Trial.” Journal of Family Planning and Reproductive Health Care 42:171–76. [DOI] [PubMed] [Google Scholar]
- Skovlund C. W., Mørch L. S., Kessling L. V., Lidegaard O.. 2016. “Association of Hormonal Contraception with Depression.” JAMA Psychiatry 73:1154–62. [DOI] [PubMed] [Google Scholar]
- Skovlund C. W., Mørch L. S., Kessling L. V., Lange T., Lidegaard O.. 2018. “Association of Hormonal Contraception with Suicide Attempts and Suicides.” American Journal of Psychiatry 175:336–42. [DOI] [PubMed] [Google Scholar]
- Soini T., Hurskainen R., Grénman S., Mäenpää J., Paavonen J., Pukkala E.. 2014. “Cancer Risk in Women Using the Levonorgestrel-releasing Intrauterine System in Finland.” Obstetrics & Gynecology 124:292–99. [DOI] [PubMed] [Google Scholar]
- Spinillo A., Capuzzo E., Nicola S., Baltaro F., Ferrari A., Monaco A.. 1995. “The Impact of Oral Contraception on Vulvovaginal Candidiasis.” Contraception 51:293–97. [DOI] [PubMed] [Google Scholar]
- Svendal G., Berk M., Pasco J. A., Jacka F. N.. 2012. “The Use of Hormonal Contraceptive Agents and Mood Disorders in Women.” Journal of Affective Disorders 140:92–96. [DOI] [PubMed] [Google Scholar]
- Toffol E., Heiknheimo O., Koponen P., Luoto R., Partonen T.. 2011. “Hormonal Contraception and Mental Health: Results of a Population Based Study.” Human Reproduction 26:3085–93. [DOI] [PubMed] [Google Scholar]
- Toffol E., Heikinheimo O., Koponen P., Luoto R., Partonen T.. 2012. “Further Evidence for Lack of Negative Associations between Hormonal Contraception and Mental Health.” Contraception 86:470–80. [DOI] [PubMed] [Google Scholar]
- Tomasicchio M., Avenant C., Toit A. Du, Ray R. M., Hapgood J. P.. 2013. “The Progestin-only Contraceptive Medroxyprogesterone Acetate, but Not Norethisterone Acetate, Enhances HIV-1 Vpr-Mediated Apoptosis in Human CD4+ T Cells through the Glucocorticoid Receptor.” PLoS One 8:e62895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai R., Schaffir J.. 2010. “Effect of Depot Medroxyprogesterone Acetate on Postpartum Depression.” Contraception 82:174–77. [DOI] [PubMed] [Google Scholar]
- Tuppurainen M., Honkanen R., Kröger H., Saarikoski S., Alhava E.. 1993. “Osteoporosis Risk Factors, Gynaecological History and Fractures in Perimenopausal Women—The Results of the Baseline Postal Enquiry of the Kuopio Osteoporosis Risk Factor and Prevention Study.” Maturitas 17:89–100. [DOI] [PubMed] [Google Scholar]
- Van Hylckama V. A., Helmerhorst F. M., Vandenbroucke J. P., Doggen C. J., Rosendaal F. R.. 2009. “The Venous Thrombotic Risk of Oral Contraceptives, Effects of Estrogen Dose and Progestogen Type: Results of the MEGA Case-control Study.” British Medical Journal 339:b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey M., Doll R., Peto R., Johnson B., Wiggins P.. 1976. “A Longterm Follow-up Study of Women Using Different Methods of Contraception—An Interim Report.” Journal of Biosocial Science 8:373–427. [DOI] [PubMed] [Google Scholar]
- Vessey M., Mant J., Painter R.. 1998. “Oral Contraception and Other Factors in Relation to Hospital Referral for Fracture. Findings in a Large Cohort Study.” Contraception 57:231–35. [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L.. 2006. “Oral Contraceptive Use and Risk of Fractures.” Contraception 73:571–76. [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L.. 2008. a. “The Effects of Depot Medroxyprogesterone Acetate and Intrauterine Device Use on Fracture Risk in Danish Women.” Contraception 78:459–64. [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L.. 2008. b. “Fracture Risk in Very Young Women Using Combined Oral Contraceptives.” Contraception 78:358–64. [DOI] [PubMed] [Google Scholar]
- Vinogradova Y., Coupland C., Hippisley-Cox J. 2015. “Use of Combined Oral Contraceptives and Risk of Venous Thromboembolism: Nested Case-control Studies Using the QResearch and CPRD Databases.” British Medical Journal 350:h2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Elm E., Altman D. G., Egger M., Pocock S. J., Gøtzsche P. C., Vandenbroucke J. P., Initiative STROBE. 2007. “The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies.” PLoS Medicine 4:1623–27 (e296). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Venn A., Ding C., Foley S., Laslett L., Jones G.. 2011. “The Association between Oral Contraceptive Use, Bone Mineral Density and Fractures in Women Aged 50-80 Years.” Contraception 84:357–62. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2017. Hormonal Contraceptive Eligibility for Women at High Risk of HIV. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Williams W. V., Carlson K., Mitchell L. A., Raviele K.. 2018. “Association of Combined Estrogen-progestogen and Progestogen Only Contraceptives with the Development of Cancer.” The Linacre Quarterly 85:412–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V. 2017. “Hormonal Contraception and the Development of Autoimmunity: A Review of the Literature.” Linacre Quarterly 84:275–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization International Agency for Research on Cancer. 1999. “IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Hormonal Contraception and Post-Menopausal Hormonal Therapy.” V72. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono72.pdf.
- World Health Organization International Agency for Research on Cancer. 2007. “IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Combined Estrogen−Progestogen Contraceptives and Combined Estrogen−Progestogen Menopausal Therapy.” http://monographs.iarc.fr/ENG/Monographs/vol91/mono91.pdf. [PMC free article] [PubMed]
- World Health Organization International Agency for Research on Cancer. 2012. “IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Combined Estrogen−Progestogen Contraceptives.” IARC Monographs 100A:283–318. http://monographs.iarc.fr/ENG/Monographs/vol100A/mono100A-19.pdf. [Google Scholar]
- Yager J. D., Davidson N. E.. 2006. “Estrogen Carcinogenesis in Breast Cancer.” New England Journal of Medicine 354:270–82. [DOI] [PubMed] [Google Scholar]
- Young E. A., Kornstein S. G., Harvey A. T., Wisniewski S. R., Barkin J., Fava M., Trivedi M. H., Rush A. J.. 2007. “Influences of Hormone-based Contraception on Depressive Symptoms in Premenopausal Women with Major Depression.” Psychoneuroendocrinology 32:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A., Giannone T., Scheffler P., Doonan R. J., Egiziano G., Gomez Y. H., Papaioannou T. G., Daskalopoulou S. S.. 2014. “The Effect of Oral Contraceptive Pills and the Natural Menstrual Cycle on Arterial Stiffness and HemodynamICs (CYCLIC).” Journal of Hypertension 32:100–107. [DOI] [PubMed] [Google Scholar]
- Yusuf A., Chowdhury A. Q., Sattar A. N. I., Rahman M.. 2007. “Evaluation of the Effect of Contraceptives in Prevalence of Candida Species on Vaginal Candidiasis in Dhakam Bangladesh.” Bangladesh Journal of Medical Microbiology 01:61–64. [Google Scholar]
- Zahran M. M., Osman M. I., Kamel M., Fayad M., Mooro H., Youssef A. F.. 1976. “Effects of Contraceptive Pills and Intrauterine Devices on Urinary Bladder.” Urology 8:567–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-lqr-10.1177_0024363920982709 for Hormonally Active Contraceptives Part I: Risks Acknowledged and Unacknowledged by William V. Williams, Joel Brind, Laura Haynes, Michael D. Manhart, Hanna Klaus, Angela Lanfranchi, Gerard Migeon, Mike Gaskins, Elvis I. Seman, Lester Ruppersberger and Kathleen M. Raviele in The Linacre Quarterly