Key Points
Question
How can modern radiation techniques reduce the risk of severe esophagitis (Common Terminology Criteria for Adverse Events grade 3 or higher) in the treatment of locally advanced lung cancers?
Findings
In this phase 1 nonrandomized clinical trial, esophagitis rates were examined among 25 participants with locally advanced non–small cell or small cell lung carcinoma treated with intensity-modulated radiation therapy and concurrent chemotherapy using a novel contralateral esophagus–sparing technique. Despite the delivery of high-dose radiation to 70 Gy and the requirement for gross tumor within 1 cm of the esophagus, no participant developed grade 3 or higher esophagitis.
Meaning
The use of this contralateral esophagus–sparing technique was associated with a reduced risk of severe esophagitis among patients with locally advanced lung cancer receiving high-dose intensity-modulated radiation therapy and may be translated into clinical practice.
Abstract
Importance
Severe acute esophagitis occurs in up to 20% of patients with locally advanced lung cancer treated with chemoradiation therapy to at least 60 Gy once daily and represents a dose-limiting toxic event associated with poor outcomes.
Objective
To assess whether formalized sparing of the contralateral esophagus (CE) is associated with reduced risk of severe acute esophagitis.
Design, Setting, and Participants
This single-center phase 1 nonrandomized clinical trial assessing an empirical CE-sparing technique enrolled patients from July 2015 to January 2019. In total, 27 patients with locally advanced non–small cell lung carcinoma (with or without solitary brain metastasis) or limited-stage small cell lung carcinoma with gross tumor within 1 cm of the esophagus were eligible.
Interventions
Intensity-modulated radiation therapy to 70 Gy at 2 Gy/fraction concurrent with standard chemotherapy with or without adjuvant durvalumab. The esophageal wall contralateral to gross tumor was contoured as an avoidance structure to guide a steep dose falloff gradient. Target coverage was prioritized over CE sparing, and 99% of internal and planning target volumes had to be covered by 70 Gy and at least 63 Gy, respectively.
Main Outcomes and Measures
The primary end point was the rate of at least grade 3 acute esophagitis as assessed by Common Terminology Criteria for Adverse Events, version 4.
Results
Of 27 patients enrolled, 25 completed chemoradiation therapy. Nineteen patients had non–small cell lung carcinoma, and 6 had small cell lung carcinoma. The median age at diagnosis was 67 years (range, 51-81 years), and 15 patients (60%) were men. Thirteen patients (52%) had stage IIIA cancer, 10 (40%) had stage IIIB cancer, and 2 (8%) had stage IV cancer. The median CE maximum dose was 66 Gy (range, 44-71 Gy); the median volume of CE receiving at least 55 Gy was 1.4 cm3 (range, 0-5.3 cm3), and the median volume of CE receiving at least 45 Gy was 2.7 cm3 (range, 0-9.2 cm3). The median combined percentage of lung receiving at least 20 Gy was 25% (range, 11%-37%). The median follow-up was 33.3 months (range, 11.1-52.2 months). Among the 20 patients who had treatment breaks of 0 to 3 days and were thus evaluable for the primary end point, the rate of at least grade 3 esophagitis was 0%. Other toxic events observed among all 25 patients included 7 (28%) with grade 2 esophagitis, 3 (12%) with at least grade 2 pneumonitis (including 1 with grade 5), and 2 (8%) with at least grade 3 cardiac toxic event (including 1 with grade 5). There was no isolated local tumor failure. The 2-year progression-free survival rate was 57% (95% CI, 33%-75%), and the 2-year overall survival rate was 67% (95% CI, 45%-82%).
Conclusions and Relevance
This phase 1 nonrandomized clinical trial found that the CE-sparing technique was associated with reduced risk of esophagitis among patients treated uniformly with chemoradiation therapy (to 70 Gy), with no grade 3 or higher esophagitis despite tumor within 1 cm of the esophagus. This technique may be translated into clinical practice.
Trial Registration
ClinicalTrials.gov Identifier: NCT02394548
This phase 1 nonrandomized clinical trial assesses whether sparing the contralateral esophagus is associated with a reduced risk of severe acute esophagitis for patients with locally advanced non–small cell lung carcinoma or limited-stage small cell lung carcinoma treated with intensity-modulated radiation therapy concurrent with standard chemotherapy.
Introduction
The standard treatment of locally advanced non–small cell lung cancer (NSCLC) and limited-stage (LS) small cell lung cancer (SCLC) is concurrent chemoradiation therapy (CRT).1,2 Because of the high rates of local tumor failure with standard radiation therapy, treatment intensification has been explored. In NSCLC, dose escalation to 74 Gy is associated with severe esophagitis (Common Terminology Criteria for Adverse Events [CTCAE] grade ≥3) in 17.4% of patients.3 In LS-SCLC, current regimens result in severe esophagitis rates of almost 20%.2 Severe esophagitis is a serious dose-limiting toxic event requiring hospital admission, feeding tube, or total parenteral nutrition, which may lead to treatment interruptions and poor outcomes.4
Intensity-modulated radiation therapy (IMRT), including volumetric-modulated arc therapy, increases dose conformality and provides greater sparing of normal tissues than traditional 3-dimensional conformal radiation. However, grade 3 esophagitis remains a problem.5,6,7 The mean esophageal radiation dose has been evaluated for limiting esophagitis, but it remains unclear how to optimize IMRT for esophageal sparing.8
We have empirically derived a technique to spare the esophageal wall contralateral to gross disease.9 This technique involves contouring the contralateral esophagus (CE) as an avoidance structure to guide a steep dose falloff gradient across the esophagus. In our experience of using this CE-sparing technique (CEST) for CRT of locally advanced thoracic malignant neoplasms, no patient experienced grade 3 or higher esophagitis, and only 20% had grade 2 esophagitis despite a high median dose of 70.2 Gy (range, 63.0-72.2 Gy).9 We subsequently designed this phase 1 nonrandomized clinical trial to prospectively examine the frequency of esophagitis in patients with locally advanced lung cancer treated with CRT using CEST. A moderate-dose escalation to 70 Gy was chosen to address the high rates of local failure associated with 60 Gy3,10 and in keeping with protocols such as Radiation Therapy Oncology Group (RTOG) 1308 (NCT01993810).11
Methods
Patient Selection
Patients 18 years of age or older with histologically confirmed locally advanced NSCLC (with or without solitary brain metastasis) or LS-SCLC with gross primary or nodal tumor within 1 cm of the esophagus were eligible (study protocol in Supplement 1). The single-center phase 1 study protocol was approved by the institutional review board of the Dana-Farber/Harvard Cancer Center, Boston, Massachusetts. All patients provided written informed consent that was obtained in a manner consistent with the Declaration of Helsinki.12 No one received compensation or was offered any incentive for participating in this study.
Study Design and Treatment
Participants received standard-of-care CRT to 70 Gy at 2 Gy/fraction with or without adjuvant durvalumab for NSCLC. Radiation was planned with IMRT or volumetric-modulated arc therapy (RayStation; RaySearch Laboratories) using custom immobilization, 4-dimensional CT planning, and daily image guidance. A shrinking-field technique was used with a boost to the internal target volume after 44 Gy (eMethods and eTable 1 in Supplement 2). The CE was contoured as a distinct avoidance structure for promoting a steep dose falloff across the esophagus (Figure; eFigure 1 in Supplement 2). Target coverage was prioritized over CE sparing, and 99% of the internal target volume and of the planning target volume were required to be covered by 100% and 90% of prescription dose, respectively. Participants received concurrent chemotherapy per their treating medical oncologist. To be analyzable for the primary end point, participants were required to have received concurrently 5 or more cycles of weekly combined carboplatin plus paclitaxel or 2 or more cycles of platinum and pemetrexed or etoposide and to have had 3 or fewer days of unplanned treatment interruption unrelated to esophagitis. We followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Figure. Axial Computed Tomographic Images Illustrating the Contralateral Esophageal–Sparing Technique.
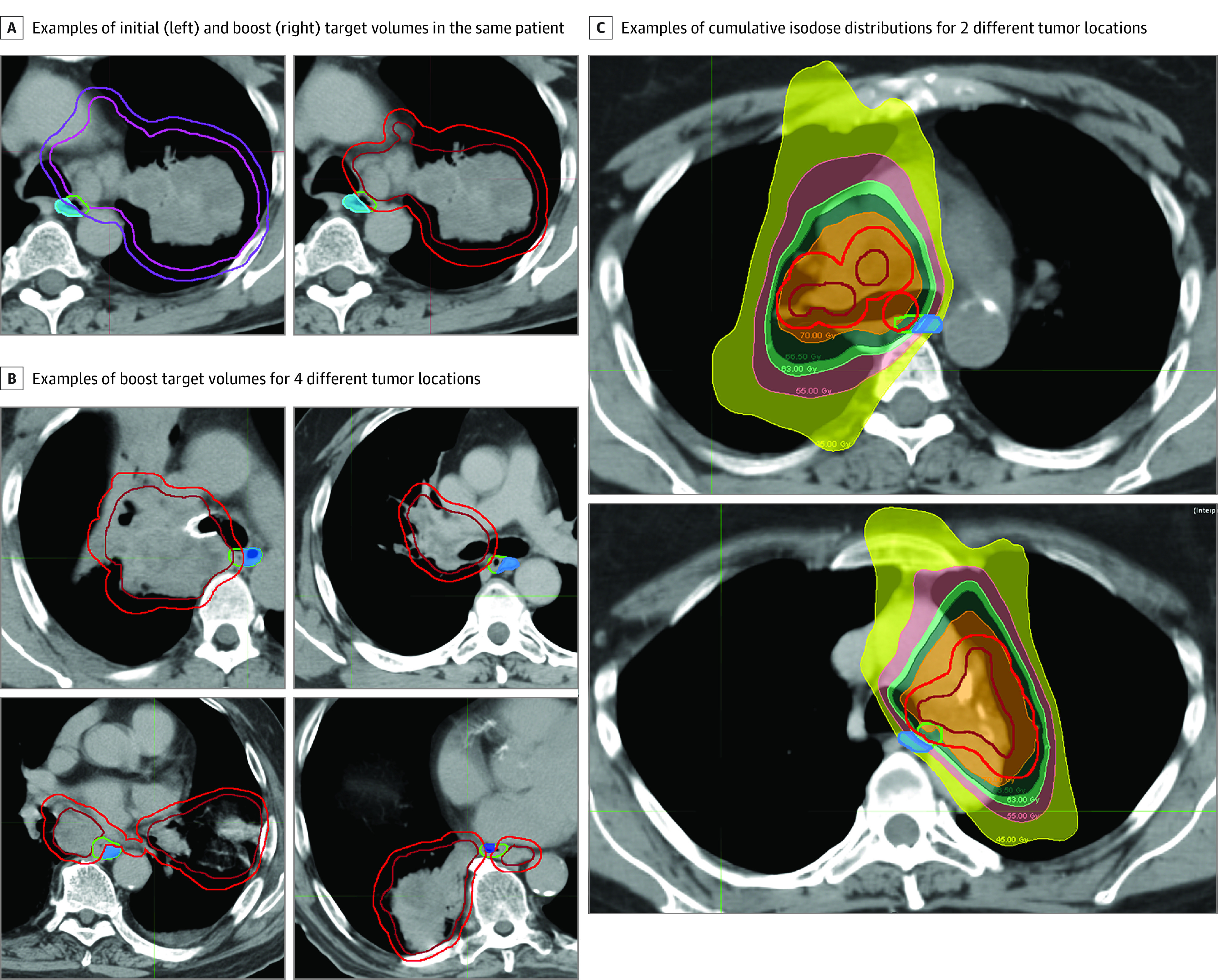
A, Contralateral esophagus (shaded blue), esophagus (green), and target contouring. Left, clinical target volume (pink) and associated planning target volume (purple) treated to 44 Gy. Right, boost internal target volume (red) and associated planning target volume (dark red) treated to 26 Gy. B, Four additional cases with different anatomical tumor locations. C, Two participants with isodose distributions for internal target volume treated to 70 Gy (orange) and planning target volume treated to a minimum of 63 Gy (light green).
Statistical Analysis
See the eMethods in Supplement 2 for clinical outcome assessments and failure definitions. Overall survival and progression-free survival rates were estimated using the Kaplan-Meier method with 95% CIs based on the log-log transformation. The primary objective was to describe the rate of grade 3 or higher esophagitis assessed using CTCAE, version 4.0. Secondary end points included the rate of esophagitis using RTOG criteria (eTable 2 in Supplement 2), general toxic events assessed using CTCAE, and 2-year clinical outcomes. We hypothesized that CEST would limit the risk of grade 3 or higher esophagitis to 5% or less of patients. A sample size of 20 participants was chosen to ensure that if no more than 1 participant was observed with grade 3 or higher esophagitis, the 1-sided upper limit of the 90% CI would not exceed 20%. All analyses were conducted from July to October 2020 using R, version 4.0.1 (R Foundation for Statistical Computing).
Results
Patient Characteristics
Between July 2015 and January 2019, 27 participants were enrolled in the trial (eFigure 2 in Supplement 2). Two participants were removed. Patient and treatment characteristics are given in Table 1. The median age at diagnosis was 67 years (range, 51-81 years), and 15 patients (60%) were men. Nineteen participants had NSCLC, and 6 participants had SCLC. Two participants had solitary brain metastases, and all others had stage IIIA cancer (13 participants [52%]) or stage IIIB cancer (10 participants [40%]). The total dose was 70 Gy for 24 participants (96%). The median CE maximum dose was 66 Gy (range, 44-71 Gy); the median volume of CE receiving at least 55 Gy was 1.4 cm3 (range, 0-5.3 cm3), and the median volume of CE receiving at least 45 Gy was 2.7 cm3 (range, 0-9.2 cm3). The median combined percentage of lung receiving at least 20 Gy was 25% (range, 11%-37%).
Table 1. Patient, Tumor, and Treatment Characteristics for 25 Eligible Participants.
| Characteristic | No. (%) |
|---|---|
| Age at diagnosis, median (range), y | 67 (51-81) |
| Sex | |
| Male | 15 (60) |
| Female | 10 (40) |
| Clinical AJCC stage (7th edition) | |
| IIIA | 13 (52) |
| IIIB | 10 (40) |
| IV (solitary brain metastasis) | 2 (8) |
| Histologic results | |
| NSCLC | 19 (76) |
| SCLC | 6 (24) |
| ITV size, cm3 | |
| Mean | 96 |
| Median | 70 |
| Range | 8-385 |
| Chemotherapy received | |
| Cisplatin plus etoposide | 7 (28) |
| Cisplatin plus pemetrexed | 7 (28) |
| Carboplatin plus paclitaxel | 9 (36) |
| Carboplatin plus pemetrexed | 1 (4) |
| Carboplatin plus etoposide | 1 (4) |
| Total dose of radiation received, Gy | |
| 70 | 24 (96) |
| 68 | 1 (4) |
Abbreviations: AJCC, American Joint Committee on Cancer; ITV, internal treatment volume; NSCLC, non–small cell lung carcinoma; SCLC, small cell lung carcinoma.
Toxic Events
The rate of grade 3 or higher esophagitis was 0% (95% CI, 0%-16%) among 20 patients eligible for the primary end point analysis (Table 2). Five participants were ineligible for primary end point analysis owing to unplanned treatment breaks of more than 3 days unrelated to esophagitis (eFigure 2 in Supplement 2). Grade 2 esophagitis was detected in 24% to 28% of patients depending on the scoring criteria and number of participants analyzed. Other common toxic events included cardiopulmonary events, fatigue, and dermatitis (Table 2). Among 25 patients, the rate of grade 2 or higher pneumonitis was 12% (3 patients), and the rate of grade 3 or higher cardiac toxic events was 8% (2 patients). There were 2 grade 5 events—1 participant had fatal pneumonitis, and 1 participant who had preexisting congestive heart failure died of it shortly after radiation therapy. Hematologic toxic events are given in eTable 3 in Supplement 2.
Table 2. Nonhematologic Toxic Eventsa.
| Toxic event | No. (%) [95% CI] of patients | |||
|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| CTCAE v 4.0 (primary end point) (20 patients) | ||||
| Esophagitis | 5 (25) [11-47] | 0 (0) [0-16] | 0 | 0 |
| RTOG (secondary end point) (25 patients) | ||||
| Esophagitis | 6 (24) [12-43] | 0 (0) [0-13] | 0 | 0 |
| CTCAE v 4.0 (secondary end points) (25 patients) | ||||
| Esophagitis | 7 (28) [14-48] | 0 (0) [0-13] | 0 | 0 |
| Dysphagia | 2 (8) | 0 | 0 | 0 |
| Dyspepsia | 4 (16) | 0 | 0 | 0 |
| Pneumonitis | 2 (8) | 0 | 0 | 1 (4) |
| Hypoxia | 0 | 1 (4) | 0 | 0 |
| Pneumonia | 1 (4) | 0 | 0 | 0 |
| Hoarseness | 1 (4) | 0 | 0 | 0 |
| Dyspnea | 4 (16) | 1 (4) | 0 | 0 |
| Nausea | 1 (4) | 0 | 0 | 0 |
| Fatigue | 6 (24) | 0 | 0 | 0 |
| Dermatitis | 5 (20) | 0 | 0 | 0 |
| Heart failure | 0 | 0 | 0 | 1 (4) |
| Atrial fibrillation | 2 (8) | 0 | 0 | 0 |
| Ventricular tachycardia | 0 | 1 (4) | 0 | 0 |
Abbreviations: CTCAE v 4.0, Common Terminology Criteria for Adverse Events, version 4.0; RTOG, Radiation Therapy Oncology Group.
Toxic events grade 2 or higher possibly, likely, or definitely associated with high-dose radiation therapy.
Clinical End Points
The median follow-up was 33.3 months (range, 11.1-52.2 months) among surviving patients. There was no isolated local tumor failure. The 2-year progression-free survival rate was 57% (95% CI, 33%-75%), and the 2-year overall survival rate was 67% (95% CI, 45%-82%) (eFigure 3 in Supplement 2).
Discussion
Severe esophagitis is a dose-limiting toxic event that complicates intensification of treatments in locally advanced NSCLC and LS-SCLC.2,4 We prospectively tested a simple method to avoid exposing the entire esophagus cross section to high doses.9 We observed no grade 3 esophagitis and grade 2 events in 24% to 28% of participants despite all participants having gross tumor adjacent to the esophagus and 96% receiving the full 70 Gy. To explain how CE sparing may reduce the risk of severe esophagitis, we consider that the esophagus may be regarded as an organ with functional subunits arranged in a serial fashion such that high-dose irradiation of the entire cross section of the esophagus may result in whole-organ dysfunction.9,13 Our data suggest that near-normal esophageal function may be maintained by preserving the function of approximately half of the esophageal cross section (ie, by converting the esophagus from a serial organ to a parallel organ) (eFigure 4 in Supplement 2). Mucosa may respond to radiation injury with accelerated repopulation during a course of several weeks of radiation.13 Esophageal mucosal regeneration cannot compensate for high-dose, full-circumference irradiation but may be able to do so if parts of the adjacent mucosa are exposed to a lower dose.
Limitations
There are limitations to this study. Because this is a single-institution trial with a small sample size conducted at a tertiary academic center, the results may not be representative of a broader patient population. We used multicriteria optimization, which may optimize CE sparing but is not available everywhere.14 The mean internal target volume was somewhat smaller than that in other series (Table 1), which may have impacted esophagitis.7 However, we included only patients with tumor within 1 cm of the esophagus, which is expected to increase esophagitis rates. Finally, we did not observe any isolated local tumor failures at a median follow-up of close to 3 years, suggesting that the steep dose gradients resulting from the CE avoidance structure did not compromise target coverage; however, this should be validated in larger series.
Conclusions
In this phase 1 trial, CEST was associated with reduced risk of esophagitis among patients treated uniformly with chemoradiation therapy (to 70 Gy), with no grade 3 or higher esophagitis despite tumor within 1 cm of the esophagus. The method requires validation by other institutions and may be included in prospective trials evaluating the escalation of radiation dose or other treatment intensifications. CEST may be translated into clinical practice where it can support current guidelines for esophagus sparing.15
Study Protocol
eMethods
eTable 1. OAR Constraints
eTable 2. Esophagitis Scoring Criteria
eTable 3. Hematologic Toxicities Grades 3-5
eFigure 1. CE Contouring
eFigure 2. CONSORT Flow Diagram
eFigure 3. Survival Outcomes
eFigure 4. CE Sparing
Data Sharing Statement
References
- 1.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 2.Faivre-Finn C, Snee M, Ashcroft L, et al. ; CONVERT Study Team . Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116-1125. doi: 10.1016/S1470-2045(17)30318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG Oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38(7):706-714. doi: 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62. doi: 10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijsman R, Dankers F, Troost EGC, et al. Comparison of toxicity and outcome in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-)radiotherapy using IMRT or VMAT. Radiother Oncol. 2017;122(2):295-299. doi: 10.1016/j.radonc.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 7.Jiang ZQ, Yang K, Komaki R, et al. Long-term clinical outcome of intensity-modulated radiotherapy for inoperable non-small cell lung cancer: the MD Anderson experience. Int J Radiat Oncol Biol Phys. 2012;83(1):332-339. doi: 10.1016/j.ijrobp.2011.06.1963 [DOI] [PubMed] [Google Scholar]
- 8.Thor M, Deasy J, Iyer A, et al. Toward personalized dose-prescription in locally advanced non-small cell lung cancer: validation of published normal tissue complication probability models. Radiother Oncol. 2019;138:45-51. doi: 10.1016/j.radonc.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Halabi H, Paetzold P, Sharp GC, Olsen C, Willers H. A contralateral esophagus-sparing technique to limit severe esophagitis associated with concurrent high-dose radiation and chemotherapy in patients with thoracic malignancies. Int J Radiat Oncol Biol Phys. 2015;92(4):803-810. doi: 10.1016/j.ijrobp.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 10.Willers H, Azzoli CG, Santivasi WL, Xia F. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J. 2013;19(3):200-207. doi: 10.1097/PPO.0b013e318292e4e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phase III randomized trial comparing overall survival after photon versus proton chemoradiotherapy for inoperable stage II-IIIB NSCLC. ClinicalTrials.gov identifier: NCT01993810. Updated December 1, 2020. Accessed February 26, 2021. https://clinicaltrials.gov/ct2/show/NCT01993810
- 12.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Dörr W, Herrmann T, Trott KR. Normal tissue tolerance. Transl Cancer Res. 2017;6(suppl 5):S840-S51. doi: 10.21037/tcr.2017.06.45 [DOI] [Google Scholar]
- 14.Kamran SC, Mueller BS, Paetzold P, et al. Multi-criteria optimization achieves superior normal tissue sparing in a planning study of intensity-modulated radiation therapy for RTOG 1308-eligible non-small cell lung cancer patients. Radiother Oncol. 2016;118(3):515-520. doi: 10.1016/j.radonc.2015.12.028 [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network . Non-small cell lung cancer (version 6.2020). Published 2020. Accessed August 25, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eMethods
eTable 1. OAR Constraints
eTable 2. Esophagitis Scoring Criteria
eTable 3. Hematologic Toxicities Grades 3-5
eFigure 1. CE Contouring
eFigure 2. CONSORT Flow Diagram
eFigure 3. Survival Outcomes
eFigure 4. CE Sparing
Data Sharing Statement


