Abstract
Background & Aims:
Esophageal biopsies in children with eosinophilic esophagitis (EoE) are often inadequate for assessment of lamina propria and lamina propria fibrosis (LPF). For children with EoE, little is known about the factors associated with adequate lamina propria (aLP) sampling or the relationship among epithelial features in esophageal biopsies with and without LPF. We aimed to evaluate aLP in esophageal biopsies from children with and without EoE, identify factors associated with aLP and LPF, and examine the relationship among epithelial features in biopsies with and without LPF in children with EoE.
Methods:
In a retrospective study, we analyzed clinical, endoscopic, and histologic data from 217 children (124 with EoE and 94 without EoE [controls]) using descriptive statistics, logistic regression, Spearman’s correlation, and receiver operating characteristic curve analysis. Active and inactive EoE were defined per the 2011 consensus guidelines.
Results:
aLP was observed in biopsies from higher proportion of children with EoE (69%) than controls (31%) (P=.0001). Active EoE was independently associated with aLP (adjusted odds ratio [aOR], 4.23; 95% CI, 1.00–18.13; P=.05). Patient sex (aOR for boys, 8.37; 95% CI, 1.23–56.74; P=.03) and peak eosinophil count (aOR, 1.02; 95% CI, 1.01–1.04; P=.01) were independently associated with LPF. Epithelial features were strongly interrelated in biopsies with LPF, and the presence of specific epithelial features was associated with LPF.
Conclusions:
aLP was observed in a higher proportion of esophageal biopsies from children with EoE than controls. EoE status, patient sex, and peak eosinophil count were associated with aLP sampling and LPF. Given the intricate relationship between epithelial features and LPF, computational models can be developed to identify children with esophageal biopsies without aLP who are at risk for LPF.
Keywords: epithelial inflammation, subepithelial fibrosis, PEC, diagnostic
Introduction:
Eosinophilic esophagitis (EoE) is an increasingly prevalent, allergen-mediated condition affecting all ages. EoE patients present with symptoms of esophageal dysfunction including feeding difficulties, dysphagia and esophageal food impaction and their esophageal mucosal biopsies reveal intense eosinophilic inflammation [defined as peak eosinophil count (PEC) of ≥ 15 eosinophils per high power field (eos/hpf)].1 Uncontrolled eosinophilic inflammation, or a delay in diagnosis can lead to progressive tissue damage, resulting in subepithelial fibrosis and esophageal remodeling.2,3
Sampling the esophageal mucosa using conventional biopsy forceps during esophagogastroduodenoscopy (EGD) and identifying characteristic histologic changes in esophageal biopsies is central to defining EoE.4 However, approximately half of the esophageal mucosal biopsies, especially in children, may not be suitable for studying subepithelial activity, including fibrotic changes in the lamina propria.5 Understanding epithelial and subepithelial activity in EoE patients is important for determining the severity of disease, extent of involvement and remodeling in order to inform therapeutic plans and monitoring strategies.6
Only few studies have examined the impact of endoscopic sampling techniques and role of the size of biopsy forceps in obtaining esophageal biopsy samples with adequate LP (aLP),7,8 and very little is known about the demographic and clinical factors associated with presence of aLP and lamina propria fibrosis (LPF) in esophageal biopsies obtained from children with EoE. The aims of this study were (1) to examine the rate of aLP in esophageal biopsies obtained from children with EoE compared to that of children without EoE, and (2) in children with EoE, identify the factors associated with aLP and LPF in esophageal biopsies, and investigate the relationship among EoE-relevant epithelial histologic features in esophageal biopsies with and without LPF. Given the relationship between histologic features and symptoms9,10 and the correlation between degree of eosinophilic inflammation in the epithelium and the subepithelial fibrosis in EoE11, we hypothesized that aLP and LPF would be present in distinct subgroups of children with EoE and that the degree of pathology in the epithelium would vary in biopsies with and without LPF.
Methods:
Study subjects and Case definitions
In this retrospective study, we included children (1–18 years old) with known EoE or with upper gastrointestinal symptoms concerning for EoE undergoing EGD with biopsies at Vanderbilt Children’s Hospital between May 2017 and April 2018. EoE was defined per the 2011 Consensus recommendations wherein they were required to have symptoms of esophageal dysfunction and PEC of ≥ 15 eosinophils per high power field (eos/hpf) in at least one of the multiple esophageal biopsies after 8 weeks of proton-pump inhibitor (PPI) therapy and in the absence of other causes of esophageal eosinophilia. Active (aEoE) was defined as PEC ≥ 15 eos/hpf and inactive EoE (iEoE) as PEC < 15 eos/hpf.12 Children without a previous diagnosis of EoE, undergoing EGD for upper gastrointestinal symptoms, and their biopsies revealing a PEC < 10 eos/hpf were considered as non-EoE controls as this cut-off has been previously shown to reliably exclude EoE.13 Also, we wanted to include all eligible subjects with esophageal eosinophilia and not limit our comparison to healthy controls (PEC ≤ 1) to simulate common clinical scenario. Subjects with eosinophilic gastroenteritis, inflammatory bowel disease, connective tissue disorders, esophageal varices, and/or previous esophageal surgery were excluded. This study was approved by the Vanderbilt University Institutional Review Board (protocol number 180086).
Data collection
Demographics (i.e. age at endoscopy, gender, ethnicity), indication(s) for the procedure (nausea, vomiting, reflux, dysphagia, abdominal pain, duration of EoE in children with EoE), presence of allergic comorbidities (such as allergic rhinitis, eczema, asthma), and medication exposure (e.g., antihistamines, nasal topical steroids, PPI, oral topical corticosteroids) data were gathered from the electronic medical records.
Endoscopic findings
Upon completion of the EGD, esophageal signs were scored per the endoscopic reference score (EREFS) [edema (0–2), rings (0–3), exudates (0–2), furrows (0–2) and strictures (0–1)]. Minor features were also evaluated. Cumulative EREFS scores (range 0–10) were calculated by combining individual values with higher scores indicitating more severe endoscopic findings.14 All endoscopies were performed by a single endoscopist (G.H.)
Esophageal biopsies and Histologic evaluation
We planned to obtain 6–8 esophageal mucosal biopsies (3–4 each from proximal and distal esophagus) from each subject during EGD using a disposable EndoJaw (Alligator Jaw Step Fenestrated with needle) biopsy forceps (Olympus Medical Systems Corp. Japan). The biopsies, each approximately 2 mm3, were submitted for H&E staining per our institutional protocol. After examining all the fragments, PEC (gold-standard) was determined by counting the number of eosinophils in each HPF (HPF = 0.237 mm2) in the fragment with highest number of eosinophils in the squamous epithelium.
For this study, all fragments were re-assessed for eosinophil counts and evaluated for other features. The worst area of each feature was scored by our expert pediatric pathologist (H.C.) for the degree of abnormality (grade score) per the Histology Scoring System (EoEHSS)15 (see supplementary material).
Statistical analysis
Descriptive statistics including counts and percentages [n (%)] for categorical variables, and means and standard deviations (mean±SD) for continuous variables were used to summarize the cohort characteristics. Bonferroni correction was applied to account for multiple comparisons and a P value of ≤ 0.002 was used to ascertain statistical significance. Logistic regression was used to estimate odds ratios (OR) and adjusted odds ratio (aOR) for presence of aLP in children with EoE and non-EoE controls and presence of LPF in children with EoE after adjusting for potential confounders. The conventional P value of ≤ 0.05 was used to assess statistical significance. Spearman rho (r) was used to develop correlation matrix of the interrelationship among epithelial features in EoE patients with and without LPF. A coefficient of: 0.00–0.30 was considered as negligible, 0.31–0.50 as weak, 0.51–0.70 as moderate, 0.71–0.90 as high, and 0.91–1.00 as very high correlation. We performed logistic regression analysis to assess the OR (95% CI) of each of the epithelial features to predict the probability of presence LPF [dependent variable = LPF absent (0) or present (1)] and identified the optimal threshold and the area under the curve for each of the epithelial features using receiver operating characteristic curve (ROC) analysis. All statistical analyses were performed using Stata version 16.0 (College Station, TX).
Results
Characteristics of the entire cohort
Overall, 217 children were included with 123 (57%) as EoE and 94 (43%) as non-EoE controls. The non-EoE controls primarily comprised of children with abdominal pain (63%), nausea (15%), and vomiting (15%). Most of the subjects were male (65%) and Caucasians (82%). The age at EGD was 11±5 years. Half (50%) had allergic rhinitis and 38% had asthma. Concomitant exposure to antihistamines was reported by 48%, PPI by 24% and swallowed topical corticosteroids by 25% (Table 1).
Table 1:
Characteristics of cohort
| Overall (N=217) | EoE (N=123) | Non-EoE (N=94) | P value | ||
|---|---|---|---|---|---|
|
| |||||
| Gender [n (%)] | |||||
| Female | 77 (35) | 34 (28) | 43 (46) | 0.006 | |
| Male | 140 (65) | 89 (72) | 51 (54) | ||
| Ethnicity [n (%)] | |||||
| White | 179 (82) | 96 (78) | 83 (88) | 0.05 | |
| African American | 24 (11) | 19 (15) | 5 (5) | 0.01 | |
| Hispanic | 4 (2) | -- | 4 (4) | ||
| Others | 10 (5) | 8 (7) | 2 (2) | 0.08 | |
| Age at EGD (years) | |||||
| Mean ± SD | 11 ± 5 | 10 ± 4 | 12 ± 5 | 0.001 | |
| Median (IQR) | 11 (8 – 15) | 10 (7 – 13) | 14 (9 – 16) | ||
| Atopic comorbidities [n (%)] | |||||
| Allergic rhinitis | 108 (50) | 89 (72) | 19 (20) | < 0.0001 | |
| Eczema | 56 (26) | 49 (40) | 7 (7) | < 0.0001 | |
| Asthma | 82 (38) | 61 (50) | 21 (22) | < 0.0001 | |
| Clinical symptoms [n (%)] | |||||
| Nausea | 21 (10) | 7 (6) | 14 (15) | 0.02 | |
| Vomiting | 25 (12) | 11 (9) | 14 (15) | 0.17 | |
| Reflux | 14 (6) | 7 (6) | 7 (7) | 0.76 | |
| Dysphagia | 35 (16) | 24 (19) | 11 (12) | 0.16 | |
| Abd pain | 78 (36) | 19 (15) | 59 (63) | < 0.0001 | |
| Symptomatic | 125 (56) | 45 (47) | 80 (85) | < 0.0001 | |
| Duration of EoE (N=117) [n (%)] | |||||
| ≤ 6 months | 35 (30) | -- | |||
| > 6 months - ≤ 12 months | 17 (15) | -- | |||
| > 12 months - ≤ 24 months | 13 (11) | -- | |||
| > 24 months | 52 (44) | -- | |||
| Medications [n (%)] | |||||
| Antihistamines | 102 (48) | 76 (62) | 26 (28) | < 0.0001 | |
| Nasal topical steroids | 75 (35) | 63 (52) | 12 (13) | < 0.0001 | |
| Proton pump inhibitors | 53 (24) | 25 (20) | 28 (30) | 0.08 | |
| Topical steroids | 14 (25) | 14 (26) | -- | ||
| Endoscopic signs [n (%)] | |||||
| Edema | 0 | 165 (76) | 72 (58) | 93 (99) | < 0.0001 |
| 1 | 52 (24) | 51 (41) | 1 (1) | < 0.0001 | |
| Rings | 0 | 204 (94) | 111 (90) | 99 (99) | 0.006 |
| 1 | 11 (5) | 10 (8) | 1 (1) | 0.01 | |
| 2 | -- | -- | -- | ||
| 3 | 2 (1) | 2 (2) | -- | ||
| Exudates | 0 | 202 (93) | 108 (88) | 94 (100) | 0.0009 |
| 1 | 9 (4) | 9 (7) | -- | ||
| 2 | 6 (3) | 6 (5) | -- | ||
| Furrows | 0 | 154 (71) | 63 (51) | 91 (97) | < 0.0001 |
| 1 | 63 (29) | 60 (49) | 3 (3) | < 0.0001 | |
| Stricture | 0 | 216 (99) | 122 (99) | 94 (100) | 0.33 |
| 1 | 1 (1) | 1 (1) | -- | ||
| Total EREFS | |||||
| Mean ± SD | 0.71 ± 1.21 | 1.22 ± 1.39 | 0.05± 0.33 | < 0.0001 | |
| Median (IQR) | 0 (0 – 1) | 1 (0 – 2) | 0 (0 – 0) | ||
| Number of biopsies | |||||
| Mean ± SD | 5 ± 2 | 6 ± 2 | 3 ± 1 | < 0.0001 | |
| Median (IQR) | 5 (3 – 6) | 6 (5 – 6) | 3 (2 – 4) | ||
| Peak eosinophil count (eos/hpf) | |||||
| Mean ± SD | 20 ± 35 | 35 ± 41 | 0.4 ± 1.41 | < 0.0001 | |
| Median (IQR) | 0 (1 – 27) | 22 (2 – 58) | 0 (0 – 0) | ||
Differences between children with EoE and non-EoE controls
Children with EoE had the typical characteristics of EoE population. Most of them were male (72% vs. 54%; P=0.006) with a higher prevalence of allergic rhinitis (72% vs. 20%; P <0.001) and asthma (50% vs. 22%; P <0.001) compared to non-EoE controls. A higher proportion of children with EoE were on antihistamines (62% vs. 28%; P <0.001) and nasal topical corticosteroids (52% vs. 13%; P <0.001). Most of the EoE subjects carried their diagnosis for > 24 months (44%). Endoscopically, edema (41% vs. 1%; P <0.001), exudates (12% vs. 0%; P <0.001), and furrows (49% vs. 3%: P <0.001) were noted in children with EoE when compared to non-EoE controls, and the EREFS was higher for children with EoE compared to non-EoE controls (1.22±1.39 vs. 0.05±0.33; P <0.001). The number of esophageal biopsies was also higher in EoE group when compared to non-EoE controls (6±2 vs. 3±1; P <0.001), as was the PEC (35±41 vs. 0.4±1.41; P <0.001) (Table 1). Of the 123 children with EoE, 71 (58%) had aEoE, and 52 (42%) had iEoE.
Rates and factors associated with adequate lamina propria in esophageal biopsies
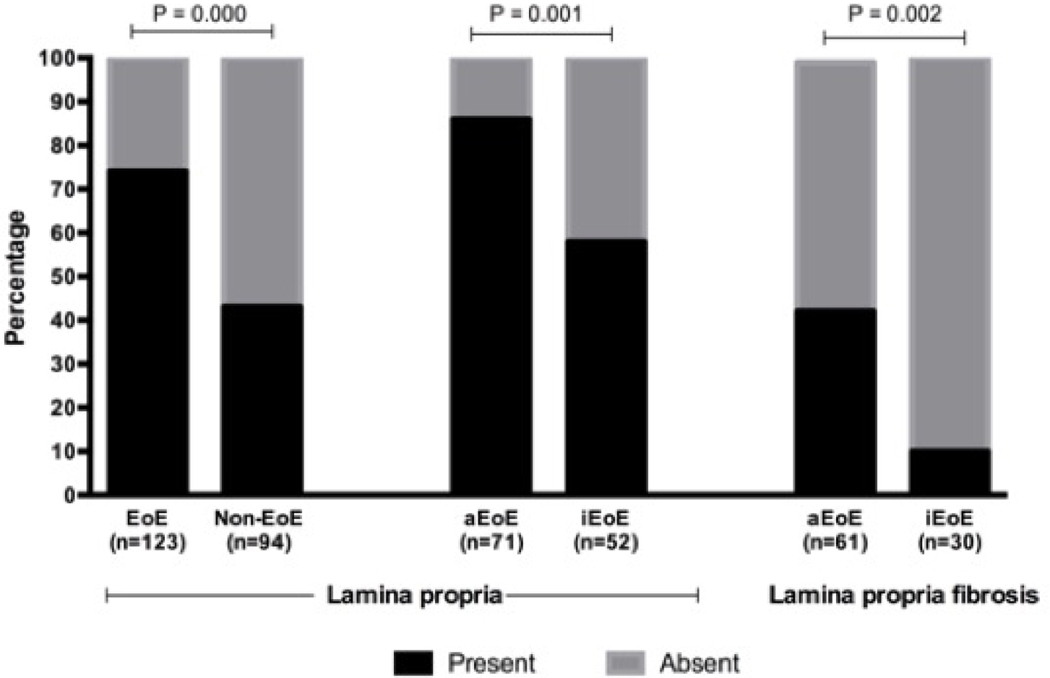
Of the 217 esophageal biopsies analyzed, aLP was present in 131 (60%) biopsies. aLP was frequently present in biopsies from children with EoE when compared to non-EoE controls (69% vs. 31%; P <0.001), and within the EoE group it was higher in children with aEoE compared to iEoE (86% vs. 58%; P =0.001) (Figure 1). Multivariate logistic regression showed that only aEoE [aOR: 4.23 (95% CI: 1.00–18.13); P =0.05] had a borderline association with presence of aLP (Table 2).
Figure 1:
Percentage of adequate lamina propria in esophageal biopsies
Table 2:
Factors associated with the presence of lamina propria and lamina propria fibrosis in esophageal biopsies obtained from children with and without EoE
| Category | Factors | Presence of lamina propria (N=217) | Factors | Presence of lamina propria fibrosis (N=123) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| OR (95% CI) | P value | aOR (95% CI) | P value | OR (95% CI) | P value | aOR (95% CI) | P value | |||
| Demographics | Male | 3.11 (1.74–5.54) | <0.001 | Male | 6.51 (1.46–29.02) | 0.01 | 8.37 (1.23–56.74) | 0.03 | ||
| Age | 0.89 (0.83–0.95) | 0.001 | ||||||||
| Clinical | Antihistamines | 2.13 (1.21–3.73) | 0.008 | Allergic rhinitis | 2.29 (1.02–5.14) | 0.04 | ||||
| Nasal topical steroids | 3.03 (1.62–5.66) | 0.001 | Asthma | 2.35 (1.03–5.35) | 0.04 | |||||
| Abdominal pain | 0.36 (0.20–0.64) | 0.001 | Dysphagia | 3.18 (1.22–8.25) | 0.01 | |||||
| Endoscopy | Edema | 3.58 (1.68–7.62) | 0.001 | Edema | 6.44 (2.68–15.45) | <0.001 | ||||
| Furrows | 3.93 (1.94–7.94) | <0.001 | Exudates | 4.54 (1.71–12.03) | 0.002 | |||||
| EREFS | 1.88 (1.34–2.63) | <0.001 | Furrows | 4.90 (2.06–11.64) | <0.001 | |||||
| EREFS | 2.00 (1.43–2.79) | <0.001 | ||||||||
| Histology | Number of biopsies | 1.25 (1.07–1.46) | 0.004 | Number of biopsies | 1.39 (1.10–1.75) | 0.005 | ||||
| PEC | 1.02 (1.01–1.03) | <0.001 | PEC | 1.03 (1.01–1.04) | <0.001 | 1.02 (1.00–1.04) | 0.01 | |||
| Activity | Active EoE | 4.47 (1.88-10.63) | 0.001 | 4.23 (1.00-18.13) | 0.05 | Active EoE | 6.68 (1.82-24.44) | 0.004 | ||
OR: Odds ratio; aOR: adjusted odds ratio; CI: confidence interval; EREFS: endoscopic reference score; PEC: peak eosinophil counts
Factors associated with presence of lamina propria fibrosis in children with EoE
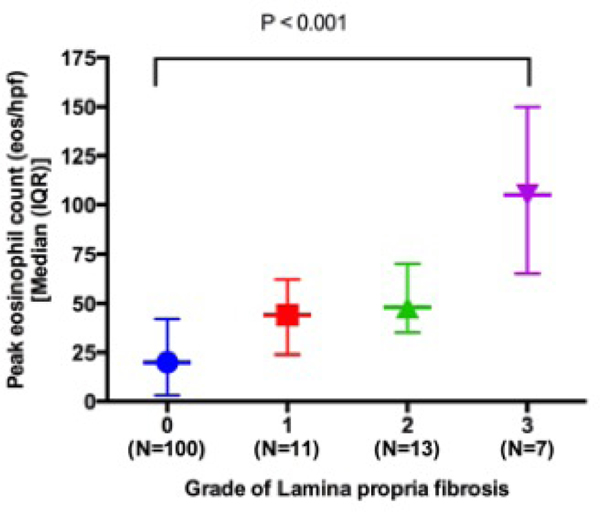
Of 131 children with aLP, 31 (24%) had LPF and 100 (76%) did not have LPF. Of the 31 children with LPF 26 (84%) had aEoE and 5 (16%) had iEoE, and of the 100 without LPF 35 (35%) had aEoE and 65 (65%) had iEoE (Figure 1). Multivariate logistic regression revealed that gender (male) [aOR (95% CI): 8.37 (1.23–56.74); P =0.03] and PEC [aOR (95% CI): 1.02 (1.01–1.04); P =0.01] independently predicted the presence of LPF in children with EoE (Table 2 and Figure 2).
Figure 2:
Relationship between peak eosinophil count and lamina propria fibrosis in biopsies with adequate lamina propria obtained from children with EoE
Relationship between epithelial features and lamina propria fibrosis in children with EoE
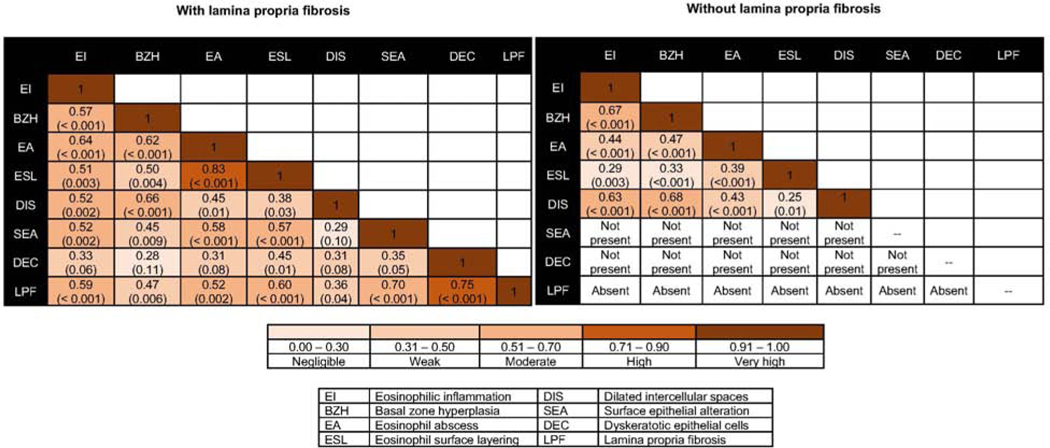
Spearman’s correlation matrix revealed a high correlation between dyskeratotic epithelial cells (DEC) and LPF (r=0.75; P <0.001), and a moderate correlation between surface epithelial alteration (SEA) (r=0.70; P <0.001), eosinophil surface layering (ESL) (r=0.60; P <0.001), eosinophilic inflammation (EI) (r=0.59; P =0.001) and eosinophilic abscess (EA) (r=0.52; P =0.002) with LPF. Both BZH (r=0.47; P =0.006) and DIS weakly correlated with LPF. In the biopsies without LPF, SEA or DEC was absent in all samples [SEA = 0 (not present); DEC = 0 (not present)]. Moderate correlation was noted between EI and BZH (r=0.67; P <0.001) and DIS (r=0.63; P <0.001), and between BZH and DIS (r=0.68; P <0.001) (Figure 3). Logistic regression revealed that the presence of SEA and DEC strongly predicted the presence of LPF, and the operating characteristics revealed that at the EoEHSS threshold value of ≥ 1 the ROC area for SEA was 0.91 (0.85–0.98) and for DEC was 0.90 (0.83–0.97), respectively (Table 3).
Figure 3.
Spearman correlation between epithelial features in biopsies with and without lamina propria fibrosis in children with EoE
Table 3:
Odds ratio and receiver operating characteristics of epithelial features to predict the presence of lamina propria fibrosis in children with EoE
| Odds ratio (95% CI) | Threshold EoEHSS score | Sensitivity (%) | Specificity (%) | Correctly classified (%) | ROC area (95% CI) | |
|---|---|---|---|---|---|---|
| EI | 3.98 (2.35 – 6.75) | ≥ 3 | 55 | 91 | 82 | 0.84 (0.76 – 0.91) |
| BZH | 3.49 (2.14 – 5.70) | ≥ 3 | 55 | 87 | 79 | 0.83 (0.76 – 0.90) |
| EA | 5.80 (2.79 – 12.03) | ≥ 2 | 39 | 99 | 85 | 0.79 (0.70 – 0.89) |
| ESL | 7.40 (2.76 – 19.83) | ≥ 1 | 58 | 96 | 87 | 0.77 (0.68 – 0.86) |
| DIS | 3.71 (2.17 – 6.34) | ≥ 3 | 71 | 79 | 77 | 0.83 (0.76 – 0.90) |
| SEA | Perfectly predicts LPF | ≥ 1 | 84 | 100 | 96 | 0.91 (0.85 – 0.98) |
| DEC | Perfectly predicts LPF | ≥ 1 | 81 | 100 | 95 | 0.90 (0.83 – 0.97) |
| EI | Eosinophilic inflammation | DIS | Dilated intercellular spaces |
| BZH | Basal zone hyperplasia | SEA | Surface epithelial alteration |
| EA | Eosinophil abscess | DEC | Dyskeratotic epithelial cells |
| ESL | Eosinophil surface layering | LPF | Lamina propria fibrosis |
Discussion:
EoE is a progressive disease complicated by fibrostenotic transformation and esophageal remodeling. Ongoing subepithelial eosinophilic inflammation even when the epithelial compartment has no eosinophilis can result in persistent symptoms.16 As such, monitoring subepithelial compartment may be an important way to ultimately prevent these complications.17 Esophageal mucosal biopsies can be inadequate for examination of the subepithelial space including the lamina propria, especially in children. Endoscopic ultrasound and functional luminal imaging has shown promise to indirectly assess the subepithelial activity and LPF. However, these are invasive and can be cumbersome in children.18–20 Deep esophageal sampling with four different biopsy forceps models in adults with EoE has been previously reported,8 but the clinical impact, feasibility, and safety of this approach in children is unknown. Tissue biomarkers such as periostin, tenacin C, fibronectin, and epithelial plasminogen activator inhibitor −1 have shown promise as markers of lamina propria fibrosis; however, their utility in clinical practice remains to be tested.21–23
We examined the prevalence of aLP in esophageal biopsies obtained from children with and without EoE and investigated the factors associated with presence of aLP. Next, in children with EoE we investigated the factors associated with presence of aLP and LPF and explored the relationship between epithelial features in biopsies with and without LPF. Of the 217 esophageal biopsies included in our study, 60% of them had aLP and most of them were from children with EoE. A recent study involving children with EoE and patients without esophageal eosinophilia reported that 42% of their esophageal pinch biopsies contained aLP for evaluation of fibrosis.7 In EoE patients, aLP rates between 37% and 75% has been previously reported with the higher values noted in single endoscopists’ experience.15,24,25,13 We also observed that presence of aLP was independently related to the aEoE and not with the demographic, clinical, endoscopic and histologic factors. Perhaps, longitudinal studies could allow us to understand if the inflammation occurring in aEoE results in thinner epithelium, partly related to the epithelial barrier dysfunction,26 and if this relationship persists over time. Also, it is unclear if the swallowed topical steroids could make the epithelium thinner and access to lamina propria easier as epidermal thinning has been reported with prolonged topical corticosteroid use for eczema.27 Epithelial biology based studies might address this question.
Of the 123 children with EoE who had aLP, less than 25% had LPF. The presence of LPF was significantly associated with gender (male) and the intensity of eosinophilic inflammation (or the PEC). It was not associated with the age at EGD, clinical factors or endoscopic features, and with the duration of EoE in particular. It is known that males prone to develop EoE and adult males report more fibrostenotic symptoms compared to adult females who often report more inflammatory symptoms at the time of diagnosis.28 It would be interesting to study if gender has any effect on the biology of esophageal histology and explain the propensity for LPF among male EoE subjects. Our observation of PEC being associated with LPF is consistent with current understanding that eosinophils act as major effector cells of tissue fibrosis in eosinophil mediated inflammatory conditions. An increased level of subepithelial fibrosis and increased expression of TGF-ß1 by eosinophils and its signaling molecule phospho-SMAD2/3 has been previously shown in children with EoE when compared to children without EoE.29,30 We noted that the degree of correlation within epithelial features was high-moderate in biopsies with LPF whereas it was moderate-weak in the biopsies without LPF. Similarly, the probability of predicting LPF increased with intensification of abnormality in each of epithelial features. These observations suggest that the intensity of eosinophilic inflammation and the extent of epithelial changes can be linked with the health of the lamina propria in EoE. This is important because early fibrosis can be reversed once epithelial inflammation including the intensity of eosinophilic inflammation is controlled.31,32
There was a high concordance between presence of SEA and DEC and presence of LPF in our study. In SEA the surface epithelial cells exhibit altered tinctorial properties and manifest as dark staining, with or without intraepithelial eosinophils, and in DEC the individual epithelial cells have deeply eosinophilic cytoplasm and hyperchromatic nuclei. Previous studies, and own our experience indicates that both SEA and DEC are less frequently noted when compared to features such as EI or DIS in EoE patients.15,33 However, when present, SEA and DEC may serve as epithelial markers of LPF. We postulate this could be related to the esophageal epithelial and mesenchymal cross-talk wherein epithelial cells can acquire mesenchymal features.23,34 Future research will be needed to validate this observation and to unravel the relationship between SEA, DEC, and LPF.
This study has some limitations. We used a retrospective design and used patient reported symptoms, medication exposure, duration of EoE (in known EoE cases), and allergic comorbidity information making misclassification and recall bias conceivable. Our cohort comprised of children with EoE and the majority of them had an inflammatory phenotype characterized by edema, furrows and exudates. Therefore, our results may not be directly applicable to adult EoE patients and patients with fibrostenotic phenotype characterized by presence of rings and strictures. We assessed the degree of tissue abnormality (grade score) and did not assess the extent of pathology (stage score) However, results from a recent study evaluating the histopathological effects of single therapy in EoE reveals that the grade score and the stage score tend to track together.35 Relatively more biopsies were obtained from the EoE subjects and quite a few of the subjects had a known history of EoE at the time of EGD. It is conceivable that the endoscopist might have unintentionally taken deeper biopsies in those children with marked endoscopic changes and/or obtained more tissue in EoE patients and this might have resulted in higher proportion of aLP in EoE subjects. However, it is important to note that the number of biopsies, EREFS, and duration of diagnosis did not hold up in the multivariate analysis as predictors of aLP. Finally, the sample size of the subgroup of EoE children with LPF was relatively small as evidenced by wide confidence intervals in correlation analysis and logistic regression analyses.
Despite these limitations, our study has several strengths. We consecutively enrolled patients and used well defined inclusion criteria to limit selection bias, and overall this study reports on a large group of children. All the EREFS were observed and recorded at the time of the EGD and this eliminated any recall bias on the part of the endoscopist and provided real-time assessment rather than re-assessment from archived photos. We used the current definition to define aLP and used the validated EoEHSS rating to grade the LPF, with all biopsies undergoing a dedicated research read. The histologic assessments were done by a single pediatric pathologist and this allowed us to minimize inter-observer variability. We accounted for multiple comparisons and used a rigorous cut-off to ascertain statistical significance.
In conclusion, disease activity status and the PEC are independently associated with presence of aLP and LPF in children with EoE. Novel to our study is the observation that the epithelial changes are intricately correlated with each other in the biopsies containing LPF, and presence of SEA and DEC appears to prognosticate presence of LPF. Efforts are underway to develop computational models to predict LPF in esophageal biopsies without aLP. Future research to understand if the LPF is patchy or uniform throughout the esophagus if found in one biopsy and the variability in histology among multiple biopsies from an individual with EoE is warranted.
Supplementary Material
Need to Know.
Background:
Esophageal biopsies in children with eosinophilic esophagitis (EoE) are often inadequate for assessment of lamina propria and lamina propria fibrosis. Little is known about the factors associated with adequate lamina propria collection and the relationship among epithelial features in esophageal biopsies with and without lamina propria fibrosis.
Findings:
Adequate lamina propria was found in higher proportions of esophageal biopsies from children with EoE compared with controls. The authors identified factors associated with adequate lamina propria sampling and fibrosis.
Implications for patient care:
Given the association between epithelial features and lamina propria fibrosis, computational models can be developed to identify children with esophageal biopsies without adequate lamina propria sampling who are at risk for fibrosis.
Acknowledgements:
We acknowledge Dr. Margaret Collins, Professor, University of Cincinnati, Department of Pathology and Laboratory Medicine for her advice on interpreting histology data. We also acknowledge Regina Tyree’s assistance with data collection and management.
Funding support:
G.H. is supported by the American College of Gastroenterology Junior Faculty Development Award, the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number K12HD087023, Katherine Dodd Faculty Scholars Program at Vanderbilt University Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Y.A.C. is supported by Department of Veterans Affairs Career Development Award IK2BX004648, Vanderbilt Digestive Disease Research Center Pilot Grant P30058404, and a Vanderbilt SCRIPS Burroughs Welcome Fund Fellowship.
Footnotes
Conflict of Interest Statement:
Authors have no conflict of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med 2015.373:1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol 2016;50:134–140. [DOI] [PubMed] [Google Scholar]
- 3.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013;145:1230–1236. [DOI] [PubMed] [Google Scholar]
- 4.Hirano I. Eosinophilic esophagitis. Gastroenterol Clin North Am 2014;43:297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menard-Katcher C, Benitez AJ, Pan Z, et al. Influence of Age and Eosinophilic Esophagitis on Esophageal Distensibility in a Pediatric Cohort. Am J Gastroenterol 2017;112:1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirano I. Clinical relevance of esophageal subepithelial activity in eosinophilic esophagitis. J Gastroenterol 2019;55:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Park JY, Huang R, et al. Obtaining adequate lamina propria for subepithelial fibrosis evaluation in pediatric eosinophilic esophagitis. Gastrointest Endosc 2018;87:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bussmann C, Schoepfer AM, Safroneeva E, et al. Comparison of different biopsy forceps models for tissue sampling in eosinophilic esophagitis. Endoscopy 2016;48:1069–1075. [DOI] [PubMed] [Google Scholar]
- 9.Aceves SS, King E, Collins MH, et al. Alignment of parent- and child-reported outcomes and histology in eosinophilic esophagitis across multiple CEGIR sites. J Allergy Clin Immunol 2018;142:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins MH, Martin LJ, Wen T, et al. Eosinophilic Esophagitis Histology Remission Score: Significant Relations to Measures of Disease Activity and Symptoms. J Pediatr Gastroenterol Nutr 2020;70:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chehade M, Sampson HA, Morotti RA, et al. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2007;45:319–328. [DOI] [PubMed] [Google Scholar]
- 12.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3–20. [DOI] [PubMed] [Google Scholar]
- 13.Dellon ES, Speck O, Woodward K, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol 2015;28:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut 2013;62:489–495. [DOI] [PubMed] [Google Scholar]
- 15.Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoepfer AM, Simko A, Bussmann C, et al. Eosinophilic esophagitis: Relationship of subepithelial eosinophilic inflammation with epithelial histology, endoscopy, blood eosinophils, and symptoms. Am J Gastroenterol 2018;113:348–357. [DOI] [PubMed] [Google Scholar]
- 17.O’Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2018;154:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamabe A, Irisawa A, Shibukawa G, et al. Clinical effects of eosinophilic esophagitis observed using endoscopic ultrasound. Clin J Gastroenterol 2014;7:305–309. [DOI] [PubMed] [Google Scholar]
- 19.Read AJ, Pandolfino JE. Biomechanics of esophageal function in eosinophilic esophagitis. J Neurogastroenterol Motil 2012;18:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JW, Pandolfino JE, Lin Z, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy 2016;48:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Politi E, Angelakopoulou A, Grapsa Di, et al. Filaggrin and Periostin Expression Is Altered in Eosinophilic Esophagitis and Normalized with Treatment. J Pediatr Gastroenterol Nutr 2017;65:47–52. [DOI] [PubMed] [Google Scholar]
- 22.Rawson R, Yang T, Newbury RO, et al. TGF-β1–induced PAI-1 contributes to a profibrotic network in patients with eosinophilic esophagitis. J Allergy Clin Immunol 2016;138:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muir AB, Wang JX, Nakagawa H. Epithelial-stromal crosstalk and fibrosis in eosinophilic esophagitis. J Gastroenterol 2019;54:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults with Eosinophilic Esophagitis. Gastroenterology 2016;150:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreae DA, Hanna MG, Magid MS, et al. Swallowed fluticasone propionate is an effective long-term maintenance therapy for children with eosinophilic esophagitis. Am J Gastroenterol 2016;111:1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rochman M, Azouz NP, Rothenberg ME. Epithelial origin of eosinophilic esophagitis. J Allergy Clin Immunol 2018;142:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol 2016;82:371–378. [DOI] [PubMed] [Google Scholar]
- 28.Lynch KL, Dhalla S, Chedid V, et al. Gender is a determinative factor in the initial clinical presentation of eosinophilic esophagitis. Dis Esophagus 2016;29:174–178. [DOI] [PubMed] [Google Scholar]
- 29.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2007;119:206–212. [DOI] [PubMed] [Google Scholar]
- 30.Aceves SS. Remodeling and fibrosis in chronic eosinophil inflammation. Dig Dis 2014;32:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieberman JA, Morotti RA, Konstantinou GN, et al. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: A historical cohort. Allergy Eur J Allergy Clin Immunol 2012;67:1299–1307. [DOI] [PubMed] [Google Scholar]
- 32.Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy Eur J Allergy Clin Immunol 2010;65:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.S T, W T, K J, et al. Correlation of the eosinophilic histopathological scoring system with esophageal gene expression in patients with eosinophilic esophagitis. J Allergy Clin Immunol 2018;3:477–488. [Google Scholar]
- 34.Kagalwalla AF, Akhtar N, Woodruff SA, et al. Eosinophilic esophagitis: Epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol 2012;129:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins MH, Dellon ES, Katzka DA, Hirano I, Williams J LL. Budesonide Oral Suspension Significantly Improves Eosinophilic Esophagitis Histology Scoring System Results: Analyses From a 12-Week, Phase 2, Randomized, Placebo-controlled Trial. Am J Surg Pathol 2019;43:1501–1509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.