Abstract
Background
Previous data suggest that the immune microenvironment plays a critical role in human epidermal growth factor receptor 2 (HER2) -positive breast cancer; however, there is little known about the immune profiles of small HER2-positive tumors. In this study, we aimed to characterize the immune microenvironment of small HER2-positive breast cancers included in the Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer (APT) trial and to correlate the immune markers with pathological and molecular tumor characteristics.
Patients and methods
The APT trial was a multicenter, single-arm, phase II study of paclitaxel and trastuzumab in patients with node-negative HER2-positive breast cancer. The study included 406 patients with HER2-positive, node-negative breast cancer, measuring up to 3 cm. Exploratory analysis of tumor infiltrating lymphocytes (TIL), programmed death-ligand 1 (PD-L1) expression (by immunohistochemistry), and immune gene signatures using data generated by nCounter PanCancer Pathways Panel (NanoString Technologies, Seattle, WA), and their association with pathological and molecular characteristics was carried out.
Results
Of the 406 patients, 328 (81%) had at least one immune assay carried out: 284 cases were evaluated for TIL, 266 for PD-L1, and 213 for immune gene signatures. High TIL (≥60%) were seen with greater frequency in hormone-receptor (HR) negative, histological grades 2 and 3, as well in HER2-enriched and basal-like tumors. Lower stromal PD-L1 (≤1%) expression was seen with greater frequency in HR-positive, histological grade 1, and in luminal tumors. Both TIL and stromal PD-L1 were positively correlated with 10 immune cell signatures, including Th1 and B cell signatures. Luminal B tumors were negatively correlated with those signatures. Significant correlation was seen among these immune markers; however, the magnitude of correlation did not indicate a monotonic relationship between them.
Conclusion
Immune profiles of small HER2-positive breast cancers differ according to HR status, histological grade, and molecular subtype. Further work is needed to explore the implication of these findings on disease outcome.
Clinical trial registration
clinicaltrials.gov identifier: NCT00542451.
Key words: HER2-postitive breast cancer; immune profile; PD-L1 expression, TIL; tumor-inflamed signature; stage I
Key Message
The immune profile of small HER2-positive breast cancers from patients included in the APT trial differs according to pathological and molecular characteristics. High levels of tumor infiltrating lymphocytes and of stromal PD-L1 expression were seen with greater frequency in hormone-receptor negative, higher grade tumors as well as in HER2-enriched and basal-like tumors.
Alt-text: Unlabelled Text
Introduction
Approximately 20% of breast cancers overexpress human epidermal growth factor receptor 2 (HER2) and are classified as HER2 positive [1, 2]. Increasing evidence suggests that the interaction between tumor and immune cells is critical in HER2-positive disease [3]. These findings include data suggesting the following: (i) tumor-infiltrating lymphocytes (TIL) are prognostic and predictive of benefit to systemic therapy [4, 5]; (ii) preclinical and clinical data have shown that both the innate and adaptive immune system participate in trastuzumab’s antitumor activity [6., 7., 8., 9., 10.]; (iii) the expression of programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1) or both, in the tumor microenvironment may act as a mechanism of immune evasion, contributing to resistance against anti-HER2 agents [9]; and (iv) anti-HER2 monoclonal antibodies synergize with anti-PD-1, improving the therapeutic activity of trastuzumab in immunocompetent mice [9].
Despite this preponderance of data, little is known about the immune microenvironment of small HER2-positive breast tumors. Most of the information regarding TIL in early-stage HER2-positive breast cancer comes from patients with larger tumors (>2 cm) and/or with node-positive disease. In addition, there is little information on how the molecular characterization of HER2-positive tumors correlates with the immune landscape of small HER2-tumors. Therefore, we carried out an exploratory analysis to characterize the immune microenvironment of tumors from HER2-positive patients included in the Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer (APT) trial, as well as to correlate these immune markers with pathological and molecular characteristics.
Methods
APT study design
The APT trial (NCT00542451) design and results have been previously reported [11, 12] and are presented in the supplementary Methods, available at Annals of Oncology online. The institutional review board at each site approved the study and all participants signed informed consent.
Supplementary Data.

Tissue evaluation
Formalin-fixed paraffin-embedded tissue from the core biopsy or surgical excision was available for 381 patients. A total of 328 (81%) patients had at least one of the immune assays carried out and are included in the current analysis. Reasons for not performing assays are shown in the REMARK diagram (supplementary Figure S1, available at Annals of Oncology online).
Histological assessment (TIL evaluation)
TIL were evaluated according to the International TIL Working Group guidelines [13] The TIL level was evaluated as a continuous measure, and as three ordinal levels of low, intermediate, and high expression (≤10%, 10% to 60%, and >60%,). Details about TIL assessment are provided in the supplementary Materials, available at Annals of Oncology online.
Immunohistochemistry for PD-L1
A total of 266 cases had PD-L1 expression assessed. Immunohistochemical expression was evaluated separately for cancer cells and stromal immune cells using a modified H-score (intensity of staining [0 to 3+] multiplied by percent of cells with positive staining in each category [0% to 100%]). PD-L1 levels were evaluated as a continuous measure, and as three ordinal levels of low, intermediate, and high expression (<1%, 1% to 10%, and >10%, respectively). Details about PD-L1 assessment are provided in the supplementary Material, available at Annals of Oncology online.
mRNA expression: assignment of intrinsic subtype, immune cell signatures and tumor-inflamed signature
Gene expression analysis was conducted on the nCounter® gene expression platform (NanoString Technologies, Seattle, WA). Intrinsic subtyping by PAM50 [14, 15] (Prosigna®, NanoString Technologies, Seattle, WA) and immune signatures [16] by the PanCancer Immune Profiling Panel were determined for 270 and 213 patients, respectively. Using data generated by the PanCancer Immune Profiling Panel, an estimated tumor-inflamed signature (TIS), evaluating 16 out of 18 genes of the original TIS [17] was also carried out (supplementary Table S1, available at Annals of Oncology online). Details about the RNA acquisition and quality assurance are provided in the supplementary Material, available at Annals of Oncology online.
Statistical analysis
Descriptive statistics were used to summarize the distribution of patient and disease characteristics (HR status, histological grade, tumor size) and molecular phenotypes. Proportions were reported with Clopper–Pearson 95% confidence intervals (95% CIs). Statistical inferences on association were made using odds ratios (OR) and Fisher and McNemar’s exact tests for categorical variables, Cochran–Mantel–Haenszel (CMH) chi-squared tests for ordinal variables. Wilcoxon and Kruskal–Wallis rank sum tests and Spearman correlation coefficients (ρ) were used for continuous variables. Statistical significance was determined using nominal P < 0.05, and corrections for multiple testing with the multiplex Immune Profile Panel were made using the Benjamini–Hochberg (BH) step-up procedure to control the false discovery rate [18]. All statistical analyses were carried out in R v3.4.3. (www.r-project.org).
Results
Patients characteristics
The subset of 328 patients with at least one assay carried out comprises the population included in this analysis. These patients presented with similar baseline characteristics as compared with the whole APT population (Table 1). Intrinsic subtype was available for 278 cases, which were classified as HER2-enriched (66%), luminal B (14%), luminal A (12%), and basal-like (8%). A greater percentage of HR-negative then HR-positive patients were classified as HER2-enriched and basal-like (P < 0.0001). There was no relationship between PAM50 subtype and tumor size (P = 0.99).
Table 1.
Baseline characteristics of patients included in the APT trial, and in the current analysis
| Characteristics | Total study population | Subpopulation with any assay | Subpopulation with all assays |
|---|---|---|---|
| Patients | N = 406 | N = 328 | N = 147 |
| Age | |||
| Mean (SD) | 55.2 (10.5) | 56.0 (10.7) | 55.6 (10.6) |
| Race | |||
| White | 351 (86%) | 279 (85%) | 130 (88%) |
| Black or AA | 28 (7%) | 27 (8%) | 7 (5%) |
| Asian | 11 (3%) | 10 (3%) | 5 (3%) |
| Other | 16 (4%) | 12 (3%) | 5 (3%) |
| Primary size (cm) | |||
| Mean (SD) | 1.17 (0.64) | 1.22 (0.64) | 1.26 (0.55) |
| Histologic grade | |||
| 1 | 44 (11%) | 30 (9%) | 15 (10%) |
| 2 | 131 (32%) | 109 (33%) | 49 (33%) |
| 3 | 228 (56%) | 189 (58%) | 83 (56%) |
| Missing | 3 (1%) | 0 | 0 |
| ER status | |||
| Positive | 265 (65%) | 219 (67%) | 100 (68%) |
| Negative | 141 (35%) | 109 (33%) | 47 (32%) |
| PR status | |||
| Positive | 209 (51%) | 176 (54%) | 81 (55%) |
| Negative | 197 (49%) | 152 (46%) | 66 (45%) |
| HR status | |||
| Positive | 272 (67%) | 226 (69%) | 102 (69%) |
| Negative | 134 (33%) | 102 (31%) | 45 (31%) |
| Molecular subtype | |||
| Basal-like | n.a. | 21 (8%) | 9 (6%) |
| Her2-enriched | n.a. | 176 (65%) | 98 (67%) |
| Luminal A | n.a. | 35 (13%) | 18 (12%) |
| Luminal B | n.a. | 38 (14%) | 22 (15%) |
AA, African American; ER, estrogen receptor; HR, hormonal receptor; PR, progesterone receptor.
TIL
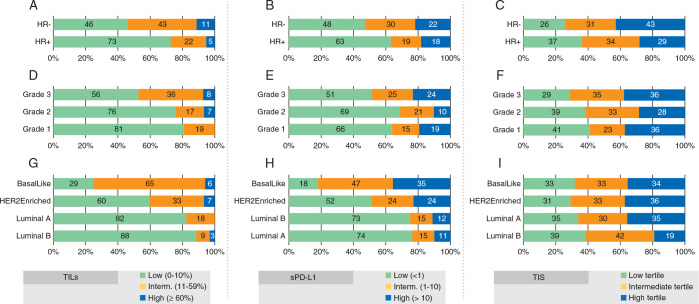
The median TIL was 5% [interquartile range (IQR) 5–20], and 184 (65%) tumors had low, 81 (28%) had intermediate, and 19 (7%) had high TIL. High TIL were seen with greater frequency in HR-negative cases (11%) than in HR-positive cases (5%) (OR = 2.5, 95% CI 0.9–7.3), and a significant ordinal relationship was observed (P < 0.0001; Figure 1A). High TIL were seen in grade 2 and 3 tumors (7% and 8%, respectively), and not seen in the 27 cases of grade 1 tumors (0%) (P = 0.0041; Figure 1D). Additionally, high TIL were seen with greater frequency in the HER2-enriched (7%) and basal-like subsets (6%) (P < 0.001; Figure 1G). Although there was a slight trend of higher TIL with increasing tumor size, it failed to reach statistical significance (P = 0.20).
Figure 1.
Tumor-infiltrating lymphocyte (TIL) (A, D and G), stromal PD-L1 (B, E, and H), and tumor-inflamed signature (TIS) (C, F, I), respectively, according to hormonal receptor (HR) status, histologic grade and molecular subtypes.
PD-L1 expression
The median stromal PD-L1 (sPD-L1) expression was 0% (IQR 0–5), and 154 (58%) tumors had low, 61 (23%) had intermediate, and 51 (19%) had high sPD-L1 expression. Low sPD-L1 levels were seen with greater frequency in HR-positive cases (63%) than in HR-negative cases (48%) (OR = 1.8, 95% CI 1.1–3.2, P = 0.024; Figure 1B), in grade 1 and 2 tumors (66% and 69%, respectively) than in grade 3 tumors (51%) (P = 0.018; Figure 1E), and in the luminal A (74%) and luminal B subsets (73%) compared with the other subtypes (P = 0.0008; Figure 1H). Of note, there was a slight positive correlation between lower sPD-L1 and smaller tumor size (ρ = 0.13, P = 0.033) which was sustained in the HER2-enriched subgroup (ρ = 0.18, P = 0.025). Data on the association of tumor PD-L1 (tPD-L1) expression with disease characteristics is in supplementary Figure S2, available at Annals of Oncology online.
Immune cell signature
We also evaluated expression signatures representing 14 immune cell types, and their association with pathological and immune characteristics (Figure 2). TIL were strongly positively correlated with 10 immune cell signatures [CD8 T, Th1, B cells, exhausted T cells, T cells, T regulatory (Treg) cells, macrophages, CD45-positive cells, cytotoxic cells and natural killer (NK) CD56+ diminished cells; adjusted P < 0.05]. To a lesser degree, both sPD-L1 and tPD-L1 also correlated with these signatures. None of the signatures were significantly associated with HR status or with tumor grade. Basal-like subtype was positively correlated with Th1 cells, B cells, NK cells, cytotoxic cells, and NK CD56 diminished cells signatures and negatively correlated with neutrophils and mast cells signatures (adjusted P < 0.05). In contrast, luminal B subtype tumors had a negative correlation with seven of these signatures [CD8 T cells, B cells, T cells, Treg cells, dendritic cells (DC), cytotoxic cells, and NK CD56 diminished cells; adjusted P < 0.05]. The HER2-enriched subtype was positively correlated with Treg cells (adjusted P = 0.039). Notably, the size of tumor was positively associated with exhausted CD8 T-cell signature and negatively associated with DC signature (adjusted P < 0.05).
Figure 2.
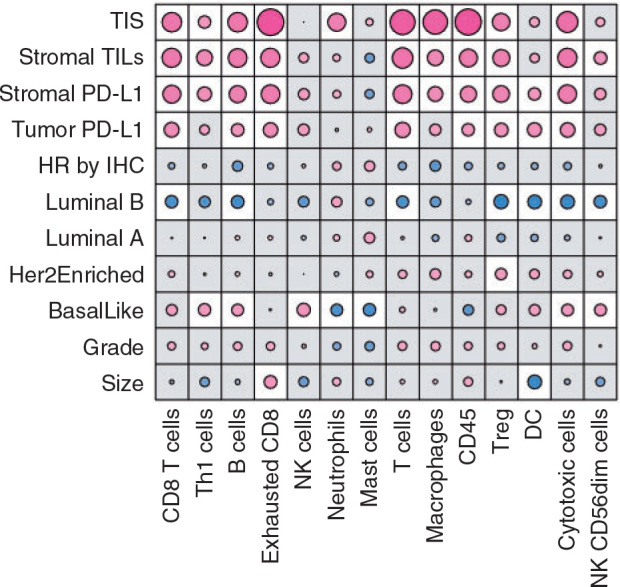
Association between immune cell gene signatures, and tumor-inflamed signature (TIS) tumor-infiltrating lymphocyte (TIL) stromal and tumor PD-L1, hormonal-receptor (HR) status, and molecular subtype. Correlation coefficients (red, positively correlated; blue, negatively correlated). The size of the circle is proportional to the strength of the correlation. White cells reach statistical significance after correcting for multiple testing (false discovery rate at the 5% level using a Benjamini–Hochberg adjustment). DC, dendritic cells; HR, hormonal receptor status; NK, natural killer.
T-cell inflamed signature
TIS scores were divided into tertiles. Higher TIS was seen in the HR-negative tumors and lower TIS in luminal B subsets (Figure 1C, F and I); however, neither reached statistical significance (P = 0.16 and P = 0.36, respectively). No relationship was observed between TIS and tumor grade (P = 0.38), while a slight positive correlation with tumor size was detected (ρ = 0.15, P = 0.028).
Correlation between different immune markers
There was moderate correlation between sPD-L1 and tPD-L1, with greater frequency of medium and high levels of staining in the stroma (P < 0.0001). Moreover, there was a significant correlation between TIL and sPD-L1 (P < 0.0001), and to a much lesser extent with tPD-L1 (P = 0.0074). TIS positively correlated with TIL, sPD-L1, and tPD-L1. Supplementary Figure S3, available at Annals of Oncology online displays the pairwise correlation and scatterplot of continuous values for each immune marker.
Outcomes
After a median follow-up of 6.5 years, 21 patients experienced an invasive disease-free survival (DFS) event (7%) and only nine patients experienced an recurrence-free interval (RFI) event (3%). The low event rate prevents making statistical inferences on the prognostic value of the immune profiles. Supplementary Table S2, available at Annals of Oncology online summarizes the pathological and immune characteristics of individuals with cases of distant recurrence.
Discussion
In this study, we show that the immune profile of small HER2-positive breast cancers, as assessed by TIL, PD-L1 expression, TIS, and immune cell signature, differs according to pathological and molecular disease characteristics. While high TIL are seen at a higher frequency in HR-negative tumors, high-grade tumors, and in HER2-enriched and basal-like subtypes, more HR-positive, low grade, and luminal tumors present with low stromal and tumor PD-L1 expression. Additionally, immune cell signatures differ according to molecular subtype.
The NeoSphere [19] and NeoALTTO [20] studies have showed that tumor with low baseline TIL had lower pathological complete response (pCR) rates. Additionally, both NeoALTTO [20] and TRYPHAENA [21] studies showed that TIL was associated with improved event-free survival when systemic therapy was given in the neoadjuvant setting. Loi et al. [22] reported that TIL was predictive of benefit to adjuvant trastuzumab in the FinHER study A pooled analysis of six prospective neoadjuvant clinical trials showed that increased TIL were associated with higher pCR rates, and improved DFS in HER2-positive breast cancer [5]. However, the analysis did not show an association between increased TIL and OS. In contrast, data from the adjuvant N9831 study suggested that the presence of high TIL was associated with an improvement in recurrence-free survival in patients who received chemotherapy alone, but not among patients treated with chemotherapy plus trastuzumab [23].
Compared with other studies [5, 19, 20, 23., 24., 25.], we found a higher frequency (65%) of tumors with low TIL (≤ 10%) and a lower frequency of tumors with lymphocyte predominant breast cancer (≥60% TIL). It is important to highlight that the APT trial [11] population is very different from the other studies (Table 2) and was comprised only patients with small (≤3 cm), node-negative HER2-positive tumors; in addition, the APT population was enriched for HR+ tumors. Similar to the PAMELA trial [26], we found a statistically significant association between intrinsic subtypes and TIL. This association was not observed in the TRYPHAENA trial though [21].
Table 2.
TIL in HER2+ tumors according to different clinical trials
| Studies | APT trial | N9831 trial | NeoSphere trial | Pooled analysis of GEPAR trials | NeoALTTO trial | PAMELA trial | TRYPHAENA trial |
|---|---|---|---|---|---|---|---|
| Number of patients | 284 patients | 945 patients | 350 patients | 1379 patients | 387 patients | 148 patients | 213 patients |
| Disease setting | Adjuvant | Adjuvant | Neoadjuvant | Neoadjuvant | Neoadjuvant | Neoadjuvant | Neoadjuvant |
| HR+ tumors, % | 68 | 54 | 46 | 53 | 50 | 52 | 50 |
| Node negative tumors, % | 100 | 14 | 28 | 40 | NR | 64 | 29 |
| Tumors measuring ≤ 2 cm, % | 89 | 39 | 0 | 12 | 2 | 40 | 0 |
| TIL as categories | ≤10%: 65% | 0–9%: 33.7% | 0–5%: 13.6% | ≤10%: 44% | NR | NR | NR |
| 10–60%: 28% | 10–60%: 56.5% | 5–50%: 69.5% | 10–60%: 37% | ||||
| ≥60%: 7% | ≥60%: 9.9% | >50%: 16.9% | ≥60%: 19% | ||||
| TIL as median (IQR) | 5% (5–20%) | NR | NR | NR | 12.5% (5–30%) | 10% (5–20%) | 14% (7–32%) |
TIL, tumor-infiltrating lymphocytes; HR+, hormone-receptor positive; IQR, interquartile range; NR, not reported.
The PD-1/PD-L1 axis is an important target for anticancer therapy with agents targeting the axis revolutionizing cancer therapy [27]. In our population, 58% of tumors had low sPD-L1 expression (<1%). This low prevalence is similar to that seen in previous work [24, 28]. Furthermore, our study also confirmed an association between low tumor grade, HR-positive status, and luminal subtypes, and low sPD-L1 expression.
Gene expression profile has been used to further evaluate the biology of tumor and host immune cell interactions within the tumor microenvironment. A recently validated 18-gene TIS, assayed on the NanoString nCounter platform, was shown to be predictive of response to PD-1 inhibitor pembrolizumab across multiple solid tumors [17]. In our study, a higher estimated TIS was seen in the HR-negative tumors, and lower TIS in luminal B subsets, though neither reached statistical significance. Furthermore, our work showed a significant correlation among TIL, PD-L1 expression, and the TIS. Similar results to this association between an immune gene signature and TIL has been showed in TRYPHAENA trial. These findings are in agreement with the known mechanisms of anticancer T-cell immunity which releases interferon-γ leading to an adaptive immune response with upregulation of PD-L1 expression [29]. However, the magnitude of correlation in the current study does not indicate a monotonic relationship between them.
Immune cell signatures (Th1, B cells, and DC) have been shown to be predictive of pCR to chemotherapy plus pembrolizumab in patients with HER2-negative breast cancer [30]. To our knowledge, this is the first study to explore the association between these signatures with pathological and molecular characteristics in HER2-positive breast cancer. We showed that while HR status and tumor grade were not associated with any immune cell signature, molecular subtypes were differently associated with them. Importantly, basal-like tumors positively correlated with Th1 and B cells signatures, while luminal B tumors negatively correlated with B cells and DC signature. Lastly, we observed a significant association between tumor size and an exhausted CD8 T-cell signature. This finding, together with the one showing a slight trend of higher TIL with increasing tumor size, and increased sPD-L1 expression in larger tumors, corroborates with the hypothesis that adaptive resistance mechanisms [29] play a role in the anti-tumor immunity escape of HER2-positive tumors. The implications of these findings require further investigation as they may have an important impact on management of early-stage HER2-positive breast cancer, and the development of new therapies for this subset of patients.
The strengths of this study include the fact that all patients were treated in a prospective manner on a clinical trial, it is a unique set of small HER2-positive tumors, incorporates TIL assessment using consensus guidelines, and explored the correlation of the immune profile with the intrinsic subtype of these tumors. However, our study has some limitations. First, the low number of recurrence events impairs the ability to establish any association between our findings and outcomes. Second, this analysis was not pre-planned in the APT trial, and only a subset of the enrolled patients in the APT trial were included in this analysis. However, there were not significant differences in patient and tumor characteristics for those with or without tissue analyzed. Finally, the lack of consensus about the most meaningful cutoff and methodology for PD-L1 expression analysis, and the use of an estimated TIS, preclude us from comparing the data to other tumor subtypes.
Conclusion
The immune profile of small HER2-positive breast cancer differs according to pathological and molecular characteristics. Patients with tumors that are HR-positive, low grade, and have a luminal molecular subtype were found to have lower TIL and lower PD-L1 expression. Immune cell signatures differ according to molecular subtype, with luminal B tumors being negatively correlated with signatures classically associated with good prognosis. Further work is needed to evaluate if these markers are associated with benefit to standard HER2-directed therapies and immunotherapy.
Acknowledgements
ST had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We kindly thank Francisco Edson Bezerra Filho for his art support to this work.
Funding
The APT trial was funded by Genentech (no grant number applies).
Disclosure
RB-S has served as an advisor/consultant to Eli Lilly and has received honoraria from Roche for participation in Speakers Bureau. SMT reported receiving institutional research support from Merck, Bristol-Myers Squibb, Exelixis, Eli Lilly, Pfizer, Novartis, AstraZeneca, Eisai, Nektar, and Genentech and has served on advisory boards for Genentech, Eli Lilly, Novartis, Pfizer, Nektar, Immunomedics, Nanostring, AstraZeneca, Eisai, Puma, and Merck. EAM has served on advisory boards for Merck. WTB reported receiving research support from Pfizer. IEK reported receiving research support from Genentech and Pfizer. All remaining authors have declared no conflicts of interest.
Genentech
References
- 1.Pathmanathan N., Provan P.J., Mahajan H. Characteristics of HER2-positive breast cancer diagnosed following the introduction of universal HER2 testing. Breast. 2012;21(6):724–729. doi: 10.1016/j.breast.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Slamon D.J., Clark G.M., Wong S.G. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G., Senovilla L., Galluzzi L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21(10):1128–1138. doi: 10.1038/nm.3944. [DOI] [PubMed] [Google Scholar]
- 4.Loi S., Michiels S., Salgado R. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Annals of Oncology. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 5.Denkert C., von Minckwitz G., Darb-Esfahani S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 6.Gennari R., Menard S., Fagnoni F. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10(17):5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 7.Clynes R.A., Towers T.L., Presta L.G., Ravetch J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 8.Park S., Jiang Z., Mortenson E.D. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18(2):160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stagg J., Loi S., Divisekera U. Anti–ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti–PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci USA. 2011;108(17):7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knutson K.L., Clynes R., Shreeder B. Improved survival of HER2+ breast cancer patients treated with trastuzumab and chemotherapy is associated with host antibody immunity against the HER2 intracellular domain. Cancer Res. 2016;76(13):3702–3710. doi: 10.1158/0008-5472.CAN-15-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolaney S.M., Barry W.T., Dang C.T. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolaney S.M., Barry W.T., Guo H. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC) Journal of Clinical Oncology. 2017;35(Suppl 15):511. 511. [Google Scholar]
- 13.Salgado R., Denkert C., Demaria S. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker J.S., Mullins M., Cheang M.C. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen T., Wallden B., Schaper C. Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer. 2014;14:177.. doi: 10.1186/1471-2407-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danaher P., Warren S., Dennis L. Gene expression markers of tumor infiltrating leukocytes. J Immunother Cancer. 2017;5:18.. doi: 10.1186/s40425-017-0215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayers M., Lunceford J., Nebozhyn M. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 19.Bianchini G., Pusztai L., Pienkowski T. Immune modulation of pathologic complete response after neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann Oncol. 2015;26(12):2429–2436. doi: 10.1093/annonc/mdv395. [DOI] [PubMed] [Google Scholar]
- 20.Salgado R., Denkert C., Campbell C. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ignatiadis M., Van den Eynden G., Roberto S. Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: a TRYPHAENA substudy. J Natl Cancer Inst. 2019;111(1):69–77. doi: 10.1093/jnci/djy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loi S., Michiels S., Salgado R. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 23.Perez E.A., Ballman K.V., Tenner K.S. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the n9831 adjuvant trial in patients with early-stage her2-positive breast cancer. JAMA Oncol. 2016;2(1):56–64. doi: 10.1001/jamaoncol.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim A., Lee S.J., Kim Y.K. Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci Rep. 2017;7(1):11671.. doi: 10.1038/s41598-017-11905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamy A.S., Pierga J.Y., Sabaila A. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann Oncol. 2017;28(9):2233–2240. doi: 10.1093/annonc/mdx309. [DOI] [PubMed] [Google Scholar]
- 26.Nuciforo P., Pascual T., Cortes J. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol. 2018;29(1):170–177. doi: 10.1093/annonc/mdx647. [DOI] [PubMed] [Google Scholar]
- 27.Brahmer J.R., Tykodi S.S., Chow L.Q. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Opyrchal M., Yao S. The role of programmed death ligand-1 and tumor-infiltrating lymphocytes in breast cancer overexpressing HER2 gene. Breast Cancer Res Treat. 2018;170(2):293–302. doi: 10.1007/s10549-018-4745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell M., Yau C., Borowsky A. Abstract PD6-08: analysis of immune infiltrates (assessed via multiplex fluorescence immunohistochemistry) and immune gene expression signatures as predictors of response to the checkpoint inhibitor pembrolizumab in the neoadjuvant I-SPY 2 trial. Cancer Res. 2018;78(Suppl 4):PD6–08. [Google Scholar]