Abstract
Although text-messaging interventions are effective for smoking cessation, few target teens in the USA and little is known about their effectiveness. The purpose of this manuscript is to examine correlates of dropout, response to smoking status prompts, and abstinence rates among subscribers of SmokeFreeTeen, a free, publicly available text-messaging smoking cessation intervention sponsored by the National Cancer Institute’s SmokeFree.Gov initiative, on quit day through 1 month follow-up. In a sample of teens (N = 2,685), aged 13–19, we examined demographics, smoking frequency, cigarettes smoked per day, prequit intervention time (i.e., maximum of 14 days of prequit day preparation), and number of quit attempts as correlates of response and abstinence rates among program initiators (i.e., participants who reached quit day but dropped out on or before intervention end) and completers (i.e., participants who reached quit day and completed the intervention). We also conducted Cox regression analysis of time from quit day to dropout by daily and nondaily smoking status. Two-thirds (n = 1,733, 64.54%) dropped out before the intervention ended, with dropout rates peaking on quit day (n = 289, 13.10%). Response rate to smoking status prompts remained below 30% throughout the intervention. At intervention end and 1 month follow-up, abstinence was 2.63% and 2.55% among program initiators, whereas abstinence was 6.09% and 6.01% among program completers. Dropout, response, and abstinence rates did not consistently differ by subscriber characteristics. Prequit time was associated with decreased likelihood of dropping out (adjusted hazards ratio: 0.94, confidence interval [CI]: 0.93–0.95), responding to smoking status prompts (adjusted odds ratio [aOR]: 0.94, CI: 0.92–0.96), and being abstinent (aOR: 0.96, CI: 0.93–0.99) on quit day. Two or more quit attempts were associated with increased response (aOR: 1.61, CI: 1.16–2.23) and abstinence (aOR: 1.91, CI: 1.25–2.92) rates on Day 7. In a first assessment of SmokeFreeTeen outcomes, we document high dropouts and low response and abstinence rates. SmokeFreeTeen produced abstinence rates lower than comparable text-messaging interventions targeting teens and young adults. Improving SmokeFreeTeen’s reach, engagement, and effectiveness is needed.
Keywords: Smoking cessation, Teens, mHealth, Text-messaging interventions
Implications.
Practice: Teen-focused smoking cessation text-messaging interventions can be used to reduce teen smoking.
Policy: Promoting SmokeFreeTeen in schools and pediatric practices and on social media platforms popular among teens can potentially increase the reach of SmokeFreeTeen.
Research: Future research is needed to identify and test strategies to engage tech-savvy teens in text-messaging interventions.
INTRODUCTION
Adolescence marks cigarette smoking initiation for 90% of U.S. adult smokers [1]. Defined primarily based on age (10–19 years old), adolescence is characterized by physical, neurodevelopmental, psychological, and social changes [2]. In 2018, cigarettes were the second most used tobacco product among high- and middle-school students with 8.1% and 1.8% past 30 day cigarette use, respectively, which is equivalent to ~1.4 million teen smokers [3]. Teen smoking is associated with nicotine dependency, continued smoking into adulthood, and other substance use, as well as health, psychological, and social problems [1]. Roughly 41% of teen tobacco users have tried to quit tobacco, including cigarettes [4], suggesting a need for effective interventions.
Text messaging is an affordable and efficient platform for smoking cessation interventions [5]. In 2018, percentages of teens who have or have access to a smartphone or cellphone were 95 and 29, respectively [6]. Texting is a key communication mode among teens, who, on average, send 39 texts a day, not including social media messaging nor texting mobile applications [6]. Traditionally, teen-focused smoking prevention and cessation interventions have been school, community, mass media based, or policy related [7]. There is a dearth of text-messaging smoking cessation interventions that exclusively target teens [7–9]. In a U.S. study of teens aged 14–18, teens who received smoking cessation text messages reported significantly fewer cigarettes smoked in the past month at 6 month follow-up compared to those who received general health text messages [10]. Alternatively, text-messaging smoking cessation interventions that include teens alongside young adults or adults elude basic audience segmentation strategies for public health interventions [11]. For two text-messaging smoking interventions targeting teens and young adults in Switzerland and Denmark, 1 month abstinence rate was 18% (vs. 15% in their respective comparator groups) [12, 13]. However, subgroup analyses by age were not available.
Although text-messaging interventions increase smoking cessation among adults [5], little is known about their effectiveness among teens. In this study, we examined dropout, response, and abstinence rates among subscribers of SmokeFreeTeen, a 6–8 week intervention that uniquely targets U.S. teens under the National Cancer Institute’s SmokeFree.gov initiative.
Intervention description
SmokeFreeTeen is a smoking cessation intervention that is freely available to teens, aged 13–19 years old, in the USA with a text-messaging-enabled phone. Teens can sign up for the intervention online or by texting TEEN to 47848. The intervention is grounded in social cognitive theory and established smoking cessation behavioral change strategies [14]. SmokeFreeTeen subscribers are recommended to select a quit date within 2 weeks of signup (i.e., prequit time) during which they receive messages to prepare them for smoking cessation. Starting on quit day, subscribers receive three to five messages per day that include tips and motivational content over 42 days. Subscribers receive messages such as “Feeling cranky is normal & some days will be hard. You got this! We know you have a lot going on but take a time out & blow off tension w/o cigs.” During the intervention, subscribers also receive weekly questions to assess their smoking status, as well as at 1, 3, and 6 months postintervention end. Subscribers can reset their quit date online or by texting NEW. To unenroll, they can text STOP.
METHODS
Study population
Data came from teens who self-enrolled in SmokeFreeTeen between January 2016 and December 2018 (N = 4,311). For subscribers who reset their quit date, we used the latest quit attempt and excluded past attempts (n = 995 records). We excluded subscribers who set a quit date before signup (n = 30), dropped out before start of prequit time (n = 5), had less than 42 days from quit day to study end on December 31, 2018 (n = 197), or were outside the 13–19 age range (n = 399). The final analytic sample was 2,685 subscribers.
Measures
At signup, subscribers reported their age, gender, zip code, smoking frequency, and cigarettes smoked per day. Zip codes were recoded into U.S. states and categorized into Census Bureau regions. The intervention automatically records each subscriber’s signup, quit, and dropout (when applicable) dates, which we used to calculate “signup to quit day” and “quit day to dropout” variables. All subscribers whose “signup to quit day” exceeded the recommended 2 weeks from sign up to quit day were defaulted to 14 days of “prequit time,” whereas for those whose “signup to quit day” was between 0 and 14 days, “prequit time” remained unchanged. The decision to default longer prequit times to 14 days is because the intervention delivers support messages only 2 weeks prior to the self-determined quit day. For teens who reset their quit date, we used their signup date to calculate “signup to quit day” and “prequit time” rather than the reset date(s). This decision was made because the signup date was the most reliable, having been captured for all subscribers, whereas reset date(s) was captured differently for subscribers who reset their date online versus on their mobile phones. Furthermore, using the signup date allowed us to capture repeated exposure to prequit messages among participants who attempted to quit more than once (multiple quit attempts ranged from 2 to 21, M = 2.57, standard deviation [SD] = 1.53).
We created four binary variables: (a) “number of quit attempts” to capture whether a subscriber had one or two or more quit attempts; (b) “completion status” to capture whether subscribers dropped out on or before intervention end (Day 42; i.e., noncompleters) or stayed in the intervention until Day 42 (i.e., completers); (c) “smoking status” to capture whether subscribers were smokers or nonsmokers if they responded “yes” or “no,” respectively, to the once-a-week smoking status prompt; and (d) “response status” to capture whether subscribers responded to the smoking status prompt with either yes = smoker or no = nonsmoker (i.e., responders) or did not respond (i.e., nonresponders). Smoking and response statuses were determined at each assessment time point independent of a participant’s previous or following responses.
Data analysis
Half of the subscribers (n = 1,294, 48.19%) had missing data on one to four subscriber characteristics (Supplementary Table 1). Data were not missing completely at random (Little’s χ 2(df = 16) = 44.55, p < .0001) [15]. To preserve sample size and reduce bias from excluding subscribers with incomplete data [16], we imputed missing data for gender, census region, smoking frequency, and cigarettes smoked per day using multiple imputations (n = 20) for data that met our inclusion criteria. We used a logistic prediction model for binary categorical variables (i.e., gender) and a generalized logit model for nonordinal categorical variables (i.e., region). Age, number of quit attempts, and prequit time had no missing data but were used as covariates.
In SAS 9.4, we conducted Cox regression survival analysis and logistic regressions to examine subscriber characteristics associated with dropout, response, and abstinence rates on quit day through 1 month follow-up. Using an intent-to-treat approach, abstinence models were conducted for (a) program initiators (n = 2,205; those who reached quit day) where nonresponders and dropouts were considered smokers and (b) program completers (n = 952; those who reached quit day and completed the intervention) where nonresponders were considered smokers. Smoking statuses were captured on Days 0 (quit day), 7, 14, 21, 28, 35, 42 (intervention end), and 72 (1 month follow-up).
RESULTS
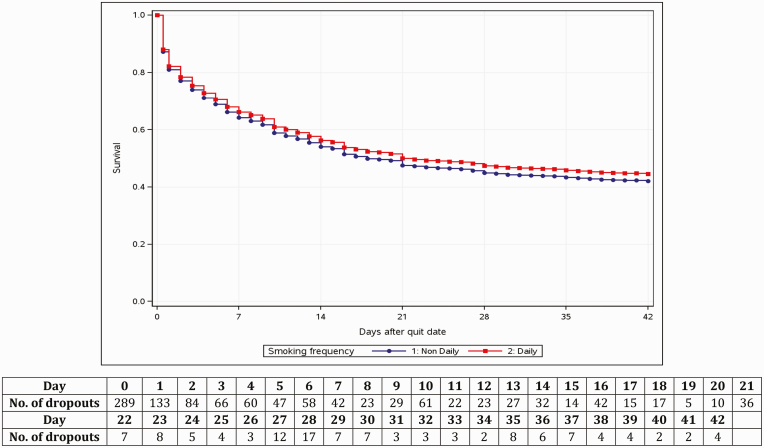
Among noncompleters (n = 1,733, 64.54%), 27.69% dropped out prior to their quit day and 72.27% dropped out on or after quit day (Table 1). Time from quit day to dropout averaged 8 days. The survival rate was around 45% for nondaily and daily smokers by intervention end (Day 42; Fig. 1). Response rates to smoking status prompts fluctuated throughout the intervention with the highest being on Day 7 at 27.99% and 22.68% and the lowest being on Day 42 at 9.41% and 9.34% among program initiators and completers, respectively (Supplementary Table 2). The 7 day point prevalence abstinence rate was 2.63% and 6.09% by intervention end (Day 42) and 2.55% and 6.01% at 1 month follow-up among program initiators and completers, respectively (Supplementary Table 2). Among program initiators, longer prequit time was associated with lower odds of dropout (hazard ratio [HR]: 0.94) but also lower odds of response (adjusted odds ratios [aORs]: 0.94, 0.94) and abstinence (aORs: 0.96, 0.95) on quit day and Day 7 (Table 2). Those with two or more (vs. one) quit attempts were associated with higher odds of responding to smoking status prompts (aOR: 1.79, 1.61) on quit day and Day 7 and of being abstinent on Day 7 (aOR: 1.91). Subscribers from the Midwest and West census regions had higher odds of responding to smoking status prompts (aORs: 1.50, 1.63) and of being abstinent (aORs: 1.53, 2.28) on quit day than subscribers from the South. Correlates of dropout, response, and abstinence rates for days 14, 21, 28, and 35 appear in Supplementary Table 3. Among program completers, baseline characteristics did not consistently contribute to abstinence outcomes (Supplementary Table 4). We found fairly comparable results with complete case analysis (Supplementary Table 5).
Table 1.
SmokeFreeTeen sample characteristics, imputed data
| Total | Noncompletersa | Completersb | |||||
|---|---|---|---|---|---|---|---|
| N | SS | n | SS | n | SS | p value | |
| Agec | |||||||
| Mean (SD) | 2,685 | 17.33 (1.59) | 1,733 | 17.29 (1.59) | 952 | 17.38 (1.56) | .13 |
| Trimmed mean (SD) | 2,415 | 17.44 (1.61) | 1,559 | 17.41 (1.62) | 856 | 17.51 (1.61) | |
| Range | 2,685 | 13–19 | 1,733 | 13–19 | 952 | 13–19 | |
| Median (IQR) | 2,685 | 18.00 (3.00) | 1,733 | 18.00 (3.00) | 952 | 18.00 (3.00) | |
| Gender, %d | |||||||
| Male | 1,313 | 48.91 | 845 | 48.77 | 468 | 49.18 | .83 |
| Female | 1,372 | 51.09 | 888 | 51.23 | 484 | 50.82 | |
| Region, %d,e | |||||||
| Northeast | 415 | 15.45 | 246 | 14.20 | 169 | 17.74 | .02 |
| Midwest | 573 | 21.34 | 359 | 20.72 | 214 | 22.47 | |
| South | 1,166 | 43.42 | 765 | 44.14 | 401 | 42.12 | |
| West | 531 | 19.79 | 363 | 20.95 | 168 | 17.67 | |
| Smoking frequency, %d | |||||||
| Nondaily | 745 | 27.73 | 505 | 29.13 | 240 | 25.19 | .03 |
| Daily | 1,940 | 72.27 | 1,228 | 70.87 | 712 | 74.81 | |
| Cigarettes smoked per day, %d | |||||||
| Light (≤10) | 1,590 | 59.23 | 1,025 | 59.13 | 566 | 59.42 | .33 |
| Moderate (11–20) | 638 | 23.75 | 399 | 23.04 | 238 | 25.04 | |
| Heavy (>20) | 457 | 17.02 | 309 | 17.83 | 148 | 15.54 | |
| Time of dropout, %c | |||||||
| Prior to quit day | 480 | 27.69 | 480 | 27.69 | – | – | – |
| On or after quit day | 1,253 | 72.30 | 1,253 | 72.30 | – | – | |
| Time from sign up to quit day (days)c | |||||||
| Mean (SD) | 2,685 | 11.17 (31.36) | 1,733 | 7.54 (17.37) | 952 | 17.78 (46.46) | <.0001 |
| 5% trimmed mean (SD) | 2,415 | 6.14 (9.50) | 1,559 | 5.42 (6.52) | 856 | 9.14 (21.72) | |
| Range | 2,685 | 0–479 | 1,733 | 0–360 | 952 | 0–479 | |
| Median (IQR) | 2,685 | 3.00 (13.00) | 1,733 | 3.00 (13.00) | 952 | 4.50 (14.00) | |
| Prequit time (days)c | |||||||
| Mean (SD) | 2,685 | 4.45 (5.52) | 1,733 | 3.38 (4.84) | 952 | 6.40 (6.13) | <.0001 |
| 5% trimmed mean (SD) | 2,415 | 4.16 (5.82) | 1,559 | 2.97 (5.10) | 856 | 6.33 (6.46) | |
| Range | 2,685 | 0–14 | 1,733 | 0–14 | 952 | 0–14 | |
| Median (IQR) | 2,685 | 1.00 (9.00) | 1,733 | 1.00 (6.00) | 952 | 4.50 (14.00) | |
| Time from quit day to dropout (days)c | |||||||
| Mean (SD) | 1,253 | 8.04 (9.45) | 1,253 | 8.04 (9.45) | – | – | – |
| 5% trimmed mean (SD) | 1,127 | 7.00 (8.98) | 1,127 | 7.00 (8.98) | – | – | |
| Range | 1,253 | 0–42 | 1,253 | 0–42 | – | – | |
| Median (IQR) | 1,253 | 4.00 (12.00) | 1,253 | 4.00 (12.00) | – | – | |
| Number of quit attempts, %c | |||||||
| One attempt | 2,301 | 85.70 | 1,551 | 89.50 | 750 | 78.78 | <.0001 |
| Two or more attemptsf | 384 | 14.30 | 182 | 10.50 | 202 | 21.22 | |
N = 2,685. Age, prequit time, and number of quit attempts had no missing values. p-values reflect differences between completers and noncompleters on sample characteristics. P is considered significant at the ≤.05 level. t-test was used for continuous variables and chi-square was used for categorical variables.
IQR interquartile range; SD standard deviation; SS summary statistic.
aNoncompleters are participants who dropped any time between signup and intervention end (Day 42).
bCompleters are participants who remained in SmokeFreeTeen until Day 42.
cComplete case variable. Full complete case table is shown as a supplementary table.
dImputed variable. Imputed values have 20 records and, thus, imputed n is 1/20th of a subject rounded to the nearest integer. Imputed numbers do not add up due to rounding error in some instances.
eSubscriber zip codes were recorded into U.S. state and categorized into US Census Bureau region.
fNumber of records per subscriber ranged from 2 to 21 (M = 2.57, SD = 1.53).
Fig 1.
Survival analysis of days in SmokeFreeTeen by daily versus nondaily smokers.
Table 2.
Associations between subscriber characteristics and dropout, response, and abstinence rates on Days 0 (quit day), 7, 42 (intervention end), and 72 (1 month follow-up), imputed data
| Response ratea | Abstinence rate (intent-to-treat)b | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dropout N = 2,205 | Quit Day n = 2,205 | Day 7 n = 1,468 | Day 42 n = 956 | Day 72 n = 910 | Quit Day n = 2,205 | Day 7 n = 2,205 | Day 42 n = 2,205 | Day 72 n = 2,150 | |
| Characteristic | HR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) |
| Age | 1.01 (0.98–1.05) | 1.07 (0.99–1.15) | 0.97 (0.90–1.05) | 1.07 (0.92–1.25) | 1.02 (0.89–1.17) | 1.12 (1.00–1.24) | 0.99 (0.90–1.09) | 1.02 (0.85–1.21) | 1.00 (0.84–1.20) |
| Gender (ref: Female) | |||||||||
| Male | 0.97 (0.87–1.09) | 0.92 (0.73–1.14) | 0.80 (0.63–1.02) | 0.74 (0.47–1.16) | 0.84 (0.55–1.28) | 0.81 (0.59–1.11) | 0.91 (0.67–1.23) | 0.73 (0.43–1.26) | 0.78 (0.45–1.35) |
| Region (ref: South) | |||||||||
| Northeast | 0.75 (0.63–0.89) | 1.02 (0.73–1.43) | 0.74 (0.52–1.04) | 1.02 (0.57–1.84) | 1.06 (0.59–1.90) | 0.94 (0.56–1.58) | 0.87 (0.55–1.38) | 1.54 (0.79–3.00) | 1.11 (0.51–2.43) |
| Midwest | 0.85 (0.73–0.99) | 1.50 (1.13–2.00) | 0.88 (0.64–1.20) | 0.63 (0.33–1.18) | 0.83 (0.46–1.47) | 1.53 (1.02–2.32) | 0.84 (0.55–1.29) | 0.56 (0.24–1.30) | 0.52 (0.21–1.28) |
| West | 0.96 (0.83–1.12) | 1.63 (1.21–2.18) | 1.03 (0.75–1.41) | 0.84 (0.45–1.58) | 1.31 (0.75–2.28) | 2.28 (1.54–3.39) | 1.64 (1.13–2.37) | 0.86 (0.40–1.81) | 1.51 (0.78–2.94) |
| Smoking intensity (ref: Light) | |||||||||
| Moderate | 0.97 (0.82–1.14) | 1.08 (0.77–1.52) | 1.02 (0.68–1.52) | 0.74 (0.35–1.55) | 0.75 (0.42–1.37) | 1.77 (1.09–2.86) | 0.82 (0.46–1.45) | 0.66 (0.26–1.68) | 0.74 (0.33–1.66) |
| Heavy | 1.04 (0.86–1.26) | 0.91 (0.62–1.34) | 0.83 (0.53–1.29) | 0.70 (0.26–1.88) | 0.61 (0.23–1.62) | 1.34 (0.73–2.46) | 0.66 (0.35–1.25) | 0.65 (0.19–2.20) | 0.52 (0.13–2.12) |
| Smoking frequency (ref: Nondaily) | |||||||||
| Daily | 0.93 (0.81–1.07) | 0.99 (0.74–1.33) | 0.94 (0.68–1.29) | 0.77 (0.43–1.37) | 0.89 (0.53–1.50) | 0.68 (0.45–1.03) | 0.75 (0.52–1.09) | 0.95 (0.49–1.86) | 0.89 (0.46–1.71) |
| Prequit time | 0.94 (0.93–0.95) | 0.94 (0.92–0.96) | 0.94 (0.92–0.96) | 0.98 (0.94–1.02) | 0.99 (0.95–1.02) | 0.96 (0.93–0.99) | 0.95 (0.92–0.98) | 1.01 (0.96–1.06) | 1.04 (0.99–1.09) |
| Number of quit attempts (ref: 1) | |||||||||
| Two or more | 0.88 (0.73–1.06) | 1.79 (1.29–2.48) | 1.61 (1.16–2.23) | 1.53 (0.87–2.69) | 1.14 (0.65–1.98) | 1.42 (0.89–2.24) | 1.91 (1.25–2.92) | 1.30 (0.63–2.69) | 1.03 (0.49–2.18) |
Cell represents significant result if the confidence interval does not cross the null hypothesis (1). Smoking and response statuses were captured and analyzed for eight assessment points. Quit day is Day 0, Day 42 is intervention end, and Day 72 is 1 month follow-up. Results for the other assessment points appear in Supplementary Table 3.
aOR adjusted odds ratio; CI confidence intervals; HR adjusted hazards ratio.
aResponse rate ns reflect the number of subscribers who had not dropped out and had the opportunity to respond to the once-a-week smoking status prompt.
bFor intent-to-treat analysis, n = 2,205 reflects the number of subscribers who made it to quit day. Nonresponders and dropouts were considered smokers. On Day 72, n = 2,150 because 55 subscribers did not have sufficient follow-up time to reach Day 72 before the end of the study on December 31, 2018.
DISCUSSION
Our results provide a first look at abstinence rates among teens, aged 13–19, who self-enrolled in SmokeFreeTeen, a teen-targeted text-messaging smoking cessation intervention. Teens prefer self-help, technology-based interventions over traditional ones such as in-person counseling because they reduce teens’ concerns about judgments and privacy [17]. Although texting is organic to teens’ lifestyles [6], few text-messaging smoking cessation interventions target teens. Furthermore, in text-messaging interventions that enroll teens, teens have been subsumed under different groups, often with varying or overlapping age ranges (i.e., adults defined as 18 years or older [18] or young people defined as younger than 20 years [7]). These practices resulted in uncertainty around the effectiveness of text-messaging cessation interventions among teens. SmokeFreeTeen produced a 2.55% and 6.01% abstinence rate at 1 month follow-up among program initiators and completers, where abstinence rates at all assessment time points were fairly uniform across subscriber characteristics. SmokeFreeTeen abstinence rates were lower than rates reported for comparable text-messaging interventions targeting adults [19] and those targeting both teens and young adults [12, 13]. Dropout was high and response rates were low throughout the intervention, both of which were also fairly uniform across SmokeFreeTeen subscriber characteristics.
Several associations between subscriber characteristics and intervention outcomes are noteworthy. Inconsistent with previous literature [20], smoking frequency and cigarettes per day were not consistently associated with abstinence outcomes. Furthermore, longer prequit time was associated with higher retention but with lower response and abstinence rates, whereas number of quit attempts was positively associated with response and abstinence rates on select assessment time points. Our results add to the mixed evidence in the literature on the association between intervention dose and smoking cessation outcomes [21, 22]. However, the positive association between number of quit attempts and abstinence may reflect literature showing it may take multiple quit attempts before quitting successfully [23]. The independent and antagonistic associations between prequit time and number of quit attempts with dropout and abstinence outcomes warrant additional research to parse out the mechanistic underpinnings of these associations.
Efforts to increase the reach of, engagement with, and effectiveness of SmokeFreeTeen are warranted. The 2,685 SmokeFreeTeen subscribers in our study represent merely 0.19% of 1.4 million teen smokers in 2018 [3]. To increase SmokeFreeTeen’s reach, algorithms can detect smoking-related words and images in posts on popular social media platforms among teens (i.e., Instagram) and deliver targeted SmokeFreeTeen advertisements. SmokeFreeTeen can also be promoted in schools and pediatric clinics to inform smoking teens of this resource.
High dropout and low response rates are problematic for behavioral interventions, especially for real-life, nonincentivized ones, such as SmokeFreeTeen [24]. On one hand, there is no consensus on the optimal frequency and timing of text messages for intervention retention, engagement, and effectiveness. For example, researchers found that text-messaging interventions with decreasing message frequency over time and opportunities for individualization show high efficacy [25], whereas others determined that fixed schedules for deploying text messages yield better results than decreasing or variable schedules [9]. On the other hand, engaging teens, as a tech-savvy generation, in text-messaging interventions, such as SmokeFreeTeen, and sustaining their engagement is challenging. Empirical evidence on teen-appropriate retention and engagement strategies in digital-based interventions is virtually nonexistent [26]. For example, engagement strategies propagated in narrative reviews (e.g., emphasizing the benefits of participation and enlisting support from others) are not empirically supported [26]. Furthermore, the frequency and content of such strategies should be dependent on the risk behavior in question. For example, our results show peaks in dropout on quit day through Day 7, a time when deployment of engagement strategies would improve retention and ultimately abstinence. Adopting strategies or elements from popular platforms amongst teens (e.g., humorous messages and videos in Vines) and team-driven challenges may be beneficial, although empirical evidence for proposed engagement strategies that are age and behavior appropriate is needed. Teen-focused engagement strategies are needed to make real-world interventions stand out against other stimuli vying for teens’ attention, lessen desensitization to intervention messages over time, and, ultimately, increase intervention effectiveness.
Noteworthy, lack of information (i.e., race and ethnicity, parental education, and withdrawal symptoms) limited our ability to examine their associations with dropout, response, and abstinence rates. Abstinence was self-reported with no biochemical verification. As with real-world interventions, low response rates and high number of dropouts and missing data were observed. For example, with 10 (0.37%) and 7 (0.26%) participants responding to all smoking status prompts until Days 42 and 72, respectively, we could not conduct repeated-measures analyses for response and smoking statuses. Missing data could have also masked important variables (i.e., smoking frequency and cigarettes smoked per day) from showing significant associations with cessation outcomes. Incentivized studies may limit missingness and allow for longitudinal analyses of smoking and response statuses. Besides smoking status, no variables were collected beyond baseline, which limited our ability to examine the effects of SmokeFreeTeen from a harm reduction perspective (i.e., reduction in the number of cigarettes smoked). Other variables (e.g., noncigarette tobacco products use) would have been beneficial to comprehensively examine tobacco use patterns and/or product switching either partially or completely.
The use of signup date (rather than reset date[s]) to calculate prequit time resulted in longer prequit time for teens with two or more quit attempts (n = 384, Mprequit time (days) = 10.04, SD = 5.29) versus those with one quit attempt (n = 2,301, Mprequit time (days) = 3.51, SD = 4.98; pooled t-test = −23.57, p < .0001). We elected to run the analyses on all 2,685 teens because subscribers with two or more quit attempts (Mage = 17.46, SD = 1.45) were significantly older than those with only one quit attempt (Mage = 17.30, SD = 1.60, Satterthwaite t-test = −2.02, p = .04). Furthermore, limiting our analyses to subscribers with only one attempt would have lowered response and abstinence rates (Supplementary Table 7). Finally, we chose to retain both prequit time and number of quit attempts because they were independently associated with dropout, response, and abstinence rates (Table 2 and Supplementary Tables 3). Sensitivity analyses on subscribers with one quit attempt only (n = 2,301) show generally consistent results with what we report using the whole sample (Supplementary Table 6). Noteworthy is that prequit time was associated with dropout, response, and abstinence rates as in full sample analyses.
SmokeFreeTeen represents an opportunity to reach teen smokers on a preferred platform and a challenge to devise engaging content for a tech-savvy audience. Nevertheless, SmokeFreeTeen has the potential to be an important vehicle to reduce teen smoking.
Supplementary Material
Acknowledgments
Analyses were run by Information Management Services, Inc., an information management services firm with a specialty in statistical consultation, used by the Division of Intramural Research, National Institute on Minority Health and Health Disparities.
Funding: The effort of C.C. was supported by the Office of the Director, National Institutes of Health. The effort of K.K. and S.E.-T. was supported by the Division of Intramural Research, National Institute on Minority Health and Health Disparities, National Institutes of Health. The SmokeFreeTXT program was funded by the Tobacco Control Research Branch of the National Cancer Institute, National Institutes of Health (contract #HHSN261201400002B and #HHSN261000011).
Compliance with Ethical Standards
Conflicts of Interest: All authors declare that they have no conflicts of interest.
Author contributions: C.C. drafted the manuscript; K.K. ran preliminary analyses; F.A. reviewed the manuscript; and S.E. conceptualized the study and critically edited and reviewed the manuscript for intellectual content. All authors approve the manuscript as submitted.
Ethical Approval: This project only involved the use of de-identified data, which is considered “not human subjects research.” “Not human subjects research” requires no institutional review board review or approval per National Institutes of Health policy and 45 CFR 46. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: This study is “not human subjects research” and informed consent, therefore, was not required.
References
- 1. US Department of Health and Human Services. Preventing Tobacco use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
- 2. World Health Organization. Adolescence: A period needing special attention.2014; Available at http://apps.who.int/adolescent/second-decade/section2/page2/age-not-the-whole-story.html. Accessibility verified September 16, 2019.
- 3. Gentzke AS, Creamer M, Cullen KA, et al. Vital signs: Tobacco product use among middle and high school students—United States, 2011-2018. MMWR Morb Mortal Wkly Rep. 2019;68(6):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67(8):1–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2016;4:CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rideout V, Robb MB.. The Common Sense Census: Media Use by Tweens, 2019. San Francisco, CA: Common Sense Media; 2019. [Google Scholar]
- 7. Stanton A, Grimshaw G. Tobacco cessation interventions for young people. Cochrane Database Syst Rev. 2013;( 8):CD003289. [DOI] [PubMed] [Google Scholar]
- 8. Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol. 2006;25(5):549–557. [DOI] [PubMed] [Google Scholar]
- 9. Spohr SA, Nandy R, Gandhiraj D, Vemulapalli A, Anne S, Walters ST. Efficacy of SMS text message interventions for smoking cessation: A meta-analysis. J Subst Abuse Treat. 2015;56:1–10. [DOI] [PubMed] [Google Scholar]
- 10. Mason MJ, Campbell L, Way T, et al. Development and outcomes of a text messaging tobacco cessation intervention with urban adolescents. Subst Abus. 2015;36(4):500–506. [DOI] [PubMed] [Google Scholar]
- 11. Slater M. Choosing audience segmentation strategies and methods. In: Maibach E, Parrott RL, eds. Designing Health Messages: Approaches From Communication Theory and Public Health Practice. Thousand Oaks, CA: Sage Publications. 1995; 186–198. [Google Scholar]
- 12. Haug S, Schaub MP, Venzin V, Meyer C, John U. Efficacy of a text message-based smoking cessation intervention for young people: A cluster randomized controlled trial. J Med Internet Res. 2013;15(8):e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skov-Ettrup LS, Ringgaard LW, Dalum P, Flensborg-Madsen T, Thygesen LC, Tolstrup JS. Comparing tailored and untailored text messages for smoking cessation: A randomized controlled trial among adolescent and young adult smokers. Health Educ Res. 2014;29(2):195–205. [DOI] [PubMed] [Google Scholar]
- 14. Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav. 2011;36(4):315–319. [DOI] [PubMed] [Google Scholar]
- 15. Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83(404):1198. [Google Scholar]
- 16. Dong Y, Peng CY. Principled missing data methods for researchers. Springerplus. 2013;2(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vuckovic N, Polen MR, Hollis JF. The problem is getting us to stop. What teens say about smoking cessation. Prev Med. 2003;37(3):209–218. [DOI] [PubMed] [Google Scholar]
- 18. Bock B, Heron K, Jennings E, et al. A text message delivered smoking cessation intervention: The initial trial of TXT-2-quit: Randomized controlled trial. JMIR Mhealth Uhealth. 2013;1(2):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cole-Lewis H, Augustson E, Sanders A, et al. Analysing user-reported data for enhancement of SmokefreeTXT: A national text message smoking cessation intervention. Tob Control. 2017;26(6):683–689. [DOI] [PubMed] [Google Scholar]
- 20. Sargent JD, Mott LA, Stevens M. Predictors of smoking cessation in adolescents. Arch Pediatr Adolesc Med. 1998;152(4):388–393. [DOI] [PubMed] [Google Scholar]
- 21. Siu AL; U.S. Preventive Services Task Force . Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. preventive services task force recommendation statement. Ann Intern Med. 2015;163(8):622–634. [DOI] [PubMed] [Google Scholar]
- 22. Heminger CL, Boal AL, Zumer M, Abroms LC. Text2Quit: An analysis of participant engagement in the mobile smoking cessation program. Am J Drug Alcohol Abuse. 2016;42(4):450–458. [DOI] [PubMed] [Google Scholar]
- 23. Chaiton M, Diemert L, Cohen JE, et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6(6):e011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kealey KA, Ludman EJ, Mann SL, et al. Overcoming barriers to recruitment and retention in adolescent smoking cessation. Nicotine Tob Res. 2007;9(2):257–270. [DOI] [PubMed] [Google Scholar]
- 25. Head KJ, Noar SM, Iannarino NT, Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: A meta-analysis. Soc Sci Med. 2013;97:41–48. [DOI] [PubMed] [Google Scholar]
- 26. Coday M, Boutin-Foster C, Sher TG, et al. Strategies for retaining studying participants in behavioral intervention trials: Retention experiences of the NIH behavior change consortium. Ann Behav Med. 2005;29(2):55–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.