Abstract
Physical activity (PA), including exercise, is safe and beneficial for children and adolescents affected by cancer. Yet, no efforts have been made to collate the breadth of review and experimental articles exploring the effects of PA in this cohort. Thus, a scoping review of review and experimental articles reporting on the effects of PA for children and adolescents affected by cancer was undertaken. Review and experimental articles published in English, summarizing or reporting on the effects of PA interventions for children and adolescents affected by cancer were included. Articles were identified through prior literature, systematic searching, reference list scanning, stakeholder engagement, and a database update. Data were extracted, collated, assessed for quality (reviews) or risk of bias (experimental articles), and summarized narratively. A total of 1,380 articles were identified; 20 review and 69 experimental articles were included. Articles explored PA behavior, physical, psychosocial, cognitive, and “other” outcomes. Improvements, no change, or mixed results were reported across the majority of outcomes explored. Two PA-related adverse events (e.g., a treatable injury, fatigue) were described. Included articles varied greatly in quality and risk of bias. Findings confirm that PA for children and adolescents affected by cancer is a rapidly growing field. More adequately powered research, focused on priority outcomes, adopting appropriate study designs, and adhering to reporting standards is required. Addressing these gaps will enable a better understanding of the effects of PA. Nevertheless, the literature confirms moving more is beneficial and safe for children and adolescents affected by cancer.
Keywords: Childhood cancer, Adolescent cancer, Physical activity, Narrative review, Exercise
Children and adolescents affected by cancer face a range of negative effects associated with cancer and its treatment. Physical activity (PA) is safe and beneficial for this population. Understanding the effects of PA among this cohort is important to inform clinical practice and guideline development. Therefore, a literature synthesis was undertaken to summarize findings from review and experimental articles exploring the effects of PA interventions among children and adolescents affected by cancer. Systematic strategies were used to identify articles, extract data, and assess article quality or risk of bias. A range of outcomes have been studied with children and adolescents affected by cancer including PA behavior, physical, psychosocial, cognitive, and “other” outcomes. Benefits were reported for most of the outcomes. However, no changes and mixed results following PA was also commonly reported. Across all included review and experimental articles, only two instances of PA-related adverse events (e.g., a treatable injury, fatigue) were described. Included articles varied greatly in quality and risk of bias. Overall, more research is needed in this field. Nevertheless, findings from this literature synthesis suggest moving more during and after treatment is beneficial and safe for children and adolescents affected by cancer.
Implications.
Practice: Physical activity may be used to confer benefits among children and adolescents affected by cancer.
Policy: To promote health among children and adolescents affected by cancer, policymakers should consider integrating physical activity within and beyond standard care.
Research: Future research should seek to contribute more adequately powered, high-quality evidence for the role of physical activity among children and adolescents across the cancer trajectory.
INTRODUCTION
Globally, over 300,000 children and adolescents are diagnosed with cancer each year [1]. Though 5-year survival rates now exceed 80% for many cancers [2], the burden of the disease and its treatments is intensive and long-lasting. Children and adolescents affected by cancer face a range of negative physical (e.g., increased fatigue and muscular atrophy, decreased functional capacity) and psychosocial effects (e.g., increased depression, anxiety and fear, decreased self-esteem) that occur during treatment and persist thereafter [3–5]. Consequently, this population experiences reduced quality of life [6]. As a result, researchers have sought to identify strategies to address these negative effects.
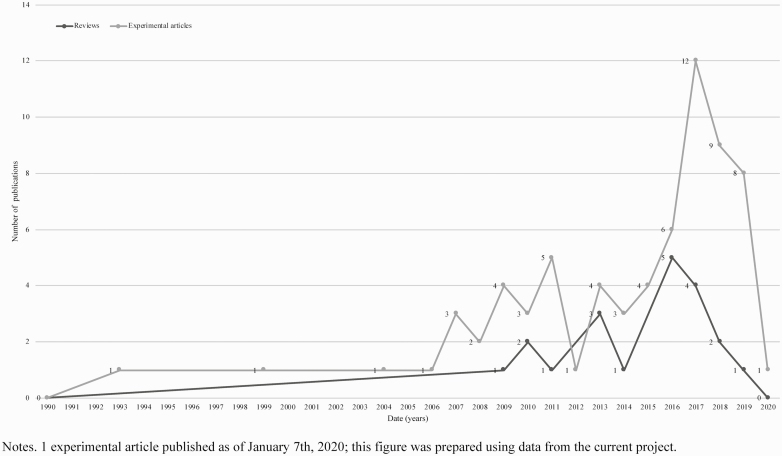
Physical activity (PA; i.e., any bodily movement produced by skeletal muscles that requires energy expenditure; [7]) and exercise (i.e., planned, structured, and repetitive PA for the purpose of conditioning any part of the body, improving health, and to maintain fitness; [7]) may enhance length and quality of life among children and adolescents affected by cancer [8–11]. For the purpose of this manuscript, the term “PA” will be used to describe any increase in energy expenditure through bodily movement, whether planned or informal. In recent years, there has been a surge in the number of experimental articles published that report on the effects of PA among children and adolescents affected by cancer (see Fig. 1). These articles report benefits including improved PA behavior, strength, quality of life, and performance on cognitive tests [12, 13].
Fig 1 |.
Number of review and experimental articles published reporting on the effects of PA with children and adolescents affected by cancer.
The ever-increasing evidence from experimental articles has been summarized in reviews (e.g., [14, 15]; see Fig. 1), which reiterate the benefits PA may confer for children and adolescents affected by cancer. Notwithstanding these benefits, reviews also highlight that no changes or mixed results are common across studies and that a range of different measurement tools have been used to assess outcomes. Meta-analyses on the topic of PA for children and adolescents affected by cancer are therefore scarce and narrative syntheses, that do not assess risk of bias, have been favored [16]. Despite the continually increasing number of reviews and experimental articles published, no attempts have been made to synthesize both types of evidence. A review of reviews and experimental articles may be an appropriate next step to explore the breadth of evidence, identify knowledge gaps, provide directions for future research, and offer end-users the information they need to make decisions [17].
Thus, the purpose of this literature synthesis was to determine the extent, range, and nature of evidence reporting on the effects of PA for children and adolescents affected by cancer. Specific objectives were to: (i) identify and summarize reviews reporting on PA interventions for children and adolescents affected by cancer; (ii) identify and synthesize experimental articles reporting on the effects of PA interventions for children and adolescents affected by cancer; (iii) determine quality and risk of bias of reviews and experimental articles, respectively; (iv) ascertain knowledge gaps to provide directions for future research and assist end-users in making PA-related decisions; and, (v) consolidate the literature to inform the international Pediatric Oncology Exercise Guidelines (iPOEG; [18]).
METHODS
This literature synthesis was performed following guidance put forth for the design, conduct, and reporting of scoping reviews [19–21], systematic reviews [22, 23], and systematic reviews of reviews [17]. Pragmatic constraints of the larger project were also considered (i.e., developing the iPOEG). A protocol covering all review methods was established and adhered to throughout.
Eligibility criteria
To be included in this literature synthesis, peer-reviewed reviews or experimental articles (as both include appropriate study designs to ascertain the effects of PA) had to meet the following inclusion criteria: (i) published in English, the language spoken by the study team; (ii) reported on the effects of PA, defined as any intervention (including programs) that increased energy expenditure through the provision of bodily movement, whether planned or informal; (iii) reported changes in PA behavior, physical, psychosocial, cognitive, and/or “other” outcomes; and, (iv) included children and adolescents affected by cancer, up to age 21. Participants could be actively receiving treatment or have completed treatment. With regards to restrictions, for reviews, articles focused on children and adolescents affected by cancer had to comprise the majority of the articles, or results for children and adolescents had to be presented separately. For experimental articles, children and adolescents had to comprise the majority of the sample. Articles were included when a PA intervention was offered ≥1 time. Articles describing interventions to enhance PA in the absence of a PA intervention (e.g., behavior change coaching, setting step goals) were excluded. No further restrictions were placed, and no comparator was defined in an attempt to include a wider range of study designs.
Data sources and search strategy for reviews and experimental articles
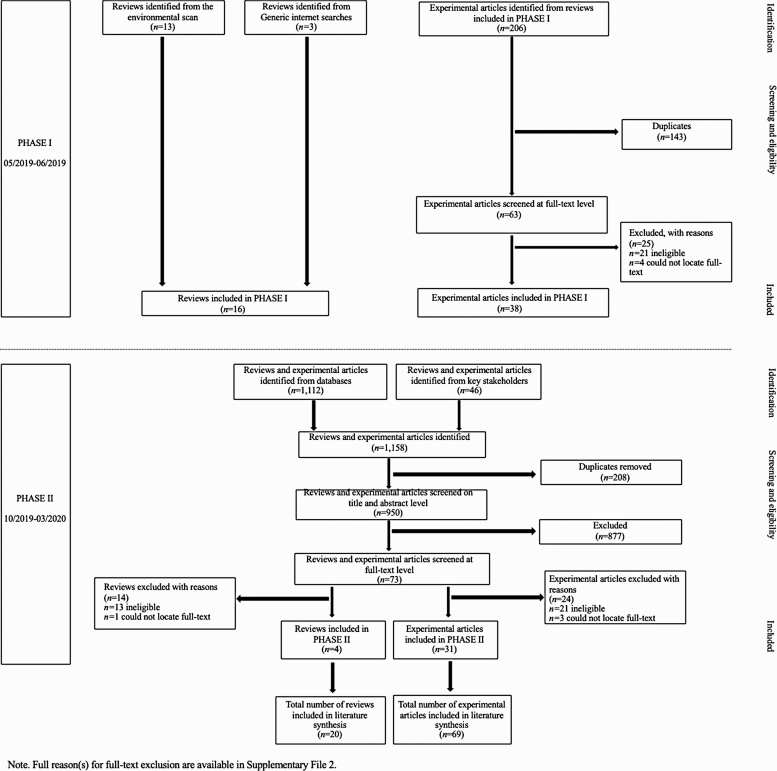
Data were identified for this project in two phases (see Fig. 2). In PHASE I, reviews were identified through: (i) a recently published environmental scan [24]; and, (ii) two independent researchers who entered combinations of keywords into Google and Google Scholar and viewed the first 20 links for possible reviews. Experimental articles were identified through scanning the reference lists of the included reviews, using the eligibility criteria described above. Discrepancies in this process were resolved via discussion. The identified reviews and experimental articles were then presented to key stakeholders comprised of the iPOEG core team (CCV, SLG, LH, MG, SK, FR, PvdT) and local healthcare providers (GG, KM). These stakeholders had the opportunity to comment on the identified literature.
Fig 2 |.
Preferred reporting items for systematic review and meta‐analysis flow diagram of search results and reasons for exclusion.
In PHASE II, stakeholders were asked to share additional reviews or experimental articles they knew of. As well, a database update was performed. A search strategy was prepared, and a combination of Medical Subject Headings and free-text words were searched in MEDLINE, PsycINFO, and SPORTDiscus (see Supplementary File 1 for search strategies) from January 2017 to January 2020. The decision to limit the search to the past 3 years was done to reduce the likelihood of gaps in the included literature (as the most recent review identified in PHASE I was published in 2017) and to ensure the timely completion of this project to inform the iPOEG [18]. Trial/study registries and grey literature were not searched. All articles identified in the database update were exported to EndNote X9. Duplicates were removed automatically and double-checked manually by two authors (EM, KE). Titles and abstracts of all identified records were independently assessed (EM and KE; 94% agreement). Full-texts of reviews and experimental articles that were potentially eligible were obtained and assessed for eligibility against the pre-specified eligibility criteria independently (EM and KE; 92% agreement). Any discrepancies throughout this process were resolved through discussion amongst authors. Reasons for exclusion were recorded and a full list of excluded studies and justification is available in Supplementary File 2.
Data extraction
Data were independently extracted from the reviews and experimental articles identified in PHASE I (by EM, CL, DC, AF, VL) and PHASE II (by EM, KE, CL) on: (i) study characteristics (i.e., author, year, country of publication, design, funding received); (ii) sample characteristics (i.e., sample size, age, cancer type, treatment status); (iii) outcomes assessed; and, (iv) results (i.e., changes in outcomes following a PA intervention that, where possible, were described as statistically or clinically significant). During this process, outcomes and results were grouped into the following five broad categories: (i) PA behavior; (ii) physical (e.g., body mass index, fatigue, strength); (iii) psychosocial (e.g., anxiety, mood, quality of life); (iv) cognitive (e.g., reaction time, structural markers of neurocognitive function); and, (v) “other” outcomes (e.g., knowledge of PA, need for pain medication). Any discrepancies during data extraction were resolved via discussion, and all data extraction was verified at study cessation by two authors (EM, KE) and an independent researcher (AC). Further, a random subset of 10% of the articles were verified by the first author (AW) who was also available to answer/address questions related to data extraction throughout.
Quality and risk of bias assessment
Reviews
The A MeaSurement Tool to Assess systematic Review (AMSTAR 2; [25]) was used by two authors to assess quality, or confidence in the results of included reviews (EM and KE; 97% agreement). The authors independently evaluated each review in 16 domains. Upon comparing and coming to agreement, they entered their ratings into the online checklist (available at: https://amstar.ca/Amstar_Checklist.php) in duplicate to produce a final rating of high, moderate, low, or critically low [25]. High ratings suggest the review provides an accurate and comprehensive summary of the literature and that confidence can be placed in findings, whereas critically low ratings suggest the review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the literature [25].
Experimental articles
The Cochrane Collaboration’s tool for assessing risk of bias provided in the Cochrane Handbook for Systematic Reviews of Interventions [26] was used to assess risk of bias of randomized controlled trials (RCTs). Two authors (EM and KE; 99% agreement) independently evaluated each article in the following six domains: (i) selection bias, (ii) performance bias, (iii) detection bias, (iv) attrition bias, (v) reporting bias, and (vi) other bias. For nonrandomized studies, the Risk of Bias Assessment tool for Nonrandomized Studies (RoBANS; [27]) was used. Two authors (EM and KE; 93% agreement) independently evaluated each article in the following five domains: (i) selection bias, (ii) performance bias, (iii) detection bias, (iv) attrition bias, (v) reporting bias. Following recommendations [26, 27], both authors made judgements independently of “low” to indicate low risk of bias, “high” to indicate high risk of bias, and “unclear” to indicate an unclear or unknown risk of bias. Judgments were compared and disagreements were resolved through discussion.
Data synthesis
Given the small and heterogenous samples, varied outcomes, and inconsistent reporting across reviews and experimental articles, a quantitative meta-analysis was not possible. Instead, results from reviews and experimental articles were summarized descriptively following recommendations for narrative synthesis [28, 29]. Specifically, extracted data from reviews and experimental articles were examined by four authors (AW, EM, CL, KE) who drafted summary tables and summary text describing key characteristics and patterns across studies. Following this, the narrative summaries were critically reviewed by one author (NCR) and an independent researcher (AC) before being sent to the iPOEG core team and local healthcare providers for review and feedback on the presentation of the results.
RESULTS
Reviews
Article characteristics
As illustrated in Fig. 2, the search yielded a total of 20 reviews. Sixteen reviews were identified in PHASE I, and four reviews were identified in PHASE II. An overview of the characteristics from each included review is provided in Supplementary File 3. All reviews were published between 2009 and 2019, with most being published from 2016 onward (n = 12, 60%). The majority of reviews (n = 7, 35%) were published in the United States of America followed by the Netherlands (n = 4, 20%), Germany (n = 3, 15%), Australia (n = 2, 10%), Italy (n = 2, 10%), Canada (n = 1, 5%), and Spain (n = 1, 5%). Four (20%) of the reviews performed meta-analysis on select outcome(s) [30, 31] or articles [32, 33]. Reviews synthesized information from 2 [34, 35] to 24 [36] experimental articles reporting on the effects of PA among children and adolescents affected by cancer. Reviews included samples comprised of children and adolescents diagnosed with acute lymphoblastic leukemia (n = 4, 20%), or mixed cancer types (n = 16, 80%) who were on-treatment (n = 3, 15%) or a combination of on- and off-treatment (i.e., mixed; n = 17, 85%).
Outcomes and results
Reviews reported on the effect of PA on one or more of the following outcomes: PA behavior (n = 16, 80%), physical (n = 20, 100%), psychosocial (n = 18, 90%), cognitive (n = 1, 5%), and/or “other” (n = 7, 35%) outcomes (see Supplementary File 3). Results were classified as positive change (i.e., increases or improvements in an outcome), negative changes (i.e., declines or worsening in an outcome), no change (i.e., no difference), or mixed results (i.e., a combination of increases, decreases, and/or no change). Five (31%) reviews reported increased PA behavior following a PA intervention, while nine (56%) reviews reported mixed results (i.e., positive, negative, and/or no change), and two (13%) reviews reported no change. Of note, the number of review articles in the denominator throughout the remaining section represents all reviews in which the category of outcome was assessed and reported.With regards to physical outcomes, the most commonly reported was strength (n = 19, 95%). Mixed results (i.e., positive and no change) were reported in 13 (68%) reviews, while positive results were reported in three (16%) reviews, and no change was reported in three (16%) reviews. Quality of life was the most commonly reported psychosocial outcome assessed (n = 18, 100%). Eight (44%) reviews reported mixed results that were positive and no change, one (6%) review reported mixed results that were negative and no change, whereas one (6%) review reported mixed results that were positive, no change, and negative. Three (17%) reviews found improvements in quality of life and five (28%) reviews found no change following PA interventions. The single (5%) review including cognitive outcomes described positive changes in reaction time and brain structure following a PA intervention [37]. In terms of “other” outcomes, reviews reported improvements, no change, or mixed results on outcomes not captured within one of the prior categories. For example, four (57%) reviews explored dietary/energy intake. Three (75%) reviews reported no change [33, 38, 39] whereas one (25%) review described mixed results (i.e., positive and no change) [36].
Some reviews described outcomes that were assessed within a single study only, as opposed to collating results across studies for the outcome [35]. Sixteen (80%) reviews reported feasibility, describing PA as feasible for children and adolescents affected by cancer. Fifteen (75%) reviews reported on adherence. Eleven (73%) reviews described acceptable levels of compliance, completion, and/or attendance, whereas four reviews (27%) described unclear/mixed adherence. Fifteen (75%) reviews reported on “adverse events.” Of these, only one (7%) review described adverse events related to PA (e.g., concerns of headache, muscle soreness, and fatigue). The remaining 14 (93%) did not report any adverse events. Across reviews, common limitations were discussed, including small and mixed samples, heterogenous study designs, different methods of analysis, variability in terms of interventions (i.e., frequency, intensity, time, and type of PA) and outcome measures used, inconsistent reporting, and a need for more research in general. Overall, findings from the reviews suggest that PA can improve PA behavior, strength, quality of life, and other outcomes (e.g., health behaviors knowledge and practices).
Quality assessment
Using AMSTAR 2 (see Supplementary File 4), 10 (50%) reviews were rated as “critically low.” In general, these reviews did not: indicate whether a protocol was established a priori, explain the selection of study design(s), use a comprehensive search strategy, perform study selection/data extraction in duplicate, perform risk of bias assessment, account for risk of bias/heterogeneity within results, and/or completely and consistently report outcomes (e.g., no reported reasons for excluding articles). Seven (35%) reviews were rated as “low” for the many of same reasons described above; however, these reviews were rated slightly higher as many included discussions regarding heterogeneity within the studies they reviewed, and the subsequent impact on their results. The remaining three (15%) reviews were rated as “moderate,” as they included randomized study designs, clearly articulated their eligibility criteria, described their methods, accounted for risk of bias in individual studies, and discussed heterogeneity. Although 16 (80%) of reviews included a conflict of interest statement, none (0%) reported on the sources of funding for the studies included in the review and only two (10%; [30, 31]) provided a list of excluded studies and justified their exclusions. No review was given a “high” rating.
Experimental articles
Article characteristics
As illustrated in Fig. 2, the search yielded a total of 69 experimental articles. Thirty-eight were identified in PHASE I, and 31 were identified in PHASE II. Twenty-six (38%) of these articles were not captured in the reviews synthesized above due to date of publication (n = 24 published ≥ 2017). An overview of the characteristics of experimental articles is included in Supplementary File 5. All articles were published from 1993 to 2020, with the majority being published from 2013 onwards (n = 47, 68%). RCTs (n = 25, 36%) and nonrandomized designs, including quasi-experimental (n = 19, 28%) and single group (n = 25, 36%) were used. A total of 2,428 participants were included and on average, participants ranged in age from 5.1 ± 1.2 [40–42] to 19.0 ± 3.0 years [43]. Cancer types included acute lymphoblastic leukemia (n = 20, 29%), brain tumors (n = 6, 9%), lower extremity sarcomas (n = 2, 3%), and mixed cancers (n = 41, 59%). In 41 (59%) articles, participants were reported as on-treatment, whereas they were off-treatment in 20 (29%) articles, and a combination of on- and off-treatment in eight (12%) articles.
PA intervention characteristics varied widely. PA types included aerobic (n = 9, 13%), mixed (n = 38, 55%), multimodal (n = 15, 22%), technology-based (n = 3, 4%), and yoga interventions (n = 4, 6%). Mixed PA interventions were those that included two or more training components (e.g., aerobic, strength, and/or mobility), multimodal were those that included PA and additional components (e.g., psychosocial support), and technology-based interventions were those that delivered PA via the use of technology (e.g., active video games). Most (n = 58, 84%) PA sessions lasted ≥30 min; however, articles described interventions ranging from 11 min/session of home-based PA [13] to a full day of multimodal (e.g., adventure-based) PA [44]. Seven (10%) articles did not report any information related to time/session. Interventions lasting ≥12 weeks were most common (n = 42, 61%), although there was a wide range with interventions lasting from one visit [45] to two and a half years [46]. Four (6%) articles did not report information related to intervention duration. Finally, with regards to supervision, most articles reported that the intervention was supervised (n = 51, 74%). Five (7%) articles described mixed supervision, wherein select components of the intervention were supervised (e.g., in-hospital PA) and others were unsupervised (e.g., home-based PA). One (1%) article reported no supervision, and 12 (17%) articles did not report on supervision.
Outcomes and results
Across experimental articles, different study designs, different methods of analysis, different populations, and differing interventions were used. Furthermore, varied populations, comparators, and outcomes were studied which are important sources of heterogeneity. Supplementary File 6 provides an overview of the outcomes assessed and results across experimental articles. PA behavior (n = 26), physical (n = 59), psychosocial (n = 39), cognitive (n = 8), and/or “other” outcomes (n = 21) were studied. Of note, some articles reported on multiple outcomes, thus n ≠ 69 here and throughout the remaining section. Similar to above, results are described as positive change, negative change, no change, or mixed results. Studies reported increased PA behavior following a PA intervention (n = 11, 42%), no changes (n = 6, 23%), or mixed results (n = 9, 35%; i.e., instances of increases, decreases, and/or no change) regardless of cancer diagnosis and treatment status (i.e., on- or off-treatment).
In the paragraphs to follow, the number of articles in the denominator represents all articles in which the category was assessed and reported. The two most commonly reported physical outcomes were strength (n = 31, 53%) and cardiorespiratory fitness (n = 30, 51%). There were mixed results observed for both outcomes, with most articles reporting improvements and no change among children and adolescents of mixed cancer types and at varying stages of treatment (i.e., on- and off-treatment). The exception to this was for cardiorespiratory fitness wherein one (3%) out of 30 articles reported within-subjects decreases in cardiorespiratory fitness following a seven-week mixed PA intervention offered in-hospital to children and adolescents undergoing hematopoietic stem cell transplant [47]. Following strength and cardiorespiratory fitness, the next most commonly reported outcomes were functional mobility (n = 16, 27%) and fatigue (n = 16, 27%; see Supplementary File 6). The most commonly explored psychosocial outcome was quality of life (n = 28, 72%). Ten (36%) of these articles reported improved quality of life, 12 (43%) articles reported no change, and five (18%) articles reported mixed results (i.e., increases and no change) across children and adolescents of mixed cancer types and at varying stages of treatment (i.e., on- and off-treatment). One (4%) of the 28 articles reported decreased quality of life among children and adolescents undergoing hematopoietic stem cell transplant in both the intervention (i.e., mixed in-hospital PA) and control group [48]. After quality of life, the second and third most commonly reported psychosocial outcomes were: emotional functioning, quality of life, mental health, and/or well-being (n = 14, 36%) and physical well-being or quality of life (n = 13, 33%; see Supplementary File 6).
Eight (12%) articles reported on cognitive outcomes. The most commonly explored cognitive outcome was self or parent-reported cognitive problems (n = 4, 50%), in which two (50%) of the four articles reported no change following PA in children and adolescents with mixed cancer types and at varying stages of treatment (i.e., on- and off-treatment; [49, 50]). The remaining two (50%) articles reported mixed results (i.e., increases, decreases, and no change) after PA among children and adolescents with acute lymphoblastic leukemia who were at varying stages of treatment (i.e., on- and off-treatment; [51, 52]). Reaction time (n = 2, 25%) and structural changes (n = 2, 25%) were the next most commonly studied among children and adolescents with brain tumors who were off-treatment. Mixed results (i.e., increases, decreases, and no change) were reported for both.
Among articles reporting on “other” outcomes (n = 21, 30%), school functioning (n = 9, 43%) was the most commonly described. One (11%) of the nine articles described improvements following PA among children and adolescents with mixed cancers who were off-treatment, whereas two (22%) articles reported mixed results (i.e., increases and no change) among children and adolescents with mixed cancers at varying states of treatment (i.e., on- and off-treatment). The remaining six (67%) of nine articles reported no change among children and adolescents with mixed cancer types at varying stages of treatment (i.e., on- and off-treatment).
Most experimental articles (n = 49, 71%) described adherence (defined as compliance, completion, and/or attendance) as acceptable (e.g., [53]). Of the 35 (51%) experimental articles that reported on adverse events, only one was reported related to PA was reported [12]. In this article, the participant suffered an injury, received immediate medical attention, recovered, and rejoined the PA intervention [12]. Finally, fifty-seven (83%) articles described sources of funding.
Risk of bias assessment
Supplementary File 7 contains the risk of bias assessments for RCTs using the Cochrane Collaboration’s tool [26] and for nonrandomized trials using the RoBANS [27]. Seventeen (68%) RCTs were classified as “high” risk of bias in at least one domain, 19 (76%) were rated as “unclear” risk of bias in at least one domain, and none (0%) were rated as “low” risk of bias across domains. Twenty-six (59%) non-RCTs were classified as “low” risk of bias across domains, four (9%) were classified as both “low” and “unclear” risk of bias across domains, 12 (27%) were classified as both “low” and “high” risk of bias across domains, and two (5%) were classified as “low” and “high” and “unclear” risk of bias.
Discussion
There is a rapidly growing body of literature documenting the benefits of PA for children and adolescents affected by cancer. Numerous reviews and experimental articles have been published; however, no efforts have been made to collate this literature. The purpose of this literature synthesis was to provide a comprehensive overview of the available evidence. Twenty reviews and 69 experimental articles collectively suggest that PA interventions may promote PA behavior, physical, psychosocial, cognitive, and “other” outcomes, for children and adolescents affected by cancer. Though the literature offers support for PA, important considerations for outcomes, samples, and study designs remain.
In terms of outcomes, physical outcomes were most commonly studied. Yet, evidence is lacking on other priority outcomes, such as the benefits of PA on symptoms of fatigue. Acquiring insight into the range of benefits PA may confer is required. In doing so researchers are urged to use validated tools and report effect sizes and confidence intervals. This will facilitate meta-analyses that have the capacity to draw firmer conclusions, ultimately enabling further assessments of the body of literature (e.g., through tools such as Grading of Recommendation Assessment, Development, and Evaluation for outcomes; [54]). Adopting patient-oriented approaches and integrated knowledge translation strategies to identify outcomes that are relevant to end-users and collating a body of psychometrically sound tools within a directory are also necessary to facilitate the next generation of impactful research (see [55] for further discussion on this topic). With regards to sample size, both reviews and experimental articles described the challenges of recruiting children and adolescents affected by cancer, which may have resulted in underpowered studies. This could partially explain the null and mixed results observed, though whether a study was adequately powered was not systematically extracted, nor considered herein, representing an important area of future inquiry. With regards to study design, only four (20%) reviews performed a meta-analysis on select outcome(s) or articles and only 25 (36%) experimental articles were RCTs—widely considered to be the gold standards in their respective domains. This can limit perceptions of the strength of evidence in the field. Therefore, researchers have been urged to conduct meta-analyses and adequality powered definitive RCTs. Nevertheless, alternative study designs are critical insight, particularly in emerging fields such as this one. Specifically, narrative syntheses that describe patterns and small-scale pilot, cohort, and case control studies, and pragmatic RCTs can contribute complimentary information to better understand the effects of PA on important outcomes. Taken together, when exploring PA among children and adolescents diagnosed with cancer, researchers should explore priority outcomes (using the same measurement tools across studies), report effect sizes and confidence intervals, and choose the most appropriate study design for their context.
This literature synthesis included evidence from published reviews in the field, which highlighted knowledge gaps, provided further evidence to support PA in this population, and offered insight into the effects of PA within specific subgroups of the population and at different points along the cancer trajectory. Collectively, this evidence enables a deeper understanding of what has been done to date in the emerging field of pediatric exercise oncology. Though most reviews were rated as “critically low” and “low” quality (n = 17, 85%), AMSTAR 2 is not intended to generate an overall score, but rather help identify reviews that offer comprehensive and accurate summaries of the available literature [25]. Since AMSTAR 2 does not allow assessors to make a rating of “unclear,” it is possible that review quality ratings may have been higher with more complete and transparent reporting. Indeed, describing whether a protocol was established and followed, clearly articulating comprehensive search strategies (using multiple databases), providing rationale for article selection, completely describing all study procedures (e.g., whether article selection was performed in duplicate), extracting information related to funding from included studies, and accounting for risk of bias are imperative. As well, performing meta-analyses, as appropriate, is recommended. However, it is important to recognize that alternative ways of summarizing and synthesizing findings comprised of different study designs (e.g., narrative review guidelines; [28]) exist. Exploring strategies to reliably rate the quality of reviews in the absence of a meta-analysis and when publication factors (e.g., word count) can impact the ability to relay additional details and information is warranted.
This literature synthesis also included published experimental articles, extending findings from previously published reviews and strengthening the evidence produced by any one article. Broader eligibility criteria and more recent evidence was collected, which bolster previous contentions regarding the benefits and safety of PA. Risk of bias within the experimental articles was generally rated as “low” risk across categories of bias. This finding highlights that authors are adhering to guidelines for the design and conduct of varied trial designs. Across articles there were few losses to follow-up and the majority reported on all assessed outcomes, even when no effect was observed. Further, articles explained risk of bias in their studies and used appropriate and validated measures and tools, and blinded assessors (where possible). This speaks to the excellence of research using varied study designs in this field. In cases where articles were rated as “high” risk of bias, this was often due to not blinding participants, personnel, and/or assessors. Since blinding is not possible in PA interventions, adjusting expectations and potentially risk of bias assessments to take this into account may need to be considered.
When interpreting the findings from this literature synthesis, there are important considerations that should be taken into account. First, there was no methodologist or biostatistician on the study team, which may have adversely impacted protocol design and quality and risk of bias assessment [56]. Second, multiple individuals were involved in this project from article inclusion through to data synthesis. Though inter-rater agreement was tracked for eligibility and quality and risk of bias assessments, it was not for data extraction nor synthesis. Consequently, the level of agreement for these aspects of the literature synthesis are unknown. That said, all those involved in data extraction and synthesis were given training in utilizing the coding guide, met as a group and independently with the first author, and proceeded in small batches to ensure adequate training, consistency, and accuracy. All disagreements were resolved through discussion and re-visiting the article. Third, only articles published in English were included, thereby omitting valuable data from reviews and experimental articles published in other languages. Fourth, though a range of strategies were used to identify literature these may not have encompassed all of the evidence on PA interventions for children and adolescents affected by cancer. Fifth, although the goal was to provide a comprehensive overview of the literature, only reviews and experimental articles were included. Other types of evidence, such as observational studies were not included. Readers are referred to [8] for a meta-analysis of observational studies reporting on PA among children and adolescents affected by cancer. Sixth, this literature synthesis included 43 (62%) experimental articles that were also included within the reviews. In addition, articles (as opposed to unique studies) were included. This may have resulted in overrepresentation of selected articles and outcomes. In an attempt to address both, notes are provided in Supplementary Files to indicate when articles emanated from the same study, and data from reviews and experimental articles are presented in a way that has not been done before (e.g., summarized review findings, collated results tables). Seventh, articles included in this literature synthesis explored the effects of PA for both children and adolescents combined. As a result, identifying differences in the effects of PA based on biological age was impeded. Moving forward it will be necessary to explore and address biological age and pertinent developmental considerations (e.g., dependence, attention span, ability to comprehend instructions, motor control, language skills) in research. Finally, there was substantial heterogeneity across reviews and experimental articles, which likely affected the results described herein and should be considered when reviewing the outcomes and results reported. Despite these considerations, findings from this literature synthesis add to the extant literature and extend previous reports that PA is beneficial and safe for children and adolescents affected by cancer.
CONCLUSION
This literature synthesis is based on available review and experimental evidence. Findings not only informed the development of the iPOEG [18], but on their own represent an important step towards consolidating information on the effects of PA for children and adolescents affected by cancer. Taken together, results from this review suggest PA may improve PA behavior, physical, psychosocial, cognitive, and “other” outcomes. Ultimately, this work suggests that “Movement is possible and important for every child and adolescent with cancer.”
Supplementary Material
Acknowledgments
The authors would like to thank the iPOEG core team (Carolina Chamorro Viña, PhD; Sarah L. Grimshaw, BPT; Lotta Hamari, PhD; Miriam Götte, PhD; Sabine Kesting, PhD; Francesca Rossi, NDT; Patrick van der Torre, MSc) and local healthcare providers (Gregory Guilcher, MD and Krista McIntyre, RN) for their input throughout this project, particularly their critical review of the articles gathered in PHASE I and identification of additional articles in PHASE II. The authors would also like to acknowledge the work of Amy Chen for verifying data extraction at study cessation. As well, the authors would like to thank Alyssa Froese, Vivien Lösse, and David Chiu for their assistance identifying, extracting, and collating evidence comprising PHASE I. This project was supported by the Daniel Family Leadership Chair in Psychosocial Oncology, Social Sciences and Humanities Research Council of Canada, the Faculty of Kinesiology at the University of Calgary, and the University Research Grants Committee.
Compliance with Ethical Standards
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Human Rights: This article does not contain any studies with human participants performed by any of the authors.
Informed Consent: This study does not involve human participants and informed consent was therefore not required.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
Authors’ Contributions: A.W. and S.N.C.R. are coleads on the iPOEG project. E.M., K.E., and C.L. are trainees engaged in pediatric oncology exercise research. All coauthors contributed meaningfully to this project and critically reviewed and approved the manuscript.
References
- 1. Steliarova-Foucher E, Colombet M, Ries LAG, . et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18(6), 719–731. doi: 10.1016/S1470-2045(17)30186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Girardi F, Atun R. Global childhood cancer survival estimates and priority-setting: a simulation-based analysis. Lancet Oncol. 2019;20(7):972–983. [DOI] [PubMed] [Google Scholar]
- 3. Bitsko MJ, Cohen D, Dillon R, Harvey J, Krull K, Klosky JL. Psychosocial late effects in pediatric cancer survivors: a report from the children’s oncology group. Pediatr Blood Cancer. 2016;63(2):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friend AJ, Feltbower RG, Hughes EJ, Dye KP, Glaser AW. Mental health of long-term survivors of childhood and young adult cancer: a systematic review. Int J Cancer. 2018;143(6):1279–1286. [DOI] [PubMed] [Google Scholar]
- 5. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eiser C. Beyond survival: quality of life and follow-up after childhood cancer. J Pediatr Psychol. 2007;32(9):1140–1150. [DOI] [PubMed] [Google Scholar]
- 7. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 8. Antwi GO, Jayawardene W, Lohrmann DK, Mueller EL. Physical activity and fitness among pediatric cancer survivors: a meta-analysis of observational studies. Support Care Cancer. 2019;27(9):3183–3194. [DOI] [PubMed] [Google Scholar]
- 9. Tonorezos ES, Ford JS, Wang L, et al. Impact of exercise on psychological burden in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2019;125(17):3059–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scott JM, Li N, Liu Q, et al. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018;4(10):1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beulertz J, Prokop A, Rustler V, Bloch W, Felsch M, Baumann FT. Effects of a 6-month, group-based, therapeutic exercise program for childhood cancer outpatients on motor performance, level of activity, and quality of life. Pediatr Blood Cancer. 2016;63(1):127–132. [DOI] [PubMed] [Google Scholar]
- 13. Braam KI, van Dijk-Lokkart EM, Kaspers GJL, et al. Effects of a combined physical and psychosocial training for children with cancer: a randomized controlled trial. BMC Cancer. 2018;18(1):1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zucchetti G, Rossi F, Chamorro Vina C, Bertorello N, Fagioli F. Exercise program for children and adolescents with leukemia and lymphoma during treatment: a comprehensive review. Pediatr Blood Cancer. 2018;65(5):e26924. [DOI] [PubMed] [Google Scholar]
- 15. Grimshaw SL, Taylor NF, Shields N. The feasibility of physical activity interventions during the intense treatment phase for children and adolescents with cancer: a systematic review. Pediatr Blood Cancer. 2016;63(9):1586–1593. [DOI] [PubMed] [Google Scholar]
- 16. Huang TT, Ness KK. Exercise interventions in children with cancer: a review. Int J Pediatr. 2011;2011:461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wurz A, McLaughlin E, Chamorro-Viña C, et al. (under review). The international Pediatric Oncology Exercise Guidelines (iPOEG). TBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. 2007. Int J Social Res Methodol. 2007;8(1):19–32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 20. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 24. Wurz A, Daeggelmann J, Albinati N, Kronlund L, Chamorro-Viña C, Culos-Reed SN. Physical activity programs for children diagnosed with cancer: an international environmental scan. Support Care Cancer. 2019;27(4):1153–1162. [DOI] [PubMed] [Google Scholar]
- 25. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [Updated March 2011]. Cochrane Collab. 2011. Available at www.handbook.cochrane.org. [Google Scholar]
- 27. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–414. [DOI] [PubMed] [Google Scholar]
- 28. Snilstveit B, Oliver S, Vojtkova M. Narrative approaches to systematic review and synthesis of evidence for international development policy and practice. J Dev Effectiveness. 2012;4(3):409–429. doi: 10.1080/19439342.2012.710641 [DOI] [Google Scholar]
- 29. Popay J, Roberts H, Sowden A, . et al. Guidance on the conduct of narrative systematic reviews. 2006:1–93. Available at https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf. [Google Scholar]
- 30. Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kasper, GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2013;4:CD008796. doi: 10.1002/14651858.CD008796.pub2 [DOI] [PubMed] [Google Scholar]
- 31. Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;3:CD008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morales JS, Valenzuela PL, Rincón-Castanedo C, et al. Exercise training in childhood cancer: a systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev. 2018;70:154–167. [DOI] [PubMed] [Google Scholar]
- 33. Mizrahi D, Wakefield CE, Fardell JE, et al. Distance-delivered physical activity interventions for childhood cancer survivors: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;118:27–41. [DOI] [PubMed] [Google Scholar]
- 34. Liu RD, Chinapaw MJ, Huijgens PC, van Mechelen W. Physical exercise interventions in haematological cancer patients, feasible to conduct but effectiveness to be established: a systematic literature review. Cancer Treat Rev. 2009;35(2):185–192. [DOI] [PubMed] [Google Scholar]
- 35. Wurz A, Brunet J. The effects of physical activity on health and quality of life in adolescent cancer survivors: a systematic review. JMIR Cancer. 2016;2(1):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raber M, Swartz MC, Santa Maria D, et al. Parental involvement in exercise and diet interventions for childhood cancer survivors: a systematic review. Pediatr Res. 2016;80(3):338–346. [DOI] [PubMed] [Google Scholar]
- 37. Runco DV, Yoon L, Grooss SA, Wong CK. Nutrition & exercise interventions in pediatric patients with brain tumors: a narrative review. J Natl Cancer Inst Monogr. 2019;2019(54):163–168. [DOI] [PubMed] [Google Scholar]
- 38. Wolin KY, Ruiz JR, Tuchman H, Lucia A. Exercise in adult and pediatric hematological cancer survivors: an intervention review. Leukemia. 2010;24(6):1113–1120. [DOI] [PubMed] [Google Scholar]
- 39. Zhang FF, Kelly MJ, Must A. Early nutrition and physical activity interventions in childhood cancer survivors. Curr Obes Rep. 2017;6(2):168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruiz JR, Fleck SJ, Vingren JL, et al. Preliminary findings of a 4-month intrahospital exercise training intervention on IGFs and IGFBPs in children with leukemia. J Strength Cond Res. 2010;24(5):1292–1297. [DOI] [PubMed] [Google Scholar]
- 41. San Juan AF, Fleck SJ, Chamorro-Viña C, et al. Early-phase adaptations to intrahospital training in strength and functional mobility of children with leukemia. J Strength Cond Res. 2007;21(1):173–177. [DOI] [PubMed] [Google Scholar]
- 42. San Juan AF, Fleck SJ, Chamorro-Viña C, et al. Effects of an intrahospital exercise program intervention for children with leukemia. Med Sci Sports Exerc. 2007;39(1):13–21. [DOI] [PubMed] [Google Scholar]
- 43. Sharkey AM, Carey AB, Heise CT, Barber G. Cardiac rehabilitation after cancer therapy in children and young adults. Am J Cardiol. 1993;71(16):1488–1490. [DOI] [PubMed] [Google Scholar]
- 44. Li HC, Chung OK, Ho KY, Chiu SY, Lopez V. Effectiveness of an integrated adventure-based training and health education program in promoting regular physical activity among childhood cancer survivors. Psychooncology. 2013;22(11):2601–2610. [DOI] [PubMed] [Google Scholar]
- 45. Ladha AB, Courneya KS, Bell GJ, Field CJ, Grundy P. Effects of acute exercise on neutrophils in pediatric acute lymphoblastic leukemia survivors: a pilot study. J Pediatr Hematol Oncol. 2006;28(10):671–677. [DOI] [PubMed] [Google Scholar]
- 46. Cox CL, Zhu L, Kaste, SC, Srivastava K, Barnes L, Nathan PC, Wells RJ, Ness KK. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatric Blood Cancer, 2018;65(4), 1–8. doi: 10.1002/pbc.26929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bogg TF, Broderick C, Shaw P, Cohn R, Naumann FL. Feasibility of an inpatient exercise intervention for children undergoing hematopoietic stem cell transplant. Pediatr Transplant. 2015;19(8):925–931. [DOI] [PubMed] [Google Scholar]
- 48. Senn-Malashonak A, Wallek S, Schmidt K, et al. Psychophysical effects of an exercise therapy during pediatric stem cell transplantation: a randomized controlled trial. Bone Marrow Transplant. 2019;54(11):1827–1835. [DOI] [PubMed] [Google Scholar]
- 49. Stein E, Rayar M, Krishnadev U, et al. A feasibility study examining the impact of yoga on psychosocial health and symptoms in pediatric outpatients receiving chemotherapy. Support Care Cancer. 2019;27(10):3769–3776. [DOI] [PubMed] [Google Scholar]
- 50. van Dijk-Lokkart EM, Braam KI, van Dulmen-den Broeder E, et al. Effects of a combined physical and psychosocial intervention program for childhood cancer patients on quality of life and psychosocial functioning: results of the QLIM randomized clinical trial. Psychooncology. 2016;25(7):815–822. [DOI] [PubMed] [Google Scholar]
- 51. Khodashenas E, Badiee Z, Sohrabi M, Ghassemi A, Hosseinzade V. The effect of an aerobic exercise program on the quality of life in children with cancer. Turk J Pediatr. 2017;59(6):678–683. [DOI] [PubMed] [Google Scholar]
- 52. Tanir MK, Kuguoglu S. Impact of exercise on lower activity levels in children with acute lymphoblastic leukemia: a randomized controlled trial from Turkey. Rehabil Nurs. 2013;38(1):48–59. [DOI] [PubMed] [Google Scholar]
- 53. Gilliam MKM, Futch L, Walsh A, Klapow J, Davis D, Whelan K, Madan-Swain A. A pilot study evaluation of a web-based token economy to increase adherence with a community-based exercise intervention in child and adolescent cancer survivors. Rehabil Oncol. 2011;29(2):16–22. [Google Scholar]
- 54. Atkins D, Best D, Briss PA, et al. ; GRADE Working Group . Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wurz A, McLaughlin E, Chamorro Viña C, et al. (accepted). Advancing the field of pediatric exercise oncology: research and innovation needs. Curr Oncol. TBD. doi: 10.3390/curroncol28010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zapf A, Rauch G, Kieser M. Why do you need a biostatistician? BMC Med Res Methodol. 2020;20(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.