Abstract
Novel synthetic compounds have been available for decades as quasi-legal alternatives to controlled substances. The hallucinogen-like effects of eight novel substituted tryptamines were evaluated to determine their potential abuse liability. Male Sprague–Dawley rats were trained to discriminate 2,5-dimethoxy-4-methylamphetamine (DOM, 0.5 mg/kg, i.p., 30 min) from saline. 4-Acetoxy-N,N-diethyltryptamine (4-AcO-DET), 4-hydroxy-N-methyl-N-ethyltryptamine (4-OH-MET), 4-hydroxy-N,N-diethyltryptamine (4-OH-DET), 4-acetoxy-N-methyl-N-isopropyltryptamine (4-AcO-MiPT), 4-acetoxy-N,N-dimethyltryptamine (4-AcO-DMT), 4-hydroxy-N,N-dimethyltryptamine (4-OH-DMT, psilocin), 5-methoxy-N-methyl-N-isopropyltryptamine (5-MeO-MiPT), 4-acetoxy-N,N-diisopropyltryptamine (4-AcO-DiPT), and 4-hydroxy-N,N-diisopropyltryptamine (4-OH-DiPT) were tested for their ability to substitute for the discriminative stimulus effects of DOM. All test compounds fully substituted for DOM with potencies less than or equal to that of DOM. 4-OH-MET, 4-OH-DET, 4-OH-DMT, and 4-AcO-DMT decreased response rate at doses that fully substituted. Because the test compounds produced DOM-like discriminative stimulus effects, they may have similar abuse liability as DOM. 4-Acetoxy substituted compounds were less potent than 4-hydroxy substituted compounds, and the N,N-diisopropyl compounds were less potent than the dimethyl, diethyl, N-methyl-N-ethyl, and N-methyl-N-isopropyl compounds.
Keywords: hallucinogens, drug discrimination, rat
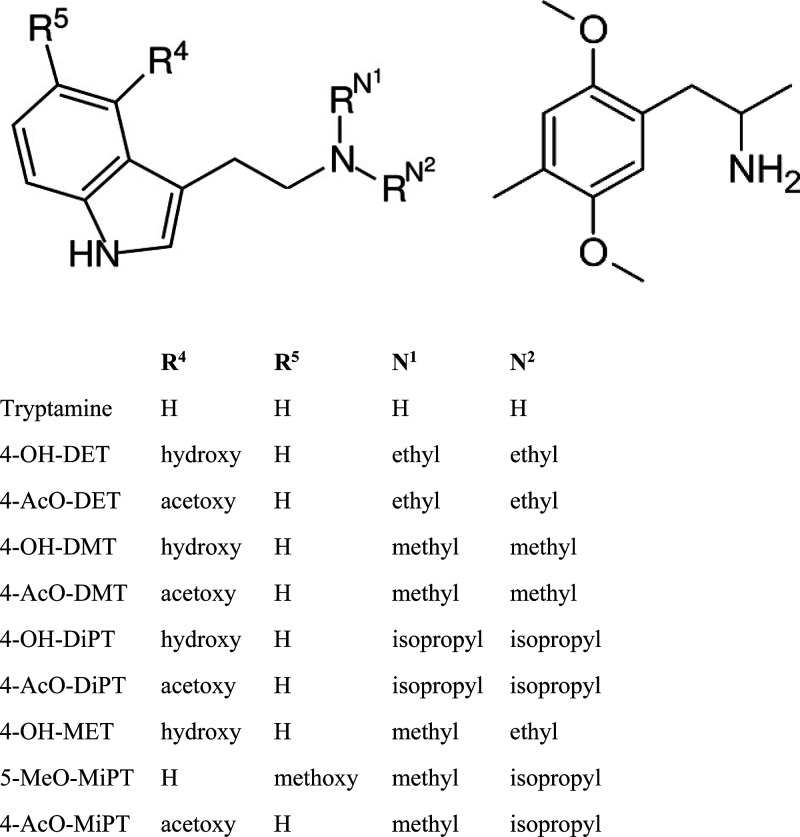
Classic serotonin-mediated hallucinogens such as psilocybin have likely been used for millennia for religious and divinatory purposes.1 In recent clinical trials, psilocybin and other classic hallucinogens have been used to treat depression and anxiety related to cancer diagnosis,2 persistent pain,3 and to aid in the cessation of drug use.4 However, they are also used in nonreligious or nonmedical settings for a myriad of reasons.5 Although classic hallucinogens such as psilocybin, lysergic acid diethylamide (LSD), N,N-dimethyltryptamine (DMT), mescaline, and 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DiPT) are still commonly used, novel synthetic hallucinogens are still coming onto the market, many of them described in Shulgin’s book TiKHAL.6 Several of these compounds have been flagged for interest by the United States Drug Enforcement Administration, including 4-acetoxy-N,N-diethyltryptamine (4-AcO-DET), 4-hydroxy-N-methyl-N-ethyltryptamine (4-OH-MET, metocin, methylcybin), 4-hydroxy-N,N-diethyltryptamine (4-OH-DET), 4-acetoxy-N-methyl-N-isopropyltryptamine (4-AcO-MiPT, mipracetin), 4-acetoxy-N,N-dimethyltryptamine (4-AcO-DMT, psilocetin, O-acetylpsilocin), 4-acetoxy-N,N-diisopropyltryptamine (4-AcO-DIPT, ipracetin), 5-methoxy-N-methyl-N-isopropyltryptamine (5-MeO-MiPT), and 4-hydroxy-N,N-diisopropyltryptamine (4-OH-DiPT) (Figure 1).
Figure 1.
Chemical structures of the test compounds. Tryptamine and its potential structural substitutions are shown on the left; DOM is shown on the right.
Several of these are easily available through Internet sources, including 4-OH-DET, 4-OH-DiPT, 4-AcO-DMT, and 4-AcO-DiPT.7 Recreational use of several tryptamines, including 4-OH-MET and 4-AcO-DMT, have been documented,8−10 and others, including 4-OH-MET, 4-OH-DET, and 4-AcO-DiPT, have been detected in blood, urine, and post-mortem samples.11−14 The most commonly found nonregulated tryptamine (2006–2015) was 4-AcO-DMT.15
Classic hallucinogens are known to act at serotonin (5-HT) receptors, particularly 5-HT2A, but have effects at a wide range of other receptors and transporters which may contribute to the hallucinogenic effects.16−18 Of the compounds tested in the current study, 4-OH-DMT, 4-OH-DiPT, 4-OH-MET, and 5-MeO-MiPT all bound to and acted as agonists at 5-HT2A.19 4-OH-DMT, 4-OH-DET, and 5-MeO-MiPT all produced potent effects on 5-HT1A, 5-HT2A, and 5-HT2B receptors.20
4-OH-DMT (psilocin) is the active metabolite of the well-known hallucinogen psilocybin,18 and after oral or intravenous administration, psilocybin is rapidly metabolized to psilocin.21 The behavioral effects of psilocybin, and hence psilocin, have been well-characterized and serve as a positive control. Very little behavioral testing has been conducted with the remainder of these test compounds. 4-AcO-DMT blocked drug-withdrawal-induced aversions in morphine and nicotine dependent mice.22 Some of the parent compounds, DMT, N,N-diethyltryptamine (DET), and N,N-diisopropyltryptamine (DiPT), have been reported to produce hallucinogen-like discriminative stimulus effects. DMT produced full substitution (>80% drug-appropriate responding) in rats trained to discriminate the hallucinogens 2,5-dimethoxy-4-methylamphetamine (DOM) or LSD in one study,23 but only 78% in LSD-trained rats in ref (24). DET fully substituted for LSD in one study25 but not another (49%).24 DiPT also fully substituted for the discriminative stimulus effects of DMT and DOM but produced only 68% LSD-like responding.26 Hallucinogenic compounds, including DOM, produce similar but not completely overlapping discriminative stimulus effects, which are mediated largely by serotonin 5-HT2A receptors,16,25 so taken together, there is good evidence that the parent compounds produce some sort of hallucinogen-like effects.
Assessment of abuse liability incorporates several factors. Compounds with chemical structures and mechanisms of action similar to known drugs of abuse are flagged for concern. Behavioral effects common to known drugs of abuse, such as the head-twitch response,27,28 help confirm related types of effects. Confirmatory tests include tests of subjective effects and rewarding/reinforcing effects. The purpose of the current study was to test whether a set of substituted tryptamine compounds identified by the DEA produce discriminative stimulus effects similar to the hallucinogen DOM. Drug discrimination is a useful animal model of the subjective effects of drugs and correlates well with human use.29,30 Currently, there are no good, consistent animal models of hallucinogen self-administration, so drug discrimination studies are useful for preclinical determination of potential hallucinogenic activity.
Results and Discussion
All of the tryptamine test compounds fully substituted for the discriminative stimulus effects of 0.5 mg/kg DOM (Figure 2). The potencies of the test compounds were less than or equal to that of DOM (Table 1). There was a 10-fold range in potency, and the rank order of potency was: DOM > 4-OH-DET > 4-OH-DMT = 4-OH-MET > 5-MeO-MiPT = 4-AcO-MiPT = 4-AcO-DMT > 4-OH-DiPT > 4-AcO-DiPT > 4-AcO-DET.
Figure 2.
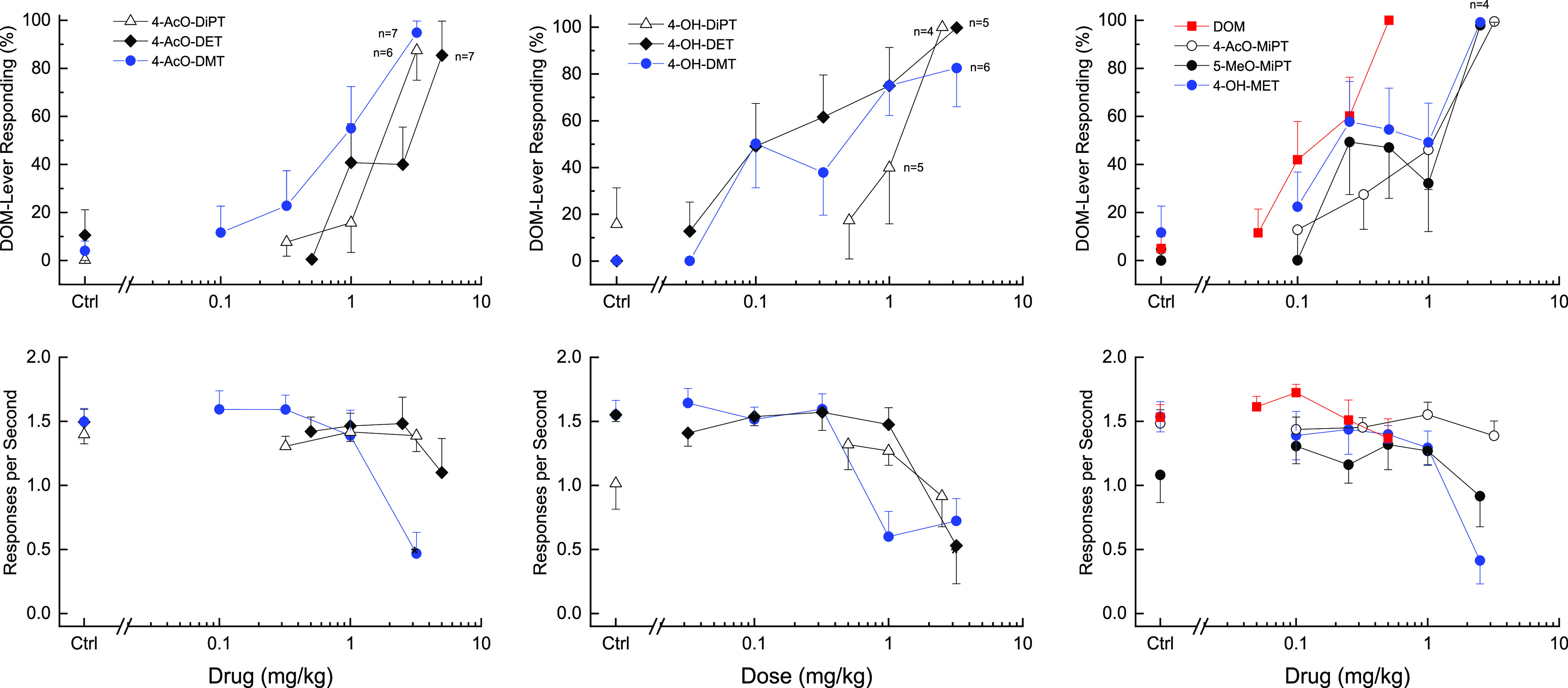
Substitution for the discriminative stimulus effects of DOM. Top panels show percentage of total responses made on the drug-appropriate lever. Bottom panels show rate of responding in responses per second (r/s). 4-AcO-DET (n = 9), 4-OH-MET (n = 9), 4-OH-DET (n = 8), 4-AcO-MiPT (n = 9), 5-MeO-MiPT (n = 6), 4-AcO-DMT (n = 9), 4-AcO-DiPT (n = 8), and 4-OH-DiPT (n = 6) unless otherwise shown. Ctrl indicates vehicle and training drug control values. * indicates response rate different from vehicle control (p < 0.05). Error bars show standard error of the mean.
Table 1. Potencies (ED50 Values Expressed in mg/kg ± Standard Error of the Mean) For the Test Compounds in the Present Study.
| test compound | potency (mg/kg) |
|---|---|
| DOM standard | 0.14 ± 0.07 |
| 4-OH-DMT (psilocin) | 0.33 ± 0.18 |
| 4-AcO-DMT | 0.68 ± 0.12 |
| 4-OH-MET | 0.38 ± 0.20 |
| 4-OH-DET | 0.18 ± 0.18 |
| 4-AcO-DET | 2.1 ± 0.10 |
| 5-MeO-MIPT | 0.61 ± 0.14 |
| 4-AcO-MIPT | 0.66 ± 0.12 |
| 4-OH-DIPT | 0.73 ± 0.14 |
| 4-AcO–DIPT | 1.47 ± 0.09 |
Rate of responding was not changed following any dose of 5-MeO-MiPT, 4-AcO-MiPT, or 4-OH-DiPT. 4-AcO-DET and 4-AcO-DiPT did not produce statistically significant decreases in response rate, but two rats failed to earn a food pellet when tested at the highest dose of each compound. 4-OH-MET decreased response rate [F(5,40) = 8.741, p < 0.001] such that 5 of 9 rats failed to earn a food pellet following 2.5 mg/kg. 4-OH-DET decreased response rate [F(5,35) = 12.699, p < 0.001] such that 3 of 8 rats failed to earn a food pellet following 3.2 mg/kg. Response rate was decreased following 1 and 3.2 mg/kg of 4-OH-DMT [F(5,35) = 12.825, p < 0.001], such that 2 of 8 rats failed to earn a food pellet following 3.2 mg/kg. 4-AcO-DMT decreased response rate [F(4,32) = 12.347, p < 0.001] such that 2 of 9 rats failed to earn a food pellet following 2.5 mg/kg.
These findings agree with earlier findings that a variety of substituted tryptamines can produce DOM-like discriminative stimulus effects,16,17,31 and are not surprising, because several of the test compounds act at 5-HT2A and 5-HT1A receptors,19,20 which are commonly thought to mediate the subjective effects of hallucinogens, including DOM.16,31 In addition, it is well-known that psilocybin produces hallucinogen-like discriminative stimulus effects, cross-substituting for LSD, DOM, and other hallucinogenic compounds.16,17,31 Psilocybin is converted to psilocin (4-OH-DMT) in the body,21,32 so it is to be expected that direct administration of 4-OH-DMT would also fully substitute for the discriminative stimulus effects of DOM, as was observed in the present study.
Comparing the potencies (Table 1), 4-AcO-DET was 10-fold less potent than 4-OH-DET, and 4-AcO-DMT and 4-AcO-DiPT were each 2-fold less potent than their respective 4-OH congeners. In the case of the N-methyl-N-isopropyltryptamine compounds, the 5-MeO substitution was not significantly different in potency compared to the 4-AcO substitution.
Taken together, eight novel substituted tryptamines produced discriminative stimulus effects comparable to those of DOM, which suggests that these compounds may have similar subjective effects as hallucinogenic tryptamines and thereby similar effects that might account for their recreational use that is currently under investigation for psilocybin and other hallucinogens. In addition, the hypothesis that psilocin is the behaviorally active metabolite of psilocybin was supported by the full substitution of psilocin in DOM-trained rats. None of the compounds produced adverse effects at the doses tested, unlike some of the compounds tested previously which produced paralysis, tremors, convulsions, and lethality.26,33
Methods
Subjects
Male Sprague–Dawley rats were obtained from Envigo. All rats were housed individually and were maintained on a 12/12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day which included the food received during operant sessions. Water was readily available. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Discrimination Procedures
Standard operant behavior-testing chambers (Coulbourn Instruments, Allentown, PA, Model E10-10) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). Response levers were positioned to the left and right of the food hopper. A house light was centered over the hopper close to the ceiling and was illuminated only when the levers were active. The computers were programmed in Med-PC for Windows, version IV (Med Associates) for the operation of the chambers and collection of data.
Using a two-lever choice methodology, 27 rats were trained to discriminate (−)-2,5-dimethoxy-4-methylamphetamine hydrochloride (0.5 mg/kg, i.p.) from saline. Rats received an injection of either saline or drug and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every 10 responses on a designated injection-appropriate lever. The pretreatment time was 30 min. Each training session lasted a maximum of 10 min, and the rats could earn up to a maximum of 20 food pellets. The rats received approximately 60 of these sessions before they were used in tests for substitution of the experimental compounds. Rats were used in testing once they had achieved 9 of 10 sessions at 85% injection-appropriate responding for both the first reinforcer and total session. The training sessions occurred on separate days in a double alternating fashion (drug–drug–saline–saline–drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one saline and one drug session occurred between each test (drug–saline–test–saline–drug–test–drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
4-AcO-DET (n = 9, saline, 60 min), 4-OH-MET (n = 9, deionized water, 30 min), 4-OH-DET (n = 8, deionized water, 30 min), 4-AcO-MiPT (n = 9, saline, 30 min), 5-MeO-MiPT (n = 6, saline, 60 min), 4-AcO-DMT (n = 9, saline, 45 min), 4-AcO-DiPT (n = 8, saline, 30 min), and 4-OH-DiPT (n = 6, deionized water, 15 min) were tested for substitution in DOM-trained rats. For dose–effect experiments, intraperitoneal (i.p.) injections (1 mL/kg) of vehicle or test compound were administered, and test sessions lasted for a maximum of 20 min. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until all 20 reinforcers were obtained or for a maximum of 20 min. A repeated-measures design was used, such that each rat was tested at all doses of a given drug, including vehicle and training-drug controls. The dose effect of each compound was tested from no effect to full effect or rate suppression (<20% of vehicle control) or adverse effects. Rats that failed to complete the first fixed ratio were excluded from the analysis of drug-appropriate responding but were used for analysis of response rate.
Drugs
4-Acetoxy-N,N-diethyltryptamine HCl, 4-hydroxy-N-methyl-N-ethyltryptamine HCl, 4-hydroxy-N,N-diethyltryptamine HCl, 4-acetoxy-N-methyl-N-isopropyltryptamine HCl, 4-acetoxy-N,N-dimethyltryptamine HCl, 4-hydroxy-N,N-dimethyltryptamine HCl, and 4-acetoxy-N,N-diisopropyltryptamine HCl were supplied by the Cayman Chemical Company (Ann Arbor, MI). (−)-2,5-Dimethoxy-4-methylamphetamine hydrochloride, 4-hydroxy-N,N-diisopropyltryptamine, and 5-methoxy-N-isopropyl-N-methyltryptamine were provided by NIDA Drug Supply. Optically active test compounds were provided as racemates. 4-OH-MET, 4-OH-DET, and 4-OH-DiPT were dissolved in deionized water. The remaining compounds were dissolved in 0.9% saline. All compounds were administered i.p. in a volume of 1 mL/kg.
Data Analysis
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Percent drug-appropriate responding was shown only if at least three rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Response rate data were analyzed by one-way repeated-measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts. The potencies (ED50 and standard errors of the mean) of the test compounds were calculated by fitting straight lines to the linear portion of the dose–response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). A two-way ANOVA was conducted on the ED50 values.
Acknowledgments
This work was supported by contract 15DDHQ18P00000735 from the Drug Enforcement Administration. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Drug Enforcement Administration. Thanks are given to Andrew Tourigny for the artwork.
The authors declare no competing financial interest.
References
- Guzmán G. (2008) Hallucinogenic mushrooms in Mexico: An overview. Econ. Bot. 62, 404–412. 10.1007/s12231-008-9033-8. [DOI] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Carducci M. A.; Umbricht A.; Richards W. A.; Richards B. D.; Cosimano M. P.; Klinedinst M. A. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 30, 1181–1197. 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]; a Shulgin A., and Shulgin A. (1997) TIHKAL: The Continuation. Transform Press, Berkeley, CA. [Google Scholar]
- Whelan A.; Johnson M. I. (2018) Lysergic acid diethylamide and psilocybin for the management of patients with persistent pain: a potential role?. Pain Manag 8, 217–229. 10.2217/pmt-2017-0068. [DOI] [PubMed] [Google Scholar]
- Bogenschutz M. P.; Johnson M. W. (2016) Classic hallucinogens in the treatment of addictions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 64, 250–8. 10.1016/j.pnpbp.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Carbonaro T. M.; Johnson M. W.; Griffiths R. R. (2020) Subjective features of the psilocybin experience that may account for its self-administration by humans: a double-blind comparison of psilocybin and dextromethorphan. Psychopharmacology (Berl) 237 (8), 2293–2304. 10.1007/s00213-020-05533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin A., and Shulgin A. (1997) TIHKAL: The Continuation. Transform Press, Berkeley, CA. [Google Scholar]
- Schifano F.; Deluca P.; Baldacchino A.; Peltoniemi T.; Scherbaum N.; Torrens M.; Ghodse A. H. (2006) Drugs on the web; the Psychonaut 2002 EU project. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 30, 640–646. 10.1016/j.pnpbp.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Kjellgren A.; Soussan C. (2011) Heaven and hell--a phenomenological study of recreational use of 4-HO-MET in Sweden. J. Psychoact. Drugs 43, 211–219. 10.1080/02791072.2011.605699. [DOI] [PubMed] [Google Scholar]
- Palamar J. J.; Barratt M. J.; Ferris J. A.; Winstock A. R. (2016) Correlates of new psychoactive substance use among a self-selected sample of nightclub attendees in the United States. Am. J. Addict. 25, 400–407. 10.1111/ajad.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito V.; Stevenson R. J. (2019) A systematic study of microdosing psychedelics. PLoS One 14, e0211023 10.1371/journal.pone.0211023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saffar Y.; Stephanson N. N.; Beck O. (2013) Multicomponent LC-MS/MS screening method for detection of new psychoactive drugs, legal highs, in urine-experience from the Swedish population. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 930, 112–20. 10.1016/j.jchromb.2013.04.043. [DOI] [PubMed] [Google Scholar]
- Álvarez-Ruiz R.; Andrés-Costa M. J.; Andreu V.; Picó Y. (2015) Simultaneous determination of traditional and emerging illicit drugs in sediments, sludges and particulate matter. J. Chromatogr A 1405, 103–15. 10.1016/j.chroma.2015.05.062. [DOI] [PubMed] [Google Scholar]
- McIntyre I. M.; Trochta A.; Gary R. D.; Storey A.; Corneal J.; Schaber B. (2015) A fatality related to two novel hallucinogenic compounds: 4-methoxyphencyclidine and 4-hydroxy-n-methyl-n-ethyltryptamine. J. Anal. Toxicol. 39, 751–755. 10.1093/jat/bkv089. [DOI] [PubMed] [Google Scholar]
- Pichini S.; Pujadas M.; Marchei E.; Pellegrini M.; Fiz J.; Pacifici R.; Zuccaro P.; Farré M.; de la Torre R. (2008) Liquid chromatography-atmospheric pressure ionization electrospray mass spectrometry determination of ″hallucinogenic designer drugs″ in urine of consumers. J. Pharm. Biomed. Anal. 47, 335–342. 10.1016/j.jpba.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Palma-Conesa Á.J.; Ventura M.; Galindo L.; Fonseca F.; Grifell M.; Quintana P.; Fornís I.; Gil C.; Farré M.; Torrens M. (2017) Something new about something old: a 10-year follow-up on classical and new psychoactive tryptamines and results of analysis. J. Psychoact. Drugs 49, 297–305. 10.1080/02791072.2017.1320732. [DOI] [PubMed] [Google Scholar]
- Nichols D. E. (2004) Hallucinogens. Pharmacol. Ther. 101, 131–181. 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Murnane K. S.; Reissig C. J. (2008) The behavioral pharmacology of hallucinogens. Biochem. Pharmacol. 75, 17–33. 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H. A.; Wurst M. G.; Daniels R. N. (2018) DARK Classics in Chemical Neuroscience: Psilocybin. ACS Chem. Neurosci. 9 (10), 2438–2447. 10.1021/acschemneuro.8b00186. [DOI] [PubMed] [Google Scholar]
- Rickli A.; Moning O. D.; Hoener M. C.; Liechti M. E. (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 26, 1327–1337. 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- McKenna D. J.; Repke D. B.; Lo L.; Peroutka S. J. (1990) Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 29 (3), 193–198. 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- Madsen M. K.; Knudsen G. M. (2020) Plasma psilocin critically determines behavioral and neurobiological effects of psilocybin. Neuropsychopharmacology 46, 257. 10.1038/s41386-020-00823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H.; Grieder T. E.; Ting-A-Kee R.; Maal-Bared G.; Chwalek M.; van der Kooy D. A. (2017) A single administration of the hallucinogen, 4-acetoxy-dimethyltryptamine, prevents the shift to a drug-dependent state and the expression of withdrawal aversions in rodents. Eur. J. Neurosci. 45, 1410–1417. 10.1111/ejn.13572. [DOI] [PubMed] [Google Scholar]
- Gatch M. B.; Rutledge M.; Carbonaro T.; Forster M. J. (2009) Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology 204, 715–724. 10.1007/s00213-009-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsley S.; Fiorella D.; Rabin R. A.; Winter J. C. (1998) A comparison of N,N-dimethyltryptamine, harmaline, and selected congeners in rats trained with LSD as a discriminative stimulus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 22, 649–663. 10.1016/S0278-5846(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Blair J. B.; Kurrasch-Orbaugh D.; Marona-Lewicka D.; Cumbay M. G.; Watts V. J.; Barker E. L.; Nichols D. E. (2000) Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J. Med. Chem. 43, 4701–4710. 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- Gatch M. B.; Forster M. J.; Janowsky A.; Eshleman A. J. (2011) Abuse liability profile of three substituted tryptamines. J. Pharmacol. Exp. Ther. 338, 280–289. 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro T. M.; Eshleman A. J.; Forster M. J.; Cheng K.; Rice K. C.; Gatch M. B. (2015) The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berl) 232 (1), 275–84. 10.1007/s00213-014-3658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Chatha M.; Klein A. K.; Wallach J.; Brandt S. D. (2020) Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933. 10.1016/j.neuropharm.2019.107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L. P.; Griffiths R. R. (2009) Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 105, S14–S25. 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton D. B.; Potter D. M.; Mead A. N. (2013) A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav. Pharmacol. 24, 10–36. 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Nichols D. E. (2016) Psychedelics. Pharmacol. Rev. 68, 264–355. 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen M. K.; Fisher P. M.; Burmester D.; Dyssegaard A.; Stenbæk D. S.; Kristiansen S.; Johansen S. S.; Lehel S.; Linnet K.; Svarer C.; Erritzoe D.; Ozenne B.; Knudsen G. M. (2019) Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44, 1328–1334. 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman A. J.; Forster M. J.; Wolfrum K. M.; Johnson R. A.; Janowsky A.; Gatch M. B. (2014) Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: Mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology 231, 875–888. 10.1007/s00213-013-3303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]