Abstract
Psychedelics represent one of the most promising classes of experimental medicines for the treatment of neuropsychiatric disorders due to their ability to promote neural plasticity and produce both rapid and sustained therapeutic effects following a single administration. Conventional wisdom holds that peak mystical experiences induced by psychedelics are a critical component of their therapeutic mechanisms of action, though evidence supporting that claim is largely correlational. Here, I present data suggesting that the subjective effects induced by psychedelics may not be necessary to produce long-lasting changes in mood and behavior. Understanding the role of subjective effects in the therapeutic mechanisms of psychedelics will have important implications for both basic neuroscience and for increasing patient access to the next generation of medicines developed as a result of psychedelic research.
Keywords: psychedelic, psychoplastogen, neural plasticity, hallucinogen, neuropsychiatric disorder, post-traumatic stress disorder, substance use disorder, antidepressant, depression, 5-HT2A receptor
Neuropsychiatric diseases such as depression, post-traumatic stress disorder (PTSD), and addiction are among the greatest contributors to disability worldwide,2 but unfortunately, traditional treatments have proven largely ineffective with long therapeutic lag times and a significant portion of the patient population remaining treatment-resistant.3 The advent of psychoplastogens4—compounds with the abilities to rapidly rewire neural circuitry by engaging plasticity mechanisms—represents a new era in the development of neurotherapeutics focused on fixing neural circuits rather than rectifying chemical imbalances. Because psychoplastogens are quite effective at promoting the growth of cortical pyramidal neurons, they are particularly well suited to treat illnesses characterized by the atrophy of neurons in the prefrontal cortex (PFC) such as stress-related neuropsychiatric diseases.
Ketamine is one of the best known psychoplastogens, and the recent approval of esketamine (the S-enantiomer of ketamine) for treatment-resistant depression represents an important milestone for psychiatry given that ketamine’s mechanism of action is unlike anything previously found in psychiatry’s arsenal. By producing long-lasting changes in neuronal structure and function, psychoplastogens have the potential to produce sustained behavioral effects after a single administration.
Dissociative, deliriant, and hallucinogenic drugs have emerged as some of the most effective psychoplastogens.4 Among these mind-altering substances, psychedelics have distinguished themselves by their dramatic, long-lasting effects on cortical neuron structure and function5 and sustained (>6 months) antidepressant, anxiolytic, and antiaddictive effects in the clinic.6 A critical question for the field to address is whether or not the acute subjective effects of these drugs are necessary to produce long-lasting therapeutic responses. Not only will research in this area help us to better understand their psychological and neurobiological mechanisms of action, it has the potential to have real world consequences for how we might treat patients in the future.
While psilocybin treatment is demonstrating impressive clinical efficacy across a broad range of stress-related neuropsychiatric diseases,6 this treatment strategy is limited by the significant healthcare costs associated with it. Due to the powerful subjective effects of the drug, healthcare professionals must provide support before, during, and after treatment to prepare patients for the subjective effects of the drug, ensure that no harm comes to them during the altered state of consciousness, and help them integrate their experience. Psilocybin induces subjective effects that last for several hours, prompting the exploration of shorter-acting psychedelics to minimize the time patients must spend in the clinic.7
In addition to the substantial healthcare costs associated with using psychedelics as medicines, many patients may be reluctant to participate in psychedelic-assisted therapy given that there is a risk for short-term anxiety and/or psychological distress. Moreover, psychedelic-assisted therapy is contraindicated for those with a family history of psychotic disorders, which is problematic given the overlapping genetics and comorbidity of neuropsychiatric diseases. Patient throughput is an important consideration given the prevalence of mental illness, with some estimates suggesting that roughly 20% of the population suffers from a neuropsychiatric disease at any given time.3 If the subjective effects of psychedelics are not necessary for their long-lasting antidepressant, anxiolytic, and antiaddictive properties, new medicines and/or treatment approaches could potentially be devised to greatly improve patient access to therapies inspired by psychedelic research.
Clinical studies on psychedelics have been challenged by the unique ability of these drugs to induce profound altered states of consciousness, making the design of truly double-blind, placebo-controlled studies exceedingly difficult. Though modern clinical studies on psychedelics have made the admirable attempt to mitigate this issue and reduce expectations by incorporating “active placebos” (e.g., niacin or a low-dose of a psychedelic) into their designs,8,9 a large proportion of both patients and clinicians are still able to correctly distinguish between a high dose of a psychedelic drug and an active placebo. Thus, it is still unclear what role placebo effects play in the overall efficacy of psychedelic-assisted therapy.
Patients who are treated with psychedelic-assisted therapy often attribute the amelioration of their symptoms to a psychological breakthrough achieved during a psychedelic-induced altered state of consciousness. This is not surprising given that many people rate psychedelic experiences as being among the most profound and meaningful events in their lives that can lead to long-lasting changes in their worldviews.10 Research has consistently shown that the intensity of “mystical” or “peak” experiences induced by psychedelics positively correlates with therapeutic responses.8,9,11−13 Similar results have been reported for ketamine.14−16
While studies associating mystical experiences with improved patient outcomes are quite intriguing, it is important to remember that correlation does not imply causation, and thus, we cannot definitively say that the subjective effects induced by psychedelics cause therapeutic responses. These experiences might contribute to therapeutic responses, but they may not be necessary to produce antidepressant, anxiolytic, and antiaddictive effects. In fact, many patients treated with psychedelics who do not have full mystical experiences still find psychedelic treatment to be beneficial, while others who do have full mystical experiences do not necessarily experience substantial reduction in disease symptoms.12,17
Perhaps one of the more compelling arguments supporting the notion that “mystical” or “peak” experiences play a critical role in psychedelic-induced therapeutic responses is related to the fact that mystical-type effects better correlate with antidepressant, anxiolytic, and antiaddictive outcomes than do the intensities of general subjective drug effects.12,13 However, mystical-type effects, such as spiritual experiences, feelings of unity, and disembodiment, might simply be better indicators of 5-HT2A receptor activation than are other subjective drug effects (e.g., perceptual changes, complex imagery, synesthesia, etc.). Analogously, psilocybin increases blood pressure (but not heart rate),9 an effect likely mediated by 5-HT2A receptors expressed in vascular smooth muscle cells. While it is attractive to speculate that mystical experiences are directly linked to therapeutic effects, we must also consider that mystical experiences, like increases in blood pressure, could simply be a good biomarker for 5-HT2A receptor activation. As 5-HT2A receptors have been shown to mediate both the hallucinogenic18 and psychoplastogenic5 effects of psychedelics, positive correlations between clinical efficacy and subjective effects should be viewed as expected dose–response relationships. Further experimentation is needed to claim any causal relationship between mystical experiences or enhanced neural plasticity and therapeutic outcomes.
Despite defying conventional wisdom, increasing evidence suggests that the therapeutic properties of psychedelics and related psychoplastogens can be dissociated from their subjective effects. Thus, if enhanced neural plasticity in key circuits is driving psychedelic-induced changes in behavior, peak mystical experiences may not be necessary for these drugs to treat mental illness. It is interesting to note that while the subjective effects of ketamine treatment subside within a few hours, the antidepressant response continues to intensify for several days.19 The time course of ketamine’s antidepressant effect is consistent with our understanding of how ketamine and serotonergic psychedelics alter neuronal structure over time. An exceedingly short stimulation period (<1 h) is sufficient for psychoplastogens to activate cortical neuron growth mechanisms that can last for several days.20 Furthermore, the Liston group recently used a photoactivatable Rac1 to demonstrate the causal relationship between ketamine-induced spine growth in the PFC and the drug’s long-lasting antidepressant-like behavioral effects in rodents.21
As evidence continues to mount implicating structural plasticity in the therapeutic properties of ketamine and psychedelics, additional pieces of data indicate that the subjective effects of these drugs may not be necessary to produce long-lasting therapeutic effects. First, clinical evidence suggests that a racemic mixture of ketamine enantiomers may be more effective than the S-enantiomer, though head-to-head clinical trials are lacking. Moreover, the R-enantiomer of ketamine is a more potent psychoplastogen than the S-enantiomer, producing longer-lasting antidepressant-like effects in preclinical animal studies22 despite having lower affinity for the NMDA receptor and reduced psychotomimetic effects.23 Furthermore, (2R,6R)-hydroxynorketamine—a metabolite of R-ketamine lacking dissociative properties—has demonstrated robust antidepressant-like effects in rodents.24 Future clinical trials using R-ketamine and (2R,6R)-hydroxynorketamine will help to shed light on the role of ketamine’s subjective effects in its antidepressant properties.
Like R-ketamine, other psychoplastogens that do not produce psilocybin-like mystical effects have also proven effective treatments for mental illness. Perhaps the most well-known is 3,4-methylenedioxymethamphetamine (MDMA)—an atypical psychedelic of the entactogen family.25 Only 20% of recreational MDMA users report experiencing any visual hallucinations, and these are often extremely mild compared to the perceptual effects induced by compounds like psilocybin or lysergic acid diethylamide (LSD).25 With the exception of its ability to induce a “blissful state,” MDMA does not produce subjective effects rivaling those of ketamine or classic serotonergic psychedelics (e.g., psilocybin and LSD) as measured using a variety of scales related to altered states of consciousness and mystical-type experiences.26,27 Despite its relatively mild subjective effects compared to classic psychedelics, MDMA potently promotes neural plasticity5 and has shown enormous potential in the clinic for treating stress-related neuropsychiatric diseases such as PTSD.25
Administration of low, subhallucinogenic doses of psychedelics also has the potential to shed light on the role of mystical-type experiences in therapeutic responses. Though anecdotal reports suggest that psychedelic microdosing—the chronic, intermittent use of subhallucinogenic doses—may produce beneficial effects and relieve symptoms of depression and anxiety,28,29 these results should be interpreted with caution given that there are currently no double-blind, placebo-controlled clinical trials related to the therapeutic effects of psychedelic microdosing.30 Our group recently demonstrated that psychedelic microdosing produced antidepressant-like and anxiolytic effects in rodents with minimal to no impact on other behavioral measures,31 but these results will need to be confirmed in humans. Unlike with clinical trials using high doses of psychedelics, it should be possible to effectively blind microdosing studies. However, the low doses required to avoid significant subjective effects may simply be insufficient to activate 5-HT2A receptors to produce long-lasting changes in neural circuitry.
Even if psychedelic microdosing ultimately proves to be efficacious, significant regulatory hurdles and issues related to abuse potential will need to be overcome if patients are to benefit from this dosing regimen. The development of nonhallucinogenic compounds capable of producing psychedelic-like therapeutic effects would solve these issues and greatly improve patient access. Recently, our group has made significant progress in this area. For example, simple transposition of the N,N-dimethylaminoethyl group of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) from the C3 to the N1 position of the indole yields 6-MeO-isoDMT. This compound exhibits significantly reduced hallucinogenic potential, as measured by the mouse head-twitch response (HTR) assay, while retaining psychoplastogenic potency comparable to its hallucinogenic congener (Figure 1).32 Because 6-MeO-isoDMT is at least equipotent to 5-MeO-DMT with respect to its ability to promote neural plasticity, it cannot simply be viewed as a less potent hallucinogen. In fact, many of the nonhallucinogenic analogues of psychedelics that our group has developed will not produce hallucinogenic behavioral responses in rodents even at extremely high doses.
Figure 1.
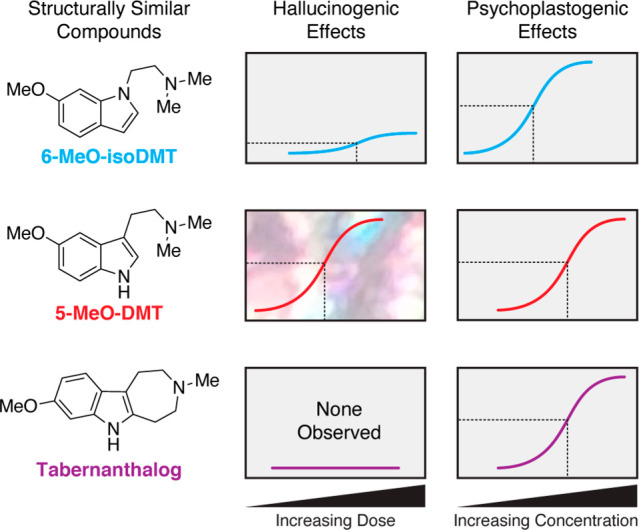
Hallucinogenic and psychoplastogenic effects can be decoupled through careful chemical design. Approximate potencies and efficacies are shown for mouse head-twitch response behavioral tests (hallucinogenic effects) and cortical neuron dendritogenesis assays (psychoplastogenic effects).
Until recently, it was unknown if nonhallucinogenic psychoplastogens could produce beneficial behavioral effects comparable to psychedelics. Through careful chemical design, we were able to engineer tabernanthalog (TBG)—a nonhallucinogenic analogue of 5-MeO-DMT (Figure 1) that promotes cortical neuron structural plasticity through activation of 5-HT2A receptors.33 Like psychedelic compounds, TBG has demonstrated preclinical therapeutic effects suggesting that it might be effective at treating a range of neuropsychiatric diseases including depression, alcohol use disorder, and heroin use disorder.33 Future work needs to address why functionally selective 5-HT2A receptor ligands such as TBG can produce plasticity and therapeutic behavioral responses without inducing behavioral effects characteristic of classic psychedelics.
Ultimately, clinical trials will be necessary to determine if psychoplastogenic analogues of psychedelics can produce therapeutic effects in humans without inducing mystical-like experiences. Additionally, there are several other experiments that could potentially be performed to elucidate the roles of both subjective effects and enhanced neural plasticity in the therapeutic properties of psychedelics. One option is to employ a compound producing mystical-like effects without promoting neuronal growth in the PFC as a true active placebo. However, no such compound has been identified yet, and such a compound might not even exist if hallucinogenic effects inevitably lead to enhanced neural plasticity.
Alternatively, psychedelics could be administered to patients under anesthesia. This would solve the blinding issue by preventing patients from experiencing the altered state of consciousness. Such an experimental design could be a powerful way to dissociate the psychological from the neurobiological effects of these drugs. However, care must be taken in the design of such studies, as several common anesthetics are known to promote neural plasticity and produce antidepressant effects themselves.
To the best of my knowledge, no clinical trial has administered a classic serotonergic psychedelic after the induction of general anesthesia. However, several studies have demonstrated that intraoperative ketamine can improve postoperative mood despite the fact that patients were unconscious during ketamine administration.34−36 While this suggests that ketamine-induced mystical experiences may not be necessary to produce therapeutic responses, the patients in these studies were not severely depressed. Thus, studies administering ketamine under general anesthesia to patients with major depressive disorder are warranted, and at least one such study is currently ongoing.37
While preliminary evidence suggests that the subjective effects of psychedelics are not necessary to produce therapeutic responses, they may be critical for achieving maximal efficacy. Though the strong positive correlation between mystical-type experiences and the strength of therapeutic responses does not imply causation, it does implicate either psychological mechanisms or perhaps an exceptionally strong placebo effect in the impressive effect sizes observed following psychedelic-assisted therapy. Thus, the combination of a pharmacologically induced state of heightened neural plasticity with a profound subjective experience could prove invaluable for treating those who are especially ill or who have attempted other treatments without success.
Despite the promising therapeutic responses produced by psychedelic-assisted therapy, the intense subjective effects of these drugs make it unlikely that they will ever become widespread treatments for disorders such as depression. In contrast, strategies for rewiring pathological neural circuitry without producing psychedelic-like mystical effects hold enormous promise as potential first-line treatments for a variety of neuropsychiatric diseases. Regardless, nonhallucinogenic analogues of psychedelics will provide a wealth of information about the fundamental neurobiology underlying both compound-induced neural plasticity and hallucinogenic effects. For an alternative perspective, please see a companion Viewpoint in this issue.1
Acknowledgments
This work was supported by funds from the National Institutes of Health (NIH) (R01GM128997 to D.E.O.). The author would like to thank Boris Heifets as well as Max Vargas, Lindsay Cameron, and the rest of the Olson Lab for helpful discussions. Special thanks to Lindsay Cameron and Lee Dunlap for providing the image of DMT crystals used in the graphical abstract.
Author Contributions
D.E.O. wrote the manuscript.
The author declares the following competing financial interest(s): DEO is the president and chief scientific officer of Delix Therapeutics, Inc.
References
- Whiteford H. A.; Degenhardt L.; Rehm J.; Baxter A. J.; Ferrari A. J.; Erskine H. E.; Charlson F. J.; Norman R. E.; Flaxman A. D.; Johns N.; et al. (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Berton O.; Nestler E. J. (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 7, 137–151. 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Olson D. E. (2018) Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 12, 117906951880050 10.1177/1179069518800508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C.; Greb A. C.; Cameron L. P.; Wong J. M.; Barragan E. V.; Wilson P. C.; Burbach K. F.; Soltanzadeh Zarandi S.; Sood A.; Paddy M. R.; Duim W. C.; Dennis M. Y.; McAllister A. K.; Ori-McKenney K. M.; Gray J. A.; Olson D. E. (2018) Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 23, 3170–3182. 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E.; Johnson M. W.; Nichols C. D. (2017) Psychedelics as Medicines: An Emerging New Paradigm. Clin. Pharmacol. Ther. 101, 209–219. 10.1002/cpt.557. [DOI] [PubMed] [Google Scholar]
- Nutt D.; Erritzoe D.; Carhart-Harris R. (2020) Psychedelic Psychiatry’s Brave New World. Cell 181, 24–28. 10.1016/j.cell.2020.03.020. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Carducci M. A.; Umbricht A.; Richards W. A.; Richards B. D.; Cosimano M. P.; Klinedinst M. A. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 30, 1181–1197. 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.; Bossis A.; Guss J.; Agin-Liebes G.; Malone T.; Cohen B.; Mennenga S. E.; Belser A.; Kalliontzi K.; Babb J.; Su Z.; Corby P.; Schmidt B. L. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol. 30, 1165–1180. 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. K.; Clifton J. M.; Weaver E. G.; Hurwitz E. S.; Johnson M. W.; Griffiths R. R. (2020) Survey of entity encounter experiences occasioned by inhaled N,N-dimethyltryptamine: Phenomenology, interpretation, and enduring effects. J. Psychopharmacol. 34, 1008–1020. 10.1177/0269881120916143. [DOI] [PubMed] [Google Scholar]
- Bogenschutz M. P.; Forcehimes A. A.; Pommy J. A.; Wilcox C. E.; Barbosa P. C.; Strassman R. J. (2015) Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol. 29, 289–299. 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A.; Griffiths R. R.; Johnson M. W. (2015) Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr. Drug Abuse Rev. 7, 157–164. 10.2174/1874473708666150107121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L.; Nutt D. J.; Carhart-Harris R. L. (2018) Quality of Acute Psychedelic Experience Predicts Therapeutic Efficacy of Psilocybin for Treatment-Resistant Depression. Front. Pharmacol. 8, 974. 10.3389/fphar.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sos P.; Klirova M.; Novak T.; Kohutova B.; Horacek J.; Palenicek T. (2013) Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro. Endocrinol. Lett. 34, 287–293. [PubMed] [Google Scholar]
- Luckenbaugh D. A.; Niciu M. J.; Ionescu D. F.; Nolan N. M.; Richards E. M.; Brutsche N. E.; Guevara S.; Zarate C. A. (2014) Do the dissociative side effects of ketamine mediate its antidepressant effects?. J. Affective Disord. 159, 56–61. 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakwar E.; Anerella C.; Hart C. L.; Levin F. R.; Mathew S. J.; Nunes E. V. (2014) Therapeutic infusions of ketamine: do the psychoactive effects matter?. Drug Alcohol Depend. 136, 153–157. 10.1016/j.drugalcdep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson E. M.; May D. G.; Forcehimes A. A.; Bogenschutz M. P. (2018) The Psychedelic Debriefing in Alcohol Dependence Treatment: Illustrating Key Change Phenomena through Qualitative Content Analysis of Clinical Sessions. Front. Pharmacol. 9, 132. 10.3389/fphar.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F. X.; Vollenweider-Scherpenhuyzen M. F.; Babler A.; Vogel H.; Hell D. (1998) Psilocybin induces schizophrenia- like psychosis in humans via a serotonin-2 agonist action. NeuroReport 9, 3897–3902. 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Berman R. M.; Cappiello A.; Anand A.; Oren D. A.; Heninger G. R.; Charney D. S.; Krystal J. H. (2000) Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354. 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Ly C., Greb C. A., Vargas M. V., Duim W. C., Grodzki A. C. G., Lein P. J., and Olson D. E. (2020) Transient Stimulation with Psychoplastogens is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci., 10.1021/acsptsci.0c00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moda-Sava R. N.; Murdock M. H.; Parekh P. K.; Fetcho R. N.; Huang B. S.; Huynh T. N.; Witztum J.; Shaver D. C.; Rosenthal D. L.; Always E. J.; Lopez K.; Meng Y.; Nellissen L.; Grosenick L.; Milner T. A.; Deisseroth K.; Bito H.; Kasai H.; Liston C. (2019) Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364, eaat8078 10.1126/science.aat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C; Shirayama Y; Zhang J-c; Ren Q; Yao W; Ma M; Dong C; Hashimoto K (2015) a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry 5, e632 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F. X.; Leenders K. L.; Oye I.; Hell D.; Angst J. (1997) Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur. Neuropsychopharmacol. 7, 25–38. 10.1016/S0924-977X(96)00042-9. [DOI] [PubMed] [Google Scholar]
- Zanos P.; Moaddel R.; Morris P. J.; Georgiou P.; Fischell J.; Elmer G. E.; Alkondon M.; Yuan P.; Pribut H. J.; Singh N. S.; Dossou K. S. S.; Fang Y.; Huang X.-P.; Mayo C. L.; Wainer I. W.; Albuquerque E. X.; Thompson S. M.; Thomas C. J.; Zarate C. A. Jr; Gould T. D. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap L. E.; Andrews A. A.; Olson D. E. (2018) Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine. ACS Chem. Neurosci. 9, 2408–2427. 10.1021/acschemneuro.8b00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E.; Gamma A.; Vollenweider F. X. (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5, e12412 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holze F.; Vizeli P.; Müller F.; Ley L.; Duerig R.; Varghese N.; Eckert A.; Borgwardt S.; Liechti M. E. (2020) Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology 45, 462–471. 10.1038/s41386-019-0569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T.; Petranker R.; Rosenbaum D.; Weissman C. R.; Dinh-Williams L.-A.; Hui K.; Hapke E.; Farb N. A. S. (2019) Microdosing psychedelics: personality, mental health, and creativity differences in microdosers. Psychopharmacology (Berl) 236, 731–740. 10.1007/s00213-018-5106-2. [DOI] [PubMed] [Google Scholar]
- Cameron L. P.; Nazarian A.; Olson D. E. (2020) Psychedelic Microdosing: Prevalence and Subjective Effects. J. Psychoact. Drugs 52, 113–122. 10.1080/02791072.2020.1718250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers K. P.; Ng L.; Erritzoe D.; Knudsen G. M.; Nichols C. D.; Nichols D. E.; Pani L.; Soula A.; Nutt D. (2019) Microdosing psychedelics: More questions than answers? An overview and suggestions for future research. J. Psychopharmacol. 33, 1039–1057. 10.1177/0269881119857204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L. P.; Benson C. J.; DeFelice B. C.; Fiehn O.; Olson D. E. (2019) Chronic, Intermittent Microdoses of the Psychedelic N,N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem. Neurosci. 10, 3261–3270. 10.1021/acschemneuro.8b00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap L. E.; Azinfar A.; Ly C.; Cameron L. P.; Viswanathan J.; Tombari R. J.; Myers-Turnbull D.; Taylor J. C.; Grodzki A. C.; Lein P. J.; Kokel D.; Olson D. E. (2020) Identification of Psychoplastogenic N,N-Dimethylaminoisotryptamine (isoDMT) Analogs Through Structure-Activity Relationship Studies. J. Med. Chem. 63, 1142–1155. 10.1021/acs.jmedchem.9b01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L. P.; Tombari R. J.; Lu J.; Pell A. J.; Hurley Z. Q.; Ehinger Y.; Vargas M. V.; McCarroll M. N.; Taylor J. C.; Myers-Turnbull D.; Liu T.; Yaghoobi B.; Laskowski L. J.; Anderson E. I.; Zhang G.; Viswanathan J.; Brown B. M.; Tjia M.; Dunlap L. E.; Rabow Z. T.; Fiehn O.; Wulff H.; McCorvy J. D.; Lein P. J.; Kokel D.; Ron D.; Peters J.; Zuo Y.; Olson D. E. (2020) A Non-Hallucinogenic Psychedelic Analog with Therapeutic Potential. Nature 10.1038/s41586-020-3008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh A.; Takahira Y.; Katagai H.; Takazawa T. (2002) Small-dose ketamine improves the postoperative state of depressed patients. Anesth. Analg. 95, 114–118. 10.1097/00000539-200207000-00020. [DOI] [PubMed] [Google Scholar]
- Jiang M.; Wang M.-H.; Wang X.-B.; Liu L.; Wu J.-L.; Yang X.-L.; Liu X.-R.; Zhang C.-X. (2016) Effect of intraoperative application of ketamine on postoperative depressed mood in patients undergoing elective orthopedic surgery. J. Anesth. 30, 232–237. 10.1007/s00540-015-2096-7. [DOI] [PubMed] [Google Scholar]
- Xu R.; Zhan Y.; Chen S. (2017) Effect of intraoperative single administration of sub-anesthesia ketamine on breast cancer patients with depression. Biomedical Res. S552–S556. [Google Scholar]
- Intraoperative Ketamine Versus Saline in Depressed Patients Undergoing Anesthesia for Non-cardiac Surgery. September 25, 2019; https://clinicaltrials.gov/ct2/show/NCT03861988 (accessed 2020).
- Yaden D. B., and Griffiths R. R. (2020) The Subjective Effects of Psychedelics Are Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci.. 10.1021/acsptsci.0c00194 [DOI] [PMC free article] [PubMed] [Google Scholar]


